A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Proposed Biological Mechanisms of Cancer-Related Fatigue
3. An Alternative Model of Cancer-Related Fatigue: The 3P Factors Model
3.1. Predisposing Factors
3.2. Precipitating Factors
3.3. Perpetuating Factors
4. Clinical Implications
5. Future Directions for Research
6. Conclusions
Funding
Conflicts of Interest
References
- Cella, D.; Davis, K.; Breitbart, W.; Curt, G. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J. Clin. Oncol. 2001, 19, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Canc. Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Borneman, T.; Piper, B.F.; Koczywas, M.; Munevar, C.M.; Sun, V.; Unman, G.C.; Ferrell, B.R. A qualitative analysis of cancer-related fatigue in ambulatory oncology. Clin. J. Oncol. Nurs. 2012, 16, E26–E32. [Google Scholar] [CrossRef] [PubMed]
- Poulson, M.J. Not just tired. J. Clin. Oncol. 2001, 19, 4180–4181. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.M.; Irwin, M.R.; Hinds, P.; Miller, A.; Berger, A.; Jacobsen, P.; Ancoli-Israel, S.; Reeve, B.B.; Mustian, K.; O’Mara, A.; et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J. Natl. Cancer Inst. 2013, 105, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Liu, L.N.; Miaskowski, C.; Chen, S.C.; Lin, Y.C.; Wang, J.S. Presurgical symptom profiles predict quality of life 2 years after surgery in women with breast cancer. Support Care Cancer 2016, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Byar, K.L.; Berger, A.M.; Bakken, S.L.; Cetak, M.A. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol. Nurs. Forum. 2006, 33, E18–E26. [Google Scholar] [CrossRef]
- Lindbohm, M.L.; Kuosma, E.; Taskila, T.; Hietanen, P.; Carlsen, K.; Gudbergsson, S.; Gunnarsdottir, H. Early retirement and non-employment after breast cancer. Psychooncology 2014, 23, 634–641. [Google Scholar] [CrossRef]
- Duijts, S.F.; Faber, M.M.; Oldenburg, H.S.; van Beurden, M.; Aaronson, N.K. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—A meta-analysis. Psychooncology 2011, 20, 115–126. [Google Scholar] [CrossRef]
- Goedendorp, M.M.; Gielissen, M.F.; Verhagen, C.A.; Bleijenberg, G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst. Rev. 2009, 2009, CD006953. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Donovan, K.A.; Vadaparampil, S.T.; Small, B.J. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007, 26, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.; Bovbjerg, D.H.; Montgomery, G.H. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol. Bull. 2008, 134, 700–741. [Google Scholar] [CrossRef] [PubMed]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Yellin, O.; Shamasunder, H.K.; Chen, C.S.; Charu, V.; Woliver, T.B.; Sanani, S.; Schlutz, M.; Nassir, Y.; Swift, R.A.; et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015, 23, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, P.; Morrow, G.R.; Roscoe, J.A.; Heckler, C.; Mohile, S.; Janelsins, M.; Peppone, L.; Hemstad, A.; Esparaz, B.T.; Hopkins, J.O. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: A University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010, 116, 3513–3520. [Google Scholar]
- Morrow, G.R.; Hickok, J.T.; Roscoe, J.A.; Raubertas, R.F.; Andrews, P.L.; Flynn, P.J.; Hynes, H.E.; Banerjee, T.K.; Kirshner, J.J.; King, D.K. Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J. Clin. Oncol. 2003, 21, 4635–4641. [Google Scholar] [CrossRef]
- Roscoe, J.A.; Morrow, G.R.; Hickok, J.T.; Mustian, K.M.; Griggs, J.J.; Matteson, S.E.; Bushunow, P.; Qazi, R.; Smith, B. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res. Treat. 2005, 89, 243–249. [Google Scholar] [CrossRef]
- Spathis, A.; Fife, K.; Blackhall, F.; Dutton, S.; Bahadori, R.; Wharton, R.; O’Brien, M.; Stone, P.; Benepal, T.; Bates, N.; et al. Modafinil for the treatment of fatigue in lung cancer: Results of a placebo-controlled, double-blind, randomized trial. J. Clin. Oncol. 2014, 32, 1882–1888. [Google Scholar] [CrossRef]
