Review of Electrochemical Testing to Assess Corrosion of Post-Tensioned Tendons with Segregated Grout
Abstract
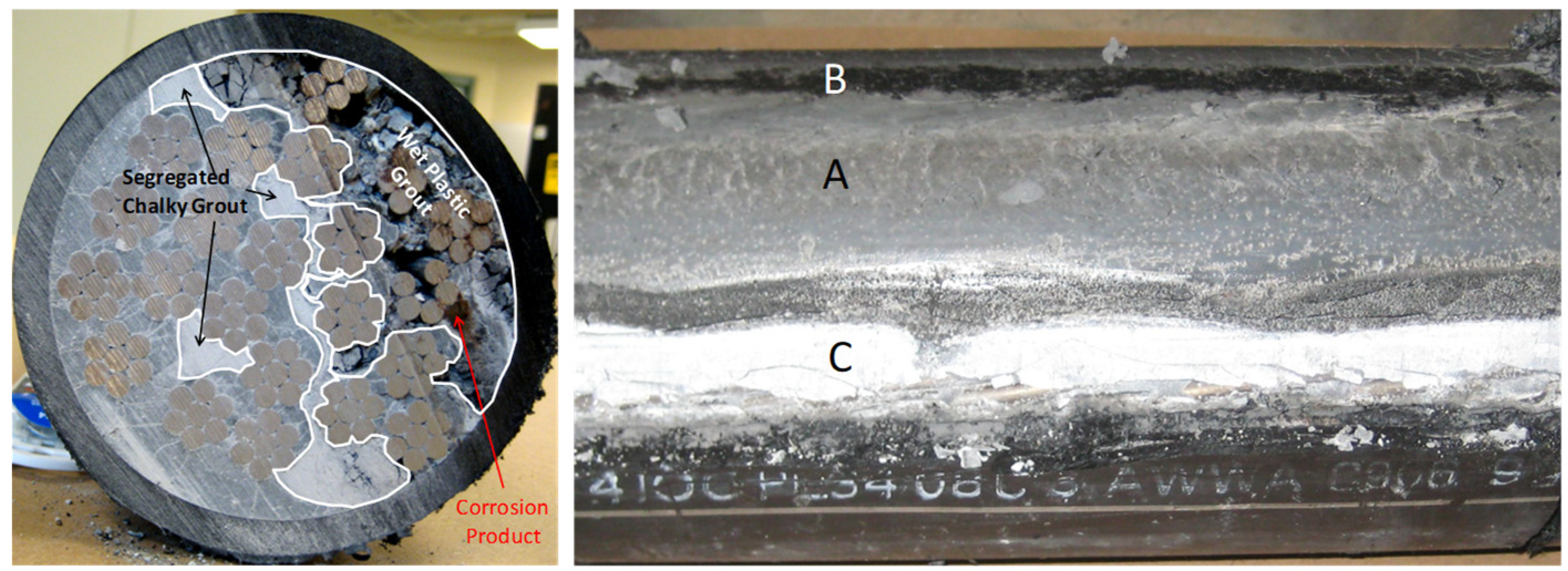
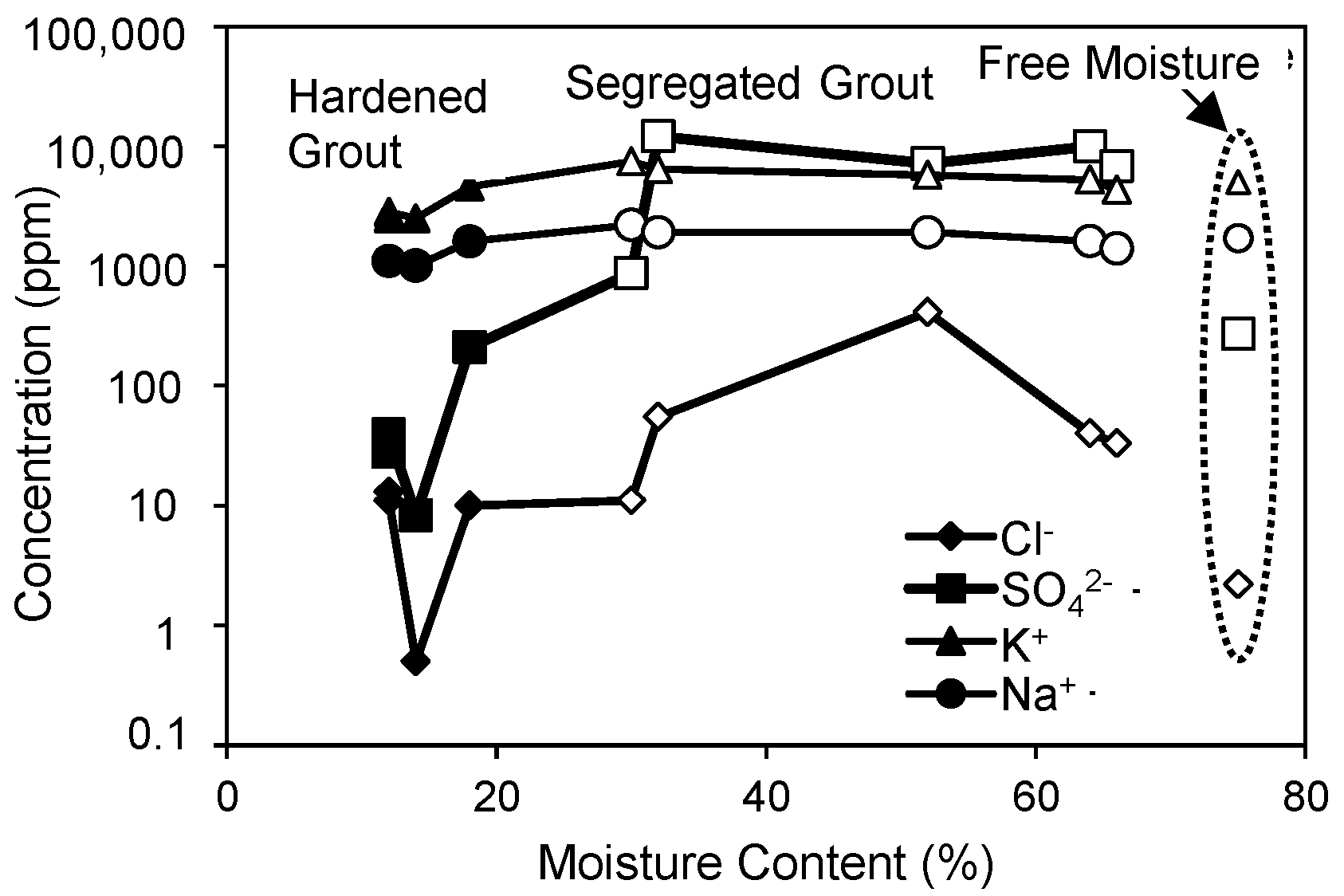
1. Introduction
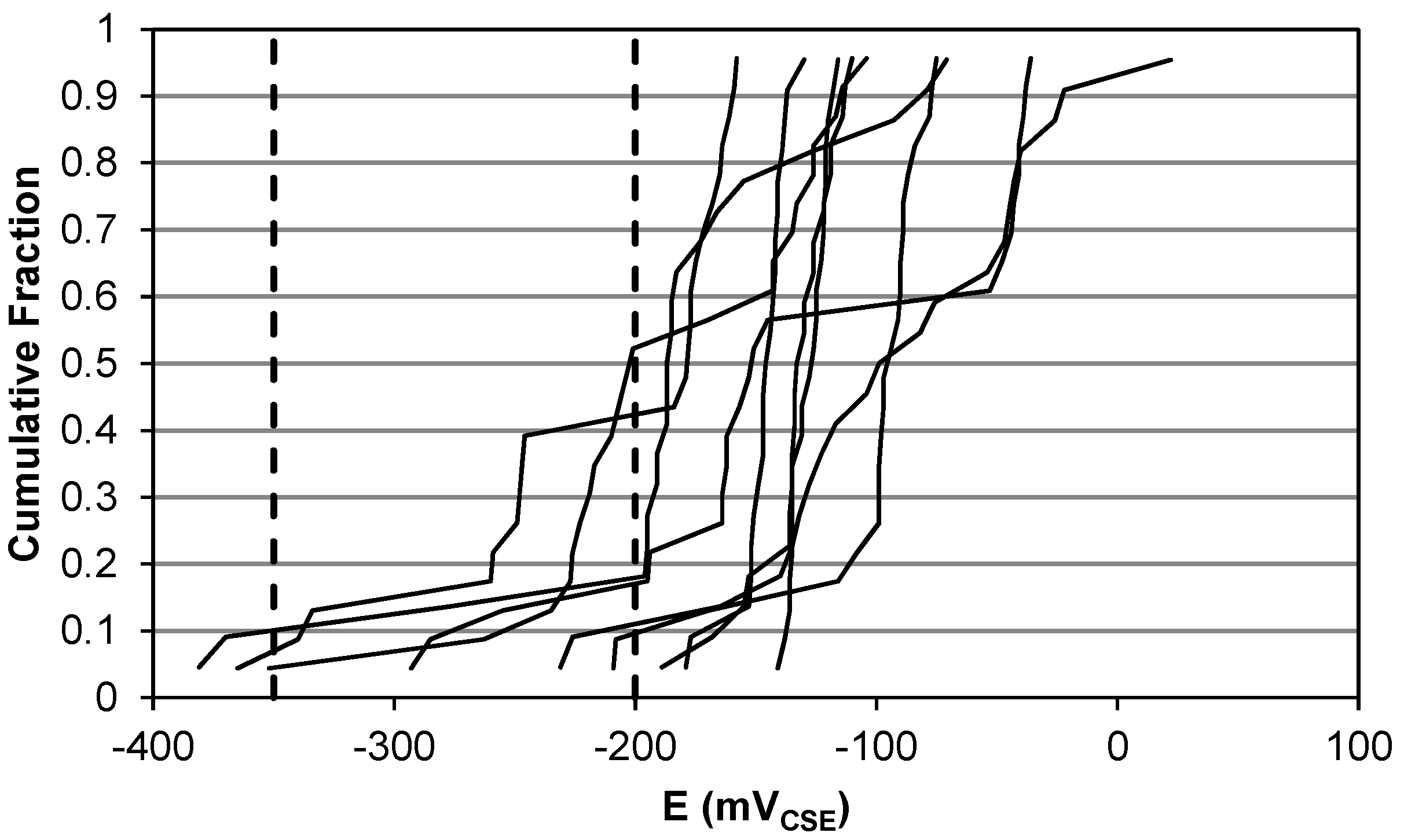
