Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of the Research Question
2.2. Systematic Searching Strategies
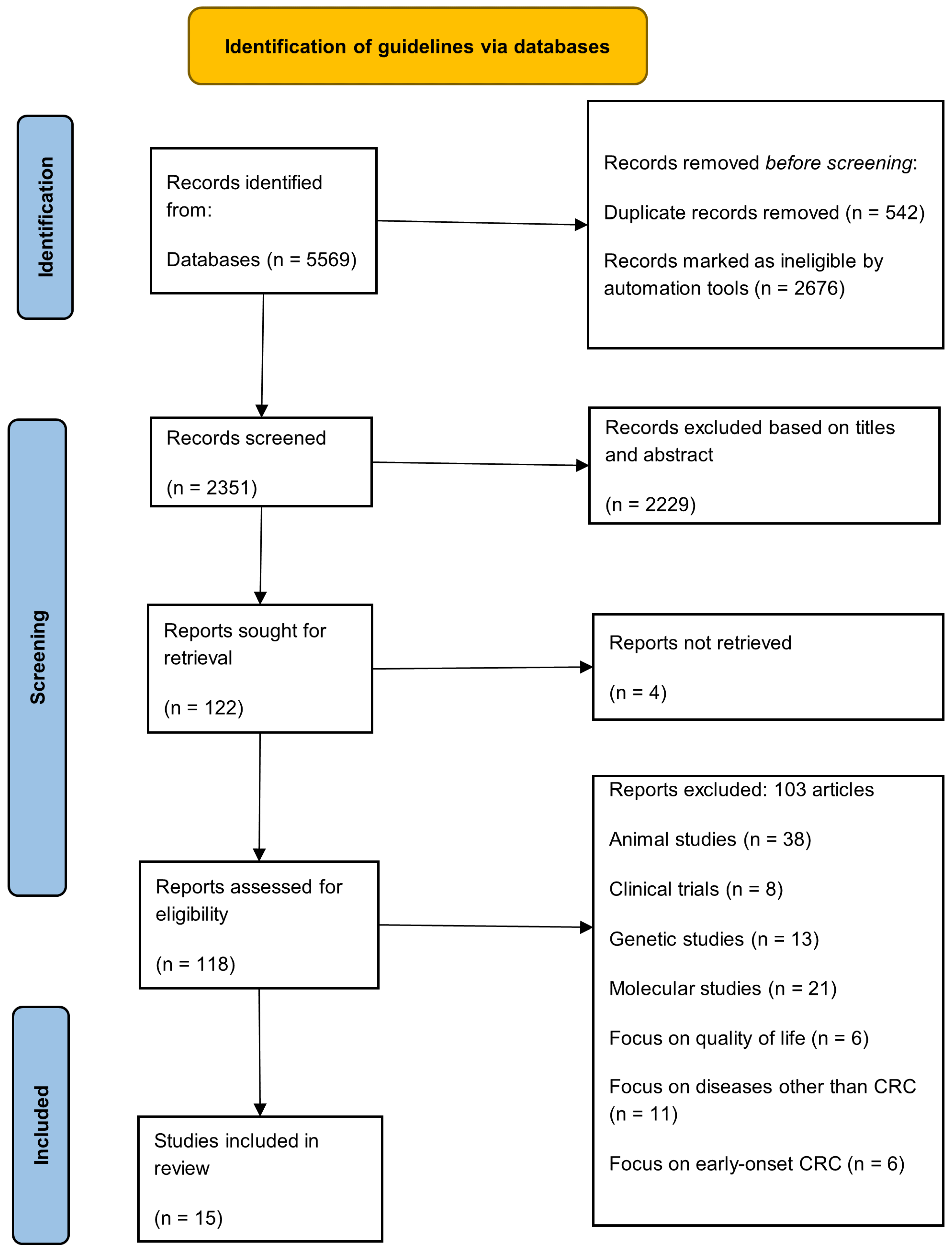
2.3. Identification
2.4. Screening
2.5. Eligibility
2.6. Quality Appraisal
2.7. Data Abstraction and Analysis
3. Results
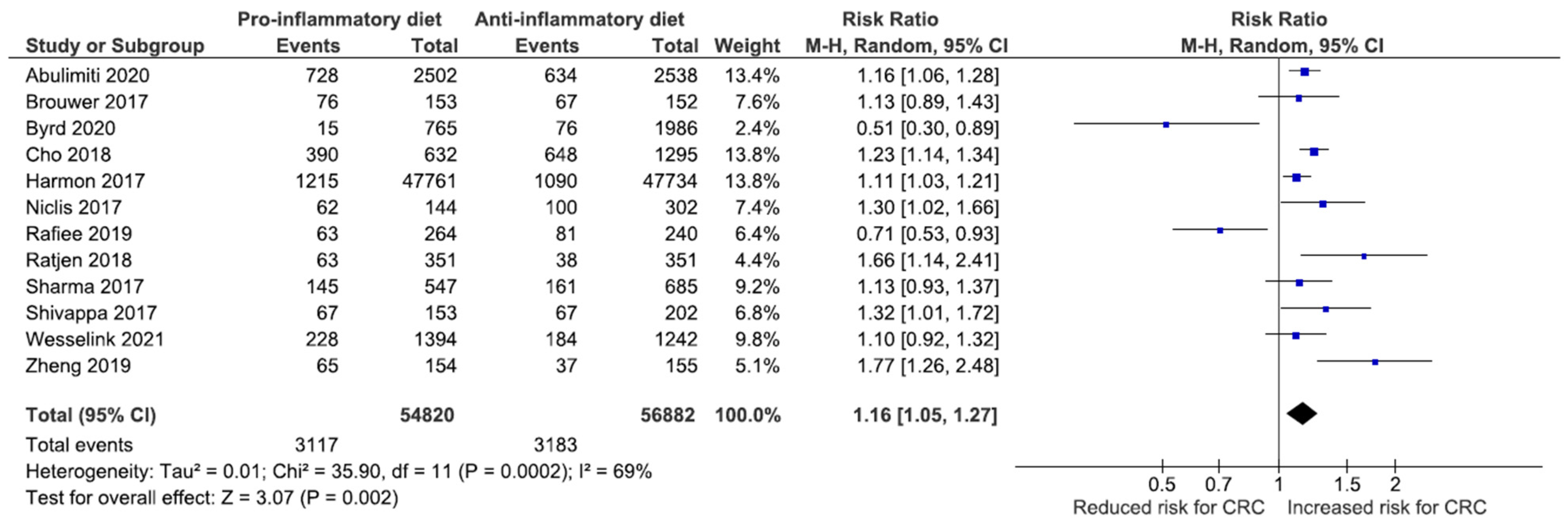
Association between DII and the Risk of CRC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. GLOBOCAN 2020-Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 23 December 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.C.; Prochaska, J.D.; Yu, X.; Kaul, S. An examination between census tract unhealthy food availability and colorectal cancer incidence. Epidemiol. Cancer 2020, 67, 101761. [Google Scholar] [CrossRef]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. et Biophys. Acta-Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef]
- Atef, N.; Alieldin, N.; Sherif, G.; Loay, I.; Mahmoud, A.M.; Mohamed, G. Microsatellite instability and life style factors in sporadic colorectal cancer. Asian Pac. J. Cancer Prev. 2020, 21, 1471–1480. [Google Scholar] [CrossRef]
- Seow, A.; Quah, S.R.; Nyam, D.; Straughan, P.T.; Chua, T.; Aw, T.C. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002, 95, 2390–2396. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary patterns and colorectal cancer risk in a Korean population. Medicine 2016, 95, e3759. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Hur, J.; Otegbeye, E.; Joh, H.-K.; Nimptsch, K.; Ng, K.; Ogino, S.; A Meyerhardt, J.; Chan, A.T.; Willett, W.C.; Wu, K.; et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021, 70, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Niclis, C.; Pou, S.A.; Shivappa, N.; Hébert, J.R.; Steck, S.E.; Díaz, M.D.P. Proinflammatory dietary intake is associated with increased risk of colorectal cancer: Results of a case-control study in Argentina using a multilevel modeling approach. Nutr. Cancer 2018, 70, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.E.; Akinyemiju, T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 372. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Cote, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Abulimiti, A.; Zhang, X.; Shivappa, N.; Hébert, J.R.; Fang, Y.-J.; Huang, C.-Y.; Feng, X.-L.; Chen, Y.-M.; Zhang, C.-X. The dietary inflammatory index is positively associated with colorectal cancer risk in a Chinese case-control study. Nutrients 2020, 12, 232. [Google Scholar] [CrossRef]
