Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Drying of Red Beetroot Peels
2.3. Preparation of Dried Red Beetroot Peels Aqueous Extract
2.4. Estimation of Total Phenol Content
2.5. Estimation of Total Flavonoids Content (TFC)
2.6. Phenols and Flavonoids Characterization
2.7. Estimation of Betalain Content
2.8. Estimation of Radical-Scavenging Activity by (DPPH IC50%)
2.9. Preparation of Fish Filets
2.10. Determination of TBA and pH
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Profile of DBRP
3.2. Identification and Quantification of Phenolic Compounds and Flavonoids in DBRP by HPLC
3.3. DPPH Radical-Scavenging Capacity of DBRP
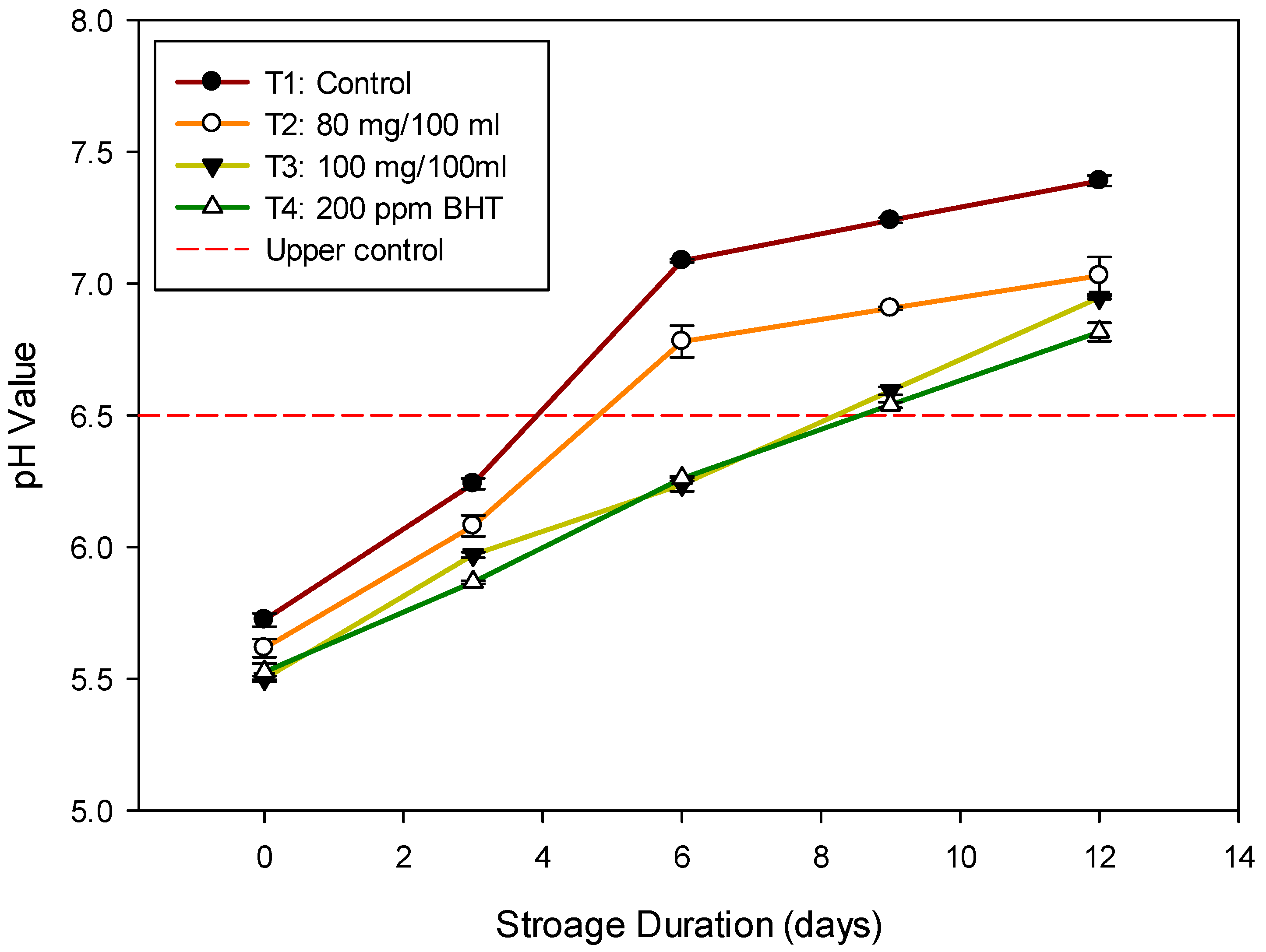
3.4. Change in pH Values of Nile Tilapia Fish Fillet
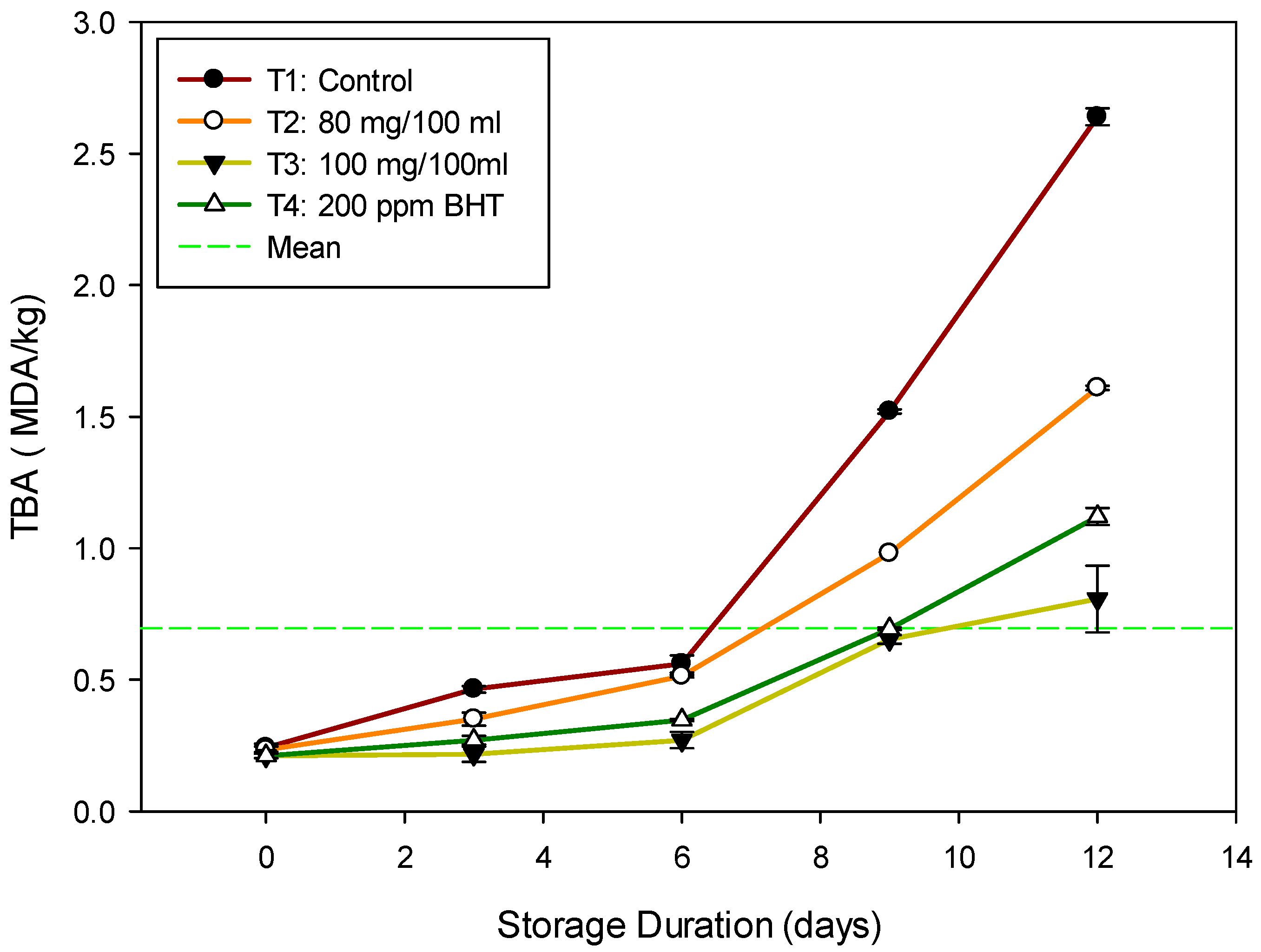
3.5. Changes in Thiobarbituric Acid (TBA) Value of Nile Tilapia Fish Fillet
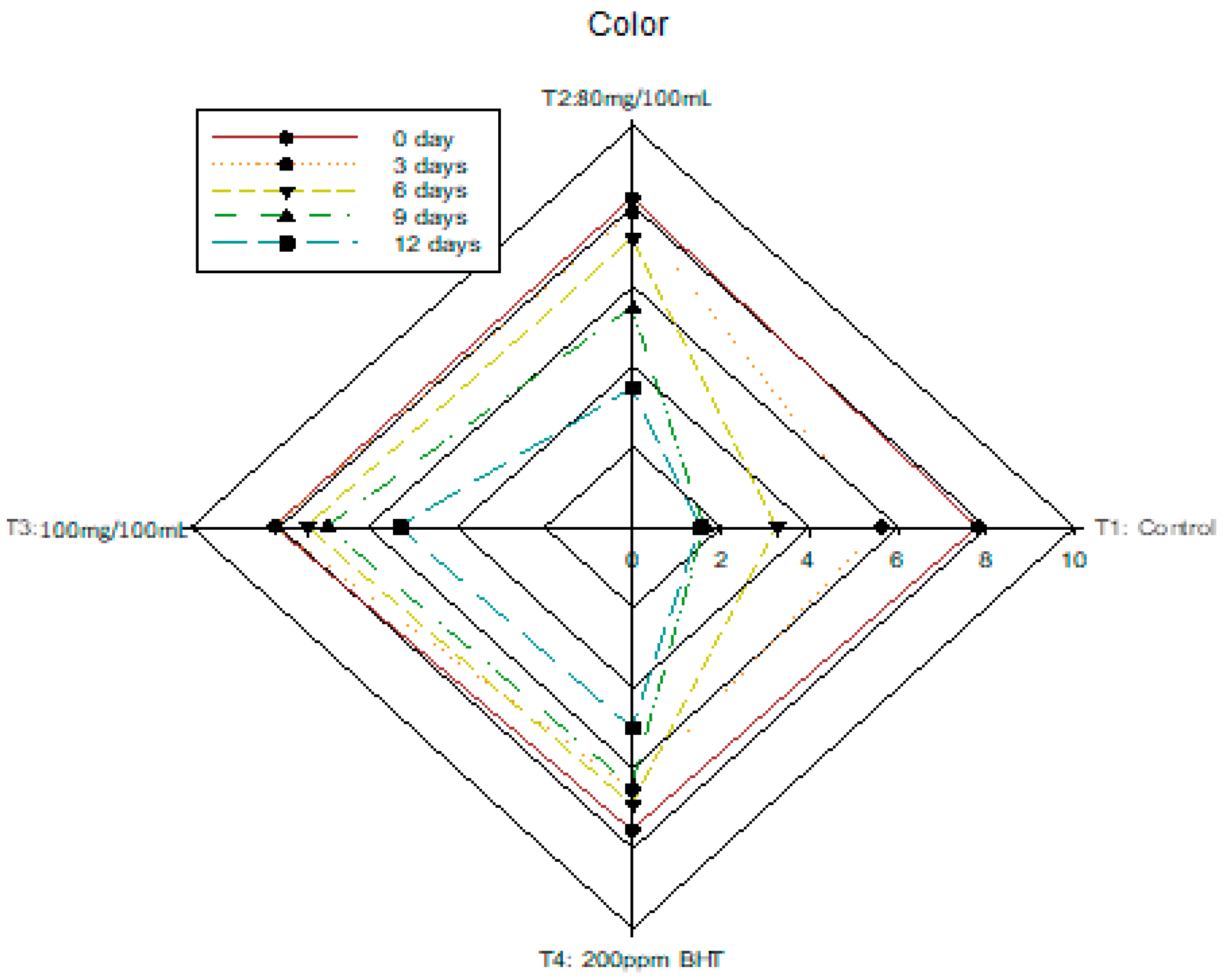
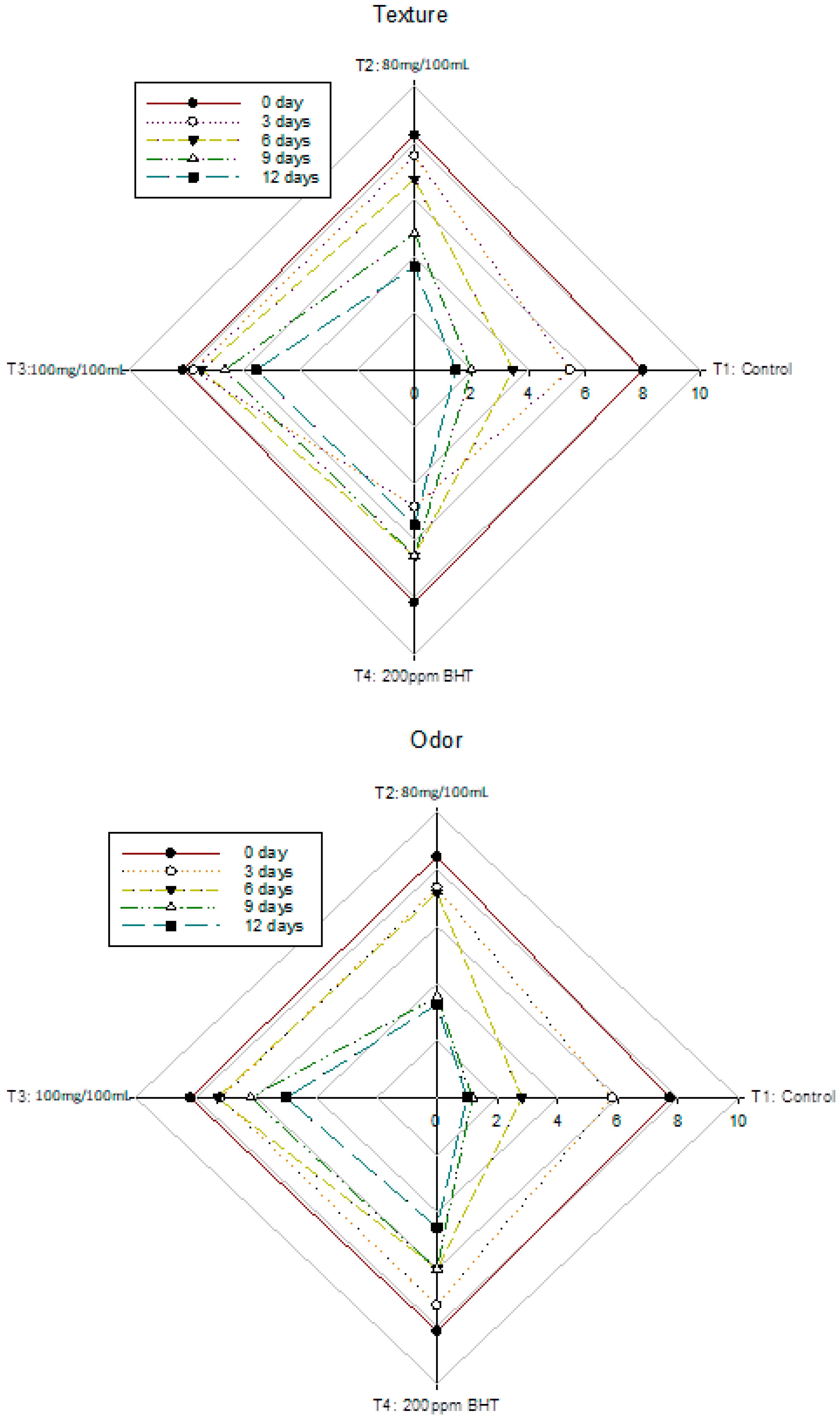
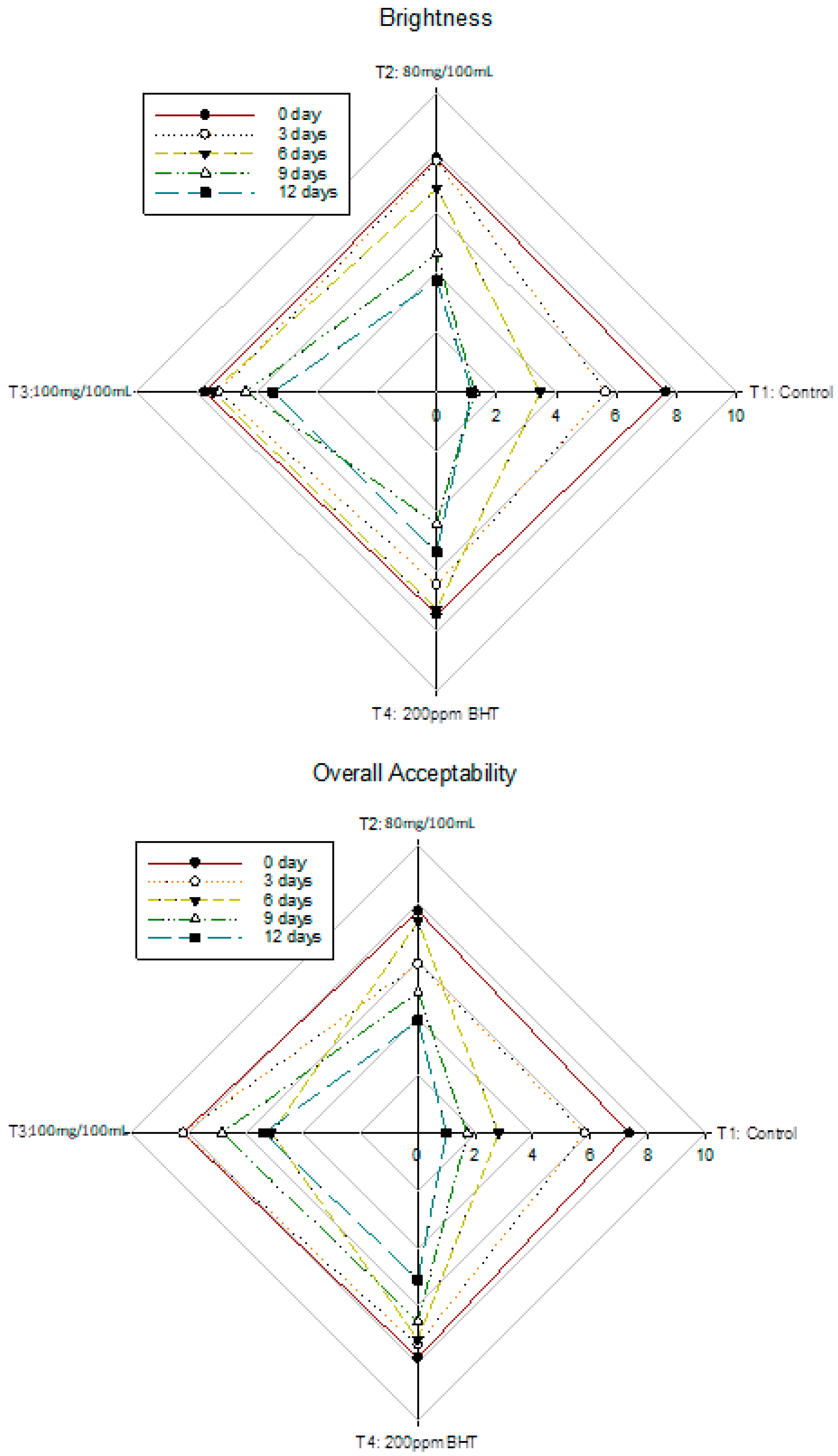
3.6. Changes in Sensory Analysis of Nile Tilapia Fish Fillet
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abouel-Yazeed, A.M. Maintaining quality and extending shelf-life of Tilapia Oreochromis niloticus fish during storage at 4 °C. J. Arab. Aquac. Soc. 2013, 8, 293–305. [Google Scholar]