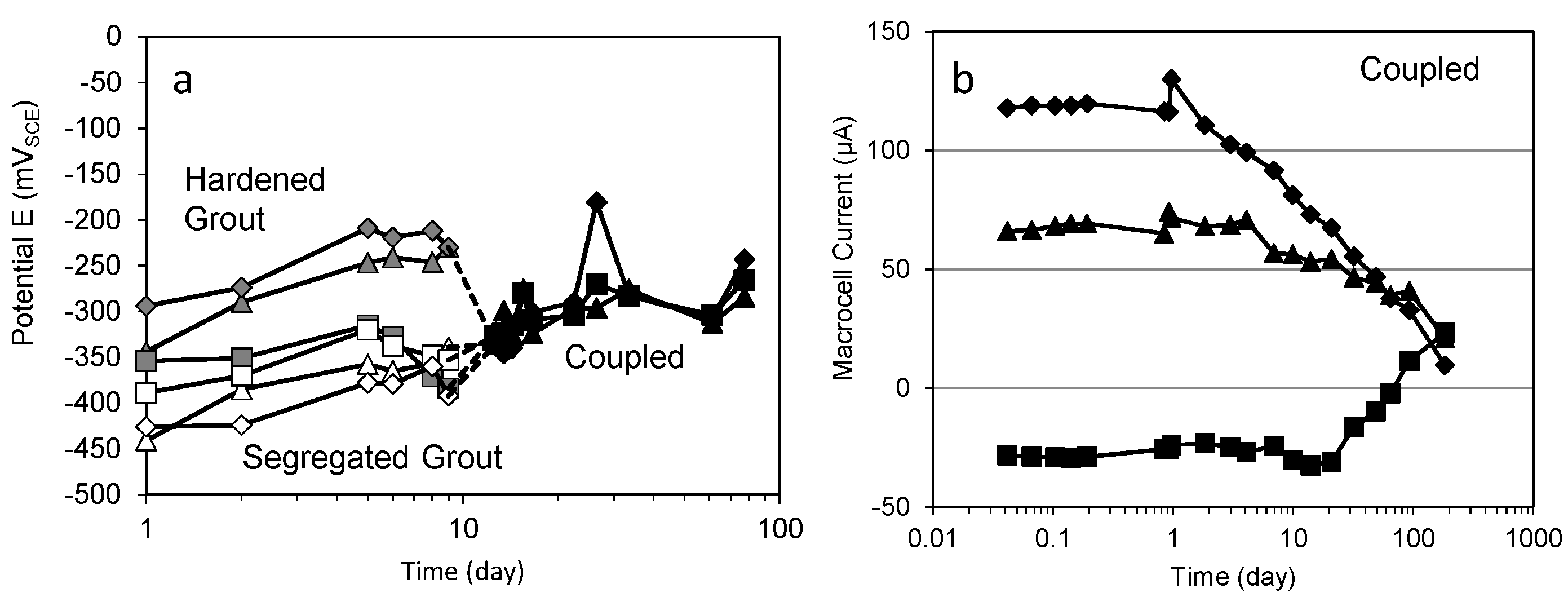
2. Open-Circuit Potential
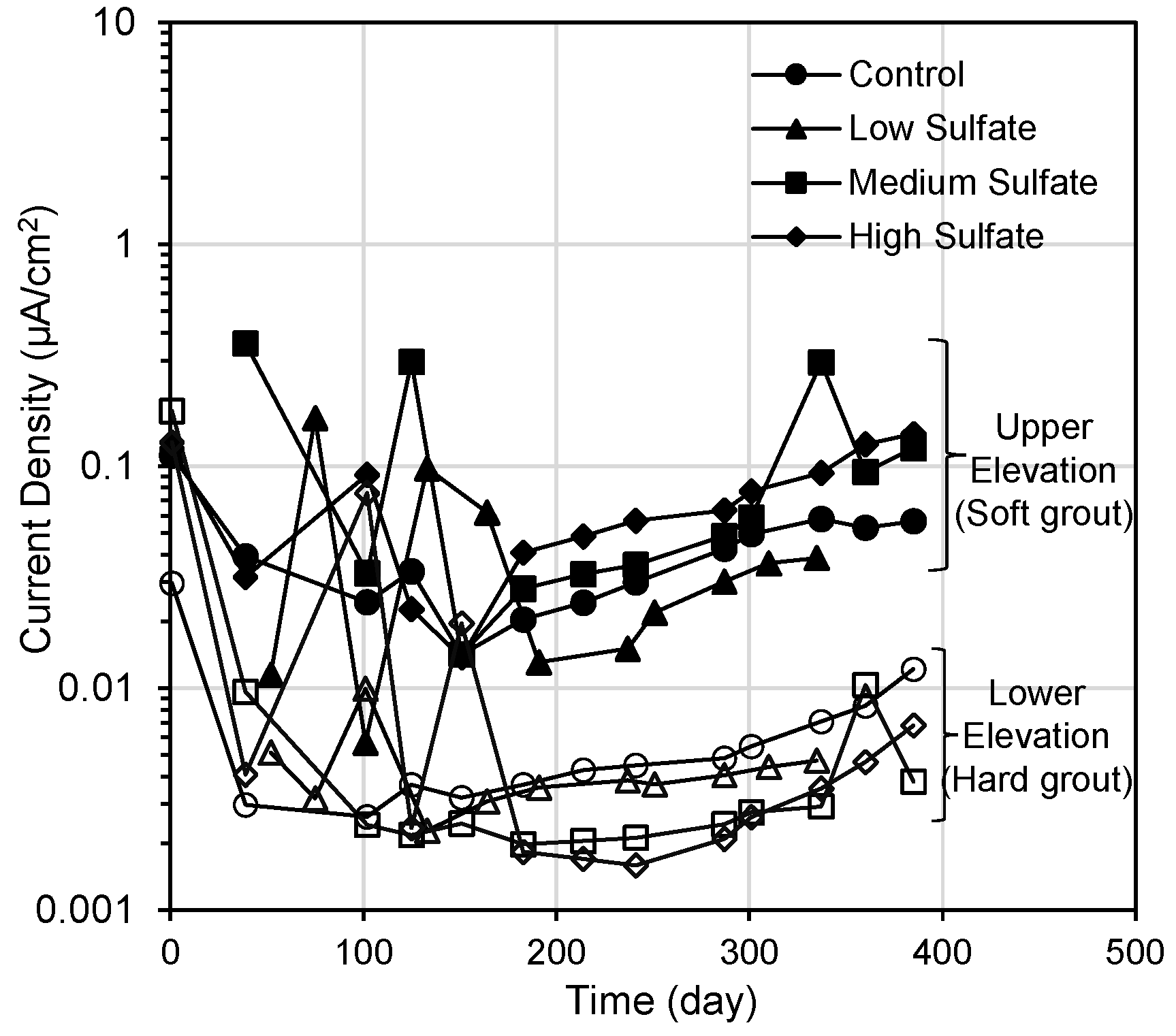
3. Linear Polarization Resistance
4. Macrocell Corrosion
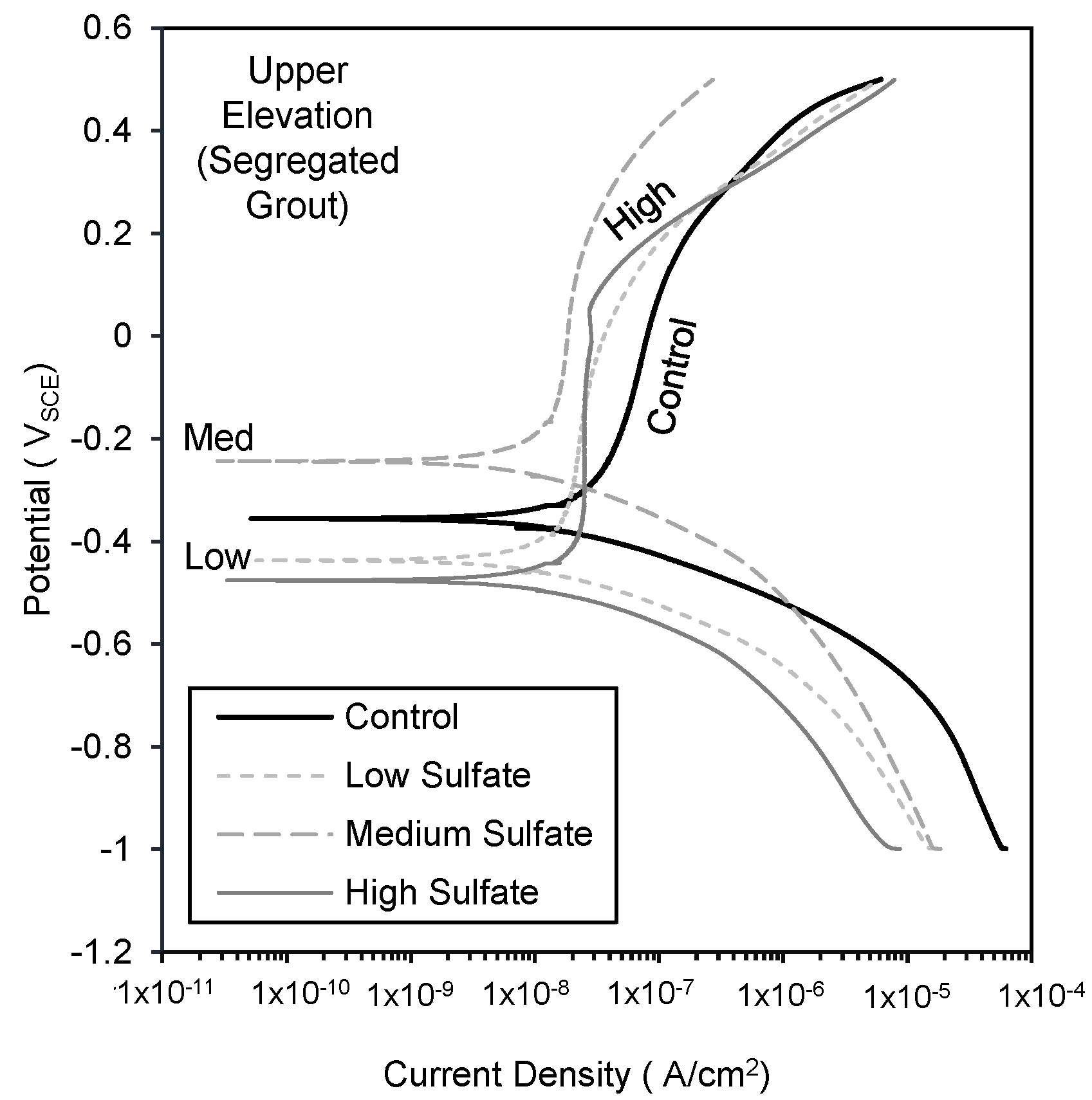
5. Anodic Potentiodynamic Polarization
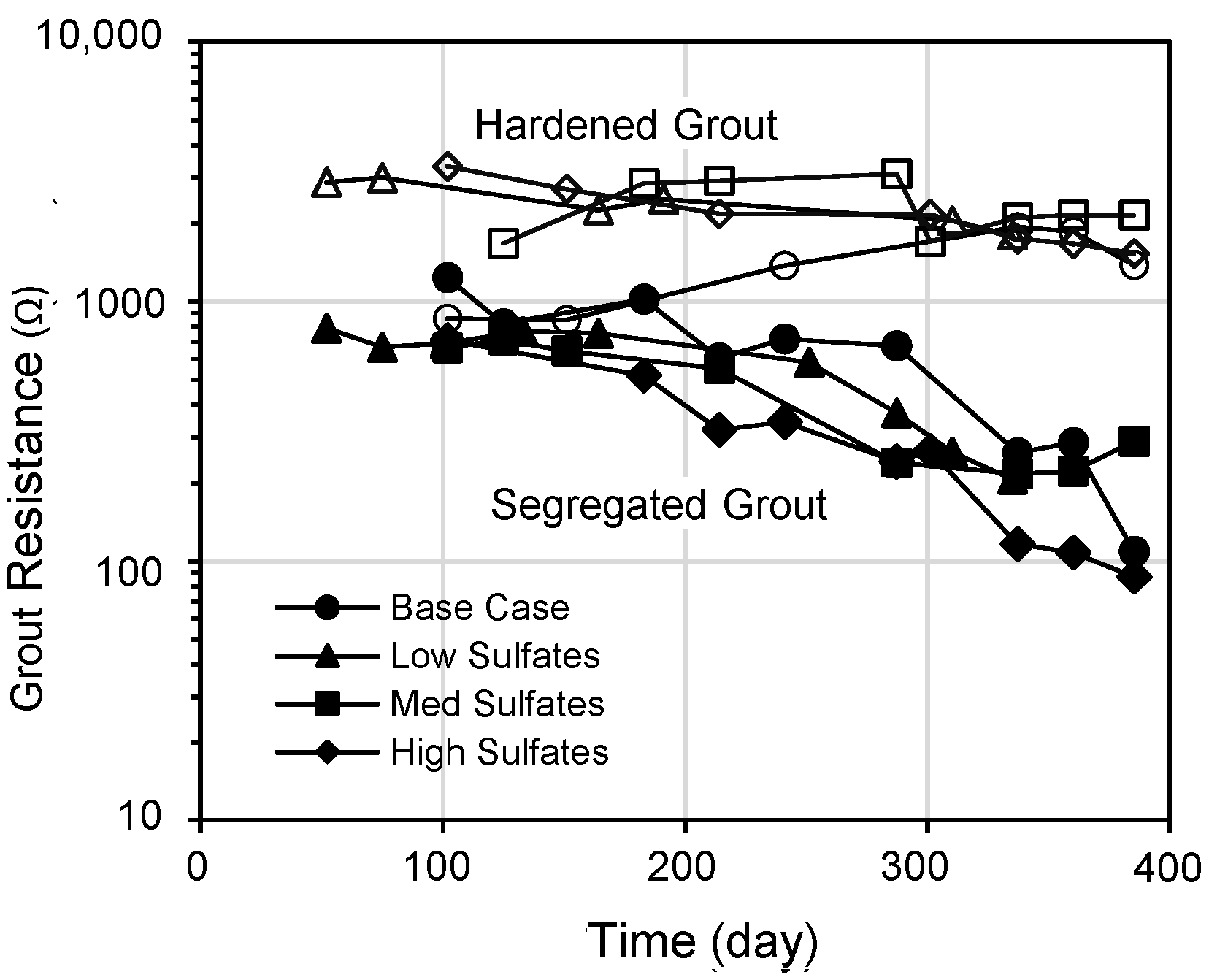
