Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. DPPH Assay and ABTS Assay
2.3. Cellular Uptake Analysis
2.4. Pulse–Chase Assay
2.5. Estimation of ROS Production
2.6. Live Imaging
2.7. Assessment of Cell Death
2.8. Data Analysis
3. Results
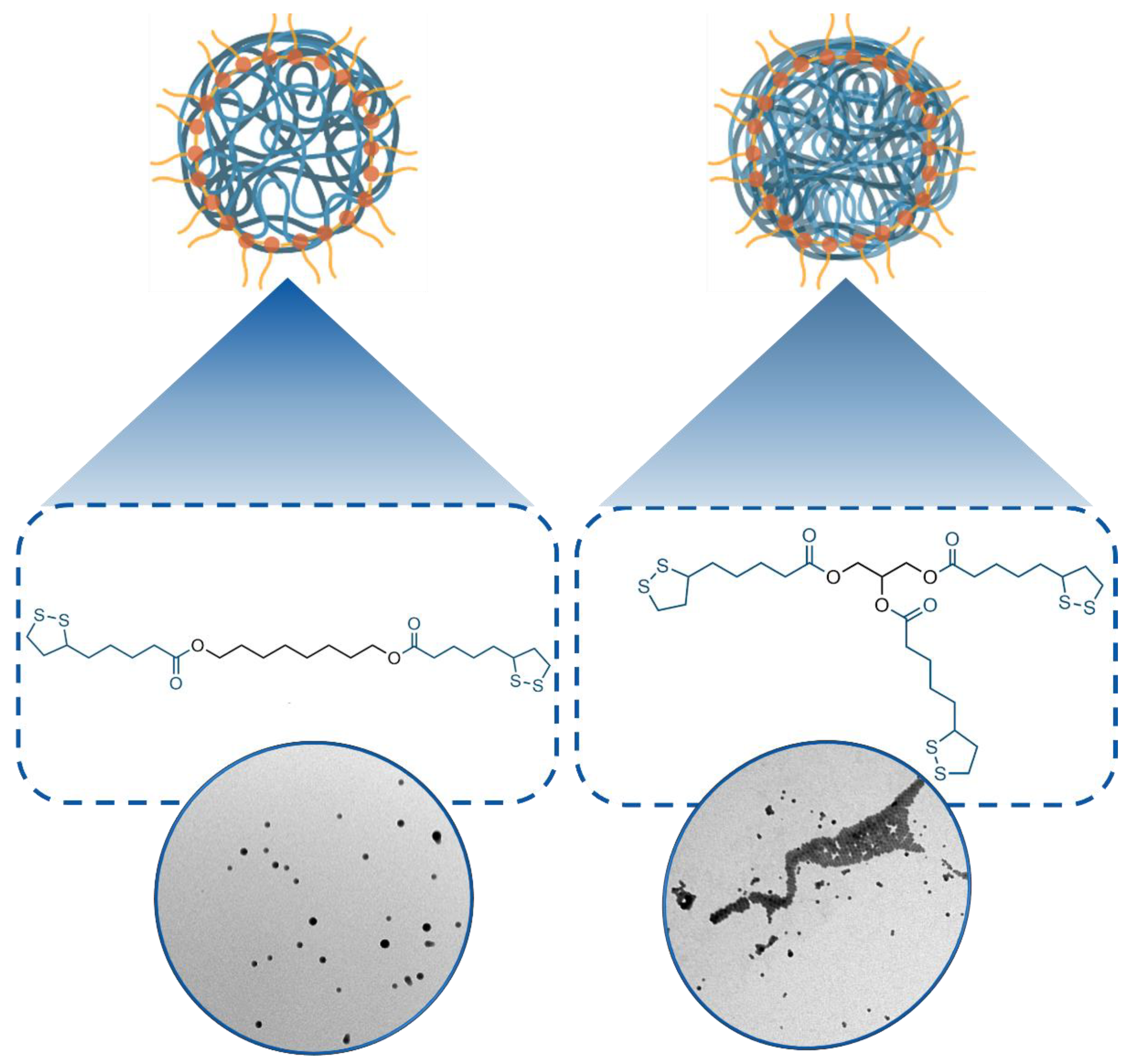
3.1. Synthesis and Characterization of 1- and 2-Poly(Lipoic Acid) NPs
3.2. Biocompatibility of Poly(Lipoic Acid) NPs
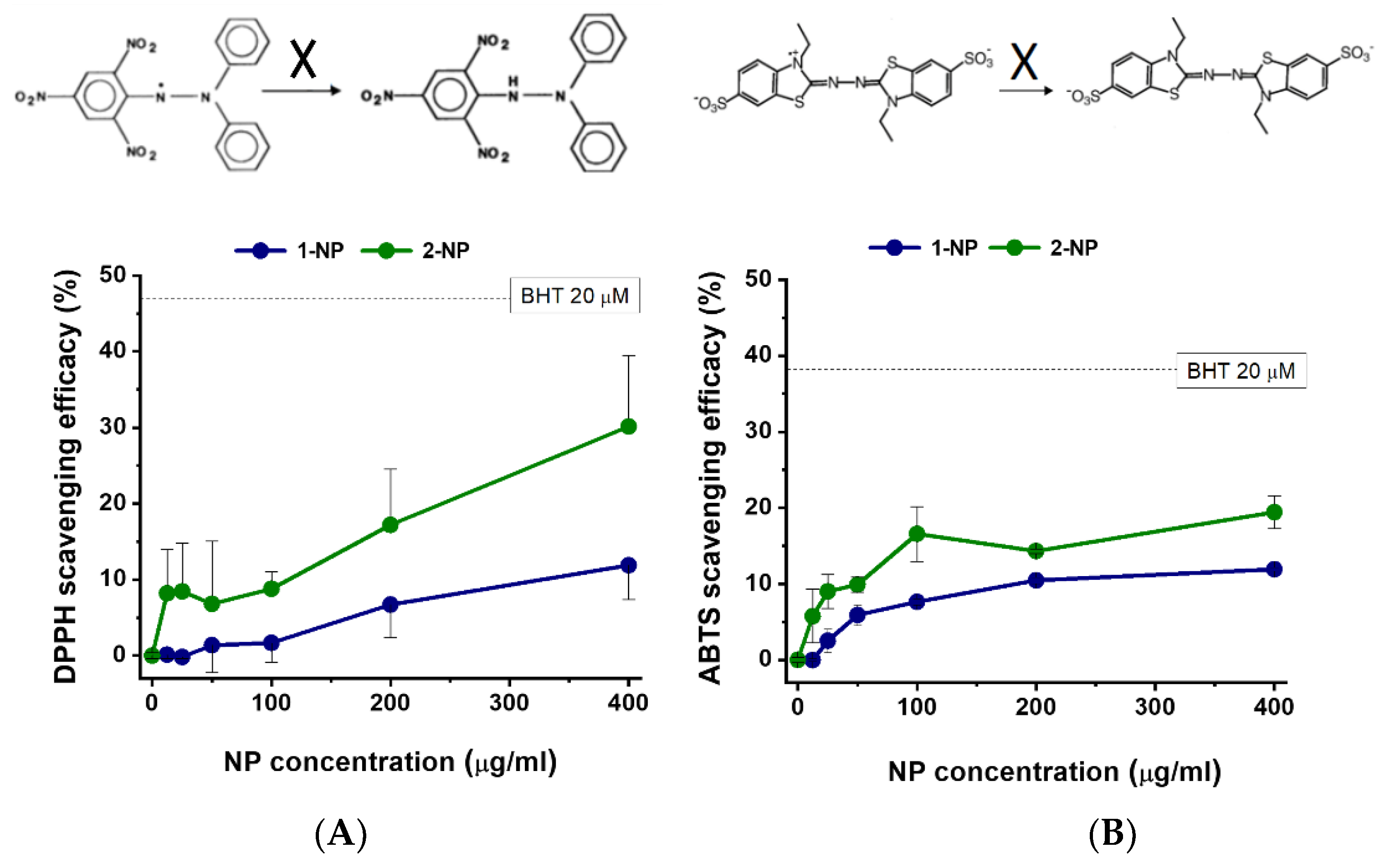
3.3. Radical Scavenging Activity of Poly(Lipoic Acid) NPs Compared with Free Lipoic Acid and Pluronic Acid in A-Cellular Assays
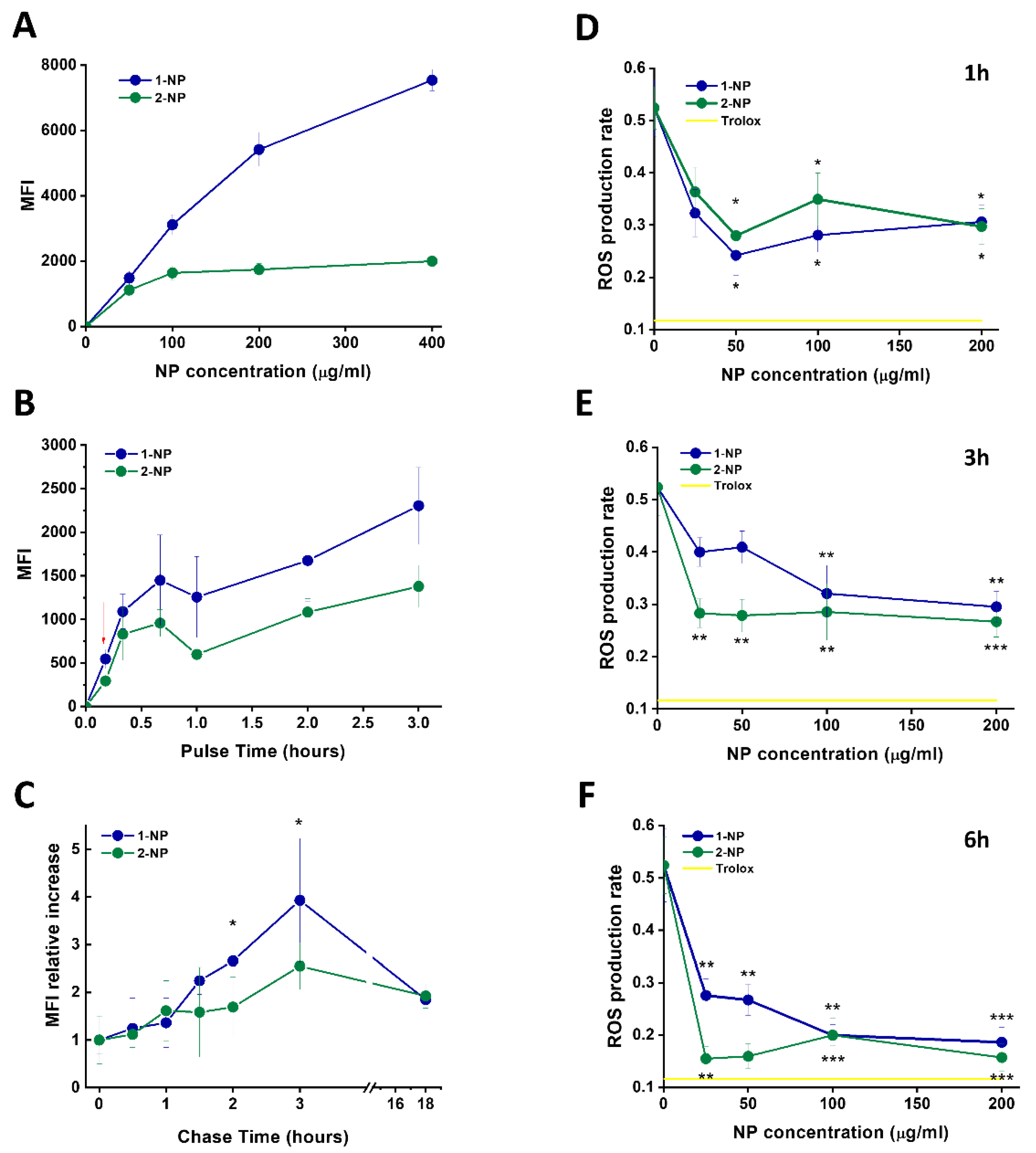
3.4. Capture of Poly(Lipoic Acid) NPs by Different Cell Lines
3.5. Capture and Antioxidative Effect of Poly(Lipoic Acid) NPs in Human Macrophage and HeLa Cells
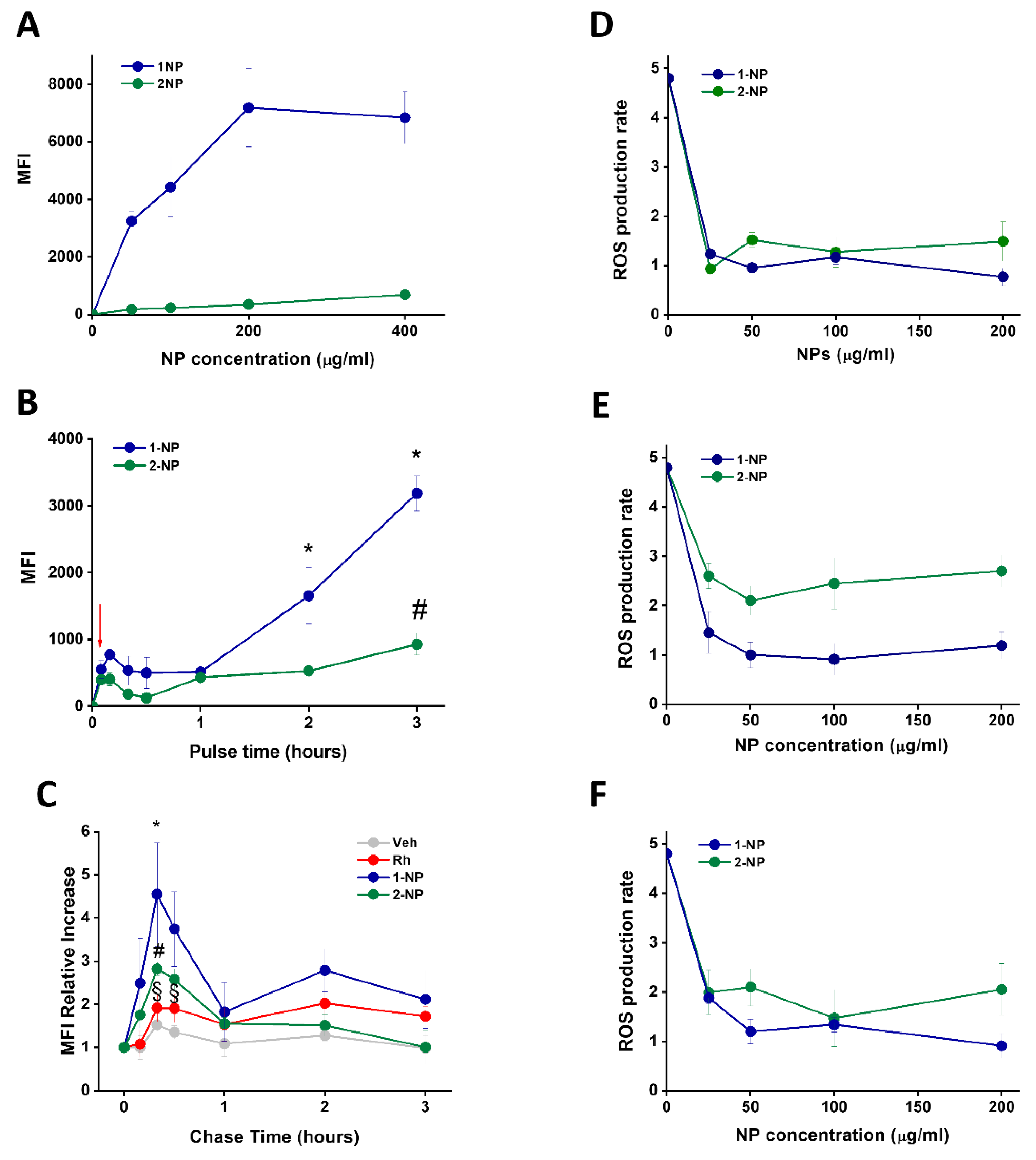
3.6. Uptake and Antioxidative Effects of Poly(Lipoic Acid) NPs in Neonatal Rat Ventricular Myocytes (NRVMs)
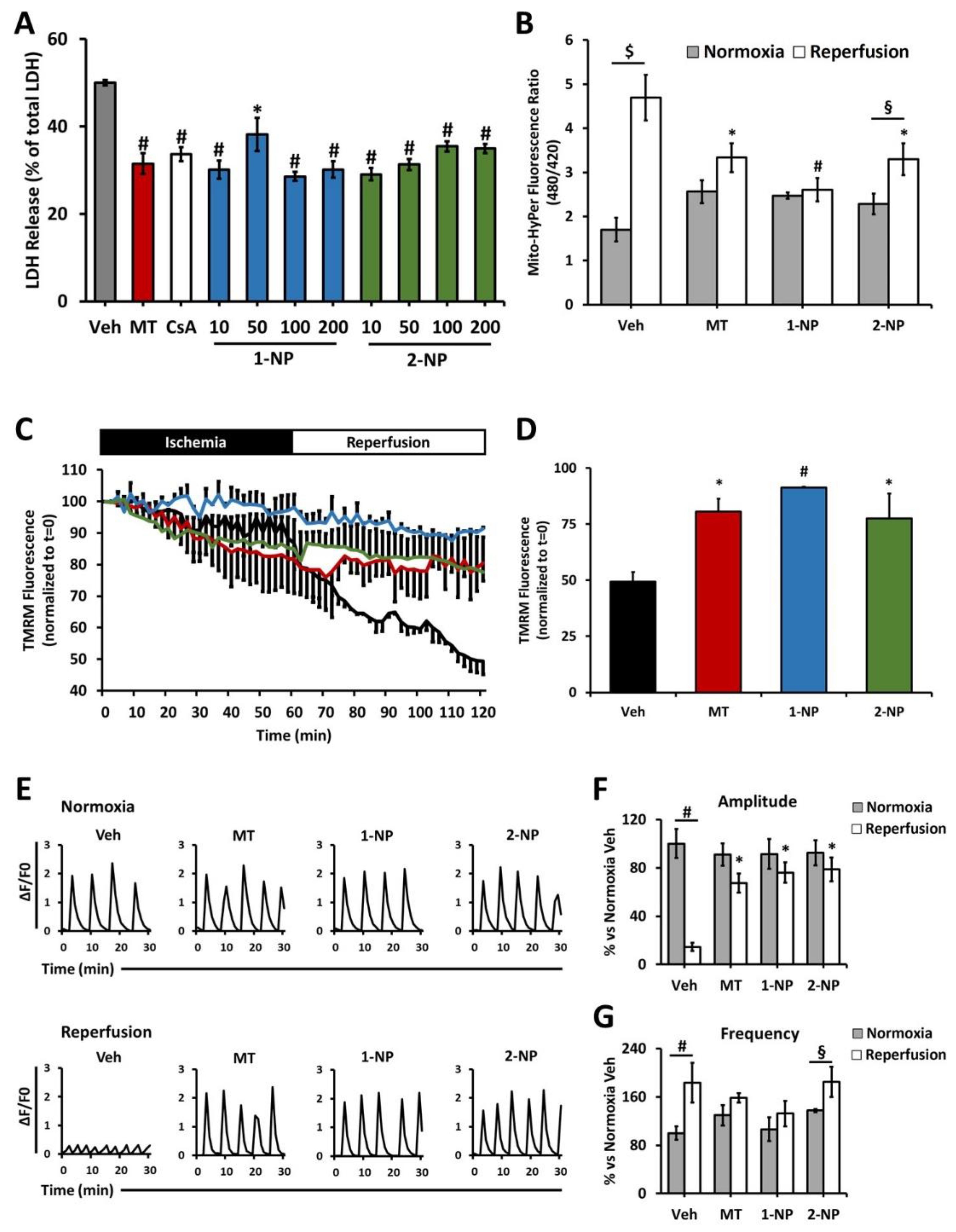
3.7. Poly(Lipoic Acid) NPs Protect NRVMs from Ischemia/Reperfusion Oxidative Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Puspita, L.; Chung, S.Y.; Shim, J.-W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Ucar, G.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Pharmacological Protection against Ischemia-Reperfusion Injury by Regulating the Nrf2-Keap1-ARE Signaling Pathway. Antioxidants 2021, 10, 823. