Mannose Receptor-Mediated Carbon Nanotubes as an Antigen Delivery System to Enhance Immune Response Both In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Cell Line
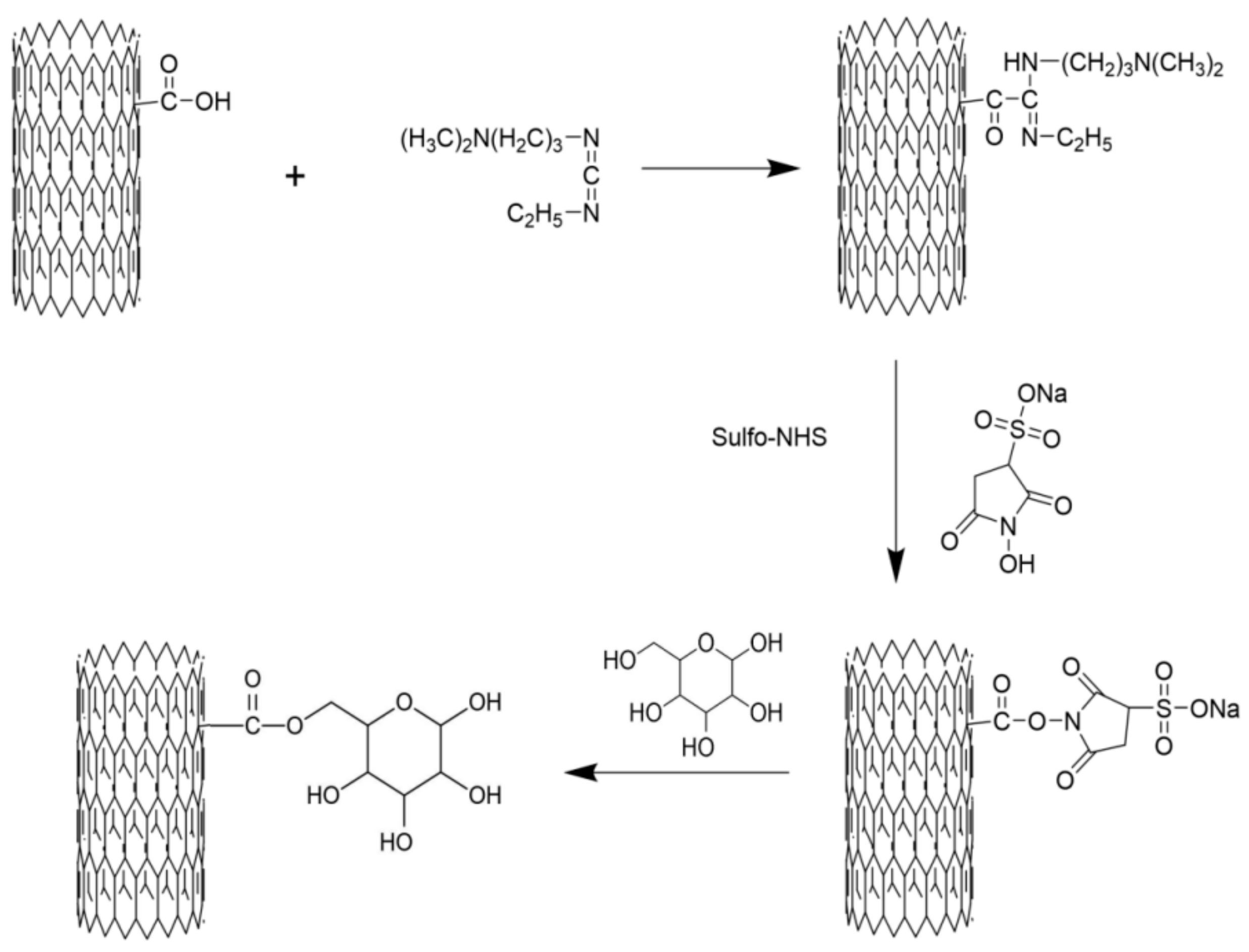
2.2. Preparation of M-MWCNT
2.3. Characterization of M-MWCNT Composites
2.4. In Vitro Test
2.4.1. Cytotoxicity on Macrophages
2.4.2. Effects of M-MWCNT on Cytokine Secretion of Macrophages
2.4.3. TEM of Macrophages
2.4.4. Antigen Internalization Assessment of RAW264.7 by CLSM
2.5. In Vivo Test
2.5.1. Mice Grouping and Treatment
2.5.2. Analysis of Activation and Maturation of Splenic Dendritic Cells
2.5.3. The Effect of M-MWCNT on Proliferation of Splenocytes in Mice
2.5.4. The Effects of M-MWCNT on the Serum Contents of IFN-γ and IL-6 in Mice
2.5.5. The OVA-Specific Antibody Titer in the Mice Serum
2.5.6. In Vivo Antigen Release Assay
2.5.7. Histological Toxicity Analysis of M-MWCNT
2.5.8. Statistical Analysis
3. Results
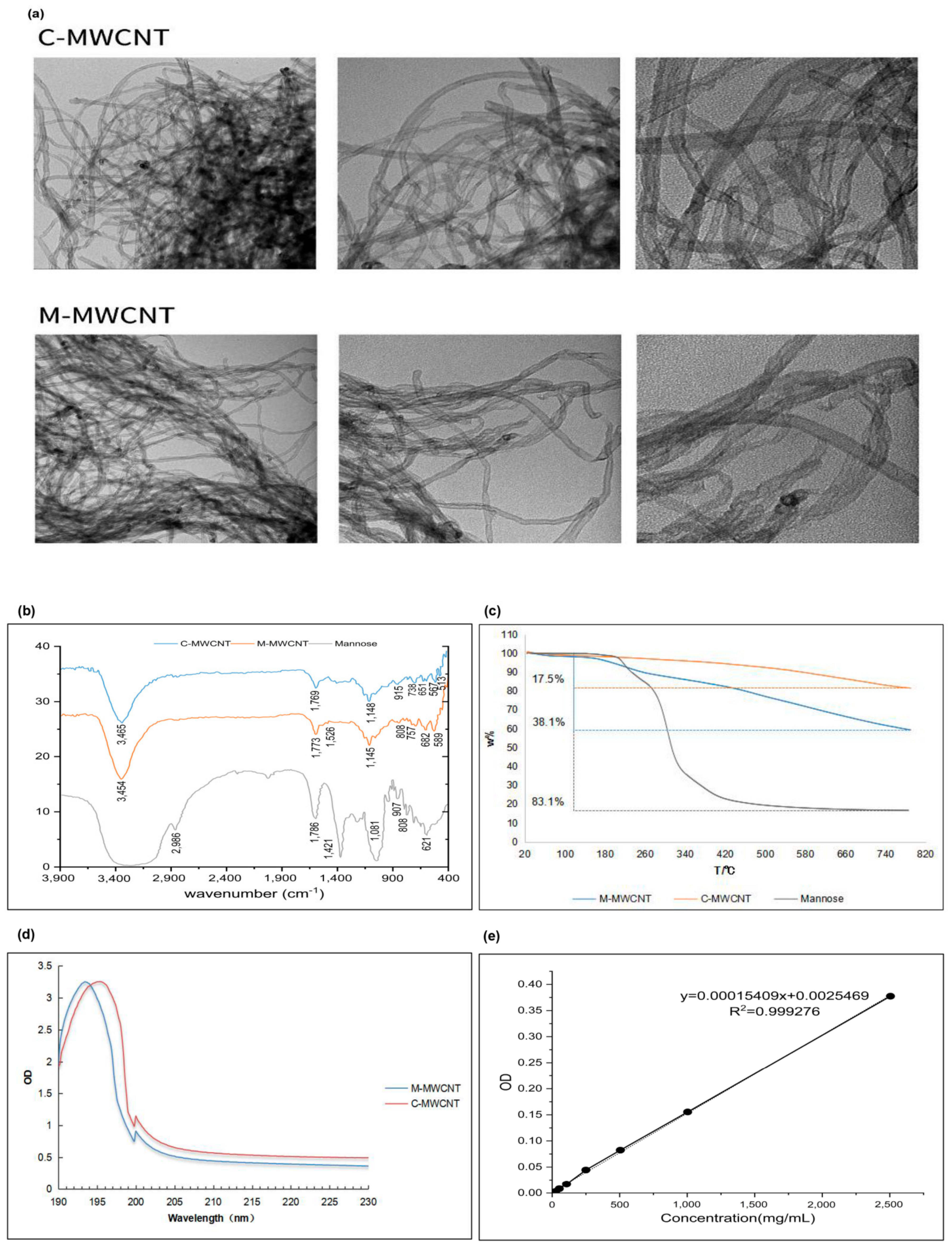
3.1. Characterization of M-MWCNT
3.1.1. TEM Morphologies of M-MWCNTs
3.1.2. FT-IR Spectroscopy
3.1.3. UV Spectrum Analysis
3.1.4. Thermogravimetric Analysis
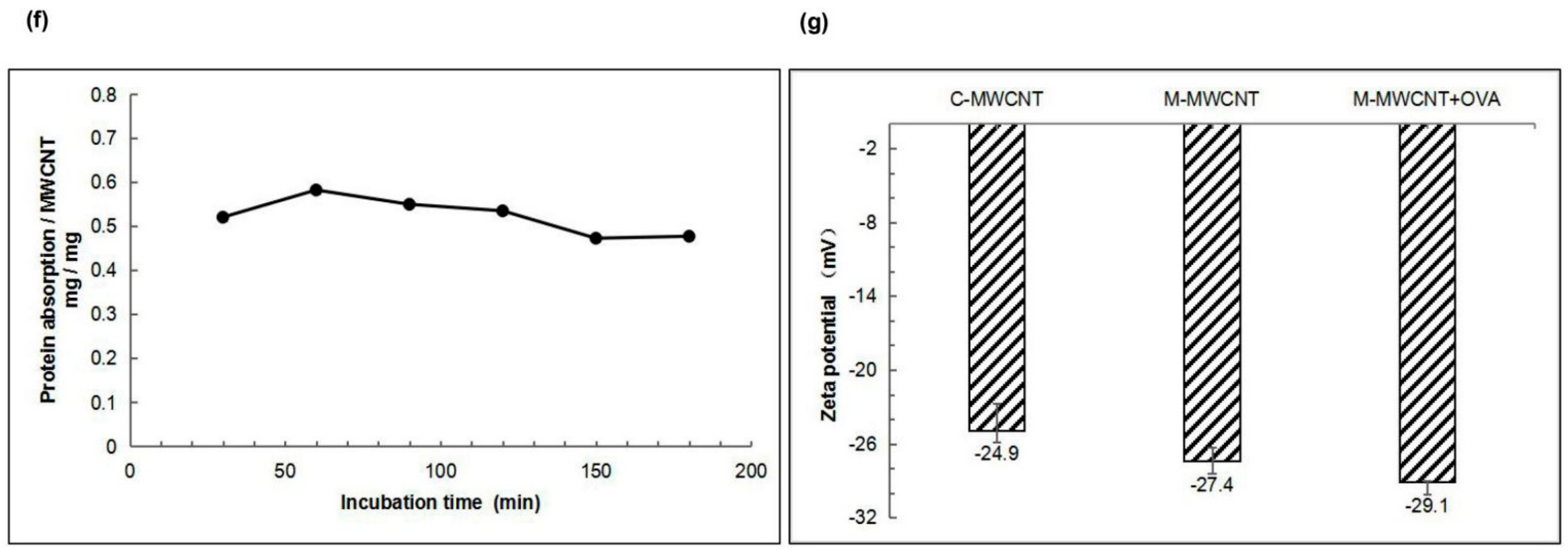
3.1.5. OVA Adsorption of Mannose Carbon Nanotubes
3.1.6. Zeta Potential Analysis
3.1.7. XPS Analysis
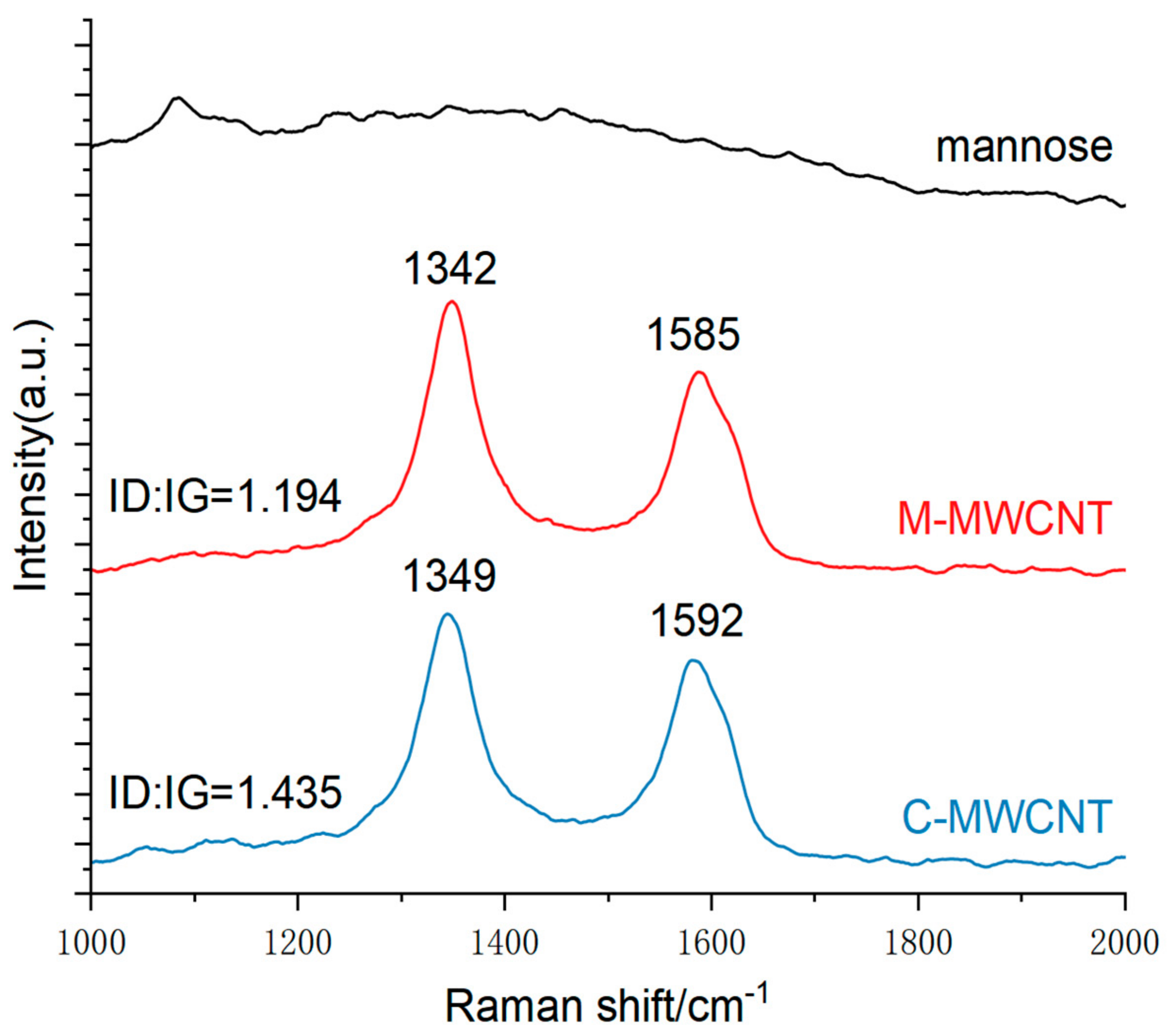
3.1.8. Raman Spectra Analysis
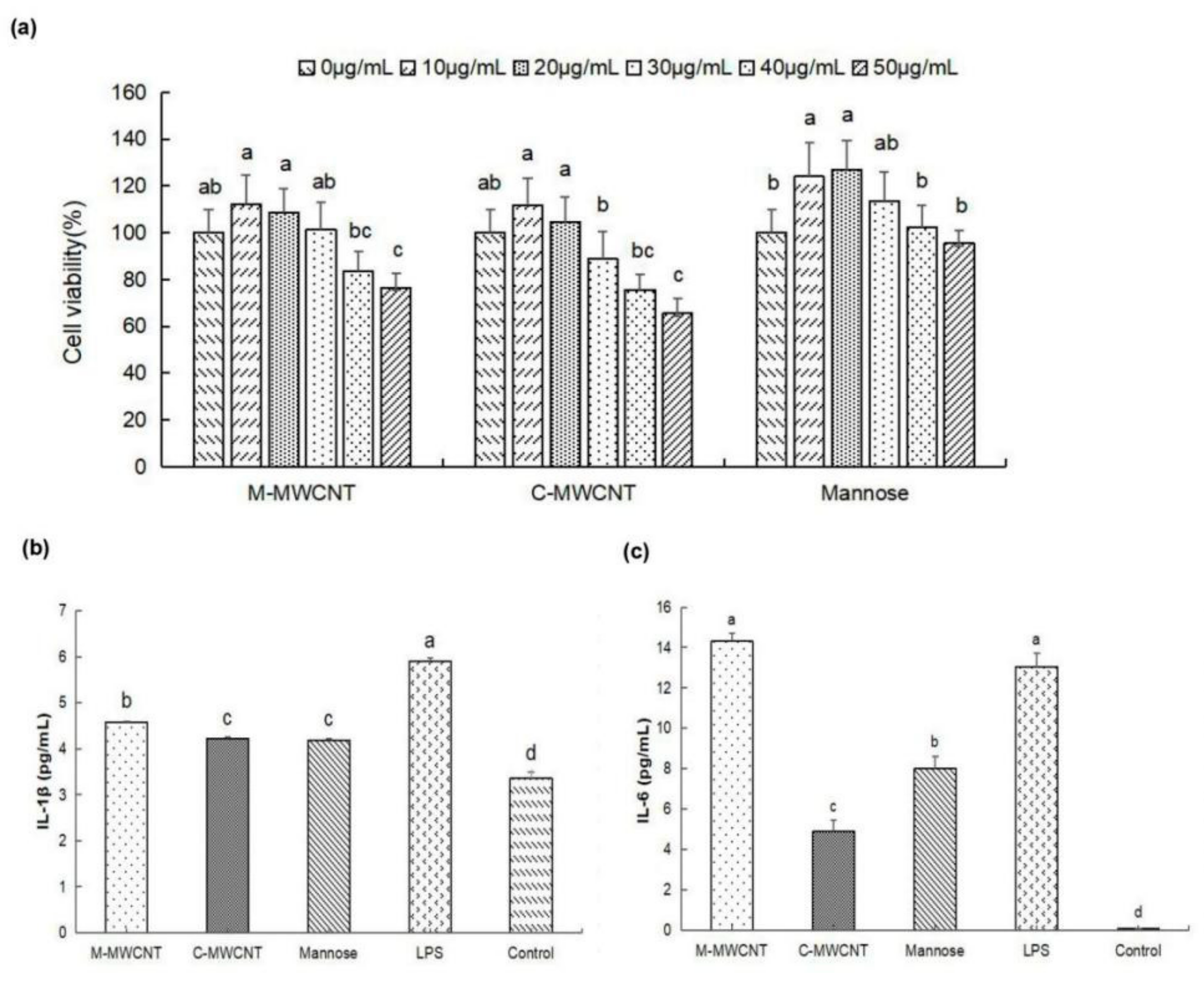
3.2. M-MWCNT Cytotoxicity on Macrophages
3.3. Effects M-MWCNT on Cytokine Secretion of Macrophages
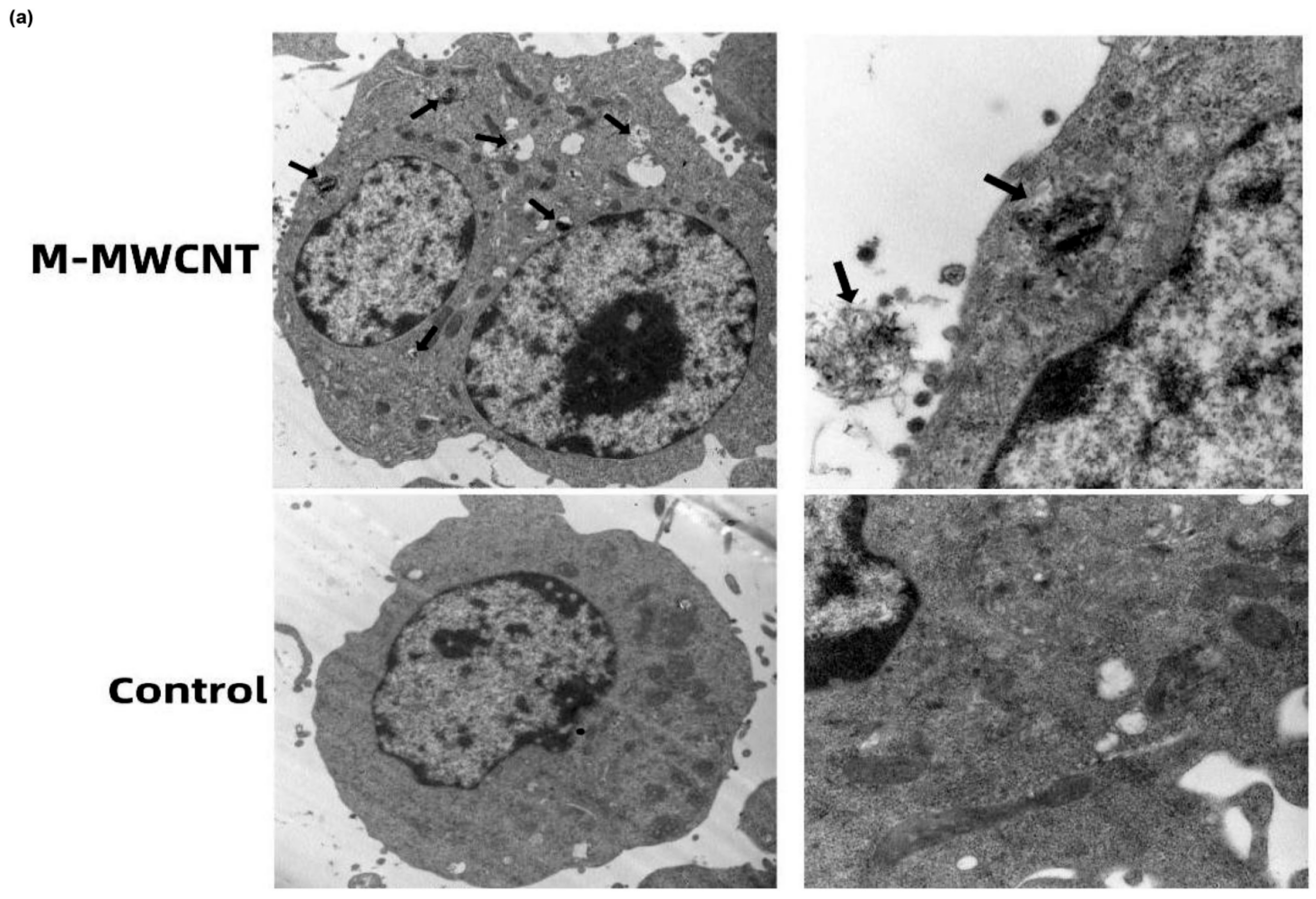
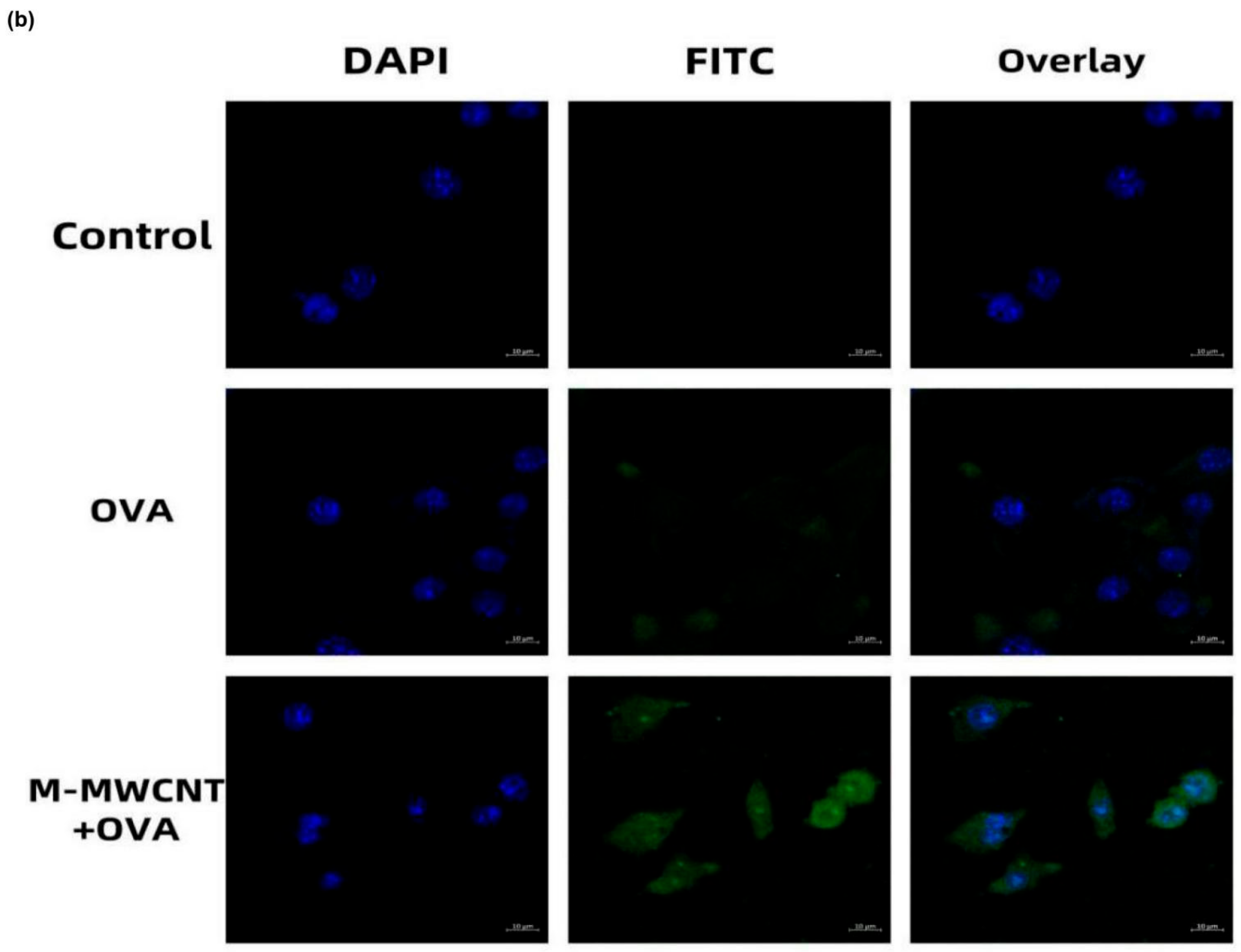
3.4. The Ultrastructure and Confocal Morphology of Macrophages
3.5. Activation and Maturation of Splenic Dendritic Cells
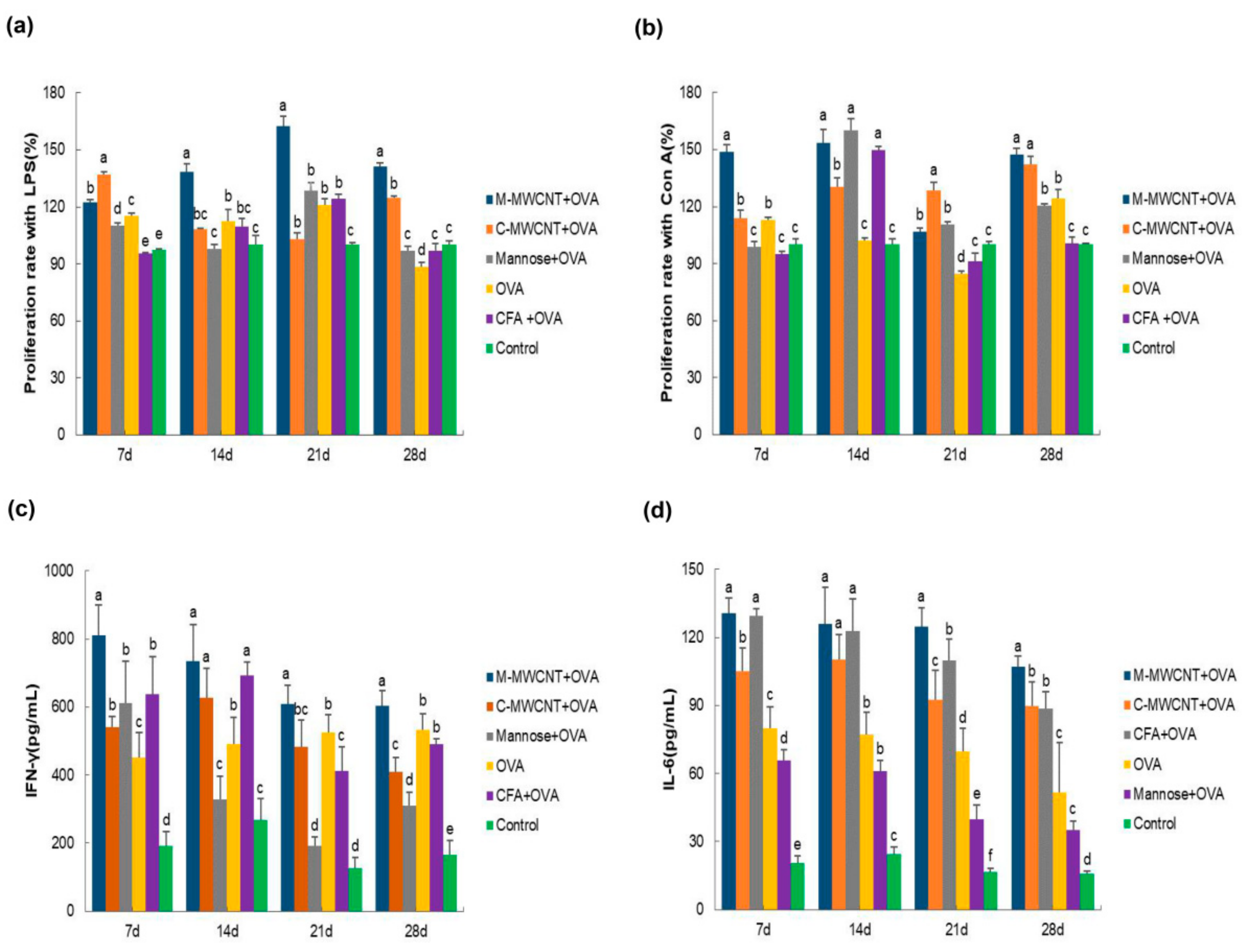
3.6. The Effects of M-MWCNT on Splenocytes Proliferation