6. Electrochemical Impedance Spectroscopy
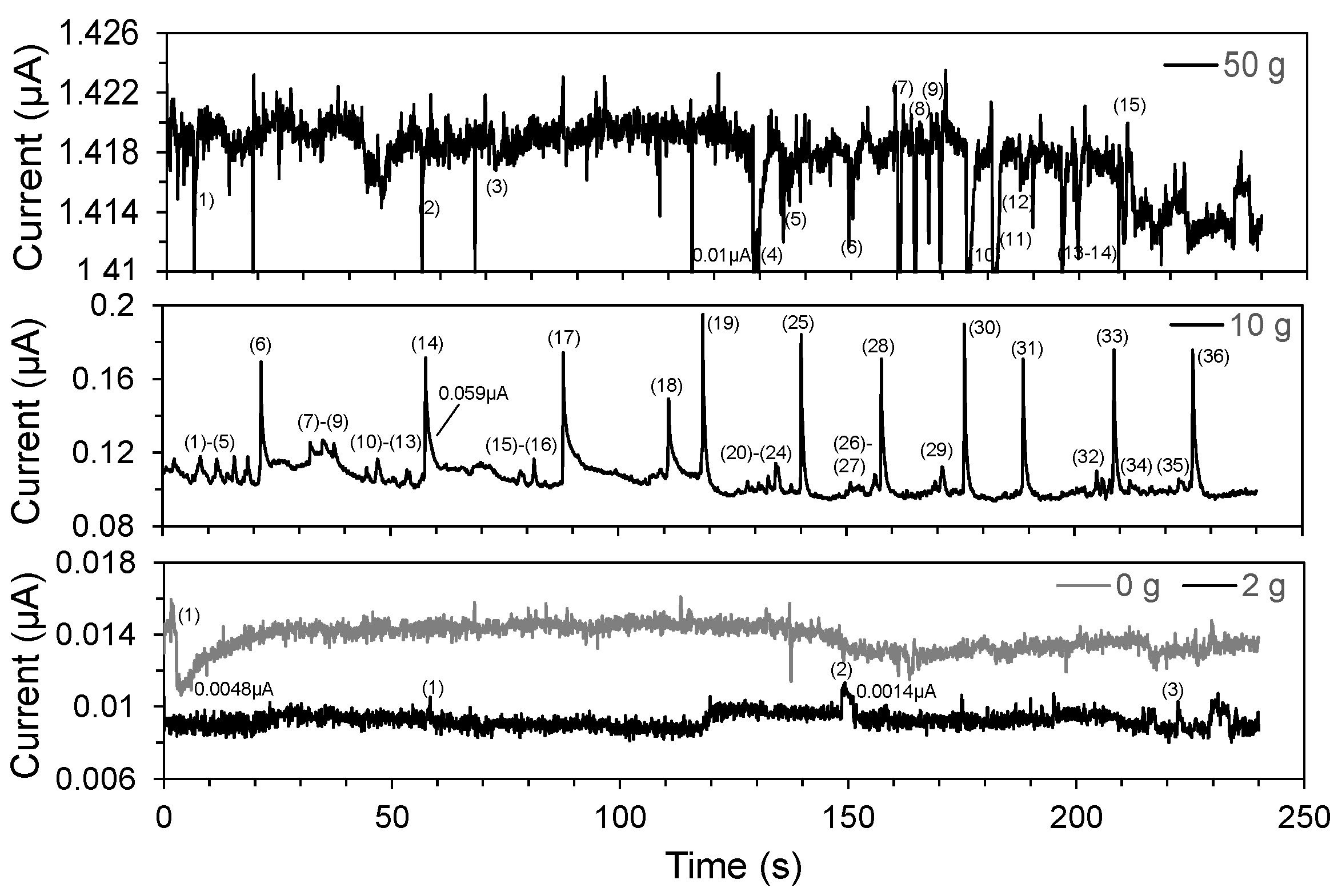
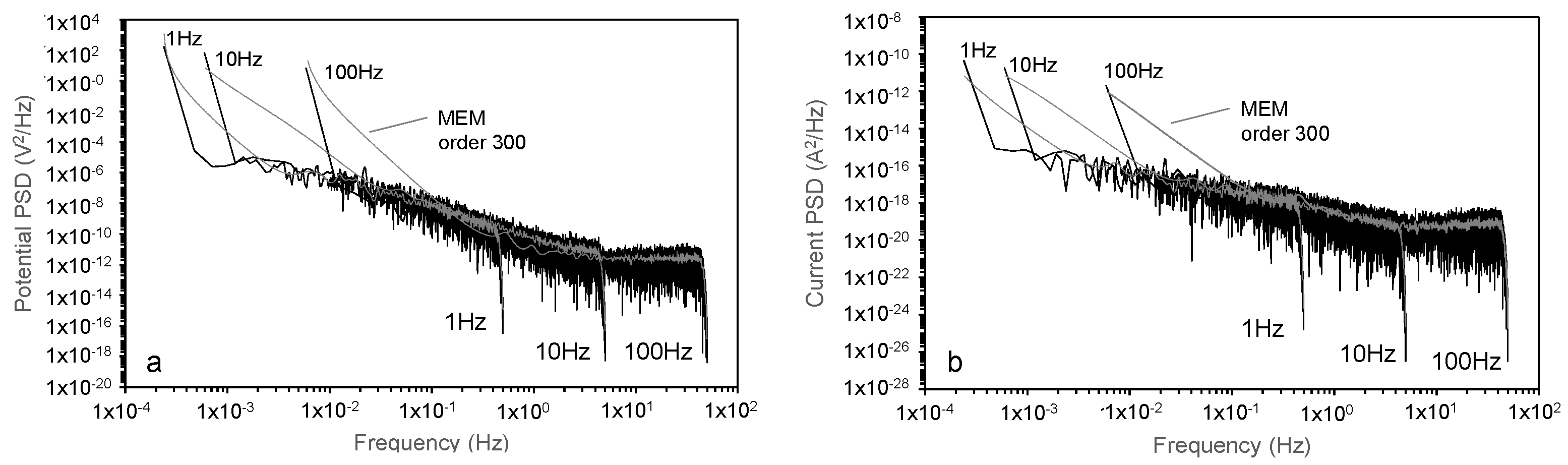
7. Electrochemical Noise
8. Overview of Steel Strand Corrosion in Deficient Grout
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hewson, N.R. Prestressed Concrete Bridges: Design and Construction; Thomas Telford Publishing: London, UK, 2003. [Google Scholar]
- Lau, K.; Lasa, I. Corrosion of prestress and post-tension reinforced-concrete bridges. In Corrosion of Steel in Concrete Structures; Poursaee, A., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 37–57. [Google Scholar] [CrossRef]
- Powers, R.G. Corrosion Evaluation of Post-Tensioned Tendons on the Niles Channel Bridge; Florida Department of Transportation: Tallahassee, FL, USA, 1999.
