Abstract
Background: The genus Achillea is rich in essential oil (EO) with high chemical diversity. In this study, eight EO samples obtained from flowers and leaves of Achillea ligustica All. collected on the Mediterranean mainland and island locations were analyzed to evaluate their possible chemical diversity. Methods: Sixteen samples of EO were analyzed by GC-MS, leading to the identification of 95 compounds in the leaves and 86 compounds in the flowers; a statistical analysis was performed to determine the chemical polymorphism. Results: Monoterpenes, such as β-pinene, borneol, ɑ-terpineol and isobornyl acetate, were more abundant in the continental samples, while the insular samples were richer in 1,8-cineole. Fragranyl acetate and fragranol were detected in remarkable concentrations in sample 8. The fruits of sample 8 were then cultivated under controlled agronomic conditions, providing plants rich in these compounds (sample 9). The geographical variability influenced the EO compositions, with unique observed chemotypes and a high degree of diversity among samples collected in various areas (mainland or island). Statistical analyses did not reveal any pattern between the geographical provenience and the compositions. Conclusion: Samples were distributed based on the plant organ, confirming the already reported high degree of chemical polymorphism of this species. Sample 8 could be used as a source of fragranol and fragranyl acetate, with potential applications in the insecticidal and pheromone industries.
Keywords:
Ligurian yarrow; fragranyl acetate; fragranol; GC-MS; NMR; Asteraceae; chemical polymorphism 1. Introduction
Essential oils are complex mixtures of volatile components, belonging to different chemical classes, produced as secondary metabolites in both normal or pathological conditions [1,2]. Essential oils are attracting increasing attention due to their importance in several applications in sectors such as aromatherapy, pharmaceuticals, cosmetics, foods, flavours and fragrances [3,4]. They have a range of pharmacological properties, such as antibacterial, antifungal, insecticidal, anti-inflammatory, antioxidant and cytotoxic activities [5,6,7,8].
Different species of Achillea are known for their pharmaceutical, cosmetic and aromatic properties [9,10]. Achillea ligustica All. (Asteraceae, Anthemideae), commonly known as Ligurian yarrow, is an aromatic plant that grows on arid slopes of the Mediterranean regions at altitudes between 0 and 800 m above sea level (a.s.l.), mainly along the Tyrrhenian coasts, from Liguria to Sicily [11]. This plant has been used in several traditional systems, such as Italian folk medicine, for skin disorders and rheumatism [9]. It is also used in Sicily against the intestinal worms [12]. In Sardinia, an infusion of A. ligustica is traditionally used for gastralgia and neuralgia [13]. In addition, it has traditionally been used to relieve sprains and insect bites, as well as to stop bleeding [14].
A. ligustica contains several secondary metabolites, such as essential oils, flavonoids and sesquiterpene lactones, piperidine amides and guaianolides, with the essential oils used as traditional herbal remedies as well as in food and cosmetics [9,10,15]. There are phytochemical studies on the essential oils of A. ligustica obtained from plants that grow in different locations, for example Greece [16], Northern Italy [17], Corsica [14], Sardinia [18], Sicily [19] and Central Italy [9].
It has been found that the ethanolic extracts of the flowering aerial parts of A. ligustica from Italy contain flavonoids and flavonoids glycosides based on luteolin, apigenin and kaempferol aglycones [20]. Other studies have reported various chemical classes, including piperidine amides, guaianolides (e.g., matricarin, chrysartemin A and B and isoapressin), novel sesquiterpene lactones with rare 5/6/5 skeletons, named ligustolide-A and ligustolide-B, 1,10-seco-guaianolides and a chlorine-containing sesquiterpene lactone [21,22,23].
It has been reported that several biological activities are due to essential oils or methanolic extracts of A. ligustica. Essential oils have been found to possess antifungal properties, as well as antibacterial activity towards both Gram-positive and -negative bacteria [18]. Moreover, anti-proliferative activities have been reported for essential oils, which could make them candidates for anti-carcinogenic formulations [9]. Both the essential oils and the methanolic extract showed anti-oxidant activity, while, for the n-hexane fraction, anti-diabetic properties due to α-amylase inhibition are reported [9,10].