3.7. The Effects of M-MWCNT on the Serum Concentration of IL-6 and IFN-γ
3.8. OVA-Specific IgG1, IgG, IgG2a, and IgG2b Serum Levels in Mice
3.9. In Vivo Biodistribution Analysis
3.10. In Vivo Histological Toxicity Analysis of M-MWCNT+OVA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Jogi, H.; Maheshwari, R.; Raval, N.; Kuche, K.; Tambe, V.; Mak, K.K.; Pichika, M.R.; Tekade, R.K. Carbon nanotubes in the delivery of anticancer herbal drugs. Nanomedicine 2018, 13, 1187–1220. [Google Scholar] [CrossRef]
- Panigrahi, B.K.; Nayak, A.K. Carbon Nanotubes: An Emerging Drug Delivery Carrier in Cancer Therapeutics. Curr. Drug Deliv. 2020, 17, 558–576. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef]
- Pasban, S.; Raissi, H.; Pakdel, M.; Farzad, F. Enhance the efficiency of 5-fluorouracil targeted delivery by using a prodrug approach as a novel strategy for prolonged circulation time and improved permeation. Int. J. Pharm. 2019, 568, 118491. [Google Scholar] [CrossRef]
- Pondman, K.M.; Salvador-Morales, C.; Paudyal, B.; Sim, R.B.; Kishore, U. Interactions of the innate immune system with carbon nanotubes. Nanoscale Horiz. 2017, 2, 174–186. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green LH, M.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; Mcdevitt, M.R.; Dao, T.; Justin, M.J.; Evan, F.; Simone, A. Carbon nanotubes as vaccine scaffolds. Adv. Drug Deliv. Rev. 2013, 65, 2016–2022. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis. Nanotoxicology 2018, 12, 153–168. [Google Scholar] [CrossRef]
- Ceballos-Alcantarilla, E.; Abad-Somovilla, A.; Agulló, C.; Abad-Fuentes, A.; Mercader, J.V. Protein-Free Hapten-Carbon Nanotube Constructs Induce the Secondary Immune Response. Bioconj. Chem. 2017, 28, 1630–1638. [Google Scholar] [CrossRef]