- Byrd, D.A.; Judd, S.E.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Agurs-Collins, T.; Bostick, R.M. Associations of Novel Dietary and Lifestyle Inflammation Scores with Incident Colorectal Cancer in the NIH-AARP Diet and Health Study. JNCI Cancer Spectr. 2020, 4, pkaa009. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Obón-Santacana, M.; Romaguera, D.; Gracia-Lavedan, E.; Molinuevo, A.; Molina-Montes, E.; Shivappa, N.; Hebert, J.R.; Tardón, A.; Castaño-Vinyals, G.; Moratalla, F.; et al. Dietary inflammatory index, dietary non-enzymatic antioxidant capacity, and colorectal and breast cancer risk (MCC-Spain study). Nutrients 2019, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, P.; Shivappa, N.; Hébert, J.R.; Nasab, S.J.; Bahrami, A.; Hekmatdoost, A.; Rashidkhani, B.; Sadeghi, A.; Houshyari, M.; Hejazi, E. Dietary inflammatory index and odds of colorectal cancer and colorectal adenomatous polyps in a case-control study from Iran. Nutrients 2019, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Zhu, Y.; Woodrow, J.R.; Mulay, S.; Parfrey, P.S.; Mclaughlin, J.R.; Hebert, J.R.; Shivappa, N.; Li, Y.; Zhou, X.; et al. Inflammatory diet and risk for colorectal cancer: A population-based case–control study in Newfoundland, Canada. Nutrition 2017, 42, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Steck, S.E.; Hofseth, L.J.; Shehadah, I.; Bani-Hani, K.E.; Al-Jaberi, T.; Al-Nusairr, M.; Heath, D.; Tayyem, R. Dietary Inflammatory Index and odds of colorectal cancer in a case-control study from Jordan. Appl. Physiol. Nutr. Metab. 2017, 42, 744–749. [Google Scholar] [CrossRef]
- Yuan, F.; Deng, L.; Sun, X.; Chen, Z.; Shivappa, N.; Sheth, A.K.; Cooper, G.S.; Hebert, J.R.; Li, L. Dietary inflammatory index and risk of colorectal adenoma: Effect measure modification by race, nonsteroidal anti-inflammatory drugs, cigarette smoking and body mass index? Cancer Causes Control. 2021, 32, 837–847. [Google Scholar] [CrossRef]
- Brouwer, J.G.M.; Makama, M.; van Woudenbergh, G.J.; Vasen, H.F.; Nagengast, F.M.; Kleibeuker, J.H.; Kampman, E.; Van Duijnhoven, F.J. Inflammatory potential of the diet and colorectal tumor risk in persons with Lynch syndrome. Am. J. Clin. Nutr. 2017, 106, 1287–1294. [Google Scholar] [CrossRef]
- Harmon, B.E.; Wirth, M.D.; Boushey, C.J.; Wilkens, L.R.; Draluck, E.; Shivappa, N.; Steck, S.E.; Hofseth, L.; Haiman, C.A.; Marchand, L.L. The Dietary Inflammatory Index is associated with colorectal cancer risk in the multiethnic cohort. J. Nutr. 2017, 147, 430–438. [Google Scholar] [CrossRef]