- Stockler, M.R.; O’Connell, R.; Nowak, A.K.; Goldstein, D.; Turner, J.; Wilcken, N.R.; Wyld, D.; Abdi, E.A.; Glasgow, A.; Beale, P.J.; et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: A placebo-controlled double-blind randomised trial. Lancet Oncol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Qu, D.; Zhang, Z.; Yu, X.; Zhao, J.; Qiu, F.; Huang, J. Psychotropic drugs for the management of cancer-related fatigue: A systematic review and meta-analysis. Eur. J. Cancer Care 2015, 25, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Sheng, P.; Jin, H.; He, H.; Qi, E.; Chen, W.; Dong, Y.; Hou, L. Effect of methylphenidate in patients with cancer-related fatigue: A systematic review and meta-analysis. PLoS ONE 2014, 9, e84391. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Yennurajalingam, S.; Palmer, J.L.; Perez-Cruz, P.E.; Frisbee-Hume, S.; Allo, J.A.; Williams, J.L.; Cohen, M.Z. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: A randomized, placebo-controlled, phase II trial. J. Clin. Oncol. 2013, 31, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Moraska, A.R.; Sood, A.; Dakhil, S.R.; Sloan, J.A.; Barton, D.; Atherton, P.J.; Suh, J.J.; Griffin, P.C.; Johnson, D.B.; Ali, A.; et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J. Clin. Oncol. 2010, 28, 3673–3679. [Google Scholar] [CrossRef]
- Ho, R.T.; Fong, T.C.; Cheung, I.K. Cancer-related fatigue in breast cancer patients: Factor mixture models with continuous non-normal distributions. Qual. Life Res. 2014, 23, 2909–2916. [Google Scholar] [CrossRef][Green Version]
- Minton, O.; Stone, P.C. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat. Care 2012, 2, 231–238. [Google Scholar] [CrossRef]
- Saligan, L.N.; Olson, K.; Filler, K.; Larkin, D.; Cramp, F.; Sriram, Y.; Escalante, C.P.; Del Giglio, A.; Kober, K.M.; Kamath, J.; et al. The biology of cancer-related fatigue: A review of the literature. Support Care Cancer 2015, 23, 2461–2478. [Google Scholar] [CrossRef]
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of cancer-related fatigue. Oncologist 2007, 12 (Suppl. S1), 22–34. [Google Scholar] [CrossRef]
- Jim, H.S.; Park, J.Y.; Permuth-Wey, J.; Rincon, M.A.; Phillips, K.M.; Small, B.J.; Jacobsen, P.B. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: Preliminary findings. Brain Behav. Immun. 2012, 26, 1030–1036. [Google Scholar] [CrossRef]
- Black, D.S.; Cole, S.W.; Christodoulou, G.; Figueiredo, J.C. Genomic mechanisms of fatigue in survivors of colorectal cancer. Cancer 2018, 124, 2637–2644. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Arevalo, J.M.; Cole, S.W. Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav. Immun. 2011, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Cole, S.W.; Pace, T.W.; Hu, F.; Woolwine, B.J.; Doho, G.H.; Raison, C.L.; Miller, A.H. Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: Relationship with depression and fatigue. Psychol. Med. 2012, 42, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Castellon, S.; Arevalo, J.; Cole, S.W. Cytokine genetic variations and fatigue among patients with breast cancer. J. Clin. Oncol. 2013, 31, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef]
- Clevenger, L.; Schrepf, A.; Christensen, D.; DeGeest, K.; Bender, D.; Ahmed, A.; Goodheart, M.J.; Penedo, F.; Lubaroff, D.M.; Sood, A.K.; et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav. Immun. 2012, 26, 1037–1044. [Google Scholar] [CrossRef]
- Collado-Hidalgo, A.; Bower, J.E.; Ganz, P.A.; Cole, S.W.; Irwin, M.R. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006, 12, 2759–2766. [Google Scholar] [CrossRef]
- Collado-Hidalgo, A.; Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Cole, S.W. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav. Immun. 2008, 22, 1197–1200. [Google Scholar] [CrossRef]
- Cuneo, M.G.; Schrepf, A.; Slavich, G.M.; Thaker, P.H.; Goodheart, M.; Bender, D.; Cole, S.W.; Sood, A.K.; Lutgendorf, S.K. Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology 2017, 84, 139–142. [Google Scholar] [CrossRef]
- de Raaf, P.J.; Sleijfer, S.; Lamers, C.H.; Jager, A.; Gratama, J.W.; van der Rijt, C.C. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: An explorative study. Cancer 2012, 118, 6005–6011. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, C.; Fillion, L. Factors related to persistent fatigue following completion of breast cancer treatment. Oncol. Nurs. Forum. 2004, 31, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.H.; Stout, N.; McGarvey, C.; Soballe, P.; Shieh, C.Y.; Diao, G.; Springer, B.A.; Pfalzer, L.A. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer 2011, 19, 1581–1591. [Google Scholar] [CrossRef]
- Hamre, H.; Zeller, B.; Kanellopoulos, A.; Ruud, E.; Fosså, S.D.; Loge, J.H.; Aukrust, P.; Halvorsen, B.; Mollnes, T.E.; Kiserud, C.E. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain Behav. Immun. 2013, 30, 80–87. [Google Scholar] [CrossRef]