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Lisa, F.; Kaludercic, N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium 2021, 94, 102344. [Google Scholar] [CrossRef]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed]
- Bround, M.J.; Bers, D.M.; Molkentin, J.D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 2020, 144, A3–A13. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Di Lisa, F.; Fogolari, F.; Lippe, G. From ATP to PTP and Back. Circ. Res. 2015, 116, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, C.; Liu, G.S.; Downey, J.M.; Cohen, M.V. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc. Res. 1998, 38, 676–684. [Google Scholar] [CrossRef][Green Version]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Badimon, J.J.; Pottecher, J.; Diemunsch, P.; Geny, B.; Zografos, T.A.; Katritsis, D.G.; Mewton, N.; Bergerot, C.; Ovize, M.; et al. Cyclosporine before PCI in Acute Myocardial Infarction. N. Engl. J. Med. 2016, 374, 88–90. [Google Scholar] [CrossRef]
- Monassier, L.; Ayme-Dietrich, E.; Aubertin-Kirch, G.; Pathak, A. Targeting myocardial reperfusion injuries with cyclosporine in the CIRCUS Trial—Pharmacological reasons for failure. Fundam. Clin. Pharmacol. 2015, 30, 191–193. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, J.; Zhang, X.; Han, Y.; Chen, X. Ischemia Reperfusion Injury: Opportunities for Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 6528–6539. [Google Scholar] [CrossRef]