- Huang, X.P.; Tian, Y.J.; Shi, W.J.; Chen, J.K.; Yan, L.; Ren, L.J.; Zhang, X.F.; Zhu, J.B. Role of inflammation in the malignant transformation of pleural mesothelial cells induced by multi-walled carbon nanotubes. Nanotoxicology 2020, 14, 947–967. [Google Scholar] [CrossRef]
- Fukai, E.; Sato, H.; Watanabe, M.; Nakae, D.; Totsuka, Y. Establishment of an in vivo simulating co-culture assay platform for genotoxicity of multi-walled carbon nanotubes. Cancer Sci. 2018, 109, 1024–1031. [Google Scholar] [CrossRef]
- Santamaria, J.; Darrigues, J.; MVan Meerwijk, J.P.; Romagnoli, P. Antigen-presenting cells and T-lymphocytes homing to the thymus shape T cell development. Immunol. Lett. 2018, 204, 9–15. [Google Scholar] [CrossRef]
- Pescatori, M.; Bedognetti, D.; Venturelli EMénard-Moyon, C.; Bernardini, C.; Muresu, E.; Piana, A.; Maida, G.; Manetti, R.; Sgarrella, F.; Bianco, A.; et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 2013, 34, 4395–4403. [Google Scholar] [CrossRef]
- Fadel, T.R.; Fahmy, T.M. Immunotherapy applications of carbon nanotubes: From design to safe applications. Trends Biotechnol. 2014, 32, 198–209. [Google Scholar] [CrossRef]
- Bai, W.; Raghavendra, A.; Podila, R.; Brown, J.M. Defect density multiwalled carbon nanotubes influences ovalbumin adsorption and promotes macrophage activation and CD4(+) T-cell proliferation. Int. J. Nanomed. 2016, 11, 4357–4371. [Google Scholar]
- Xing, J.; Liu, Z.H.; Huang, Y.F.; Qin, T.; Bo, R.N.; Zheng, S.S.; Luo, L.; Huang, Y.E.; Niu, Y.L.; Wang, D.Y. Lentinan-Modified Carbon Nanotubes as an Antigen Delivery System Modulate Immune Response In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 19276–19283. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with Peptide-Functionalized Carbon Nanotubes Enhances Virus-Specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef]
