Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Samples
2.3. Sample Preparation and Optimization of Extraction Conditions
2.4. Analytical Methods
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Antioxidant Capacity (AC)
2.5. Microencapsulation by Spray Drying
2.6. Characterization of the Microcapsules
2.6.1. Retention and Encapsulation Efficiency
2.6.2. Moisture Content and Water Activity (aw)
2.6.3. Particle Size Distribution
2.6.4. Morphology of Microcapsules
2.7. In Vitro Digestion
2.8. Statistical Analysis
3. Results and Discussion
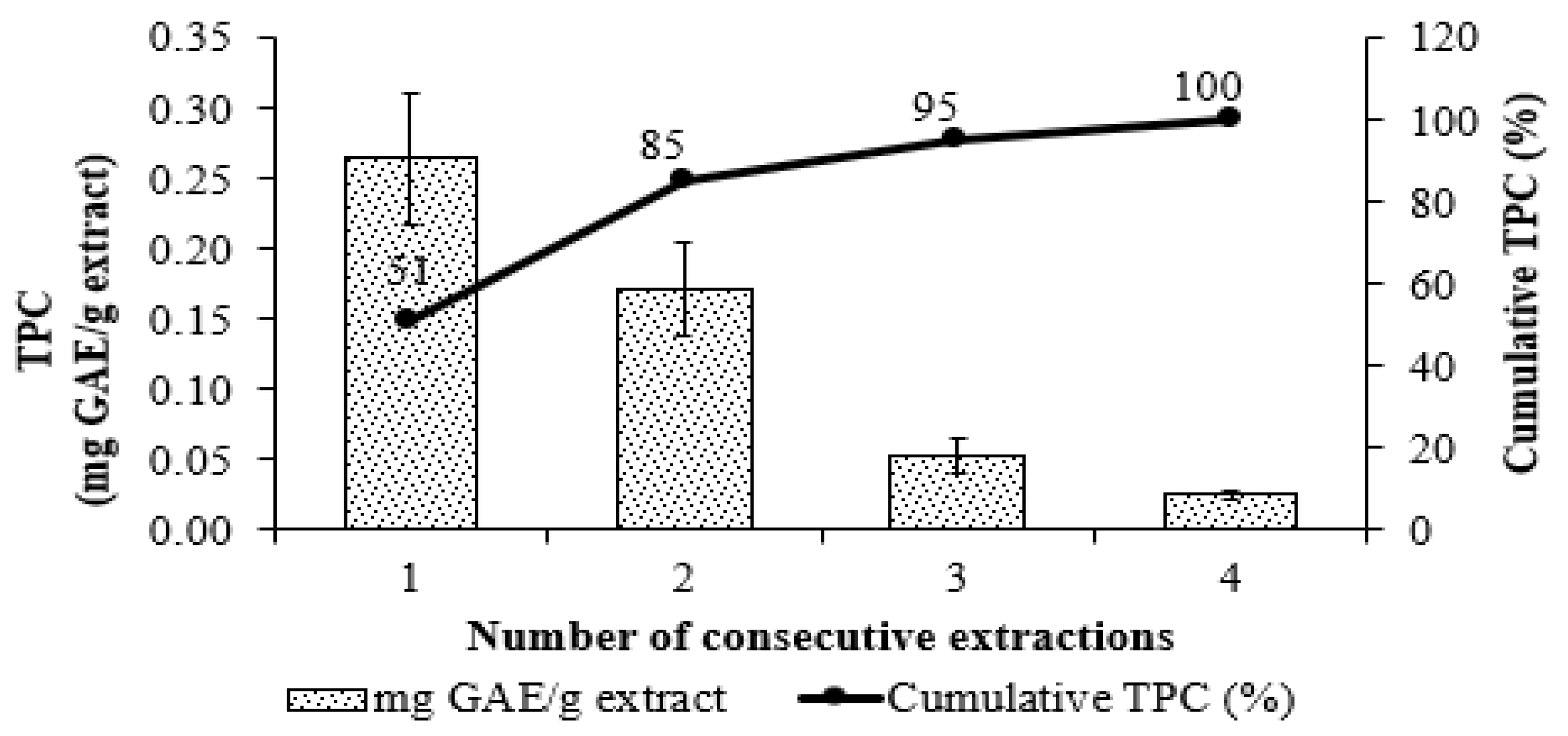
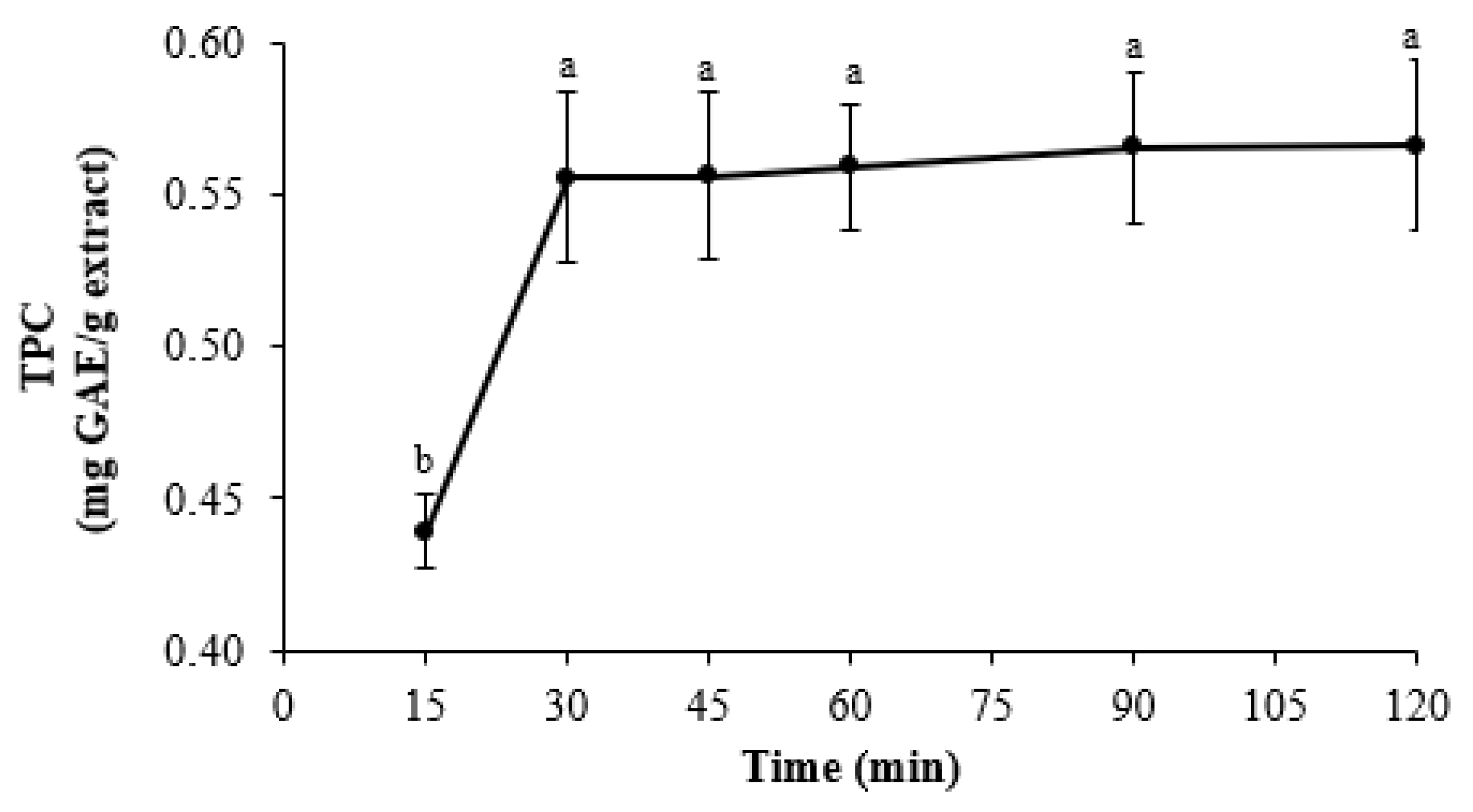
3.1. Optimization of Extraction Conditions for TPC
3.2. Red Banana Peel Characterization
3.3. Microencapsulation by Spray Drying
3.4. Moisture Content and Water Activity (aw)
3.5. Particle Size Distribution and Morphology of Microcapsules
3.6. In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neto, J.J.L.; de Almeida, T.S.; de Medeiros, J.L.; Vieira, L.R.; Moreira, T.B.; Maia, A.L.V.; Ribeiro, P.R.V.; Brito, E.S.; Farias, D.F.; Calvalho, A.F.U. Impact of bioaccessibility and bioavailability of phenolic compounds in biological systems upon the antioxidant activity of the ethanolic extract of Triplaris gardneriana seeds. Biomed. Pharm. 2017, 88, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Passo Tsamo, C.V.; Herent, M.F.; Tomekpe, K.; Happi Emaga, T.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C. Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.). Food Chem. 2015, 167, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Amyrgialaki, E.; Makris, D.; Mauromoustakos, A.; Kefalas, P. Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind. Crops Prod. 2014, 59, 216–222. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 28, 397–405. [Google Scholar] [CrossRef]
