A Facile Method for the Fabrication of Luminescent Eu3+-Doped SiO2 Nanowires
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Preparation
2.1.1. Materials
2.1.2. Preparation of Eu3+-Doped Silica Nanowires
2.2. Characterization
3. Results and Discussion
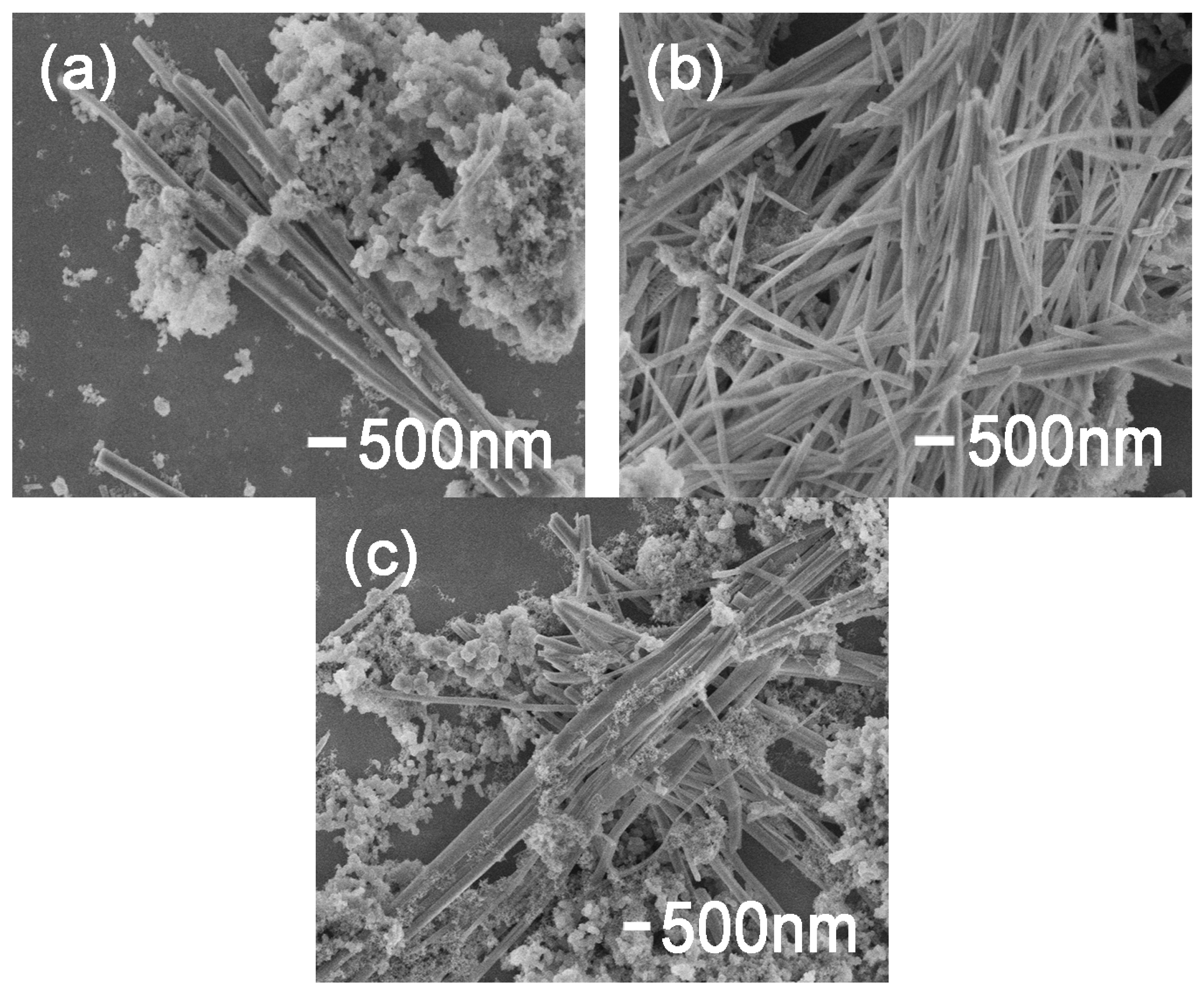
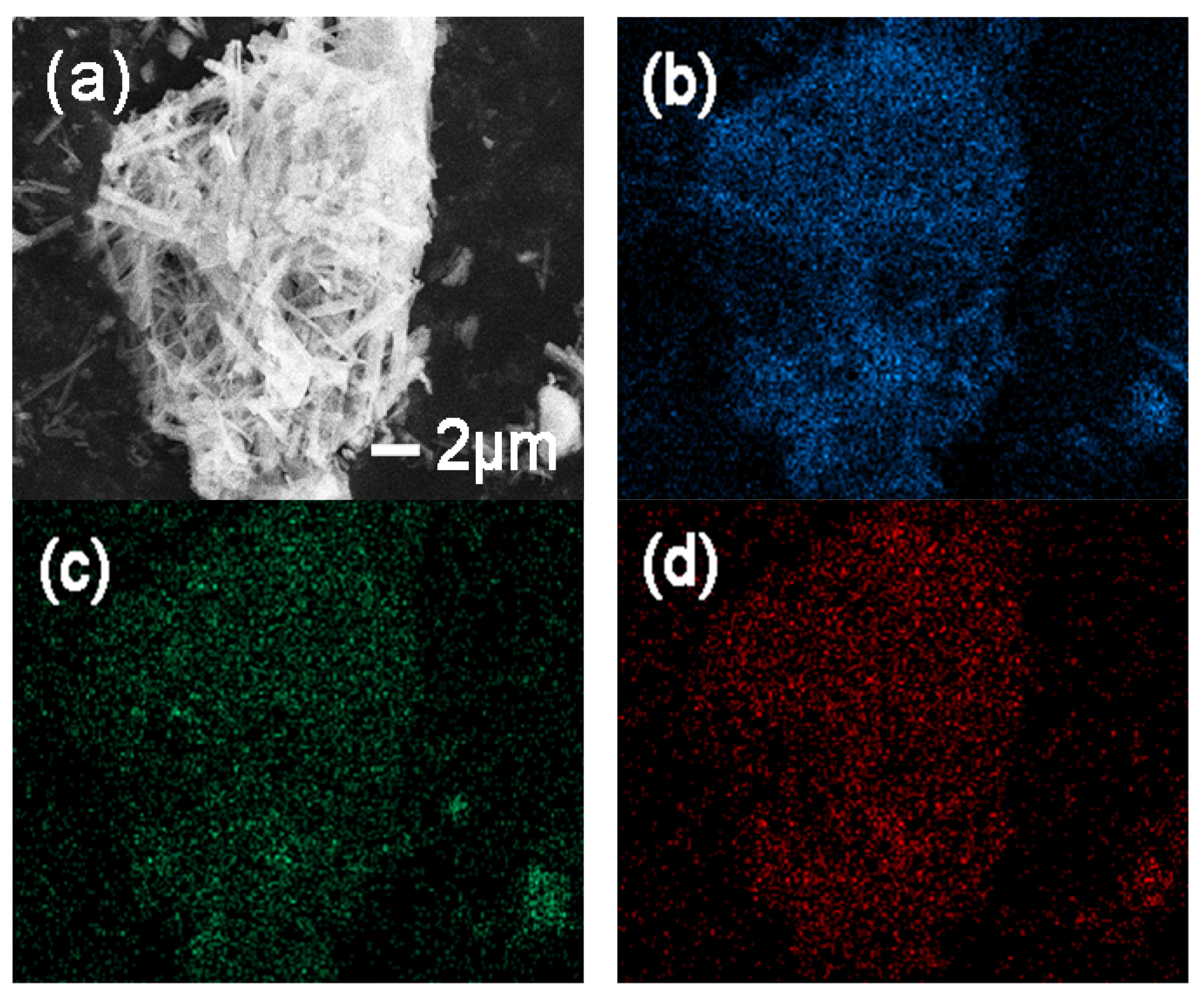
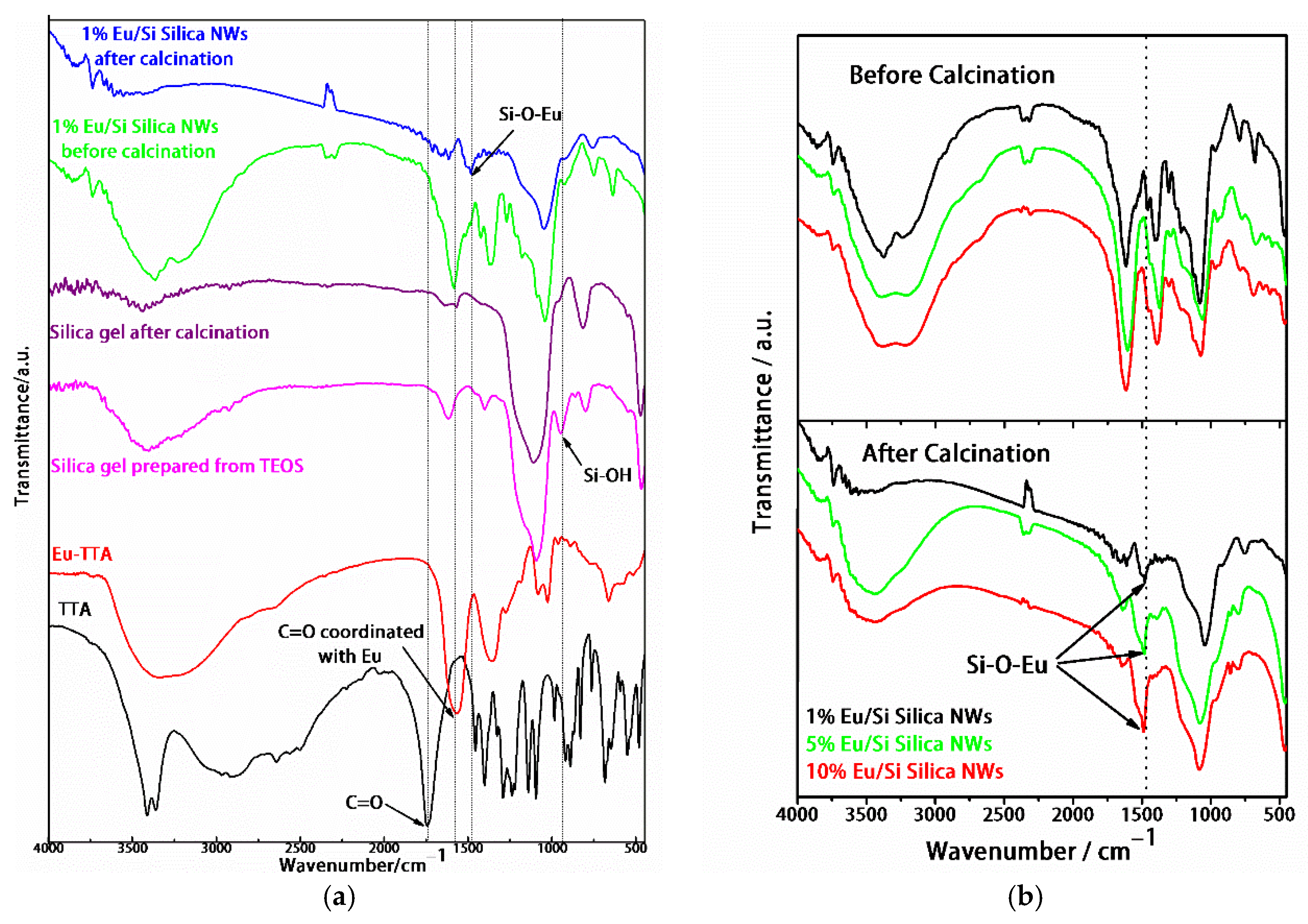
3.1. Characterization of Samples
3.2. Factors Influencing the Reaction
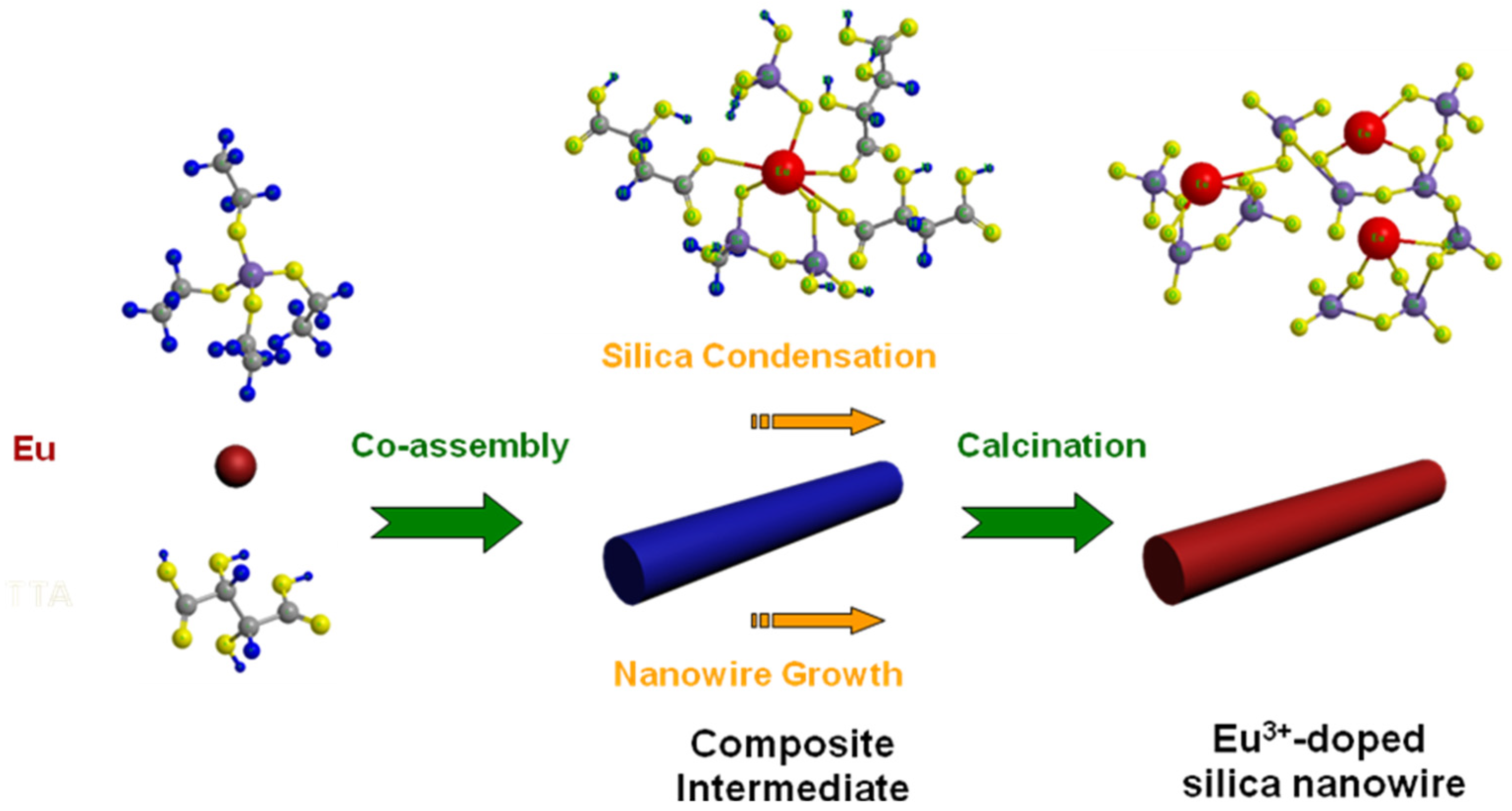
3.3. Formation Mechanism
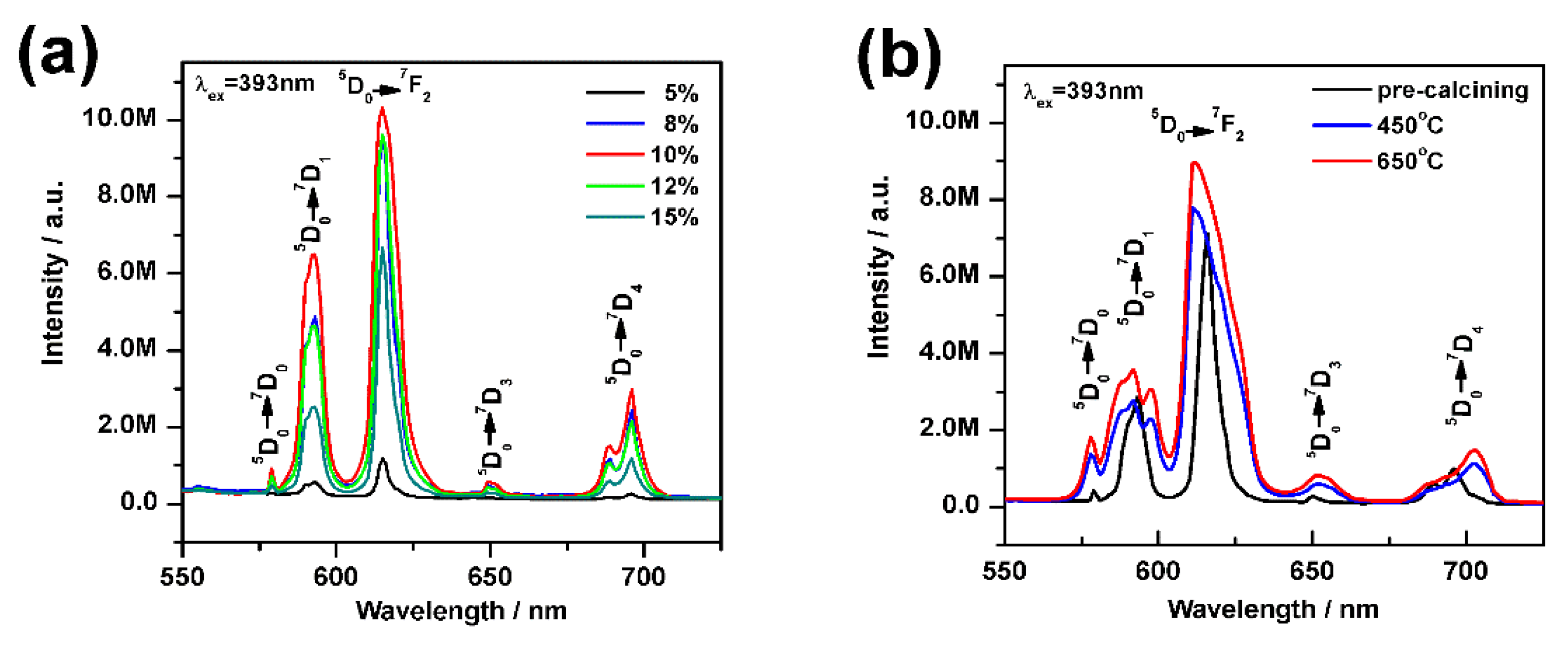
3.4. Photoluminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, J.H.; Yoshida, K.; Shimizu, T. Creation of novel double-helical silica nanotubes using binary gel system. Langmuir 2002, 18, 8724–8727. [Google Scholar] [CrossRef]
- Kyoung, G.; Lee, K.G.; Wi, R.; Imran, M.; Park, T.J.; Lee, J.; Lee, S.Y.; Kim, D.H. Functionalization Effects of SingleWalled Carbon Nanotubes as Templates for the Synthesis of Silica Nanorods and Study of Growing Mechanism of Silica. ACS Nano 2010, 4, 3933–3942. [Google Scholar]
- Jin, Y.; Lohstreter, S.; Pierce, D.T.; Parisien, J.; Wu, M.; Hall, C., III; Zhao, J.X. Silica Nanoparticles with Continuously Tunable Sizes: Synthesis and Size Effects on Cellular Contrast Imaging. Chem. Mater. 2008, 20, 4411–4419. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, T.; Yuan, X.Y.; Geng, B.Y.; Wu, G.S.; Wang, G.Z.; Meng, J.W.; Zhang, L.D. A simple route to large scale synthesis of crystalline αSi3N4 nanowires. Appl. Phys. A Mater. Sci. Process. 2005, 81, 477–479. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Zhang, J.; Gao, J.; Ding, X.; Huang, Z.; Tang, C.; Hu, L.; Chen, D. Characterization and Photoluminescence Properties of Tb-Doped SiO2 Nanowires as a Novel Green-Emitting Phosphor. Chem. Mater. 2007, 19, 2585–2588. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Javni, I.; Waddon, A.; Bánhegyi, G. Structure and properties of polyurethane–silica nanocomposites. Appl. Polym. Sci. 2000, 76, 133–150. [Google Scholar] [CrossRef]
- Gao, X.; Yu, K.M.K.; Tam, K.Y.; Tsang, S.C. Colloidal stable silica encapsulated nano-magnetic composite as a novel bio-catalyst carrier. Chem. Commun. 2003, 24, 2998–2999. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.C.; Yip, H.Y.; Wu, L.; Wong, P.K.; Lai, S.Y. A mesoporous Pt/TiO2 nanoarchitecture with catalytic and photocatalytic functions. Chem. A Eur. J. 2005, 11, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Song, W.H.; Wang, K.Y.; Hu, T.; Zhao, B.; Sun, Y.P.; Du, J.J. Preparation and photoluminescence properties of amorphous silica nanowires. Chem. Phys. Lett. 2001, 336, 53–56. [Google Scholar] [CrossRef]