- Badee, A.Z.M.; Moawad, R.K.; ElNoketi, M.M.; Gouda, M. Improving the Quality and Shelf-Life of Refrigerated Chicken Meat by Marjoram Essential Oil. J. Appl. Sci. Res. 2014, 9, 5718–5729. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.D.S.; Marsico, E.T.; Ribeiro, R.D.O.R.; Junior, C.A.C.; Alvares, T.S.; De Jesus, E.F.O. Quality attributes in shrimp treated with polyphosphate after thawing and cooking: A study using physicochemical analytical methods and low-field 1H NMR. J. Food Process Eng. 2013, 36, 492–499. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Li, H.Y.; Hao, Z.B.; Wang, X.L.; Huang, L.; Li, J.P. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol. 2009, 100, 970–974. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Moawad, R.K.; Mohamed, G.F.; Hanna, A.; El-Banna, G.F.; Mahmoud, K.F. Assessment of Hurdle Technology to Preserve Nile Tilapia Fillets During Refrigeration with the Application of Marjoram Oil/Polyphosphates Dipping. Asian J. Sci. Res. 2017, 10, 116–127. [Google Scholar] [CrossRef][Green Version]
- de Abreu, D.A.P.; Losada, P.P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int. 2010, 43, 1277–1282. [Google Scholar] [CrossRef]
- Khalafalla, F.A.; Ali, F.H.M.; Hassan, A.-R.H.A. Quality improvement and shelf-life extension of refrigerated Nile Tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 33–40. [Google Scholar] [CrossRef]
- Žitňanová, I.; Ranostajová, S.; Sobotová, H.; Demelová, D.; Pecháň, I.; Ďuračková, Z. Antioxidative activity of selected fruits and vegetables. Biologia 2006, 61, 279–284. [Google Scholar] [CrossRef]