- Guan, Y.; Yao, W.; Yi, K.; Zheng, C.; Lv, S.; Tao, Y.; Hei, Z.; Li, M. Nanotheranostics for the Management of Hepatic Ischemia-Reperfusion Injury. Small 2021, 17, 2007727. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 2021, 7, 47–72. [Google Scholar] [CrossRef]
- Chen, W.; Li, D. Reactive Oxygen Species (ROS)-Responsive Nanomedicine for Solving Ischemia-Reperfusion Injury. Front. Chem. 2020, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Nagasaki, Y. Self-Assembling Antioxidants for Ischemia & Reperfusion Injuries. Antioxid. Redox Signal. 2021, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ding, H.; Qi, G.; Guo, J.; Xu, F.; Li, C.; Puglia, D.; Kenny, J.; Ma, P. Enhancing the Radical Scavenging Activity and UV Resistance of Lignin Nanoparticles via Surface Mannich Amination toward a Biobased Antioxidant. Biomacromolecules 2021, 22, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Matoba, T.; Nakano, Y.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Funamoto, D.; Sunagawa, K.; Egashira, K. Nanoparticle-Mediated Targeting of Cyclosporine a Enhances Cardioprotection against Ischemia-Reperfusion Injury through Inhibition of Mitochondrial Permeability Transition Pore Opening. Sci. Rep. 2016, 6, 20467. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Matoba, T.; Ishikita, A.; Nagaoka, K.; Nakano, K.; Koga, J.; Tsutsui, H.; Egashira, K. Nanoparticle-Mediated Simultaneous Targeting of Mitochondrial Injury and Inflammation Attenuates Myocardial Ischemia-Reperfusion Injury. J. Am. Heart Assoc. 2021, 10, e019521. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Aljawish, A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Hassan, N.S. Chitosan nanoparticles plus quercetin suppress the oxidative stress, modulate DNA fragmentation and gene expression in the kidney of rats fed ochratoxin A-contaminated diet. Food Chem. Toxicol. 2017, 99, 209–221. [Google Scholar] [CrossRef]
- Sabourian, P.; Ji, J.; Lotocki, V.; Moquin, A.; Hanna, R.; Frounchi, M.; Maysinger, D.; Kakkar, A. Facile design of autogenous stimuli-responsive chitosan/hyaluronic acid nanoparticles for efficient small molecules to protein delivery. J. Mater. Chem. B 2020, 8, 7275–7287. [Google Scholar] [CrossRef]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-encapsulated resveratrol and quercetin in chitosan and peg modified chitosan nanoparticles: For efficient intra ocular pressure reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef]
- De Cristo Soares Alves, A.; Mainardes, R.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Mu, X.; Yan, C.; Tian, Q.; Lin, J.; Yang, S. BSA-assisted synthesis of ultrasmall gallic acid & Fe(III) coordination polymer nanoparticles for cancer theranostics. Int. J. Nanomed. 2017, 12, 7207–7223. [Google Scholar] [CrossRef]