- Ruffino, F.; Censabella, M.; Torrisi, V.; Grimaldi, M.G. Size-selected growth of ultrathin SiO2 nanowires on surface and their decoration by Au nanoparticles. Mater. Res. Express 2015, 2, 25003. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Meng, X.; Zhou, S.; Wu, S.; Shi, W.; Lee, S. Bulk Preparation of Si–SiOx Hierarchical Structures: High-Density Radially Oriented Amorphous Silica Nanowires on a Single-Crystal Silicon Nanocore. Angew. Chem. Int. Ed. 2005, 44, 6934–6937. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Sun, X.W.; Yu, M.B.; Xiong, Y.Z.; Dong, Z.L.; Chen, J.S. Magnetic nanobraids of iron-doped amorphous silica. Appl. Phys. Lett. 2004, 85, 5364–5366. [Google Scholar] [CrossRef]
- Yang, Y.; Tay, B.; Sun, X.; Fan, H.; Shen, Z. Photoluminescence and growth mechanism of amorphous silica nanowires by vapor phase transport. Phys. E Low-Dimens. Syst. Nanostructures 2006, 31, 218–223. [Google Scholar] [CrossRef]
- Huo, Q.; Zhao, D.; Feng, J.; Weston, K.; Buratto, S.K.; Stucky, G.D.; Schacht, S.; Schüth, F. Room temperature growth of mesoporous silica fibers: A new high-surface-area optical waveguide. Adv. Mater. 1997, 9, 974–978. [Google Scholar] [CrossRef]
- Yu, D.P.; Hang, Q.L.; Ding, Y.; Zhang, H.Z.; Bai, Z.G.; Wang, J.J.; Zou, Y.H.; Qian, W.; Xiong, G.C.; Feng, S.Q. Amorphous silica nanowires: Intensive blue light emitters. Appl. Phys. Lett. 1998, 73, 3076–3078. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Sun, L.; Tang, D.; Zou, X.; Li, Y.; Wang, C.; Wang, G.; Xie, S. Growth of amorphous silicon nanowires. Chem. Phys. Lett. 2001, 341, 523–528. [Google Scholar] [CrossRef]
- Zhang, M.; Bando, Y.; Wada, K.; Kurashima, K. Synthesis of nanotubes and nanowires of silicon oxide. J. Mater. Sci. Lett. 1999, 18, 1911–1913. [Google Scholar] [CrossRef]
- Shao, C.; Kim, H.; Gong, J.; Lee, D. A novel method for making silica nanofibres by using electrospun fibres of polyvinylalcohol/silica composite as precursor. Nanotechnology 2002, 13, 635. [Google Scholar] [CrossRef]
- Glinka, Y.D.; Lin, S.H.; Chen, Y.T. The photoluminescence from hydrogen-related species in composites of SiO2 nanoparticles. Appl. Phys. Lett. 1999, 75, 778–780. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Zhang, H.; Wang, L.; Li, D.; Yang, D. Magnesium catalyzed growth of SiO2 hierarchical nanostructures by a thermal evaporation process. Nanotechnology 2008, 19, 165601. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Chen, H.; Jiao, Z.; Jin, Y.; He, Y.; Gong, M.; Sun, X. Growth of amorphous SiO2 nano-wires on pre-oxidized silicon substrate via chemical vapor deposition. J. Non-Cryst. Solids 2008, 354, 1731–1735. [Google Scholar] [CrossRef]