Phytochemical studies of the essential oil (EO) of A. ligustica have shown a high variability in its constituents. The composition of leaf and flower EOs of A. ligustica from continental Greece is dominated by linalool (28.1% for leaf and 70.8% for flower) [16]. In contrast, the EOs obtained from aerial parts of specimens from northern Italy were rich in artemisia ketone (43.9%) and 2,7-dimethyl-4,6-octadien-2-ol (16.1%), and characterized by fair amounts of linalool (9.6%) [17]. Differences also emerged in the composition of EOs obtained from Mediterranean islands, with Sardinian and Corsican samples containing santolina alcohol (6.7–21.8%; 3.8–10.1%), artemisia ketone (0.3–7.6%; 3.2–7.5%) and camphor (0.7–5.8%; 17.0–17.4%) [18,24]. This high variability in the chemical composition of the EOs obtained from both the continent and the islands has an impact on their biological activity, demonstrated by different ranges of inhibition of microbial growth, which largely depend on geographical and seasonal factors [9].
Due to this variability and as a part of an ongoing study on Italian aromatic plants [25], the aim of the present study was the analysis of the essential oil composition of the leaves and flowers of A. ligustica from eight Italian locations. The samples were collected from three mainland locations in Southern Italy and five island locations, including Sicily and Vulcano Island in the Aeolian Archipelago; moreover, a specimen cultivated under a controlled condition was also included, in order to detect qualitative and quantitative differences with respect to geographical location and environmental conditions. GC-MS has been used to verify the influence of the different habitats on the production of volatile oils, and NMR was applied to confirm the detection of fragranyl acetate, the lead volatile compound only characterized in samples 8 and 9, with potential biological applications.
2. Results
2.1. Essential Oil (EO) Compositions
The essential oils (EOs) obtained by hydrodistillation of nine leaf and flower samples of A. ligustica (3 from mainland, 5 from island and 1 cultivated, Figure 1) showed degrees of variability in the content and composition of their components, with extraction yields ranging from 0.38 to 0.7% v/w.
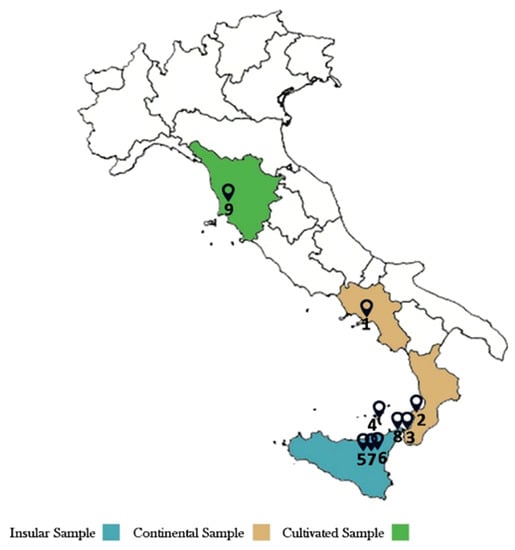
Figure 1.
Map of samples (1–9) distributions.
A total of 95 compounds were identified in all the leaves EOs, as reported in Table 1, and 86 compounds were detected in the flowers EOs Table 2. Oxygenated monoterpenes were detected as the most abundant chemical class in all the leaf EOs, with the only exception being sample 6, as they ranged from 37.6% (1) up to 70.6% (8). Among them, however, qualitative differences between the higher contributions were observed. Borneol was the most abundant monoterpene hydrocarbon in sample 1 (10.2%), while 1,8-cineole prevailed in samples 2 (11.8%), 3 (10.4%) and 5 (34.8%); cis-p-menth-2-en-1-ol was the most represented only in sample 4 (16.7%), while α-thujone was found only in sample 7 with a relative abundance of 21%; finally, fragranyl acetate was found only in samples 8 (54.3%) and 9 (24.0%). To the best of our knowledge, this is the first time that fragranyl acetate has been isolated from this species. The essential oils of sample 8 (Messina) showed a unique compositional profile because of the high percentages of fragranyl acetate and fragranol. These monoterpenes reached 54.3 and 8.5% in the leaves, and 59.7 and 16.9% in the flowers (Table 2), respectively. Fragranyl acetate identification was confirmed by purification and NMR analysis. The cultivated sample 9 differed, as fragranol and fragranyl acetate were markedly reduced in the leaf EO, with fragranyl acetate dropping to 24.0%, and fragranol to 3.5%. In the flower EO, instead, although the percentage of fragranol dropped from 16.9% (sample 8) to 4% (sample 9), the amount of its acetate rose from 59.7% to 71.4%. Interestingly, the sum of fragranol and fragranyl acetate in the flower oil of the wild plant (76.6%) was comparable to that of the cultivated one (75.4%). Fragranol was isolated for the first time from Artemisia fragrance by Bohlmann et al. in 1973 [26]. Interestingly, fragranol, which has a cyclobutane ring, is the diastereomers of grandisol (Figure 2), the main component of the aggregation pheromone, known as grandlure, of the male cotton ball weevil (Anthonomus grandis), which strongly attracts both males and females. It is also the main constituent of the aggregation or sex pheromones of other serious agricultural pests, such as bark weevils and bark beetles that cause conifer infestations in North America and Central Europe [27]. Traps filled with grandisol are used as a protective measure against heavy damage to cotton crops and its associated economic consequences [28]. Fragranol was found to be 100–200 times less active as an attractant than grandisol [29].