- Ratjen, I.; Shivappa, N.; Schafmayer, C.; Burmeister, G.; Nothlings, U.; Hampe, J.; Hebert, J.R.; Lieb, W.; Schlesinger, S. Association between the Dietary Inflammatory Index and All-Cause Mortality in Colorectal Cancer Long-Term Survivors. Int. J. Cancer 2019, 144, 1292–1301. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Lane, D.S.; Ho, G.Y.F.; Hou, L.; Snetselaar, L.; Ockene, J.K.; et al. Changes in the inflammatory potential of diet over time and risk of colorectal cancer in postmenopausal women. Am. J. Epidemiol. 2017, 186, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tabung, F.K.; Zhang, J.; Murphy, E.A.; Shivappa, N.; Ockene, J.K.; Caan, B.; Kroenke, C.H.; Hébert, J.R.; Steck, S.E. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the Women’s Health Initiative. Eur. J. Nutr. 2020, 59, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Staritsky, L.E.; van Zutphen, M.; Geijsen, A.J.; Kok, D.E.; Kruyt, F.; Veenstra, R.P.; Bilgen, E.J.S.; Kouwenhoven, E.A.; de Wilt, J.H.; et al. The association between the adapted dietary inflammatory index and colorectal cancer recurrence and all-cause mortality. Clin. Nutr. 2021, 40, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, H.; Qu, L.; Liu, H. Positive association between dietary inflammatory index and gastric cancer risk: A systematic review and meta-analysis. Nutr. Cancer 2020, 72, 1290–1296. [Google Scholar] [CrossRef]
- Eckert-dreher, R.G.; Coelho, D.; Zibetti, A.W.; Felipe, K.B.; Wilhelm-filho, D.; Pedrosa, R.C. Dietary Patterns and Empirical Dietary Inflammatory Index in Southern Brazil and Risk of Colorectal Cancer: A Case-Control Study. Food Nutr. Sci. 2020, 11, 281–300. [Google Scholar] [CrossRef][Green Version]
- Farazi, M.; Jayedi, A.; Shab-Bidar, S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: An umbrella review of meta-analyses of observational studies. Crit. Rev. Food Sci. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Association between the dietary inflammatory index and the incidence of cancer: A systematic review and meta-analysis of prospective studies. Public Health 2018, 164, 148–156. [Google Scholar] [CrossRef]
- Sun, D.; Cao, M.; Li, H.; He, S.; Chen, W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin. J. Cancer Res. 2020, 32, 129–139. [Google Scholar] [CrossRef]
- Steck, S.E.; Guinter, M.; Zheng, J.; Thomson, C.A. Index-based dietary patterns and colorectal cancer risk: A systematic review. Adv. Nutr. 2015, 6, 763–773. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary inflammatory index and colorectal cancer risk—a meta-analysis. Nutrients. 2017, 9, 1043. [Google Scholar] [CrossRef]