- Corven Engineering. Mid-Bay Bridge Post-Tensioning Evaluation; Final Report to Florida Department of Transportation; The Florida Department of Transportation Research Center: Tallahassee, FL, USA, 10 October 2001.
- Hartt, W.; Venugopalan, S. Corrosion Evaluation of Post-Tensioned Tendons on the Mid Bay Bridge in Destin, Florida; Final Report to Florida Department of Transportation; Florida Department of Environmental Protection: Tallahasse, FL, USA, April 2002.
- Hansen, B. Forensic Engineering: Tendon Failure Raises Questions about Grout in Post-Tensioned Bridges. Civ. Eng. News 2007, 77, 17–18. [Google Scholar]
- Lau, K.; Lasa, I.; Paredes, M. Corrosion Development of PT Tendons with Deficient Grout: Corrosion Failure in Ringling Causeway Bridge; Draft Report; Florida Department of Transportation State Materials Office: Gainesville, FL, USA, 2011.
- Permeh, S.; Krishna Vigneshwaran, K.K.; Lau, K. Corrosion of Post-Tensioned Tendons with Deficient Grout; Final Report to Florida Department of Transportation, Contract No. BDV29-977-04; Florida Department of Transportation: Tallahassee, FL, USA, 20 October 2016.
- Sprinkel, M.M.; Balakumaran, S.S.G. Problems with Continuous Spliced Posttensioned–Prestressed Concrete Bulb-Tee Girder Center Spans at West Point, Virginia. Transp. Res. Rec. 2017, 2642, 46–54. [Google Scholar] [CrossRef]
- Lau, K.; Powers, R.; Paredes, M. Corrosion Evaluation of Repair-Grouted Post-Tensioned Tendons in Presence of Bleed Water. In Proceedings of the CORROSION 2013, Orlando, FL, USA, 17–21 March 2013; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- Lau, K.; Rafols, J.; Paredes, M.; Lasa, I. Laboratory Corrosion Assessment of Post-Tensioned Tendons Repaired with Dissimilar Grout. In Proceedings of the CORROSION 2013, Orlando, FL, USA, 17–21 March 2013; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- Lau, K.; Paredes, M.; Lasa, I. Corrosion Failure of Post-Tensioned Tendons in Presence of Deficient Grout. In Proceedings of the CORROSION 2013, Orlando, FL, USA, 17–21 March 2013; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- Lau, K.; Lasa, I.; Paredes, M. Bridge tendon failures in the presence of deficient grout. Mater. Perform. 2013, 52, 64–68. [Google Scholar]
- Lau, K.; Lasa, I.; Paredes, M. Update on Corrosion of Post-Tensioned Tendons with Deficient Grout. In Proceedings of the CORROSION 2014, San Antonio, TX, USA, 9–13 March 2014; NACE International: Houston, TX, USA, 2014. [Google Scholar]
- Lee, S.K.; Zielske, J. An FHWA Special Study: Post-Tensioning Tendon Grout Chloride Thresholds; FHWA-HRT-14-039; U.S. Department of Transportation, Federal Highway Administration: McLean, VA, USA, May 2014.
- Theryo, T.; Hartt, W.H.; Paczkowski, P. Guidelines for Sampling, Assessing, and Restoring Defective Grout in Prestressed Concrete Bridge Post-Tensioning Ducts; FHWA-HRT-13-028; FHWA-HRT-14-039; U.S. Department of Transportation, Federal Highway Administration: McLean, VA, USA, October 2013.
- Lee, S.K. Corrosivity of Water-Soluble Sulfate Ions in Simulated Pore Water Solutions and Different Types of Grout Samples; No. FHWA-HRT-21-052; Federal Highway Administration. Office of Infrastructure Research and Development: McLean, VA, USA, 2021.
- Permeh, S.; Krishna Vigneshwaran, K.K.; Lau, K.; Lasa, I. Corrosion of PT Tendons in Deficient Grout in Presence of Enhanced Sulfate and Chloride Concentration. In Proceedings of the NACE Corrosion Risk Management Conference, Houston, TX, USA, 23–25 May 2016; Paper No. 16-18751. NACE: Houston, TX, USA, 2016. [Google Scholar]
- Permeh, S.; Krishna Vigneshwaran, K.K.; Lau, K.; Lasa, I. Corrosion of Post-Tensioned Tendons with Deficient Grout, Part 3: Segregated Grout with Elevated Sulfate and Vestigial Chloride Content. Corrosion 2019, 75, 848–864. [Google Scholar] [CrossRef]
- Sonawane, R.; Permeh, S.; Lau, K.; Duncan, M.; Simmons, R. Review on the Determination of Sulfate Ion Concentrations in Deficient Post-Tensioned Grouts. In Proceedings of the CORROSION 2021, Virtual Conference, 19–30 April 2021; NACE International: Houston, TX, USA, 2021. [Google Scholar]
- Sonawane, R.; Permeh, S.; Lau, K. Development of deficient grout and corrosion due to water and solute transport. In Proceedings of the 1st Corrosion and Materials Degradation Web Conference, Virtual Conference, 17–19 May 2021; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K.; Tansel, B. Moisture and ion mobilization and stratification in post-tensioned (PT) grout during hydration. Case Stud. Constr. Mater. 2021, 15, e00644. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K. Accelerated Corrosion Testing of Grouts for PT Steel Strand; Final Report to Florida Department of Transportation, Contract No. BDV29-977-44; Florida Department of Transportation: Tallahassee, FL, USA, April 2021.