- Mereddy, R.; Chan, A.; Fanning, K.; Nirmal, N.; Sultanbawa, Y. Betalain rich functional extract with reduced salts and nitrate content from red beetroot (Beta vulgaris L.) using membrane separation technology. Food Chem. 2017, 215, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent developments in emerging technologies for beetroot pigment extraction and its food applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Manganaris, G.A. Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar] [CrossRef]
- Hegary, A.E.; Ibrahim, I.M. Antioxidant activities of orange peels extracts. World Appl. Sci. J. 2012, 18, 684–688. [Google Scholar]
- John, S.; Monica, J.; Priyadarshini, S.; Sivaraj, C.; Arumugam, P. Antioxidant and antibacterial activities of beta vulgaris l. peel extracts. Int. J. Pharma Res. Health Sci. 2017, 5, 1974–1979. [Google Scholar]
- Miller, H.E.; Rigelhof, F.; Marquart, L.; Prakash, A.; Kanter, M. Antioxidant Content of Whole Grain Breakfast Cereals, Fruits and Vegetables. J. Am. Coll. Nutr. 2000, 19, 312S–319S. [Google Scholar] [CrossRef]
- Kujala, T.; Vienola, M.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Lazăr, S.; Constantin, O.E.; Horincar, G.; Andronoiu, D.G.; Stănciuc, N.; Muresan, C.; Râpeanu, G. Beetroot By-Product as a Functional Ingredient for Obtaining Value-Added Mayonnaise. Processes 2022, 10, 227. [Google Scholar] [CrossRef]
- Deshmukh, G.P.; Inka, P.; Sindhav, R.; Jose, N. Application of beetroot as natural coloring pigment and functional ingredient in dairy and food products. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2010–2016. [Google Scholar] [CrossRef]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef]