- Laird, B.J.; McMillan, D.C.; Fayers, P.; Fearon, K.; Kaasa, S.; Fallon, M.T.; Klepstad, P. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013, 18, 1050–1055. [Google Scholar] [CrossRef]
- Liu, L.; Mills, P.J.; Rissling, M.; Fiorentino, L.; Natarajan, L.; Dimsdale, J.E.; Sadler, G.R.; Parker, B.A.; Ancoli-Israel, S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav. Immun. 2012, 26, 706–713. [Google Scholar] [CrossRef]
- Meyers, C.A.; Albitar, M.; Estey, E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005, 104, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Orre, I.J.; Murison, R.; Dahl, A.A.; Ueland, T.; Aukrust, P.; Fossa, S.D. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav. Immun. 2009, 23, 868–874. [Google Scholar] [CrossRef]
- Orre, I.J.; Reinertsen, K.V.; Aukrust, P.; Dahl, A.A.; Fosså, S.D.; Ueland, T.; Murison, R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J. Psychosom. Res. 2011, 71, 136–141. [Google Scholar] [CrossRef]
- Pertl, M.M.; Hevey, D.; Boyle, N.T.; Hughes, M.M.; Collier, S.; O’Dwyer, A.M.; Harkin, A.; Kennedy, M.J.; Connor, T.J. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav. Immun. 2013, 34, 108–119. [Google Scholar] [CrossRef]
- Pusztai, L.; Mendoza, T.R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D.; et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Clevenger, L.; Christensen, D.; DeGeest, K.; Bender, D.; Ahmed, A.; Goodheart, M.J.; Dahmoush, L.; Penedo, F.; Lucci, I.I.I.J.A.; et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain Behav. Immun. 2013, 30, S126–S134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Shi, Q.; Williams, L.A.; Mao, L.; Cleeland, C.S.; Komaki, R.R.; Mobley, G.M.; Liao, Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav. Immun. 2010, 24, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.; Frost, M.; Zwinderman, A.; Hall, P.; Halyard, M.; Consortium, G. I’m so tired: Biological and genetic mechanisms of cancer-related fatigue. Qual. Life Res. 2010, 19, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.R.; Andrews, P.L.; Hickok, J.T.; Roscoe, J.A.; Matteson, S. Fatigue associated with cancer and its treatment. Support Care Cancer 2002, 10, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.; Hoogland, A.I.; Han, H.S.; Culakova, E.; Heckler, C.; Janelsins, M.; Williams, G.C.; Bower, J.; Cole, S.; Desta, Z.; et al. A randomized placebo-controlled trial of bupropion for Cancer-related fatigue: Study design and procedures. Contemp. Clin. Trials. 2020, 91, 105976. [Google Scholar] [CrossRef]
- Ashrafi, F.; Mousavi, S.; Karimi, M. Potential Role of Bupropion Sustained Release for Cancer-Related Fatigue: A Double-Blind, Placebo-Controlled Study. Asian Pac. J. Cancer Prev. 2018, 19, 1547–1551. [Google Scholar]
- Salehifar, E.; Azimi, S.; Janbabai, G.; Zaboli, E.; Hendouei, N.; Saghafi, F.; Borhani, S. Efficacy and safety of bupropion in cancer-related fatigue, a randomized double blind placebo controlled clinical trial. BMC Cancer 2020, 20, 158. [Google Scholar] [CrossRef]
- Burks, T.F. New agents for the treatment of cancer-related fatigue. Cancer 2001, 92, 1714–1718. [Google Scholar] [CrossRef]
- Wade, D.T.; Halligan, P.W. The biopsychosocial model of illness: A model whose time has come. Clin. Rehabil. 2017, 31, 995–1004. [Google Scholar] [CrossRef]
- Wright, C.D.; Tiani, A.G.; Billingsley, A.L.; Steinman, S.A.; Larkin, K.T.; McNeil, D.W. A Framework for Understanding the Role of Psychological Processes in Disease Development, Maintenance, and Treatment: The 3P-Disease Model. Front. Psychol. 2019, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. The role of neuro-immune interactions in cancer-related fatigue: Biobehavioral risk factors and mechanisms. Cancer 2019, 125, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Hornby, L.; Lucar, E.; Bacon, S.L.; Morais, J.A. Cancer-related fatigue: The impact of skeletal muscle mass and strength in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2010, 1, 177–185. [Google Scholar] [CrossRef] [PubMed]