- Silvestre, B.T.; Silveira, J.; Facury-Filho, E.J.; Carvalho, A.Ú.; Versiani, A.F.; Estevam, L.; Araújo, M.; Martins-Filho, O.A.; Negrão-Corrêa, D.A.; Ribeiro, M. Immune response and biochemistry of calves immunized with rMSP1a (Anaplasma mrginale) using a carbon nanotubes ascarrier molecules. Rev. Bras. Parasitol. Vet. 2018, 27, 191–202. [Google Scholar] [CrossRef]
- Engering, A.J.; Cella, M.; Fluitsma, D.M.; Hoefsmit, E.C.; Lanzavecchia, A.; Pieters, J. Mannose Receptor Mediated Antigen Uptake and Presentation in Human Dendritic Cells. Oxyg. Transp. Tissue 1997, 417, 183–187. [Google Scholar]
- Sehgal, K.; Dhodapkar, K.M.; Dhodapkar, M.V. Targeting human dendritic cells in situ to improve vaccines. Immunol. Lett. 2014, 162, 9–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, Q.; Huo, M.; Zhang, N.; Li, B.; Li, H.; Xu, Y.; Chen, M.; Hong, H.; Wang, Y. Mannose-Modified Multi-Walled Carbo Nanotubes as a Delivery Nanovector Optimizing the Antigen Presentation of Dendritic Cells. Chem. Open 2019, 8, 915–921. [Google Scholar]
- Hu JWei, P.; Seeberger, P.H.; Yin, J. Mannose-Functionalized Nanoscaffolds for Targeted Delivery in Biomedical Applications. Chem. Asian J. 2018, 13, 3448–3459. [Google Scholar]
- Zhu, D.W.; Hu, C.Y.; Fan, F.; Qin, Y.; Huang, C.H.; Zhang, Z.M. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials 2019, 206, 25–40. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, L.; Cui, L.; Zhu, X. Mannose: Good player and assister in pharmacotherapy. Biomed Pharm. 2020, 129, 110420. [Google Scholar] [CrossRef]