- Chen, L.; Lei, X.; Chen, G.; Zhong, R.; Lan, S.; Liang, X.; Lu, J. Synthesis, Characterization and Photophysical Properties of Phenanthroline Derivatives and Their Europium Complexes. J. South-Cent. Univ. Natl. (Nat. Sci. Ed.) 2015, 18, 1315–1322. [Google Scholar]
- Dos Santos, C.M.; Harte, A.J.; Quinn, S.J.; Gunnlaugsson, T. Recent developments in the field of supramolecular lanthanide luminescent sensors and self-assemblies. Coord. Chem. Rev. 2008, 252, 2512–2527. [Google Scholar] [CrossRef]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Forrest, S.R. Electroluminescence mechanisms in organic light emitting devices employing a europium chelate doped in a wide energy gap bipolar conducting host. J. Appl. Phys. 2000, 87, 8049–8055. [Google Scholar] [CrossRef]
- Miura, K.; Suzuki, T.; Hanaizumi, O. Photoluminescence Properties of Europium and Cerium Co-Doped Tantalum-Oxide Thin Films Prepared Using Co-Sputtering Method. J. Mater. Sci. Chem. Eng. 2015, 3, 30–34. [Google Scholar] [CrossRef][Green Version]
- Liu, X.-L.; Zhu, P.-X.; Gao, Y.-F.; Jin, R.-H. Synthesis of free-standing sub-10 nm Y2O3: Eu particles on silica nanowire matrix and amplified luminescence performance. J. Mater. Chem. C 2013, 1, 477–483. [Google Scholar] [CrossRef]
- Gao, F.; Sheng, Y.; Song, Y.; Zheng, K.; Lin, C.; Zhang, H.; Huo, Q.; Zou, H. Facile synthesis and luminescence properties of europium(III)-doped silica nanotubes. J. Sol-Gel Sci. Technol. 2014, 71, 313–323. [Google Scholar] [CrossRef]