- Boyano-Orozco, L.; Gallardo-Velázquez, T.; Meza-Márquez, O.G.; Osorio-Revilla, G. Microencapsulation of rambutan peel extract by spray drying. Foods 2020, 9, 899. [Google Scholar] [CrossRef]
- Toledo-Madrid, K.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Microencapsulation of Purple Cactus Pear Fruit (Opuntia Ficus Indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study. Processes 2018, 6, 189. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Chávez, S.L.; Gallardo-Velázquez, T.; Meza-Márquez, O.G.; Osorio-Revilla, G. Spray drying and spout-fluid bed drying microencapsulation of Mexican plum fruit (Spondias purpurea L.) extract and its effect on in vitro gastrointestinal bioaccessibility. Appl. Sci. 2022, 12, 2213. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying, and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Garti, N., McClements, D.J., Eds.; WP Woodhead Publishing: Oxford, UK, 2012; pp. 73–109. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Babbar, N.; Oberoi, H.S. Potential of agro-residues as sources of bioactive compounds. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S., Dhillon, G.S.C., Eds.; Springer: New York, NY, USA, 2014; pp. 261–295. [Google Scholar]
- Blasco, G.; Gómez, F.J. Propiedades funcionales del plátano (Musa sp). Rev. Med. Univ. Ver. 2014, 14, 22–26. [Google Scholar]
- González-Montelongo, R.; Gloria, L.M.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78. [Google Scholar] [CrossRef]
- Navghare, V.V.; Dhawale, S.C. In vitro antioxidant, hypoglycemic, and oral glucose tolerance test of banana peels. Alex. J. Med. 2017, 53, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Shrisakthi, S.; Sathyapriya, B.; Shyampriya, R.; Nithya, R.; Poongodi, P. Antioxidant Potential of Peel Extracts of Banana Varieties (Musa sapientum). Food Nutr. Sci. 2011, 2, 1128–1133. [Google Scholar]