- Schulpen, M.; van den Brandt, P. A Mediterranean diet adherence and risk of colorectal cancer: The prospective Netherlands Cohort Study. Eur. J. Epidemiol. 2020, 35, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Khil, H.; Kim, S.M.; Hong, S.E.; Gil, H.M.; Cheon, E.; Lee, D.H.; Kim, Y.A.; Keum, N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Jo, C.; Yoon, Y.; Jeong, J.Y.; Lee, K.T. Controversy on the correlation of red and processed meat consumption with colorectal cancer risk: An Asian perspective. Crit. Rev. Food Sci. Nutr. 2019, 59, 3526–3537. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Mahdavinia, M.; Vacca, M.; Malekzadeh, R.; Mariani-Costantini, R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014, 20, 6055–6072. [Google Scholar] [CrossRef]
- Kenkhuis, M.F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; VAN Duijnhoven, F.J.B.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Sedentary Behavior and Physical Activity with Quality of Life in Colorectal Cancer Survivors. Med. Sci. Sports Exerc. 2021, 53, 2298–2308. [Google Scholar] [CrossRef]
- Soffian, S.S.S.; Nawi, A.M.; Hod, R.; Chan, H.K.; Hassan, M.R.A. Area-level determinants in colorectal cancer spatial clustering studies: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 10486. [Google Scholar] [CrossRef]
- Sko, F.; Carlsson, A.C.; Schmidt, P.T.; Forsberg, A.M. The prediction of colorectal cancer using anthropometric measures: A Swedish population-based cohort study with 22 years of follow-up. United Eur. Gastroenterol. J. 2019, 7, 1250–1260. [Google Scholar] [CrossRef]
- Carr, P.R.; Amitay, E.L.; Jansen, L.; Alwers, E.; Roth, W.; Herpel, E.; Kloor, M.; Schneider, M.; Bläker, H.; Chang-Claude, J.; et al. Association of BMI and major molecular pathological markers of colorectal cancer in men and women. Am. J. Clin. Nutr. 2020, 111, 562–569. [Google Scholar] [CrossRef]
- Li, H.; Boakye, D.; Chen, X.; Hoffmeister, M.; Brenner, H. Association of Body Mass Index with Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. Suppl. 2021, 116, 2173–2183. [Google Scholar] [CrossRef]
- Li, J.-B.; Luo, S.; Wong, M.C.S.; Li, C.; Feng, L.-F.; Peng, J.-H.; Li, J.-H.; Zhang, X. Longitudinal associations between BMI change and the risks of colorectal cancer incidence, cancer-relate and all-cause mortality among 81,388 older adults. BMC Cancer 2019, 19, 1082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Du, M.; Zhang, B.; Xin, J.; Chu, H.; Ni, M.; Zhang, Z.; Gu, D.; Wang, M. Body mass index (BMI) trajectories and risk of colorectal cancer in the PLCO cohort. Br. J. Cancer 2018, 119, 130–132. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Mitchell, J.S.; Law, P.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br. J. Cancer 2016, 115, 266–272. [Google Scholar]