- Ni, D.; Wei, H.; Chen, W.; Bao, Q.; Rosenkrans, Z.T.; Barnhart, T.E.; Ferreira, C.A.; Wang, Y.; Yao, H.; Sun, T.; et al. Ceria Nanoparticles Meet Hepatic Ischemia-Reperfusion Injury: The Perfect Imperfection. Adv. Mater. 2019, 31, e1902956. [Google Scholar] [CrossRef] [PubMed]
- Rana, T. Prospects and future perspectives of selenium nanoparticles: An insight of growth promoter, antioxidant and anti-bacterial potentials in productivity of poultry. J. Trace Elem. Med. Biol. 2021, 68, 126862. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, S.; Gupta, G.; Martin, S.; Fadeel, B. Multi-walled carbon nanotubes trigger lysosome-dependent cell death (pyroptosis) in macrophages but not in neutrophils. Nanotoxicology 2021, 15, 1125–1150. [Google Scholar] [CrossRef] [PubMed]
- Mal, J.; Veneman, W.J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Peijnenburg, W.J.G.M.; Vijver, M.G.; Lens, P.N.L. A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 2017, 11, 87–97. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Zhao, L.; Liu, J.; Yu, J.; Huang, Y.; Zhu, Y.; Li, Z.; Zhao, R.; Hua, S.; et al. Size-Dependent Cytotoxicity and Reactive Oxygen Species of Cerium Oxide Nanoparticles in Human Retinal Pigment Epithelia Cells. Int. J. Nanomed. 2021, 16, 5333–5341. [Google Scholar] [CrossRef]