Table 1.
Compositions of the leaf EOs obtained from the eight wild samples of Achillea ligustica (1–8) and a cultivated one (9).

Table 2.
Compositions of the flowers EOs obtained from the eight wild samples of Achillea ligustica (1–8) and a cultivated one (9).
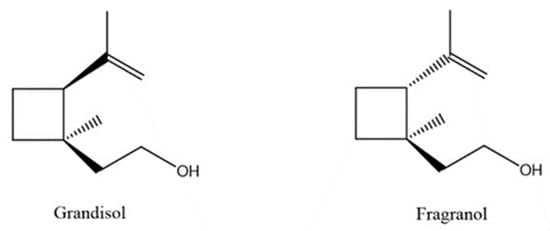
Figure 2.
Fragranol and its diastereomers grandisol.
Oxygenated monoterpenes were also the most abundant chemical group in all flower samples, exhibiting relative abundances ranging from 58.4% (sample 2) to 84.1% (sample 9). Samples 1, 3, 4, 7, 8 and 9 contained the same oxygenated monoterpenes found in the corresponding leaf EOs as the most abundant in their compositions. Linalool was the most represented compound in samples 2 (23.1%) and 5 (26.0%), but its relative abundance was quantitatively relevant in all flower samples, while its contribution in leaf samples was always lower than 1.0%. The higher relative quantity of linalool in flower EOs is consistent with its attractive power towards pollinators [30], as also reported for Scutellaria altissima L. [31]. Linalool was also found as the most abundant compound in A. ligustica EO hydrodistilled from flowers of specimens gathered in Central Italy [32]. Santolina alcohol was exclusively found (19.3%) in sample 6.
Among the non-oxygenated monoterpenes, which represent the second most abundant chemical class in the EO samples of three leaves (1, 4 and 5) and four flowers (1, 3, 4 and 7), β-pinene was detected as the main constituent in most of the leaf (1, where it was also the most abundant compound in the total composition, 2, 3, 4, 5 and 9) and flower (1–6) samples. Its relative abundance was higher in the three mainland leaf samples (1–3) than in those of the islands (4–8). Sabinene, p-cymene and γ-terpinene followed among the quantitatively relevant compounds of this group.
2.2. Statistical Analysis
Due to the high dimensionality of the resulting data (two 9 × 94 and 9 × 85 matrices for leaves and flowers, respectively), multivariate statistical analysis was used to highlight possible similarities between the different samples. In the hierarchical cluster analysis (HCA), all the EO samples were distributed in two macro-clusters, as shown in Figure 3. The first macro-cluster was further divided in three sub-groups (red, green and blue), while the second macro-cluster was composed only of yellow samples. Within each sub-cluster, samples 6, 7 and 8 showed a high degree of similarity between the compositions of the EO of leaves and flowers, as shown by their proximity in the same sub-clusters (green for sample 7, blue for sample 6 and yellow for sample 8). The highest degree of dissimilarity between the two organs was instead highlighted for sample 9, as the two oils were grouped not only into different subgroups, but even into two different macro-clusters. All the other samples were instead grouped in the red sub-cluster, where they were further distributed quite sharply according to the organ of origin.