- Thrift, A.P.; Gong, J.; Peters, U.; Chang-Claude, J.; Rudolph, A.; Slattery, M.L.; Chan, A.T.; Locke, A.E.; Kahali, B.; Justice, A.E.; et al. Mendelian randomization study of body mass index and colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Goto, A.; Nakatochi, M.; Naritaet, A.; Yamaji, T.; Sawada, N.; Katagiri, R.; Iwagami, M.; Hanyuda, A.; Hachiya, T.; et al. Body mass index and colorectal cancer risk: A Mendelian randomization study. Cancer Sci. 2021, 112, 1579–1588. [Google Scholar] [CrossRef] [PubMed]

| Database | Search String |
|---|---|
| Scopus | TITLE-ABS-KEY ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer *” OR “colorectal neoplas *” OR “colorectal tumo * r” OR “colorectal malignanc *”)) |
| Web of Science | TS = ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer *” OR “colorectal neoplas *” OR “colorectal tumo * r” OR “colorectal malignanc *”)) |
| PubMed | ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer” OR “colorectal neoplasm” OR “colorectal tumor” OR “colorectal tumour” OR “colorectal malignancy” OR “colorectal malignancies”)) |
| EBSCOHost | ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer” OR “colorectal neoplasm” OR “colorectal tumor” OR “colorectal tumour” OR “colorectal malignancy” OR “colorectal malignancies”)) |
| Studies (Case-Control Studies) | Selection (Maximum ****) | Comparability (Maximum **) | Exposure (Maximum ***) | Total Scores (Maximum 9) | |||||
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | ||
| Abulimi et al., 2020 [21] | * | * | * | * | ** | - | * | - | 7 |
| Byrd et al., 2020 [22] | * | * | * | * | ** | * | * | - | 8 |
| Cho et al., 2019 [23] | - | * | - | * | ** | * | * | - | 6 |
| Niclis et al., 2018 [14] | * | - | * | * | * | - | - | * | 5 |
| Obon-Santacana, 2019 [24] | - | - | - | * | ** | - | * | - | 4 |
| Rafiee et al., 2019 [25] | * | * | * | * | * | * | * | - | 7 |
| Sharma et al., 2017 [26] | - | * | * | * | * | * | * | - | 6 |
| Shivappa et al., 2017 [27] | * | * | - | * | * | * | * | - | 6 |
| Yuan et al., 2021 [28] | * | * | - | * | * | * | * | - | 6 |
| Studies (cohort studies) | Selection (maximum ****) | Comparability (maximum **) | Outcome (maximum ***) | Total scores (maximum 9) | |||||
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Brouwer et al., 2017 [29] | * | - | * | * | * | * | * | * | 7 |
| Harmon et al., 2017 [30] | * | - | - | * | - | * | * | - | 4 |
| Ratjen et al., 2019 [31] | * | - | * | * | - | * | * | * | 6 |
| Tabung et al., 2017 [32] | * | * | * | * | * | * | * | * | 8 |
| Zheng et al., 2020 [33] | * | - | * | * | * | * | * | * | 7 |
| Wesselink et al., 2021 [34] | * | - | * | * | * | * | * | * | 7 |