- Mathew, N.S.; Negi, P.S. Traditional uses, phytochemistry and pharmacology of wild banana (Musa acuminata Colla): A review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput microplate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Fatemeh, S.R.; Saifullah, R.; Abbas, F.M.A.; Azhar, M.E. Total phenolics, flavonoids, and antioxidant activity of banana pulp and peel flours: Influence of variety and stage of ripeness. Int. Food Res. J. 2012, 19, 1041–1046. [Google Scholar]
- Ortiz, L.; Dorta, E.; Gloria Lobo, M.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of banana (Musa acuminata Colla AAA) Peel Extract as an antioxidant source in orange juices. Plant Foods Hum. Nutr. 2017, 72, 60–66. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Fernandes, K.F.; Crizel-Cardozo, M.M.; Krumreich, F.D.; Borges, C.D.; Zambiazi, R.C. Microencapsulation of Propolis in Protein Matrix Using Spray Drying for Application in Food Systems. Food Bioprocess Technol. 2018, 11, 1422–1436. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, Y.S. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020, 269, 109744–109754. [Google Scholar] [CrossRef]
- Eroglu, E.; Tontul, İ.; Topuz, A. Optimization of aqueous extraction and spray drying conditions for efficient processing of hibiscus blended rosehip tea powder. J. Food Processing Preserv. 2018, 42, 13643–13650. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolic compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bioactivities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Ruiz Canizales, J.; Heredia, J.B.; Domínguez Avila, J.A.; Madera Santana, T.J.; Villegas Ochoa, M.A.; Robles Sánchez, R.M.; González Aguilar, G.A. Microencapsulation of blue maize (Zea mays L.) polyphenols in two matrices: Their stability during storage and in vitro digestion release. Food Meas. 2018, 13, 892–900. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Hermida, L.G.; Gallardo, G. Food applications of microencapsulated omega-3 oils. In Microencapsulation and Microspheres for Food Applications; Sagis, L., Ed.; Academic Press: Oxford, UK, 2015; pp. 271–299. [Google Scholar]