- Shui, M.; Song, Y.; Ren, Y.; Wang, X. Structural analysis of amorphous silica prepared by water glass-based precursors and its thermal, spectral characterization. Phys. B Condens. Matter 2010, 405, 1316–1320. [Google Scholar] [CrossRef]
- Gao, F.; Song, Y.; Sheng, Y.; Lin, C.; Huo, Q.; Zou, H. Growth, structure and optical properties of tartaric acid-templated silica nanotubes by sol–gel method. J. Sol-Gel Sci. Technol. 2013, 68, 204–212. [Google Scholar] [CrossRef]
- Lin, C.; Song, Y.; Gao, F.; Zhang, H.; Sheng, Y.; Zheng, K.; Shi, Z.; Xu, X.; Zou, H. Synthesis and luminescence properties of Eu (III)-doped silica nanorods based on the sol–gel process. J. Sol-Gel Sci. Technol. 2014, 69, 536–543. [Google Scholar] [CrossRef]
- Firdous, A.; Quasim, I.; Ahmad, M.M.; Kotru, P.N. Studies on gel-grown pure and strontium-modified lanthanum tartrate crystals. J. Cryst. Growth 2009, 311, 3855–3862. [Google Scholar] [CrossRef]
- Joshi, S.J.; Parekh, B.B.; Vohra, K.D.; Joshi, M.J. Growth and characterization of gel grown pure and mixed iron-manganese levo-tartrate crystals. Bull. Mater. Sci. 2006, 29, 307–312. [Google Scholar] [CrossRef][Green Version]
- Haranath, D.; Gandhi, N.; Sahai, S.; Husain, M.; Shanker, V. Highly emissive and low refractive index layers from doped silica nanospheres for solar cell applications. Chem. Phys. Lett. 2010, 496, 100–103. [Google Scholar] [CrossRef]