- Maqbool, H.; Safeena, M.P.; Abubacker, Z.; Azhar, M.; Kumar, S. Effect of beetroot peel dip treatment on the quality preservation of Deccan mahseer (Tor khudree) steaks during frozen storage (−18 °C). LWT 2021, 151, 112222. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; Awad, A.H.R.; Ali, M.R.; Ludlow, R.A.; Chen, T.; El-Mogy, M.M. Increasing the Storability of Fresh-Cut Green Beans by Using Chitosan as a Carrier for Tea Tree and Peppermint Essential Oils and Ascorbic Acid. Plants 2022, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Ali, M.R.; Smuda, S.S.; Abedelmaksoud, T.G. Utilization of sugarcane bagasse aqueous extract as a natural preservative to extend the shelf life of refrigerated fresh meat. Braz. J. Food Technol. 2021, 24, e2020167. [Google Scholar] [CrossRef]
- Singh, A.; Ganesapillai, M.; Gnanasundaram, N. Optimizaton of extraction of betalain pigments from beta vulgaris peels by microwave pretreatment. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032004. [Google Scholar] [CrossRef]
- Ali, M.R.; El Said, R.M. Assessment of the potential of Arabic gum as an antimicrobial and antioxidant agent in developing vegan “egg-free” mayonnaise. J. Food Saf. 2020, 40, e12771. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.E.; Ali, M.R.; Hammad, K.S.H.; Morsy, N.F.S. Preservative effect of carboxymethyl cellulose coating enriched with oregano (Origanum vulgare L.) essential oil on European eel (Anguilla anguilla) fillets during cold storage. Egypt. J. Nutr. 2019, 34, 1–40. [Google Scholar]