- van Baar, H.; Bours, M.J.; Beijer, S.; van Zutphen, M.; van Duijnhoven, F.J.; Kok, D.E.; Wesselink, E.; de Wilt, J.H.; Kampman, E.; Winkels, R.M. Body composition and its association with fatigue in the first 2 years after colorectal cancer diagnosis. J. Cancer Surviv. 2021, 15, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, E.C.; Van Den Hurk, R.M.; Blauwhoff-Buskermolen, S.; van der Vorst, M.J.; Becker-Commissaris, A.; de van der Schueren, M.A.; Buffart, L.M.; Verheul, H.M. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Sjøblom, B.; Wentzel-Larsen, T.; Grønberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Fløtten, Ø.; Jordhøy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Schur, E.; Afari, N.; Goldberg, J.; Buchwald, D.; Sullivan, P.F. Twin analyses of fatigue. Twin Res. Hum. Genet. 2007, 10, 729–733. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Evengard, B.; Jacks, A.; Pedersen, N.L. Twin analyses of chronic fatigue in a Swedish national sample. Psychol. Med. 2005, 35, 1327–1336. [Google Scholar] [CrossRef]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef]
- Schlauch, K.A.; Khaiboullina, S.F.; De Meirleir, K.L.; Rawat, S.; Petereit, J.; Rizvanov, A.A.; Blatt, N.; Mijatovic, T.; Kulick, D.; Palotas, A.; et al. Genome-wide association analysis identifies genetic variations in subjects with myalgic encephalomyelitis/chronic fatigue syndrome. Transl. Psychiatry 2016, 6, e730. [Google Scholar] [CrossRef]
- Smith, A.K.; Fang, H.; Whistler, T.; Unger, E.R.; Rajeevan, M.S. Convergent genomic studies identify association of GRIK2 and NPAS2 with chronic fatigue syndrome. Neuropsychobiology 2011, 64, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Deary, V.; Hagenaars, S.P.; Harris, S.E.; Hill, W.D.; Davies, G.; Liewald, D.C.; McIntosh, A.M.; Gale, C.R.; Deary, I.J. Genetic contributions to self-reported tiredness. Mol. Psychiatry 2018, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Illig, T.; Gieger, C.; Zhai, G.; Römisch-Margl, W.; Wang-Sattler, R.; Prehn, C.; Altmaier, E.; Kastenmüller, G.; Kato, B.S.; Mewes, H.W.; et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2010, 42, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yin, J.; Miller, A.H.; Xiao, C. A systematic review of the association between fatigue and genetic polymorphisms. Brain Behav. Immun. 2017, 62, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef]
- Wilson, C. Concern coronavirus may trigger post-viral fatigue syndromes. New Sci. 2020, 246, 10–11. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef]
- Smitherman, A.B.; Wood, W.A.; Mitin, N.; Ayer Miller, V.L.; Deal, A.M.; Davis, I.J.; Blatt, J.; Gold, S.H.; Muss, H.B. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty. Cancer 2020, 126, 4975–4983. [Google Scholar] [CrossRef]
- Guigni, B.A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; Toth, M.J. Skeletal muscle atrophy and dysfunction in breast cancer patients: Role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell Physiol. 2018, 315, C744–C756. [Google Scholar] [CrossRef]
- Beaudry, R.I.; Kirkham, A.A.; Thompson, R.B.; Grenier, J.G.; Mackey, J.R.; Haykowsky, M.J. Exercise Intolerance in Anthracycline-Treated Breast Cancer Survivors: The Role of Skeletal Muscle Bioenergetics, Oxygenation, and Composition. Oncologist 2020, 25, e852–e860. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Hureau, T.J.; Pagano, A.F. Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights from Other Cancers. Front. Cell Dev. Biol. 2021, 9, 719643. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Port, J.D.; Kaufman, K.R.; Jatoi, A.; Hart, C.R.; Gries, K.J.; Lanza, I.R.; Kumar, R. Skeletal muscle mitochondrial dysfunction and muscle and whole body functional deficits in cancer patients with weight loss. J. Appl. Physiol. 2022, 132, 388–401. [Google Scholar] [CrossRef]
- Forsyth, L.M.; Preuss, H.G.; MacDowell, A.L.; Chiazze, L.; Jr Birkmayer, G.D.; Bellanti, J.A. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann. Allergy Asthma Immunol. 1999, 82, 185–191. [Google Scholar] [CrossRef]
- Lane, R.J.; Barrett, M.C.; Taylor, D.J.; Kemp, G.J.; Lodi, R. Heterogeneity in chronic fatigue syndrome: Evidence from magnetic resonance spectroscopy of muscle. Neuromuscul. Disord. 1998, 8, 204–209. [Google Scholar] [CrossRef]
- McCully, K.K.; Natelson, B.H.; Iotti, S.; Sisto, S.; Leigh, J.S., Jr. Reduced oxidative muscle metabolism in chronic fatigue syndrome. Muscle Nerve 1996, 19, 621–625. [Google Scholar] [CrossRef]
- Pastoris, O.; Aquilani, R.; Foppa, P.; Bovio, G.; Segagni, S.; Baiardi, P.; Catapano, M.; Maccario, M.; Salvadeo, A.; Dossena, M. Altered muscle energy metabolism in post-absorptive patients with chronic renal failure. Scand. J. Urol. Nephrol. 1997, 31, 281–287. [Google Scholar] [CrossRef]