- Hunag, W.H.; Shieh, J.M.; Lien, Y.C.; Jhou, K.J.; Tu, C.-H.; Wang, C.; Shen, C.-H.; Hsieh, W.-H.; Kuo, H.-C.; Pan, F.-M. Novel solar down-conversion luminescent and switchable interface polarization material by europium doped Si-O polar structures. In Proceedings of the International Conference on Quantum Electronics and Laser Science, San Jose, CA, USA, 6–11 May 2012. Paper JTh4J.5. [Google Scholar]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-ray photoelectron spectroscopy of rare-earth compounds. J. Electron Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Her, J.-L.; Lin, C.-W.; Chang, K.-Y.; Pan, T.-M. Label-Free Detection of Creatinine Using a Disposable Poly-N-Isopropylacrylamide as an Encapsulating Creatinine Deiminase Based Eu2Ti2O7 Electrolyte-Insulator-Semiconductors. Int. J. Electrochem. Sci. 2012, 7, 387–404. [Google Scholar]
- Li, H.; Liu, P.; Wang, Y.; Zhang, L.; Yu, J.; Zhang, H.; Liu, B.; Schubert, U. Preparation and luminescence properties of hybrid titania immobilized with lanthanide complexes. J. Phys. Chem. C 2009, 113, 3945–3949. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, H.; Lin, Y.; Yang, Z.; Cheng, X.; Huang, J. Photoluminescent Lanthanide-Doped Silica Nanotubes: Sol−Gel Transcription from Functional Template. J. Phys. Chem. C 2011, 115, 7323–7330. [Google Scholar] [CrossRef]