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Foods 2012, 4, 670–678. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Sakac, M.B.; Pericin, D.M.; Mandic, A.I.; Kormanjos, S.M. Antioxidant properties of ethanolic extract of sugar beet pulp. Acta Period. Technol. 2004, 35, 255–264. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Maraie, N.K.; Abdul-Jalil, T.Z.; Alhamdany, A.T.; Janabi, H.A. Phytochemical study of the Iraqi Beta vulgaris leaves and its clinical application for the treatment of different dermotological diseases. World J. Pharm. Pharm. Sci. 2014, 3, 5–19. [Google Scholar]
- Lan, W. Effect of chlorogenic acid on antioxidant activity of Flos Lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Oskoueian, A.; Jaafar, H.Z.E. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 2012, 17, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J. Agric. Food Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef]
- Wu, L.-C.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.-A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Orhan, D.D.; Hartevioğlu, A.; Küpeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef]
- Hernández, M.D.; López, M.B.; Álvarez, A.; Ferrandini, E.; García García, B.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 25, 101–106. [Google Scholar] [CrossRef]
- Egyptian Organization for Standardization (EOS). Standard Specifications for Chilled and Frozen Fish Fillets (3494) and (2-889); EOS: Egypt, Cairo, 2005. [Google Scholar]
- Sallam, K.I.; Ahmed, A.M.; Elgazzar, M.M.; Eldaly, E.A. Chemical quality and sensory attributes of marinated Pacific saury (Cololabis saira) during vacuum-packaged storage at 4 °C. Food Chem. 2007, 102, 1061–1070. [Google Scholar] [CrossRef]
- Abbas, K.A.; Mohamed, A.; Jamilah, B.; Ebrahimian, M. A Review on Correlations between Fish Freshness and pH during Cold Storage. Am. J. Biochem. Biotechnol. 2008, 4, 416–421. [Google Scholar] [CrossRef]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem. 2006, 99, 83–91. [Google Scholar] [CrossRef]
- Chang, L.-W.; Yen, W.-J.; Huang, S.C.; Duh, P.-D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Megahed, B.M.H.; Gamal, M.; Safwat, G. Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of Beta vulgaris L. root. Fresenius Environ. Bull. 2018, 9, 6369–6378. [Google Scholar]

| Treatment | Details/Concentration |
|---|---|
| T1 (Control) | fish fillet cubes were dipped in distilled water |
| T2 | fish fillet cubes were dipped in aquatic DRBR extract (80 mg powder/100 mL water) |
| T3 | fish fillet cubes were dipped in an aquatic extract of DRBR (100 mg powder/100 mL water) |
| T4 | fish fillet cubes were dipped in BHT solution (200 ppm). |
| Phenolic Compounds | Flavonoids | ||
|---|---|---|---|