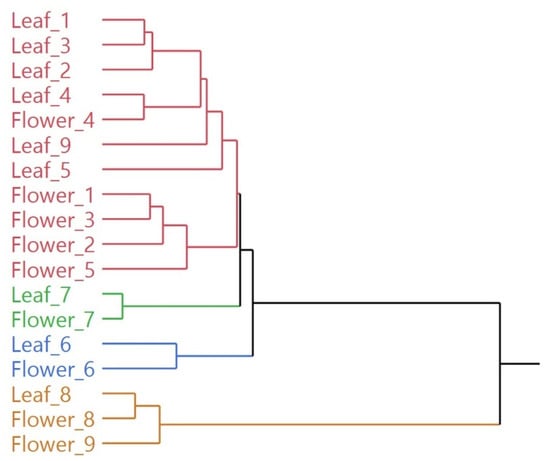
Figure 3.
Dendrogram of the hierarchical cluster analysis (HCA) of the complete EO compositions for all samples.
3. Discussion
For both organs, leaf and flower, oxygenated sesquiterpenes have always prevailed over hydrocarbons in essential oils (Table 3). In leaf sample 6, they were the most represented chemical class and, among them, β-eudesmol reached 30.8%, thus being the main constituent in this sample. Viridiflorol, instead, was the most abundant in all other leaf and flower EO samples.

Table 3.
The structure of the most abundant terpenoids of A. ligustica samples.
Within sesquiterpene hydrocarbons, germacrene D was found to be the main one in leaf samples 1, and 4–9, and in flower samples 3–6, with an overall higher presence in the island samples. β-Caryophyllene was, instead, the most represented in both leaf and flower EOs of sample 2, as well as in flower samples 7–9. Both germacrene D and β-caryophyllene biosynthesis are reported in the literature as herbivory-induced [33]; this is consistent with our findings, as both these compounds were identified in higher relative percentages in the leaves than in flowers EOs. Finally, alloaromadendrene was found as the sesquiterpene hydrocarbon with the highest relative abundance in leaf sample 3, while δ-cadinene in flower sample 1.
All the EOs showed a very varied chemotype distribution, in agreement with previous published studies [18]. Moreover, this marked variability of the EO composition among specimens belonging to the same species has also been reported for other Achillea species, such as A. millefolium [34], A. cartilaginea [35], A. biebersteinii [36], A. crithmifolia [37], A. ageratum [33] and A. wilhelmsii [34]. This chemical polymorphism, thus, appears to be a distinctive trait of this genus, whose EO profile seems to be strongly influenced by the growing environment.
The score and loading plots of the principal component analysis (PCA) are shown in Figure 4 (left and right, respectively). Only the EOs from both organs of samples 8 and 9 are plotted (Figure 4, left) in the right quadrants (PC1 > 0), due to their fragranyl acetate and fragranol content (Figure 4, right). The high degree of similarity between the compositions of the EO of leaves and flowers for samples 6, 7 and 8, demonstrated in the HCA dendrogram, is confirmed by the score plot of the PCA (Figure 4, left), as they are grouped quite close to each other. Moreover, sample 7 is grouped closer to all the red samples, mostly in the second quadrant (PC1 > 0, PC2 > 0) of the PCA, where its positioning was mainly determined by the α-thujone vector (Figure 4, right): this compound was only detected in this sample, and with high relative concentrations in both its organs. The flower and leaf EOs of sample 6 were, instead, positioned in the third quadrant (Figure 4, left) due to their β-eudesmol, camphor and santolina alcohol content (Figure 4, right). All the red samples were distributed between the second and third quadrants, quite clearly grouped according to the organ.
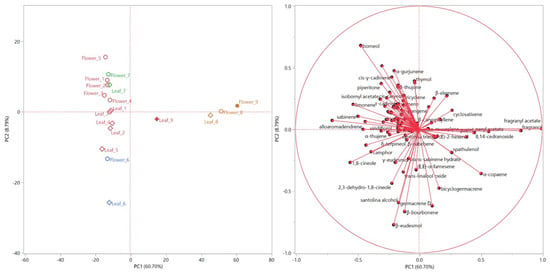
Figure 4.
Score (left) and loadings (right) plots obtained with the Principal Component Analysis (PCA) performed on the complete compositions of all the hydrodistilled essential oils.