- Yang, H.; Shen, W.; Liu, W.; Chen, L.; Zhang, P.; Xiao, C.; Chen, X. PEGylated Poly(α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018, 19, 4492–4503. [Google Scholar] [CrossRef]
- Gu, F.; Hu, C.; Tai, Z.; Yao, C.; Tian, J.; Zhang, L.; Xia, Q.; Gong, C.; Gao, Y.; Gao, S. Tumour microenvironment-responsive lipoic acid nanoparticles for targeted delivery of docetaxel to lung cancer. Sci. Rep. 2016, 6, 36281. [Google Scholar] [CrossRef]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef]
- Picchetti, P.; Moreno-Alcántar, G.; Talamini, L.; Mourgout, A.; Aliprandi, A.; De Cola, L. Smart Nanocages as a Tool for Controlling Supramolecular Aggregation. J. Am. Chem. Soc. 2021, 143, 7681–7687. [Google Scholar] [CrossRef]
- Talamini, L.; Picchetti, P.; Ferreira, L.M.; Sitia, G.; Russo, L.; Violatto, M.B.; Travaglini, L.; Alarcon, J.F.; Righelli, L.; Bigini, P.; et al. Organosilica Cages Target Hepatic Sinusoidal Endothelial Cells Avoiding Macrophage Filtering. ACS Nano 2021, 15, 9701–9716. [Google Scholar] [CrossRef]
- Trzciński, J.W.; Morillas-Becerril, L.; Scarpa, S.; Tannorella, M.; Muraca, F.; Rastrelli, F.; Castellani, C.; Fedrigo, M.; Angelini, A.; Tavano, R.; et al. Poly(lipoic acid)-Based Nanoparticles as Self-Organized, Biocompatible, and Corona-Free Nanovectors. Biomacromolecules 2021, 22, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Bang, E.-K.; Molinard, G.; Tulumello, D.V.; Ward, S.; Kelley, S.O.; Roux, A.; Sakai, N.; Matile, S. Cellular Uptake of Substrate-Initiated Cell-Penetrating Poly(disulfide)s. J. Am. Chem. Soc. 2014, 136, 6069–6074. [Google Scholar] [CrossRef] [PubMed]