- Kurz, K.; Fiegl, M.; Holzner, B.; Giesinger, J.; Pircher, M.; Weiss, G.; Denz, H.A.; Fuchs, D. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE 2012, 7, e36956. [Google Scholar]
- Newsholme, E.A.; Blomstrand, E. Tryptophan, 5-hydroxytryptamine and a possible explanation for central fatigue. Adv. Exp. Med. Biol. 1995, 384, 315–320. [Google Scholar]
- Yamashita, M.; Yamamoto, T. Tryptophan circuit in fatigue: From blood to brain and cognition. Brain Res. 2017, 1675, 116–126. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Blomstrand, E. Branched-chain amino acids and central fatigue. J. Nutr. 2006, 136, 274S–276S. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef] [PubMed]
- Freidin, M.B.; Wells, H.R.R.; Potter, T.; Livshits, G.; Menni, C.; Williams, F.M.K. Metabolomic markers of fatigue: Association between circulating metabolome and fatigue in women with chronic widespread pain. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, I.; Gjesdal, C.G.; Jonsson, G.; Norheim, K.B.; Lundstedt, T.; Trygg, J.; Omdal, R. Metabolomics study of fatigue in patients with rheumatoid arthritis naive to biological treatment. Rheumatol. Int. 2016, 36, 703–711. [Google Scholar] [CrossRef]
- Wilson, H.E.; Rhodes, K.K.; Rodriguez, D.; Chahal, I.; Stanton, D.A.; Bohlen, J.; Davis, M.; Infante, A.M.; Hazard-Jenkins, H.; Klinke, D.J.; et al. Human Breast Cancer Xenograft Model Implicates Peroxisome Proliferator-activated Receptor Signaling as Driver of Cancer-induced Muscle Fatigue. Clin. Cancer Res. 2019, 25, 2336–2347. [Google Scholar] [CrossRef]
- Wilson, H.E.; Stanton, D.A.; Rellick, S.; Geldenhuys, W.; Pistilli, E.E. Breast cancer-associated skeletal muscle mitochondrial dysfunction and lipid accumulation is reversed by PPARG. Am. J. Physiol. Cell Physiol. 2021, 320, C577–C590. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte Ceramides—The Nexus of Inflammation and Metabolic Disease. Front. Immunol. 2020, 11, 576347. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Moylan, J.S.; Gilliam, L.A.; Smith, J.D.; Nikolova-Karakashian, M.; Reid, M.B. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am. J. Physiol. Cell Physiol. 2010, 299, C552–C560. [Google Scholar] [CrossRef]
- Marinac, C.R.; Sears, D.D.; Natarajan, L.; Gallo, L.C.; Breen, C.I.; Patterson, R.E. Frequency and Circadian Timing of Eating May Influence Biomarkers of Inflammation and Insulin Resistance Associated with Breast Cancer Risk. PLoS ONE 2015, 10, e0136240. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Hyland, K.A.; Nelson, A.M.; Eisel, S.L.; Hoogland, A.I.; Ibarz-Pinilla, J.; Sweet, K.; Jacobsen, P.B.; Knoop, H.; Jim, H.S. Fatigue Perpetuating Factors as Mediators of Change in a Cognitive Behavioral Intervention for Targeted Therapy-Related Fatigue in Chronic Myeloid Leukemia: A Pilot Study. Ann. Behav. Med. 2022, 56, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Garcia, A.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernandez-Lao, C.; Diaz-Rodriguez, L.; Arroyo-Morales, M. Influence of physical inactivity in psychophysiological state of breast cancer survivors. Eur. J. Cancer Care 2013, 22, 738–745. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, M.; Kampman, E.; Giovannucci, E.L.; van Duijnhoven, F.J.B. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr. Colorectal. Cancer Rep. 2017, 13, 370–401. [Google Scholar] [CrossRef] [PubMed]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Vareki, S.M.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef]
- Carroll, J.E.; Small, B.J.; Tometich, D.B.; Zhai, W.; Zhou, X.; Luta, G.; Ahles, T.A.; Saykin, A.J.; Nudelman, K.N.; Clapp, J.D.; et al. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: Interaction with genotype. Cancer 2019, 125, 4516–4524. [Google Scholar] [CrossRef]
- Liu, L.; Fiorentino, L.; Rissling, M.; Natarajan, L.; Parker, B.A.; Dimsdale, J.E.; Mills, P.J.; Sadler, G.R.; Ancoli-Israel, S. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav. Sleep Med. 2013, 11, 189–206. [Google Scholar] [CrossRef]
- Alfano, C.M.; Day, J.M.; Katz, M.L.; Herndon, J.E.; Bittoni, M.A.; Oliveri, J.M.; Donohue, K.; Paskett, E.D. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology 2009, 18, 128–133. [Google Scholar] [CrossRef]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the association of diet and persistent cancer-related fatigue: A pilot study. Oncol. Nurs. Forum. 2013, 40, E41–E49. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Crowder, S.L.; Sarma, K.P.; Mondul, A.M.; Chen, Y.T.; Li, Z.; Pepino, M.Y.; Zarins, K.R.; Wolf, G.T.; Rozek, L.S.; Arthur, A.E. Pretreatment Dietary Patterns Are Associated with the Presence of Nutrition Impact Symptoms 1 Year after Diagnosis in Patients with Head and Neck Cancer. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1652–1659. [Google Scholar] [CrossRef]