Both the HCA and PCA did not prove a distribution based on the provenience but rather on the hydrodistilled organ. These data further confirm the reports already published on this species regarding the high degree of chemical variability among specimens of different geographical origin [18], as well as among different species of the Achillea genus [33,34,35,36,37,38]. That geographic origin drastically affects the composition of secondary plant metabolites has also been observed for other species. For example, the populations of Peumus boldus growing in different areas of Chile have different compositions of essential oils: plants growing in the north were rich in ascaridole while the trees growing in the south were rich in p-cymene [39]. This phenomenon is also observed among the populations of the genus Achillea growing in the same country; we can cite Achillea millefolium growing in different European countries [40], and Achillea wilhelmsii growing in Iran and Turkey. The Iranian sample was rich in caryophyllene oxide (12.5%) and cis-Nerolidol (10.8%), while the Turkish sample contains mainly camphor (46.6%) [41,42].
It has been shown that plants containing fragranol and fragranyl acetate are active against several species of parasites, i.e., the essential oil of Achillea santolina growing in Egypt, rich in fragranyl acetate and fragranol (27.3 and 8.2%, respectively), resulted in being active against the Khapra beetle (Trogoderma granarium) in topical application, contact and fumigation bioassays [43]. The EO of Achillea umbellata growing in Serbia is also rich in fragranyl acetate and fragranol (44.7 and 29.9%, respectively), and has been shown to have anxiolytic, antinociceptive and antimicrobial properties. Pure fragranyl acetate was very effective against Staphylococcus aureus, Klebsiella pneumoniae ATCC 10031 and clinical isolates, with an MIC of 0.78 µg/mL. In the same study, the microorganisms were more sensitive to fragranol, with an MIC of 0.39 µg/mL against Staphylococcus aureus and the clinical isolate of Klebsiella pneumoniae; Klebsiella pneumoniae ATCC 10031 was even more sensitive, with an MIC of 0.09 µg/mL [44].
4. Materials and Methods
4.1. Plant Material
The plant material was gathered at the end of April during the flowering stage in eight different sites in Southern Italy. The plants were identified by Rosalba Villari, and a voucher specimen number (E1) was deposited in the Herbarium Messanaensis, Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Universita’ di Messina, Italy. The altitude and the pH of the soil of all sites of collection are shown in Table 4.

Table 4.
Altitude and soil pH of the sample collection sites.
4.2. Plant Cultivation
The fruits of sample 8 (Messina, Italy) were collected in June and cultivated in a completely different habitat near Pisa, under controlled agronomic conditions (sample 9). The fruits were cultivated in a field within the Centro Sperimentale di Rottaia of Dipartimento di Scienze Agrarie, Alimentari e Agro-ambientali (University of Pisa, Italy). Soil chemical-physical properties were as follows: sand 43.8%; silt 44.9%; clay 11.3%; pH 7.9; organic matter (Lotti method) 3.0%; total N (Kjeldahl method) 1.5‰; assimilable P (Olsen method) 16.6 ppm; exchangeable K (Intern. method) 222.5 mg/kg. This soil, characterized by the presence of a rather superficial phreatic water-bearing (at most 120 cm), is especially deep and cool and has a water capacity of field (−0.033 MPa) equal to 27.3% of dry weight and a withering point (−1.5 MPa) equal to 9.4% of dry weight. The plants were transplanted in May; the inter-row and inter-plant spacing were 0.2 m. Basic fertilization consisted of 50 kg ha−1 of nitrogen (from urea) and 100 kg ha−1 of phosphorous (from triple phosphate rock) and 100 kg ha-1 of potassium (from potassium sulfate), while an additional 50 kg ha−1 of nitrogen (from urea) was distributed as a surface fertilizer. Weed control was accomplished by mechanical means (hoeing and harrowing) from the time of transplanting to the complete elimination of the spaces between the rows. When all of the plants were in the full flowering phase, the flowers and leaves were harvested separately.
4.3. Essential Oil Hydrodistillation
The bulk samples of leaves and flowers were dried separately in the shade and then 50 g amounts were submitted to hydrodistillation in a Clevenger-like apparatus for 2 h. The essential oil yields varied between 0.38 to 0.7% (dry weight).
4.4. Gas Chromatography with Electron Impact Mass Spectrometry (GC-EIMS)
GC-EIMS analyses were performed with a Varian CP-3800 gas-chromatograph (Varian Inc. Palo Alto, CA, USA)equipped with a DB-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions were as reported in previous works [45,46]. Identification of the constituents was based on the comparison of their retention times with those of authentic samples, by comparing their l.r.i.s to the series of n-hydrocarbons, and by computer matching against the commercial National Institute of Standards and Technology 2014 [47] and a home-made library mass spectrum, built up from pure compounds, components of essential oils of known composition and MS literature data [48].