- Goswami, R.; Chatzikleanthous, D.; Lou, G.; Giusti, F.; Bonci, A.; Taccone, M. Mannosylation of LNP Results in Improved Potency for Self-Amplifying RNA (SAM) Vaccines. ACS Infect. Dis. 2019, 5, 1546–1558. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.C.; Tian, S.M.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.G.; Sun, X.Q.; Chen, Q.; Zhao, Y.H.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Feng, H.B.; Fan, J.; Lin, L.; Liu, Y.J.; Chai, D.K.; Yang, J. Immunomodulatory Effects of Phosphorylated Radix Cyathulae officinalis Polysaccharides in Immunosuppressed Mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef]
- Xia, C.H.; Dai, Q.; Wei, F. Study on infrared spectra of polysaccharides. J. Wuhan Univ. Technol. 2007, 29, 45–47. [Google Scholar]
- Raza, K.; Kumar, D.; Kiran, C.; Kumar, M.; Guru, S.K.; Kumar, P.; Arora, S.; Sharma, G.; Bhushan, S.; Katare, O.P. Conjugation of Docetaxel with Multiwalled Carbon Nanotubes and Codelivery with Piperine: Implications on Pharmacokinetic Profile and Anticancer Activity. Mol. Pharm. 2016, 13, 2423–2432. [Google Scholar] [CrossRef]
- Rode, A.; Sharma, S.; Mishra, D.K. Carbon Nanotubes: Classification, Method of Preparation and Pharmaceutical Application. Curr. Drug Deliv. 2018, 15, 620–629. [Google Scholar] [CrossRef]
- Biagiotti, G.; Pisaneschi, F.; Gammon, S.T.; Machetti, F.; Ligi, M.C.; Giambastiani, G.; Tuci, G.; Powell, E.; Piwnica-Worms, H.; Pranzini, E.; et al. Multiwalled Carbon Nanotubes for Combination Therapy: A Biodistribution and Efficacy Pilot Study. J. Mater. Chem. B 2019, 7, 2678–2687. [Google Scholar] [CrossRef]
- Smoleń, P.; Czujko, T.; Komorek, Z.; Grochala, D.; Rutkowska, A.; Osiewicz-Powęzka, M. Mechanical and Electrical Properties of Epoxy Composites Modified by Functionalized Multiwalled Carbon Nanotubes. Materials 2021, 14, 3325. [Google Scholar] [CrossRef]