- Argirion, I.; Arthur, A.E.; Zarins, K.R.; Bellile, E.; Crowder, S.L.; Amlani, L.; Taylor, J.M.; Wolf, G.T.; McHugh, J.; Nguyen, A.; et al. Pretreatment Dietary Patterns, Serum Carotenoids and Tocopherols Influence Tumor Immune Response in Head and Neck Squamous Cell Carcinoma. Nutr. Cancer 2021, 73, 2614–2626. [Google Scholar] [CrossRef]
- Baguley, B.J.; Skinner, T.L.; Jenkins, D.G.; Wright, O.R.L. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial. Clin. Nutr. 2021, 40, 245–254. [Google Scholar] [CrossRef]
- Shi, N.; Aroke, D.; Jin, Q.; Lee, D.H.; Hussan, H.; Zhang, X.; Manson, J.E.; LeBlanc, E.S.; Barac, A.; Arcan, C.; et al. Proinflammatory and Hyperinsulinemic Dietary Patterns Are Associated with Specific Profiles of Biomarkers Predictive of Chronic Inflammation, Glucose-Insulin Dysregulation, and Dyslipidemia in Postmenopausal Women. Front. Nutr. 2021, 8, 690428. [Google Scholar] [CrossRef]
- Aroke, D.; Folefac, E.; Shi, N.; Jin, Q.; Clinton, S.K.; Tabung, F.K. Inflammatory and Insulinemic Dietary Patterns: Influence on Circulating Biomarkers and Prostate Cancer Risk. Cancer Prev. Res. 2020, 13, 841–852. [Google Scholar] [CrossRef]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; Dragsted, L.O.; Draper, J.; et al. Perspective: Dietary Biomarkers of Intake and Exposure-Exploration with Omics Approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The path toward using microbial metabolites as therapies. EBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J. Regulating inflammation with microbial metabolites. Nat. Med. 2016, 22, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.T.; Famodu, O.A.; Olfert, M.D.; Murray, P.J.; Cuff, C.F.; Downes, M.T.; Haughey, N.J.; Colby, S.E.; Chantler, P.D.; Olfert, I.M.; et al. Efficacy of nutritional interventions to lower circulating ceramides in young adults: FRUVEDomic pilot study. Physiol. Rep. 2017, 5, e13329. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid. Res. 2006, 45, 42–72. [Google Scholar] [CrossRef]
- Holland, W.L.; Summers, S.A. Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 2008, 29, 381–402. [Google Scholar] [CrossRef]
- Summers, S.A. The ART of Lowering Ceramides. Cell Metab. 2015, 22, 195–196. [Google Scholar] [CrossRef]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef]
- George, S.M.; Neuhouser, M.L.; Mayne, S.T.; Irwin, M.L.; Albanes, D.; Gail, M.H.; Alfano, C.M.; Bernstein, L.; McTiernan, A.; Reedy, J.; et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Q.; Kang, X.; Song, Y.; Zhao, W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: A cross-sectional study. BMC Cancer 2010, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4, e12785. [Google Scholar] [CrossRef]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef]
- Mizunoya, W.; Iwamoto, Y.; Shirouchi, B.; Sato, M.; Komiya, Y.; Razin, F.R.; Tatsumi, R.; Sato, Y.; Nakamura, M.; Ikeuchi, Y. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLoS ONE 2013, 8, e80152. [Google Scholar] [CrossRef]
- Philp, L.K.; Heilbronn, L.K.; Janovska, A.; Wittert, G.A. Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice. PLoS ONE 2015, 10, e0117494. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Wirth, M.D.; Zhao, L.; Turner-McGrievy, G.M.; Ortaglia, A. Associations between Fasting Duration, Timing of First and Last Meal, and Cardiometabolic Endpoints in the National Health and Nutrition Examination Survey. Nutrients 2021, 13, 2686. [Google Scholar] [CrossRef]
- Dun, L.; Xian-Yi, W.; Xiao-Ying, J. Effects of Moderate-To-Vigorous Physical Activity on Cancer-Related Fatigue in Patients with Colorectal Cancer: A Systematic Review and Meta-Analysis. Arch. Med. Res. 2020, 51, 173–179. [Google Scholar] [CrossRef]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [PubMed]