4.5. Compound Isolation
Fragranyl acetate (60 mg) was obtained in pure form from the essential oil (400 mg) of sample 8 (from Messina) by silica gel chromatography eluting with n-hexane (100 mL), n-hexane-Et2O 98:2 (100 mL) and n-hexane-Et2O (95:5). The latter fraction contained the pure compound fragranyl acetate.
4.6. Nuclear Magnetic Resonance Spectroscopy (NMR) of Fragranyl Acetate
1H-, 13C-NMR and DEPT spectra were obtained with a Bruker Avance 400 spectrometer (Bruker Corporation, Milano, Italy) in CDCl3, using TMS as the internal standard.
1H NMR (400 MHz, CDCl3) δ: 0.87 (3H, s), 1.35 (1H, m), 1.58 (3H, s), 1.69 (H, brs), 1.71–1.88 (4H, m), 1.93 (1H, m), 1.98 (3H, s), 2.54 (1H, m), 4.04 (2H, m), 4.55 (1H, brs), 4.78 (1H, m);
13C NMR (100 MHz, CDCl3) δ: 19.1 (CH3), 19.6 (CH2), 21.0 (CH3), 22.9 (CH3), 29.9 (CH2), 40.7 (C), 41.9 (CH2), 50.3 (C), 61.7 (CH2), 109.9 (CH2), 145.2 (C), 171.1 (C).
4.7. Statistical Analysis
The hierarchical cluster (HC) and principal component (PC) analyses were performed on the complete EO compositions of all samples. The data comprising all the detected compounds in all samples comprised an 18 × 110 matrix (18 samples, 110 compounds). The HCA was conducted with the Ward’s algorithm on unscaled data, using Euclidean distances as a measure of similarity. To perform the PCA, linear regressions were operated on mean-centered, unscaled data of the covariance matrix, to select the two highest principal components (PCs). This unsupervised method reduced the dimensionality of the multivariate data of the matrix, while preserving most of the variance. The chosen PC1 and PC2 studied 60.70% and 8.79% of the variance, respectively, for a total of 69.49% observed data. The observation of the groups of samples with the HCA and the PCA methods can be applied even without reference samples to be used as a training set to establish the model.
5. Conclusions
The EO chemotypes exhibited a highly variable pattern, with no correlation with the area of origin. Indeed, samples showed a remarkable degree of variability according to each sampling site, regardless of mainland or island origin. The high variability of the essential oil composition between samples is a commonly reported trait for other species of the Achillea genus.
Since chemotypes are linked to genotype variations, these results suggest that the investigated samples are genetically different and deserve further biomolecular analyses to confirm that any genetic differences were actually responsible for this variability. Finally, such chemical diversity of essential oil can be exploited by further investigating these samples and isolating larger quantities of secondary metabolites with potential therapeutic and biological effects, such as fragranol and fragranyl acetate.
Author Contributions
Conceptualization, A.B.; methodology, A.B., P.L.C., L.C., W.A.-S. and R.A. and G.F.; software, R.A. and A.A.; validation, M.I.S.A., R.A. and G.F.; formal analysis, P.L.C. and A.B.; investigation, A.B., L.C., W.A.-S., R.A. and G.F.; resources, A.B.; data curation, M.I.S.A.; writing—original draft preparation, A.B., A.A. and R.A.; writing—review and editing, G.F.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia (Grant Code: 18-MED-1-01-0019).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work (Grant Code: 18-MED-1-01-0019).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asres, K.; Tei, A.; Moges, G.; Sporer, F.; Wink, M. Terpenoid composition of the wound-induced bark exudate of Commiphora tenuis from Ethiopia. Planta Med. 1998, 64, 473–475. [Google Scholar] [CrossRef] [PubMed]
- İşcan, G. Antibacterial and Anticandidal Activities of Common Essential Oil Constituents. Rec. Nat. Prod. 2017, 11, 374–388. [Google Scholar]