- Wu, D.P. Preparation and Preliminary Study of CSFV E2 Protein Carbon Nanotube Antigen; Henan University of Science and Technology: Zhengzhou, China, 2015; pp. 5–57. [Google Scholar]
- Wang, S.Y.; Liu, R.C.; Yu, Q.; Dong, L.; Bi, Y.J.; Liu, G.W. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Hirano, S.; Zhou, Q.; Furuyama, A.; Kanno, S. Differential Regulation of IL-1beta and IL-6 Release in Murine Macrophages. Inflammation 2017, 40, 1933–1943. [Google Scholar] [CrossRef]
- Aarreberg, L.D.; Esser-Nobis, K.; Driscoll, C.; Shuvarikov, A.; Roby, J.A.; Gale, M., Jr. Interleukin-1beta Induces mtDNA Release to Activate Innate Immune Signaling via cGAS-STING. Mol. Cell. 2019, 74, 801–815.e6. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Narazaki, M.; Kishimoto, T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Zhao, X.X.; Jiang, Y.P.; Wang, L.; Li, Z.H.; Li, Q.; Feng, X.L. Advances in Understanding the Immune Imbalance between T-Lymphocyte Subsets and NK Cells in Recurrent Spontaneous Abortion. Geburtshilfe Frauenheilkd. 2018, 78, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wang, L.X.; He, J.Y.; Tan, J.H.; Liu, W.; Zhang, S.P.; Zhang, C.W.; Wang, Z.; Jiao, S.H.; Liu, S.J.; et al. Carbon nanotubes provoke inflammation by inducing the pro-inflammatory genes IL-1βand IL-6. Gene 2012, 493, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J. Study on the Factors Influencing the Immune Safety of Carbon Nanotubes; Peking Union Medical College: Beijing, China, 2016; pp. 5–16. [Google Scholar]
- Wang, R.H.; Lohray, R.; Chow, E.; Gangupantula, P.; Smith, L.; Draper, R. Selective Uptake of Carboxylated Multi-Walled Carbon Nanotubes by Class A Type 1 Scavenger Receptors and Impaired Phagocytosis in Alveolar Macrophages. Nanomaterials 2020, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Müller, K.H.; Koziol, K.K.K.; Skepper, J.N.; Midgley, P.A.; Welland, M.E.; Porter, A.E. Toxicity imaging of multi-walled carbon nanotubes in human macrophage cells. Biomaterials 2009, 30, 4152–4160. [Google Scholar] [CrossRef]