- Wan, N.; Xu, J.; Lin, T.; Zhang, X.; Xu, L. Energy transfer and enhanced luminescence in metal oxide nanoparticle and rare earth co-doped silica. Appl. Phys. Lett. 2008, 92, 201109. [Google Scholar] [CrossRef]
- Nassar, E.J.; Ciuffi, K.J.; Ribeiro, S.J.L.; Messaddeq, Y. Europium incorporated in silica matrix obtained by sol-gel: Luminescent materials. Mater. Res. 2003, 6, 557–562. [Google Scholar] [CrossRef]
- Berry, A.; King, T. Characterisation of doped sol-gel derived silica hosts for use in tunable glass lasers. J. Phys. D Appl. Phys. 1989, 22, 1419. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Zhao, X.; Liu, J. A Facile Method for the Fabrication of Luminescent Eu3+-Doped SiO2 Nanowires. Gels 2022, 8, 286. https://doi.org/10.3390/gels8050286
Gao F, Zhao X, Liu J. A Facile Method for the Fabrication of Luminescent Eu3+-Doped SiO2 Nanowires. Gels. 2022; 8(5):286. https://doi.org/10.3390/gels8050286
Chicago/Turabian StyleGao, Fei, Xinyu Zhao, and Jinglin Liu. 2022. "A Facile Method for the Fabrication of Luminescent Eu3+-Doped SiO2 Nanowires" Gels 8, no. 5: 286. https://doi.org/10.3390/gels8050286
APA StyleGao, F., Zhao, X., & Liu, J. (2022). A Facile Method for the Fabrication of Luminescent Eu3+-Doped SiO2 Nanowires. Gels, 8(5), 286. https://doi.org/10.3390/gels8050286