| Author, Year | Study Location | Study Design | Study Period | Study Instrument | Number of Food Parameters | Sample Size | Range of DII Scores | Type of Data and Comparison | Measures of Association | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Abulimiti et al., 2020 [21] | China | Case control | 2010–2019 | 81-item FFQ 1 | 34 | 2502 cases | −5.96 to +6.01 | Categorical | OR = 1.40 (95% CI 1.16, 1.68) | Age, sex, marital status, residence, education level, occupation, income, BMI 2, smoking status, family history of CRC, comorbidities |
| 2538 controls | Quartile 4 vs. Quartile 1 | |||||||||
| Brouwer et al., 2017 [29] | Netherlands | Prospective cohort | 2006–2012 | 183-item FFQ | 28 | 457 | −11.7 to +8.4 | Categorical | HR = 1.37 (95% CI 0.80, 2.34; p > 0.05) | Age, smoking status, education level |
| Tertile 3 (0.3 to 8.4) vs. Tertile 1 (−11.7 to <−1.8) | ||||||||||
| Byrd et al., 2020 [22] | United States | Case control | 1991–2002 | 126-item FFQ | 19 | 765 cases | (controls): −0.7 ± 2.4 | Categorical | OR = 1.31 (95% CI 0.98, 1.75) | Age, sex, education, NSAIDs 3 use, hormone use, family history of CRC, smoking status, BMI, alcohol intake, physical activity |
| 1986 controls | (cases): −0.5 ± 2.4 | Quintile 5 vs. Quintile 1 | ||||||||
| Cho et al., 2019 [23] | Korea | Case control | 2010–2013 | 106-item FFQ | 35 | 632 cases | (controls): 0.94 ± 2.24 | Categorical | OR = 1.38 (95% CI 1.12, 1.71) | Age, sex, family history of CRC, education level, BMI, physical activity, smoking status, alcohol intake |
| 1295 controls | (cases): 1.77 ± 1.97 | High vs. Low | ||||||||
| Harmon et al., 2017 [30] | United States | Prospective cohort | 1993–2010 | 169-item FFQ | 28 | 190,963 | −6.64 to +4.95 | Categorical | HR = 1.21 (95% CI 1.11, 1.32) | Age, sex, race, comorbidities, smoking status, BMI, family history of CRC, education level, aspirin use, hormones use |
| Quartile 4 (−0.52 to 4.95) vs. Quartile 1 (−6.64 to −3.66) | ||||||||||
| Niclis et al., 2018 [14] | Argentina | Case control | 2008–2015 | 127-item FFQ | 22 | 144 cases | −3.15 to +3.77 | Categorical | OR = 1.56 (95% CI 1.20, 2.03) | Age, sex, BMI, smoking status, socioeconomic status, physical activity, NSAIDs use |
| 302 controls | Tertile II (0.6–1.86) vs. Tertile 1 (<0.65) | |||||||||
| Obon-Santacana et al., 2019 [24] | Spain | Case control | 2008–2013 | 140-item FFQ | 30 | 1852 cases | (men): −5.11 to 5.47 | Continuous DII (per one unit increase) | OR = 1.14 (95% CI 1.10, 1.18) | Sex, age, education level, study area, family history of CRC, smoking status, physical activity, BMI, NSAIDs use |
| 3447 controls | (women): −5.64 to 5.12 | |||||||||