| Pyrogallol | 0.151 | Rutin | 0.090 |
| Quinol | - | Naringenin | 0.146 |
| Gallic | 0.019 | Rosmarinic | 0.003 |
| Protocatchoic | 0.011 | Hesperdin | 0.040 |
| Catechol | 24.981 | Quercetin | 0.008 |
| P-Hydroxy benzoic acid | 0.018 | Kaempferol | 0.001 |
| 4-Aminobenzoic | 0.003 | Catechein | 0.003 |
| Salicylic acid | - | Myricetin | 0.002 |
| Chlorogenic | 38.870 | Apignin | 0.0001 |
| Caffeine | 0.009 | Hespertin | 0.0002 |
| Benzoic acid | 0.012 | ||
| Caffeic acid | 0.028 | ||
| Vanillic acid | - | ||
| P-Coumaric acid | 13.122 | ||
| Syringic acid | 55.012 | ||
| Ferulic acid | 2.450 | ||
| Iso-Ferulic acid | 1.971 | ||
| O-Coumaric acid | 2.042 | ||
| Coumarin | 0.691 | ||
| Cinnamic acid | 0.007 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. https://doi.org/10.3390/antiox11050906
El-Beltagi HS, El-Mogy MM, Parmar A, Mansour AT, Shalaby TA, Ali MR. Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants. 2022; 11(5):906. https://doi.org/10.3390/antiox11050906
Chicago/Turabian StyleEl-Beltagi, Hossam S., Mohamed M. El-Mogy, Aditya Parmar, Abdallah Tageldein Mansour, Tarek A. Shalaby, and Marwa Rashad Ali. 2022. "Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet" Antioxidants 11, no. 5: 906. https://doi.org/10.3390/antiox11050906
APA StyleEl-Beltagi, H. S., El-Mogy, M. M., Parmar, A., Mansour, A. T., Shalaby, T. A., & Ali, M. R. (2022). Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants, 11(5), 906. https://doi.org/10.3390/antiox11050906