- Yao, W.J.; Jiao, Y.; Luo, J.; Du, M.Z.; Zong, L. Practical synthesis and characterization of mannose-modified chitosan. Int. J. Biol. Macromol. 2012, 50, 821–825. [Google Scholar] [CrossRef]
- Xia, Q.M. Study on Anti Prostate Cancer Immunotherapy Based on Peptide Modified Multi Walled Carbon Nanotubes Loaded with CpG; Naval Medical University of the Chinese People’s Liberation Army: Shanghai, China, 2018; pp. 4–15. [Google Scholar]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar]
- Tang, H.C.; Lai, Y.Y.; Zheng, J.; Jiang, H.Y.; Xu, G.J. miR-223-3p Inhibits Antigen Endocytosis and Presentation and Promotes the Tolerogenic Potential of Dendritic Cells through Targeting Mannose Receptor Signaling and Rhob. Immunol. Res. 2020, 18, 1379458. [Google Scholar] [CrossRef]
- Guo, Z.R.; Zhao, Z.; Zhang, C.; Jia, Y.J.; Qiu, D.K.; Zhu, B.; Wang, G.X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides). Fish Shellfish Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Heinzel, S.; Marchingo, J.M.; Horton, M.B.; Hodgkin, P.D. The regulation of lymphocyte activation and proliferation. Curr. Opin. Immunol. 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Pastori, D.; Bucci, T.; Triggiani, M.; Ames, P.R.J.; Parrotto, S.; Violi, F.; Pignatelli, P.; Farcomeni, A. Immunoglobulin G (IgG) anticardiolipin antibodies and recurrent cardiovascular events. A systematic review and Bayesian meta-regression analysis. Autoimmun. Rev. 2019, 18, 519–525. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.X.; Zhu, D.W.; Zhang, H.L.; Leng, X.G. Transactivator of transcription (TaT) peptide–chitosan functionalized multiwalled carbon nanotubes as a potential drug delivery vehicle for cancer therapy. Int. J. Nanomed. 2015, 10, 3829–3841. [Google Scholar]