- Alexander, C.L.; Chen, Y.M.; Orazem, M.E. Impedance-Based Detection of Corrosion in Post-Tensioned Cables: Phase 2 from Concept to Application; Final Report to Florida Department of Transportation, Contract No. BDV31-977-35; Florida Department of Transportation: Tallahassee, FL, USA, July 2017.
- Alexander, C.L.; Orazem, M.E. Indirect electrochemical impedance spectroscopy for corrosion detection in external post-tensioned tendons: 1. Proof of concept. Corros. Sci. 2020, 164, 108331. [Google Scholar] [CrossRef]
- Taveira, L.V.; Joseph, B.; Sagüés, A.A.; Sabando, J.L. Detection of corrosion of post-tensioned strands in grouted assemblies. In Proceedings of the CORROSION 2008, New Orleans, LA, USA, 16 March 2008; NACE International: Houston, TX, USA, 2008. [Google Scholar]
- Azizinamini, A.; Gull, J. Improved Inspection Techniques for Steel Prestressing/Post-Tensioning Strand: Volume I; Contract No. BDV80-977-13; Final Report to Florida Department of Transportation; FDOT Research Center: Tallahassee, FL, USA, June 2012.
- Dukeman, D.; Freij, H.; Sagüés, A.A.; Alexander, C. Field Demonstration of Tendon Imaging Methods; Contract No. BDV25-977-52; Final Report to Florida Department of Transportation; FDOT Research Center: Tallahassee, FL, USA, July 2019.
- Hartt, W.H.; Lee, S.K. Modeling and projecting the onset and subsequent failure rate of corroding bridge post-tension tendons arising from deficient grout. Corrosion 2018, 74, 241–248. [Google Scholar] [CrossRef]
- Hartt, W.H. Effect of Modeling Variables upon Projection of Corrosion-Induced Bridge Post-Tension Tendon Failures. Corrosion 2019, 74, 768–775. [Google Scholar] [CrossRef]
- Whitmore, D.; Lasa, I. Impregnation technique provides corrosion protection to grouted post-tensioning tendons. MATEC Web Conf. 2018, 199, 05008. [Google Scholar] [CrossRef][Green Version]
- Whitmore, D.; Lasa, I. Cable Impregnation for Post-Tension Grouting Problems. In Transportation 2014: Past, Present, Future-2014 Conference and Exhibition of the Transportation Association of Canada//Transport 2014: Du passé vers l’avenir-2014 Congrès et Exposition de’Association des transports du Canada; Transportation Association of Canada: Ottawa, ON, Canada, 2014. [Google Scholar]
- Hamilton, H.R.; Piper, A.; Randell, A.; Brunner, B. Simulation of Prepackaged Grout Bleed under Field Conditions; Final Report to Florida Department of Transportation; BDK75-977-59; Department of Civil and Coastal Engineering, University of Florida: Tallahassee, FL, USA, April 2014. [Google Scholar]
- Hamilton, H.R.; Torres, E.; Aguirre, M.; Ferraro, C.C. Evaluation of Shelf Life in Post-Tensioning Grouts; BDV31-977-31; Final Report to Florida Department of Transportation; Department of Civil and Coastal Engineering, University of Florida: Tallahassee, FL, USA, November 2018. [Google Scholar]
- Hamilton, H.R. Evaluation of Techniques to Remove Defective Grout from Post-Tensioning Tendons; BDV31-977-58; Final Report to Florida Department of Transportation; University of Florida: Gainesville, FL, USA, February 2020. [Google Scholar]
- Whitmore, D.; Tore Arnesen, P.E. Evaluation and Mitigation of Post-Tensioned Steel Corrosion. In Proceedings of the Eighth Congress on Forensic Engineering, ASCE, Reston, VA, USA, 29 November–2 December 2018. [Google Scholar]
- Rehmat, S.; Lau, K.; Azizinamini, A. Development of Quality Assurance and Quality Control System for Post Tensioned Segmental Bridges in Florida: Case of Ringling Bridge—Phase II; BDV29-977-34; Final Report to Florida Department of Transportation; FDOT Research Center: Tallahassee, FL, USA, March 2019.
- Hurlebaus, S.; Hueste, M.; Karthik, M.; Terzioglu, T. Condition Assessment of Bridge Post-Tensioning and Stay Cable Systems Using NDE Methods; Final Report to National Cooperative Highway Research Program (NCHRP), Project No. 14–28; Transportation Research Board of the National Academies: Washington, DC, USA, September 2016. [Google Scholar]
- Permeh, S.; Lau, K.; Duncan, M.; Simmons, R. Identification of steel corrosion in alkaline sulfate solution by electrochemical noise. Mater. Corros. 2021, 72, 1456–1467. [Google Scholar] [CrossRef]
- Jones, D. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Bentur, A.; Diamond, S.; Berke, N.S. Steel Corrosion in Concrete: Fundamentals and Civil Engineering Practice, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- ASTM C876-(2015); Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM: West Conshohocken, PA, USA, 2015.