| Rafiee et al., 2019 [25] | Iran | Case control | 2017–2018 | 148-items FFQ | 21 | 134 cases | −4.23 to +3.89 | Categorical | OR = 2.64 (95% CI 1.40, 4.99) | Age, sex, physical activity, salt intake, comorbidities, smoking, family history of CRC, cooking method, supplement intake |
| 240 controls | Tertile 3 (>0.04) vs. Tertile 1 (<−1.13) | |||||||||
| Ratjen et al., 2019 [31] | Germany | Prospective cohort | 2009–2011 | 112-item FFQ | 27 | 1404 | −3.99 to +4.11 | Continuous DII (per one unit increase) | HR = 1.08 (95% CI 0.97, 1.20) | Sex, age at diet assessment, BMI, physical activity, survival time, tumor location, metastasis, other type of cancers, therapy, smoking status, alcohol intake |
| Sharma et al., 2017 [26] | Canada | Case control | 1999–2003 | 169-item FFQ | 29 | 547 cases | −5.19 to +6.93 | Categorical | OR = 1.65 (95% CI 1.13, 2.42) | Age, sex, BMI, physical activity, comorbidities, family history of CRC, smoking status, alcohol intake, NSAIDs use |
| 685 controls | Quartile 4 (≥0.3582) vs. Quartile 1 (<−2.036) | |||||||||
| Wesselink et al., 2021 [34] | Netherlands | Prospective cohort | 2010–2017 | 204-item FFQ | 28 | 1478 | −12.2 to +8.5 | Categorical | HR = 0.98 (95% CI 0.94, 1.04; p > 0.05) | Age, sex, staging, BMI, smoking status, NSAIDs use, comorbidities |
| Tertile 3 (1.2 to <8.5) vs. Tertile 1 (−12.2 to <−1.0) | ||||||||||
| Shivappa et al., 2017 [27] | Jordan | Case control | 2010–2012 | 90-item FFQ | 18 | 153 cases | −2.25 to +2.86 | Continuous DII (per one unit increase) | OR = 1.45 (95% CI 1.13, 1.85) | Age, sex, education level, physical activity, BMI, smoking status, family history of CRC |
| 202 controls | ||||||||||
| Tabung et al., 2017 [32] | United States | Prospective cohort | 1993–2014 | 122-item FFQ | 32 | 87,042 | −6.62 to +5.39 | Categorical | HR = 1.06 (95% CI 0.90, 1.26) | Age, race, education level, smoking status, comorbidities, regular NSAIDs use, estrogen use, BMI, physical activity |
| Quintiles 5 vs. Quintiles 1 | ||||||||||
| Yuan et al., 2021 [28] | United States | Case control | 2005–2015 | 175-item FFQ | 34 | 587 cases | −5.9 to +4.6 | Continuous DII (per one unit increase) | OR = 1.07 (95% CI 0.97, 1.19) | Age, gender, race, BMI, education level, smoking status, comorbidities, NSAIDs use, family history of CRC, supplements use |
| 1313 controls | ||||||||||
| Zheng et al., 2020 [33] | United States | Prospective cohort | 1993–2015 | 122-item FQ | 32 | 161,808 | −6.80 to +3.25 | Categorical | HR = 0.72 (95% CI 0.46, 1.12) | Age, race, smoking status, income levels, cancer staging, education level, physical activity, BMI |
| Tertile 1 | ||||||||||
| (−5.96 to −2.25) vs. Tertile 3 (−0.18 to 3.82) |