- Tavano, R.; Gabrielli, L.; Lubian, E.; Fedeli, C.; Visentin, S.; De Laureto, P.P.; Arrigoni, G.; Geffner-Smith, A.; Chen, F.; Simberg, D.; et al. C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes. ACS Nano 2018, 12, 5834–5847. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, S.; Mulvey, J.F.; Burger, N.; Di Sante, M.; Hall, A.R.; Hinchy, E.C.; Caldwell, S.; Gruszczyk, A.V.; Deshwal, S.; Hartley, R.; et al. Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radic. Biol. Med. 2019, 134, 678–687. [Google Scholar] [CrossRef]
- Tonolo, F.; Sandre, M.; Ferro, S.; Folda, A.; Scalcon, V.; Scutari, G.; Feller, E.; Marin, O.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides protect against oxidative stress in a Caco-2 cell model. Food Funct. 2018, 9, 1245–1253. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Solhjoo, S.; O’Rourke, B. Mitochondrial instability during regional ischemia & reperfusion underlies arrhythmias in monolayers of cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 78, 90–99. [Google Scholar] [CrossRef]
- Bernardi, P.; Scorrano, L.; Colonna, R.; Petronilli, V.; Di Lisa, F. Mitochondria and cell death. Mechanistic aspects and methodological issues. JBIC J. Biol. Inorg. Chem. 1999, 264, 687–701. [Google Scholar] [CrossRef]