- Buckle, J. Chapter 4-Essential Oil Toxicity and Contraindications. In Clinical Aromatherapy, 3rd ed.; Buckle, J., Ed.; Churchill Livingstone: St. Louis, MI, USA, 2015; pp. 73–94. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential oil analysis and antimicrobial evaluation of three aromatic plant species growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Maggi, F.; Giuliani, C.; Fico, G.; Ricciutelli, M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Cianfaglione, K.; Tirillini, B. Secondary metabolites, secretory structures and biological activity of water celery (Apium nodiflorum (L.) Lag.) growing in central Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2019, 153, 325–335. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.; Ascrizzi, R.; Bader, A. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1, 8-cineole against A2780 ovarian cancer cells. Molecules 2020, 25, 1582. [Google Scholar] [CrossRef] [Green Version]
- Maggi, F.; Bramucci, M.; Cecchini, C.; Coman, M.M.; Cresci, A.; Cristalli, G.; Lupidi, G.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia 2009, 80, 313–319. [Google Scholar] [CrossRef]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Bonsangue, G.; Gargano, M.L.; Venturella, G.; La Bella, S. Popular uses of wild plant species for medicinal purposes in the Nebrodi Regional Park (North-Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 157, 21–37. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Filippi, J.-J.; Lanfranchi, D.-A.; Prado, S.; Baldovini, N.; Meierhenrich, U.J. Composition, Enantiomeric Distribution, and Antibacterial Activity of the Essential Oil of Achillea ligustica All. from Corsica. J. Agric. Food Chem. 2006, 54, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands). Nat. Prod. Res. 2016, 30, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O.; Loukis, A.; Verykokidou, E.; Roussis, V. Chemical Constituents of the Essential Oil of Achillea ligustica All. from Greece. J. Essent. Oil Res. 1995, 7, 549–550. [Google Scholar] [CrossRef]
- Maffei, M.; Germano, F.; Doglia, G.; Chialva, F. Essential Oils, Chromosome Numbers and Karyotypes from Italian Achillea Species. Part II. J. Essent. Oil Res. 1993, 5, 61–70. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical Composition and Antioxidant, Antimicrobial, and Antifungal Activities of the Essential Oil of Achillea ligustica All. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar] [CrossRef]
- Bader, A.; Panizzi, L.; Cioni, P.; Flamini, G. Achillea ligustica: Composition and antimicrobial activity of essential oils from the leaves, flowers and some pure constituents. Cent. Eur. J. Biol. 2007, 2, 206–212. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessì, M.A.; Pizza, C.; Cabras, P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009, 50, 440–448. [Google Scholar] [CrossRef]
- Greger, H.; Zdero, C.; Bohlmann, F. Pyrrolidine and piperidine amides from Achillea. Phytochemistry 1984, 23, 1503–1505. [Google Scholar] [CrossRef]
- Bruno, M.; Herz, W. Guaianolides and other constituents of Achillea ligustica. Phytochemistry 1988, 27, 1871–1872. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gáti, T.; Hussein, T.A.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Tóth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1,10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar] [CrossRef]
- Muselli, A.; Pau, M.; Desjobert, J.-M.; Foddai, M.; Usai, M.; Costa, J. Volatile Constituents of Achillea ligustica All. by HS-SPME/GC/GC-MS. Comparison with Essential Oils Obtained by Hydrodistillation from Corsica and Sardinia. Chromatographia 2009, 69, 575–585. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Zdero, C.; Faass, U. Naturally occurring terpene derivatives. XXVI. Constituents of Artemisia fragrans. Chem. Ber. 1973, 106, 2904–2909. [Google Scholar] [CrossRef]
- Bernard, A.M.; Frongia, A.; Secci, F.; Delogu, G.; Ollivier, J.; Piras, P.P.; Salaün, J. Stereospecific palladium(0)-catalyzed reduction of 2-cyclobutylidenepropyl esters. A versatile preparation of diastereomeric monoterpenoids: (±)-fragranol and (±)-grandisol. Tetrahedron 2003, 59, 9433–9440. [Google Scholar] [CrossRef]
- Graham, T.J.A.; Gray, E.E.; Burgess, J.M.; Goess, B.C. An efficient synthesis of (+/-)-grandisol featuring 1,5-enyne metathesis. J. Org. Chem. 2010, 75, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Martín, T.; Rodríguez, C.M.; Martín, V.S. A new approach to functionalized cyclobutanes: Stereoselective synthesis of the enantiomers of grandisol and fraganol. Tetrahedron Asymmetry 1995, 6, 1151–1164. [Google Scholar] [CrossRef]