- Li, R.B.; Wu, R.A.; Zhao, L.; Qin, H.Q.; Wu, J.L.; Zhang, J.W.; Bao, R.Y.; Zou, H.F. In vivo detection of magnetic labeled oxidized multi-walled carbon nanotubes by magnetic resonance imaging. Nanotechnology 2014, 25, 495102. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Feng, Y.; Lin, L.; Wu, D.; Liu, Q.; Li, H.; Zhang, X.; Li, S.; Tang, F.; Liu, Z.; et al. Mannose Receptor-Mediated Carbon Nanotubes as an Antigen Delivery System to Enhance Immune Response Both In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 4239. https://doi.org/10.3390/ijms23084239
Feng H, Feng Y, Lin L, Wu D, Liu Q, Li H, Zhang X, Li S, Tang F, Liu Z, et al. Mannose Receptor-Mediated Carbon Nanotubes as an Antigen Delivery System to Enhance Immune Response Both In Vitro and In Vivo. International Journal of Molecular Sciences. 2022; 23(8):4239. https://doi.org/10.3390/ijms23084239
Chicago/Turabian StyleFeng, Haibo, Yangyang Feng, Lang Lin, Daiyan Wu, Qianqian Liu, Hangyu Li, Xinnan Zhang, Sheng Li, Feng Tang, Ziwei Liu, and et al. 2022. "Mannose Receptor-Mediated Carbon Nanotubes as an Antigen Delivery System to Enhance Immune Response Both In Vitro and In Vivo" International Journal of Molecular Sciences 23, no. 8: 4239. https://doi.org/10.3390/ijms23084239
APA StyleFeng, H., Feng, Y., Lin, L., Wu, D., Liu, Q., Li, H., Zhang, X., Li, S., Tang, F., Liu, Z., & Zhang, L. (2022). Mannose Receptor-Mediated Carbon Nanotubes as an Antigen Delivery System to Enhance Immune Response Both In Vitro and In Vivo. International Journal of Molecular Sciences, 23(8), 4239. https://doi.org/10.3390/ijms23084239