| Subgroups | No. of Studies | RR (95% CI) | Heterogeneity | Significance Test | ||
|---|---|---|---|---|---|---|
| I2 (%) | p | Z | p | |||
| Study design | ||||||
| Case-control | 7 | 1.14 (0.89, 1.45) | 81% | 0.000 | 1.03 | 0.300 |
| Cohort | 4 | 1.24 (1.06, 1.44) | 63% | 0.030 | 2.74 | 0.006 |
| Groups | ||||||
| Continuous | 4 | 0.35 (0.28, 0.41) | 0% | 0.400 | 10.12 | 0.000 |
| Categorical | 3 | 1.61 (1.26, 2.05) | 0% | 0.900 | 3.80 | 0.000 |
| Region | ||||||
| AMR | 4 | 0.32 (0.24, 0,40) | 62% | 0.050 | 8.29 | 0.000 |
| EUR | 4 | 0.40 (0.33, 0.47) | 98% | 0.000 | 10.50 | 0.000 |
| Asia | 2 | 0.44 (0.34, 0.54) | 95% | 0.000 | 8.59 | 0.000 |
| EMR | 2 | 0.36(0.21, 0.52) | 22% | 0.260 | 4.61 | 0.000 |
| Study period | ||||||
| Less than 10 years | 11 | 1.12 (0.94, 1.35) | 97% | 0.000 | 1.27 | 0.200 |
| 10 years or more | 2 | 2.95 (2.47, 3.52) | 92% | 0.001 | 12.01 | 0.000 |
| Adjustment for family history of CRC | ||||||
| Yes | 8 | 1.01 (0.82, 1.24) | 97% | 0.000 | 0.06 | 0.950 |
| No | 5 | 1.31 (1.10, 1.56) | 55% | 0.060 | 3.01 | 0.003 |
| Adjustment for education level | ||||||
| Yes | 8 | 1.11 (0.89, 1.39) | 98% | 0.000 | 0.93 | 0.350 |
| No | 5 | 1.12 (0.90, 1.39) | 75% | 0.003 | 1.04 | 0.300 |
| Adjustment for comorbidities | ||||||
| Yes | 5 | 1.08 (0.97, 1.20) | 64% | 0.030 | 1.41 | 0.160 |
| No | 8 | 1.18 (0.92, 1.50) | 96% | 0.000 | 1.28 | 0.200 |
| Adjustment for physical activity | ||||||
| Yes | 9 | 1.11 (0.89, 1.39) | 95% | 0.000 | 0.93 | 0.350 |
| No | 4 | 1.13 (1.07,1.20) | 0% | 0.890 | 4.38 | 0.000 |
| Adjustment for BMI | ||||||
| Yes | 5 | 1.60 (1.54, 1.67) | 96% | 0.000 | 23.81 | 0.000 |
| No | 2 | 0.86 (0.78, 0.96) | 92% | 0.001 | 2.84 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed Soffian, S.S.; Mohammed Nawi, A.; Hod, R.; Ja’afar, M.H.; Isa, Z.M.; Chan, H.-K.; Hassan, M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022, 14, 1555. https://doi.org/10.3390/nu14081555
Syed Soffian SS, Mohammed Nawi A, Hod R, Ja’afar MH, Isa ZM, Chan H-K, Hassan MRA. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients. 2022; 14(8):1555. https://doi.org/10.3390/nu14081555
Chicago/Turabian StyleSyed Soffian, Sharifah Saffinas, Azmawati Mohammed Nawi, Rozita Hod, Mohd Hasni Ja’afar, Zaleha Md Isa, Huan-Keat Chan, and Muhammad Radzi Abu Hassan. 2022. "Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer" Nutrients 14, no. 8: 1555. https://doi.org/10.3390/nu14081555
APA StyleSyed Soffian, S. S., Mohammed Nawi, A., Hod, R., Ja’afar, M. H., Isa, Z. M., Chan, H.-K., & Hassan, M. R. A. (2022). Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients, 14(8), 1555. https://doi.org/10.3390/nu14081555