- Castro-Alatorre, N.C.; Gallardo-Velázquez, T.; Boyano-Orozco, L.C.; Téllez-Medina, D.I.; Meza-Márquez, O.G.; Osorio-Revilla, G. Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods. Foods 2021, 10, 1747. [Google Scholar] [CrossRef]






| Parameter | Content |
|---|---|
| TPC (g GAE/100 gDW) | 0.55 ± 0.01 |
| TFC (g CE/100 gDW) | 0.22 ± 0.02 |
| ABTS (g TE/100 gDW) | 0.95 ± 0.07 |
| DPPH (g TE/100 gDW) | 1.97 ± 0.14 |
| Moisture content (%) | 3.51 |
| Encapsulated Powders | Moisture Content (%) | Water Activity (aw) |
|---|---|---|
| Microcapsules with gum arabic | 4.09 ± 0.18 a | 0.33 ± 0.39 a |
| Microcapsules with soy protein isolate | 4.14 ± 0.15 a | 0.38 ± 0.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Damián, M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Téllez-Medina, D.I.; Ramos-Monroy, O.A. Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by In Vitro Digestion. Processes 2022, 10, 768. https://doi.org/10.3390/pr10040768
Ramírez Damián M, Meza-Márquez OG, Osorio-Revilla G, Gallardo-Velázquez T, Téllez-Medina DI, Ramos-Monroy OA. Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by In Vitro Digestion. Processes. 2022; 10(4):768. https://doi.org/10.3390/pr10040768
Chicago/Turabian StyleRamírez Damián, Morayma, Ofelia Gabriela Meza-Márquez, Guillermo Osorio-Revilla, Tzayhri Gallardo-Velázquez, Darío Iker Téllez-Medina, and Oswaldo Arturo Ramos-Monroy. 2022. "Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by In Vitro Digestion" Processes 10, no. 4: 768. https://doi.org/10.3390/pr10040768
APA StyleRamírez Damián, M., Meza-Márquez, O. G., Osorio-Revilla, G., Gallardo-Velázquez, T., Téllez-Medina, D. I., & Ramos-Monroy, O. A. (2022). Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by In Vitro Digestion. Processes, 10(4), 768. https://doi.org/10.3390/pr10040768