- Di Lisa, F.; Menabò, R.; Canton, M.; Barile, M.; Bernardi, P. Opening of the Mitochondrial Permeability Transition Pore Causes Depletion of Mitochondrial and Cytosolic NAD+ and Is a Causative Event in the Death of Myocytes in Postischemic Reperfusion of the Heart. J. Biol. Chem. 2001, 276, 2571–2575. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, C.; Antonucci, S.; Morillas-Becerril, L.; Scarpa, S.; Tavano, R.; Mancin, F.; Di Lisa, F.; Papini, E. Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury. Antioxidants 2022, 11, 907. https://doi.org/10.3390/antiox11050907
Bellini C, Antonucci S, Morillas-Becerril L, Scarpa S, Tavano R, Mancin F, Di Lisa F, Papini E. Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury. Antioxidants. 2022; 11(5):907. https://doi.org/10.3390/antiox11050907
Chicago/Turabian StyleBellini, Chiara, Salvatore Antonucci, Lucía Morillas-Becerril, Sara Scarpa, Regina Tavano, Fabrizio Mancin, Fabio Di Lisa, and Emanuele Papini. 2022. "Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury" Antioxidants 11, no. 5: 907. https://doi.org/10.3390/antiox11050907
APA StyleBellini, C., Antonucci, S., Morillas-Becerril, L., Scarpa, S., Tavano, R., Mancin, F., Di Lisa, F., & Papini, E. (2022). Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury. Antioxidants, 11(5), 907. https://doi.org/10.3390/antiox11050907