- Stevenson, P.C. For antagonists and mutualists: The paradox of insect toxic secondary metabolites in nectar and pollen. Phytochem. Rev. 2020, 19, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Santagostini, L.; Papini, A.; Flamini, G.; Fico, G. A novel study approach on Scutellaria altissima L. cultivated at the Ghirardi Botanic Garden (Lombardy, Italy). Plant Biol. 2020, 22, 1013–1021. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.; Ricci, D.; Papa, F.; Maggi, F. Essential Oil of Achillea ligustica (Asteraceae) as an antifungal agent against phytopathogenic fungi. Nat. Prod. Commun. 2018, 13, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Grandi, R.; Messerotti, W.; Pagnoni, U.M. Monoterpene variation in two Achillea ageratum chemotypes. Phytochemistry 1976, 15, 1770–1771. [Google Scholar] [CrossRef]
- Saeidi, K.; Moosavi, M.; Lorigooini, Z.; Maggi, F. Chemical characterization of the essential oil compositions and antioxi-dant activity from Iranian populations of Achillea wilhelmsii K.Koch. Ind. Crops Prod. 2018, 112, 274–280. [Google Scholar] [CrossRef]
- Gudaitytė, O.; Venskutonis, P.R. Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the controlled environment. Biochem. Syst. Ecol. 2007, 35, 582–592. [Google Scholar] [CrossRef]
- Gudaitytė, O.; Venskutonis, P.; Maždžierienė, R. Distribution and chemical polymorphism of the essential oils of Achillea cartilaginea growing wild in Lithuania. Nat. Prod. Res. 2012, 26, 722–730. [Google Scholar] [CrossRef]
- Mirahmadi, S.F.; Hassandokht, M.R.; Sefidkon, F.; Hassani, M.E. Variability of the essential oil content and composition among the wild populations of Achillea biebersteinii Afan. from Iran: Occurrence of new nepetalactones chemotypes. J. Essent. Oil Res. 2012, 24, 523–531. [Google Scholar] [CrossRef]
- Németh, É.; Bernáth, J.; Héthelyi, É. Chemotypes and their stability in Achillea crithmifolia W. et K. populations. J. Essent. Oil Res. 2000, 12, 53–58. [Google Scholar] [CrossRef]
- Vogel, H.; Razmilic, I.; Muñoz, M.; Doll, U.; Martin, J.S. Studies of Genetic Variation of Essential Oil and Alkaloid Content in Boldo (Peumus boldus). Planta Med. 1999, 65, 90–91. [Google Scholar] [CrossRef]
- Orav, A.; Arak, E.; Raal, A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European Countries. Nat. Prod. Res. 2006, 20, 1082–1088. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Asgary, S.; Lockwood, G. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med. 1996, 62, 77–78. [Google Scholar] [CrossRef]
- Çakır, A.; Özer, H.; Aydın, T.; Kordali, Ş.; Çavuşoglu, A.T.; Akçin, T.; Mete, E.; Akçin, A. Phytotoxic and Insecticidal Properties of Essential Oils and Extracts of Four Achillea Species. Rec. Nat. Prod. 2016, 10, 154–167. [Google Scholar]
- Nenaah, G.E. Chemical composition, insecticidal and repellence activities of essential oils of three Achillea species against the Khapra beetle (Coleoptera: Dermestidae). J. Pest Sci. 2014, 87, 273–283. [Google Scholar] [CrossRef]
- Radulović, N.S.; Dekić, M.S.; Ranđelović, P.J.; Stojanović, N.M.; Zarubica, A.R.; Stojanović-Radić, Z.Z. Toxic essential oils: Anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm.(Asteraceae) volatiles. Food Chem. Toxicol. 2012, 50, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Flamini, G.; Cioni, P.L.; Morelli, I. The composition of the root oil of Salvadora persica L. J. Essent. Oil Res. 2002, 14, 128–129. [Google Scholar] [CrossRef]
- Bader, A.; Caponi, C.; Cioni, P.L.; Flamini, G.; Morelli, I. Acorenone in the essential oil of flowering aerial parts of Seseli tortuosum L. Flavour Fragr. J. 2003, 18, 57–58. [Google Scholar] [CrossRef]
- NIST; Wiley Technology. NIST/EPA/NIH Mass Spectral Library 2014, 1st ed.; Wiley: Hoboken, NJ, USA, 2014.
- Adams, R. Identification of Essential Oil Components by Gas Chromatography–Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, USA, 1995. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