- Krishna Vigneshwaran, K.K.; Permeh, S.; Lasa, I.; Lau, K. Corrosion of Steel in Deficient Grout with Enhanced Sulfate Content. In Proceedings of the NACE Concrete Service Life Extension Conference, Philadelphia, PA, USA, 1 July 2015; Paper No. CCC15-6942. NACE: Houston, TX, USA, 2015. [Google Scholar]
- Permeh, S.; Krishna Vigneshwaran, K.K.; Lau, K.; Lasa, I.; Paredes, M. Material and Corrosion Evaluation of Deficient PT Grout with Enhanced Sulfate Concentrations. In Proceedings of the CORROSION 2015, Dallas, TX, USA, 15–19 March 2015; NACE International: Houston, TX, USA, 2015. [Google Scholar]
- Permeh, S.; Krishna Vigneshwaran, K.K.; Echeverría, M.; Lau, K.; Lasa, I. Corrosion of Post-Tensioned Tendons with Deficient Grout, Part 2: Segregated Grout with Elevated Sulfate Content. Corrosion 2018, 74, 457–467 2018. [Google Scholar] [CrossRef]
- Feliu, S.; González, J.; Andrade, M.C.; Feliu, V. Determining polarization resistance in reinforced concrete slabs. Corros. Sci. 1989, 29, 105–113. [Google Scholar] [CrossRef]
- Feliu, S.; Galvan, J.C.; Feliu, S., Jr.; Simancas, J.; Bastidas, J.M.; Morcillo, M.; Almeida, E. Differences between apparent polarization resistance values obtained in the time and frequency domains. J. Electroanal. Chem. 1995, 381, 1–4. [Google Scholar] [CrossRef]
- Krishna Vigneshwaran, K.K.; Permeh, S.; Echeverría, M.; Lau, K.; Lasa, I. Corrosion of Post-Tensioned Tendons with Deficient Grout, Part 1: Electrochemical Behavior of Steel in Alkaline Sulfate Solutions. Corrosion 2018, 74, 362–371. [Google Scholar] [CrossRef]
- Wang, H.; Sagüés, A.A.; Powers, R.G. Corrosion of the strand-anchorage system in post-tensioned grouted assemblies. In Proceedings of the CORROSION 2005, Houston, TX, USA, April 2005; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Darwin, D.; Sturgeon, W. Rapid Macrocell Tests of Enduramet Steel Bars; The University of Kansas Center for Research, Inc.: Lawrence, KS, USA, January 2011. [Google Scholar]
- O’Reilly, M.; Darwin, D.; Browning, J. Corrosion Performance of Prestressing Strands in Contact with Dissimilar Grouts; No. KS-12-4; Department of Transportation: Washington, DC, USA, 2013.
- O’Reilly, M.; Darwin, D.; Browning, J. Corrosion Performance of Prestressing Strands in Contact with Dissimilar Grouts. ACI Mater. J. 2015, 112, 491–500. [Google Scholar] [CrossRef]
- Rafols, J.C.; Lau, K.; Lasa, I.; Paredes, M.; ElSafty, A. Approach to determine corrosion propensity in post-tensioned tendons with deficient grout. Open J. Civ. Eng. 2013, 3, 36394. [Google Scholar] [CrossRef]
- Sonawane, R.; Permeh, S.; Garber, D.; Lau, K.; Simmons, R.; Duncan, M. Test Methods to Identify Robustness of Grout Materials to Resist Corrosion. In Proceedings of the CORROSION 2020, Digital Proceedings, Lviv, Ukraine, 14–18 June 2020; NACE International: Houston, TX, USA, 2020. [Google Scholar]
- Permeh, S.; Sonawane, R.; Lau, K.; Duncan, M.; Simmons, R. Update on the Evaluation of Grout Robustness and Corrosion by Accelerated Corrosion and Rapid Macrocell Testing. In Proceedings of the CORROSION 2021, Virtual Conference, 19–30 April 2021; NACE International: Houston, TX, USA, 2021. [Google Scholar]
- Permeh, S.; Lau, K.; Tansel, B. Development of Standard Methodology to Measure Sulfate Ions in Post-Tensioned Grouts; Final Report to Florida Department of Transportation, Contract No. BDV29-977-43; Florida Department of Transportation: Tallahassee, FL, USA, April 2021.
- Permeh, S.; Lau, K.; Matthew, D. Accelerated Testing to Assess Robustness of Post-Tensioning Grout. Mater. Perform. 2021, 60, 34–38. [Google Scholar]
- Bertolini, L.; Carsana, M. High pH corrosion of prestressing steel in segregated grout. Model. Corroding Concr. Struct. 2011, 5, 147–158. [Google Scholar]
- Carsana, M.; Bertolini, L. Characterization of segregated grout promoting corrosion of posttensioning tendons. J. Mater. Civ. Eng. 2016, 28, 04016009. [Google Scholar] [CrossRef]
- Krishna Vigneshwaran, K.K.; Lau, K.; Permeh, S.; Lasa, I. Anodic Behavior of Steel in Enhanced Sulfate Solutions. In Proceedings of the CORROSION 2016, Vancouver, BC, Canada, 6–10 March 2016; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Echeverria, M.; Lau, K.; Permeh, S. Anodic Behavior of Steel in Sulfate-Rich Simulated Grout Pore Solution. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, September 2017; p. 801. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lau, K.; Paredes, M.; Rafols, J. Corrosion Evaluation of Post-Tensioned Tendons with Dissimilar Grout. In Proceedings of the CORROSION 2012, Salt Lake City, UT, USA, 11–15 March 2012; NACE International: Houston, TX, USA, 2015. [Google Scholar]
- Permeh, S.; Lau, K.; Duncan, M.; Simmons, R. Initiation of localized steel corrosion in alkaline sulfate solution. Mater. Struct. 2021, 54, 143. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K. Corrosion of post-tension tendons associated with segregated grout. In Proceedings of the 1st Corrosion and Materials Degradation Web Conference, Virtual Conference, 17–19 May 2021; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Ritter, S.; Huet, F.; Cottis, R.A. Guideline for an assessment of electrochemical noise measurement devices. Mater. Corros. 2012, 63, 297–302. [Google Scholar] [CrossRef]
- Cottis, R. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permeh, S.; Lau, K. Review of Electrochemical Testing to Assess Corrosion of Post-Tensioned Tendons with Segregated Grout. Constr. Mater. 2022, 2, 70-84. https://doi.org/10.3390/constrmater2020006
Permeh S, Lau K. Review of Electrochemical Testing to Assess Corrosion of Post-Tensioned Tendons with Segregated Grout. Construction Materials. 2022; 2(2):70-84. https://doi.org/10.3390/constrmater2020006
Chicago/Turabian StylePermeh, Samanbar, and Kingsley Lau. 2022. "Review of Electrochemical Testing to Assess Corrosion of Post-Tensioned Tendons with Segregated Grout" Construction Materials 2, no. 2: 70-84. https://doi.org/10.3390/constrmater2020006
APA StylePermeh, S., & Lau, K. (2022). Review of Electrochemical Testing to Assess Corrosion of Post-Tensioned Tendons with Segregated Grout. Construction Materials, 2(2), 70-84. https://doi.org/10.3390/constrmater2020006