- Juvet, L.K.; Thune, I.; Elvsaas, I.Ø.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 2017, 33, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Cogswell, D.T.; Bisesi, P.J.; Markwald, R.R.; Cruickshank-Quinn, C.; Quinn, K.; Melanson, E.L.; Reisdorph, N.; Wright, K.P., Jr. Developing preliminary blood metabolomics-based biomarkers of insufficient sleep in humans. Sleep 2020, 43, zsz321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Moore, S.C.; Keadle, S.K.; Xiang, Y.B.; Zheng, W.; Peters, T.M.; Leitzmann, M.F.; Ji, B.T.; Sampson, J.N.; Shu, X.O.; et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int. J. Epidemiol. 2016, 45, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Irwin, M.R.; Levine, M.; Seeman, T.E.; Absher, D.; Assimes, T.; Horvath, S. Epigenetic Aging and Immune Senescence in Women with Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol. Psychiatry 2017, 81, 136–144. [Google Scholar] [CrossRef]
- Lahtinen, A.; Puttonen, S.; Vanttola, P.; Viitasalo, K.; Sulkava, S.; Pervjakova, N.; Joensuu, A.; Salo, P.; Toivola, A.; Härmä, M.; et al. A distinctive DNA methylation pattern in insufficient sleep. Sci. Rep. 2019, 9, 1193. [Google Scholar] [CrossRef]
- Mayer, D.K.; Alfano, C.M. Personalized Risk-Stratified Cancer Follow-Up Care: Its Potential for Healthier Survivors, Happier Clinicians, and Lower Costs. J. Natl. Cancer Inst. 2019, 111, 442–448. [Google Scholar] [CrossRef]
- Jim, H.S.; Hyland, K.A.; Nelson, A.M.; Pinilla-Ibarz, J.; Sweet, K.; Gielissen, M.; Bulls, H.; Hoogland, A.I.; Jacobsen, P.B.; Knoop, H. Internet-assisted cognitive behavioral intervention for targeted therapy-related fatigue in chronic myeloid leukemia: Results from a pilot randomized trial. Cancer 2020, 126, 174–180. [Google Scholar] [CrossRef]
- Abrahams, H.J.; Gielissen, M.F.; Donders, R.R.; Goedendorp, M.M.; van der Wouw, A.J.; Verhagen, C.A.; Knoop, H. The efficacy of Internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer 2017, 123, 3825–3834. [Google Scholar] [CrossRef]
- Van Gessel, L.D.; Abrahams, H.J.; Prinsen, H.; Bleijenberg, G.; Heins, M.; Twisk, J.; Van Laarhoven, H.W.; Verhagen, S.C.; Gielissen, M.F.; Knoop, H. Are the effects of cognitive behavior therapy for severe fatigue in cancer survivors sustained up to 14 years after therapy? J. Cancer Surviv. 2018, 12, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin. Oncol. Nurs. 2015, 31, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, P.; Sabiston, C.M.; Lopez, C.; Chang, E.; Goodman, J.; Jones, J.; McCready, D.; Randall, I.; Rotstein, S.; Santa Mina, D. Feasibility of Prehabilitation Prior to Breast Cancer Surgery: A Mixed-Methods Study. Front Oncol. 2020, 10, 571091. [Google Scholar] [CrossRef] [PubMed]
- Loughney, L.; West, M.A.; Kemp, G.J.; Grocott, M.P.; Jack, S. Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: A systematic review. Eur. J. Surg. Oncol. 2016, 42, 28–38. [Google Scholar] [CrossRef]
- Abrahams, H.J.; Knoop, H.; Schreurs, M.; Aaronson, N.K.; Jacobsen, P.B.; Newton, R.U.; Courneya, K.S.; Aitken, J.F.; Arving, C.; Brandberg, Y.; et al. Moderators of the effect of psychosocial interventions on fatigue in women with breast cancer and men with prostate cancer: Individual patient data meta-analyses. Psychooncology 2020, 29, 1772–1785. [Google Scholar] [CrossRef]
- Poort, H.; Müller, F.; Bleijenberg, G.; Verhagen, S.A.; Verdam, M.G.; Nieuwkerk, P.T.; Knoop, H. Condition or cognition? Mechanism of change in fatigue in a randomized controlled trial of graded exercise therapy or cognitive behavior therapy for severe fatigue in patients with advanced cancer. J. Consult. Clin. Psychol. 2021, 89, 731–741. [Google Scholar] [CrossRef]
- Van Vulpen, J.K.; Sweegers, M.G.; Peeters, P.H.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Galvão, D.A.; Chinapaw, M.J.; Steindorf, K.; et al. Moderators of Exercise Effects on Cancer-related Fatigue: A Meta-analysis of Individual Patient Data. Med. Sci. Sports Exerc. 2020, 52, 303–314. [Google Scholar] [CrossRef]
- Gielissen, M.F.; Wiborg, J.F.; Verhagen, C.A.; Knoop, H.; Bleijenberg, G. Examining the role of physical activity in reducing postcancer fatigue. Support Care Cancer 2012, 20, 1441–1447. [Google Scholar] [CrossRef][Green Version]
- Buffart, L.M.; Sweegers, M.G.; May, A.M.; Chinapaw, M.J.; van Vulpen, J.K.; Newton, R.U.; Galvão, D.A.; Aaronson, N.K.; Stuiver, M.M.; Jacobsen, P.B.; et al. Targeting Exercise Interventions to Patients with Cancer in Need: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 1190–1200. [Google Scholar] [CrossRef]
- Howell, D.; Mayer, D.K.; Fielding, R.; Eicher, M.; Verdonck-de Leeuw, I.M.; Johansen, C.; Soto-Perez-de-Celis, E.; Foster, C.; Chan, R.; Alfano, C.M.; et al. Management of Cancer and Health After the Clinic Visit: A Call to Action for Self-Management in Cancer Care. J. Natl. Cancer Inst. 2021, 113, 523–531. [Google Scholar] [CrossRef]
- Jim, H.S.; Jacobsen, P.B.; Phillips, K.M.; Wenham, R.M.; Roberts, W.; Small, B.J. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol. 2013, 32, 768–774. [Google Scholar] [CrossRef] [PubMed]
- van der Hout, A.; van Uden-Kraan, C.F.; Holtmaat, K.; Jansen, F.; Lissenberg-Witte, B.I.; Nieuwenhuijzen, G.A.; Hardillo, J.A.; de Jong, R.B.; Tiren-Verbeet, N.L.; Sommeijer, D.W.; et al. Reasons for not reaching or using web-based self-management applications, and the use and evaluation of Oncokompas among cancer survivors, in the context of a randomised controlled trial. Internet Interv. 2021, 25, 100429. [Google Scholar] [CrossRef] [PubMed]
- Haase, K.R.; Avery, J.; Bryant-Lukosius, D.; Kryzanowska, M.; Kukretti, V.; Liu, G.; Mayo, S.J.; Jones, J.; Howell, D. Patient and clinician perspectives of desired features for a web-based self-management program (icanmanage.ca): Exposing patients “hard work” of managing acute cancer. Support Care Cancer 2021, 29, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Metabolic features of the cell danger response. Mitochondrion 2014, 16, 7–17. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Vichaya, E.G.; Christian, D.L.; Molkentine, J.M.; Vermeer, D.W.; Gross, P.S.; Vermeer, P.D.; Lee, J.H.; Dantzer, R. Tumor-Associated Fatigue in Cancer Patients Develops Independently of IL1 Signaling. Cancer Res. 2018, 78, 695–705. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Vichaya, E.G.; Gross, P.S.; Ford, B.G.; Scott, K.A.; Estrada, D.; Vermeer, D.W.; Vermeer, P.; Dantzer, R. Interleukin 6-independent metabolic reprogramming as a driver of cancer-related fatigue. Brain Behav. Immun. 2020, 88, 230–241. [Google Scholar] [CrossRef]

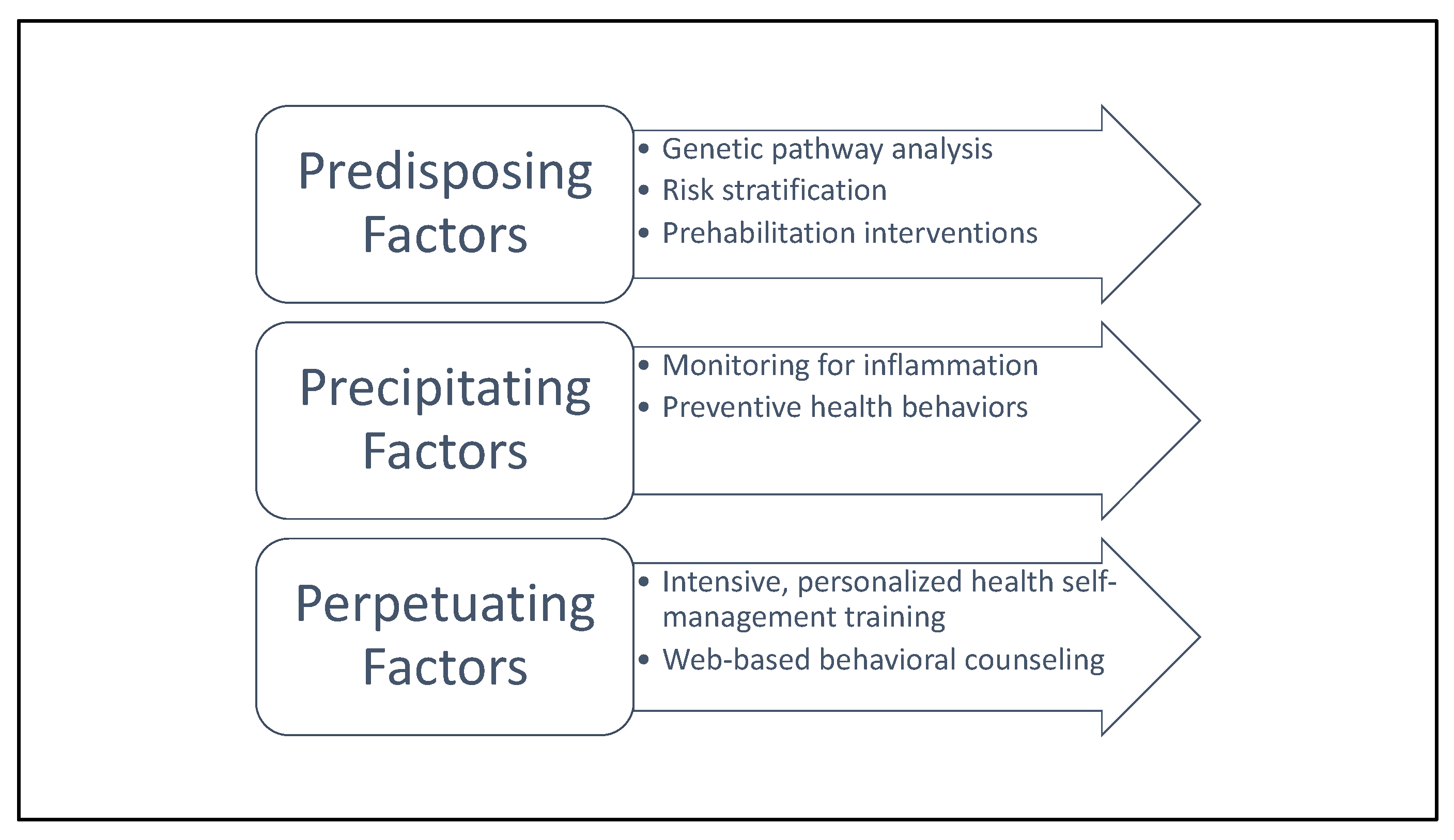
| 3P Component | Definition | Examples | Recommended Clinical Actions | |
|---|---|---|---|---|
| Predisposing Factors | Relatively stable patient characteristics that increase risk of developing cancer-related fatigue | Sex; age; genetics; circadian disruption; SNPS in circadian regulation; body composition; genetic variants altering metabolome and inflammasome | Genetic pathway analysis; risk stratification; tailored prehabilitation interventions |  Patient education about the 3P model; standardized self-health-management techniques for mitigating cancer-related fatigue.  |
| Precipitating Factors | States and traits that bring about or hasten the onset of cancer-related fatigue | Metabolic dysregulation; inflammation; biobehavioral: metabolic dysregulation, inflammation; treatment-related factors: systemic therapy, radiotherapy | Monitoring for inflammation; preventive health behaviors | |
| Perpetuating Factors | Characteristics and behaviors that worsen or prolong fatigue | Metabolic endotoxemia caused by changes in the microbiome; physical inactivity; sleep disturbance; biobehavioral: metabolic endotoxemia caused by changes in the microbiome, physical inactivity, circadian disruption, sleep disturbance. Treatment-related factors: maintenance therapy (e.g., aromatase inhibitors) Psychosocial: social isolation | Intensive, personalized health self-management training; web-based behavioral counseling | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleight, A.G.; Crowder, S.L.; Skarbinski, J.; Coen, P.; Parker, N.H.; Hoogland, A.I.; Gonzalez, B.D.; Playdon, M.C.; Cole, S.; Ose, J.; et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers 2022, 14, 1982. https://doi.org/10.3390/cancers14081982
Sleight AG, Crowder SL, Skarbinski J, Coen P, Parker NH, Hoogland AI, Gonzalez BD, Playdon MC, Cole S, Ose J, et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers. 2022; 14(8):1982. https://doi.org/10.3390/cancers14081982
Chicago/Turabian StyleSleight, Alix G., Sylvia L. Crowder, Jacek Skarbinski, Paul Coen, Nathan H. Parker, Aasha I. Hoogland, Brian D. Gonzalez, Mary C. Playdon, Steven Cole, Jennifer Ose, and et al. 2022. "A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care" Cancers 14, no. 8: 1982. https://doi.org/10.3390/cancers14081982
APA StyleSleight, A. G., Crowder, S. L., Skarbinski, J., Coen, P., Parker, N. H., Hoogland, A. I., Gonzalez, B. D., Playdon, M. C., Cole, S., Ose, J., Murayama, Y., Siegel, E. M., Figueiredo, J. C., & Jim, H. S. L. (2022). A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers, 14(8), 1982. https://doi.org/10.3390/cancers14081982







