The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms
Abstract
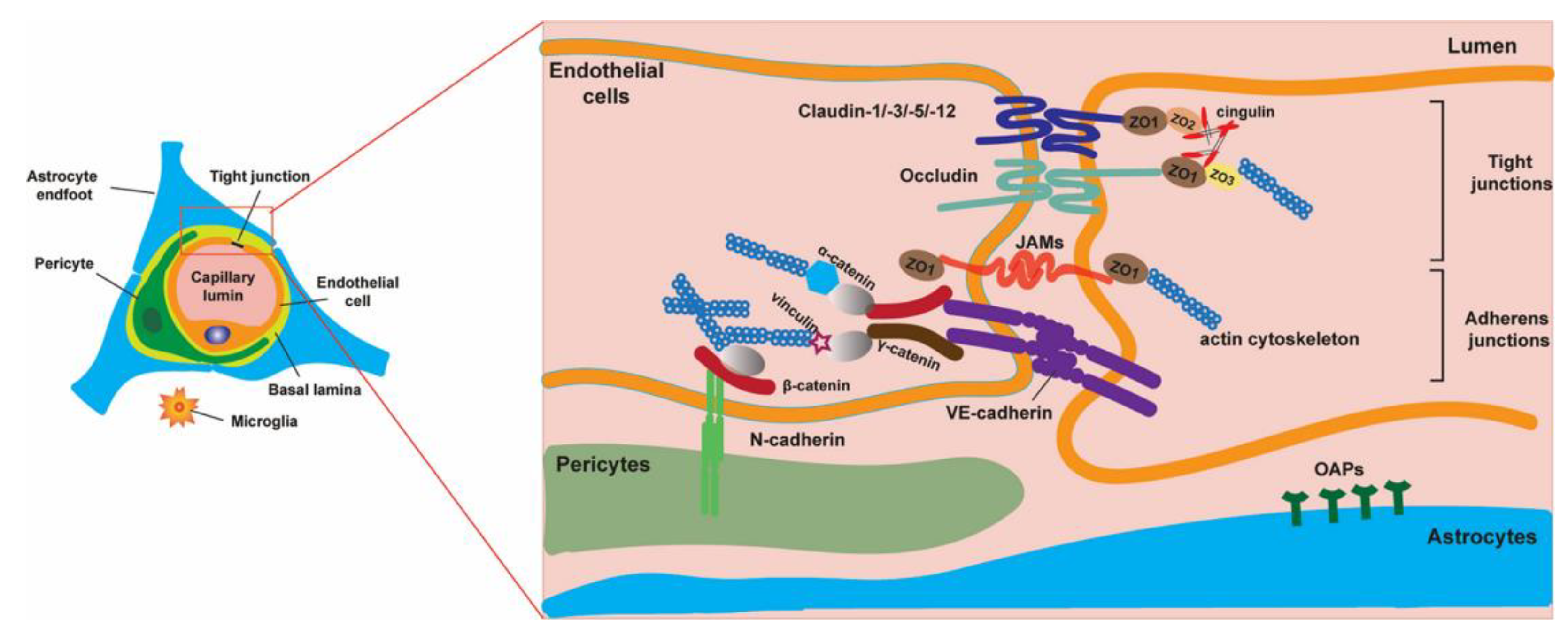
1. The Blood–Brain Barrier—Structure and Physiological Functions
1.1. Brain Microvascular Endothelial Cells
1.2. Smooth Muscle Cells and Brain Pericytes
1.3. Astrocytes
1.4. Oligodendrocytes
1.5. Microglia
1.6. Neurons
1.7. Extracellular Matrix
1.8. The Physiological Roles of the BBB
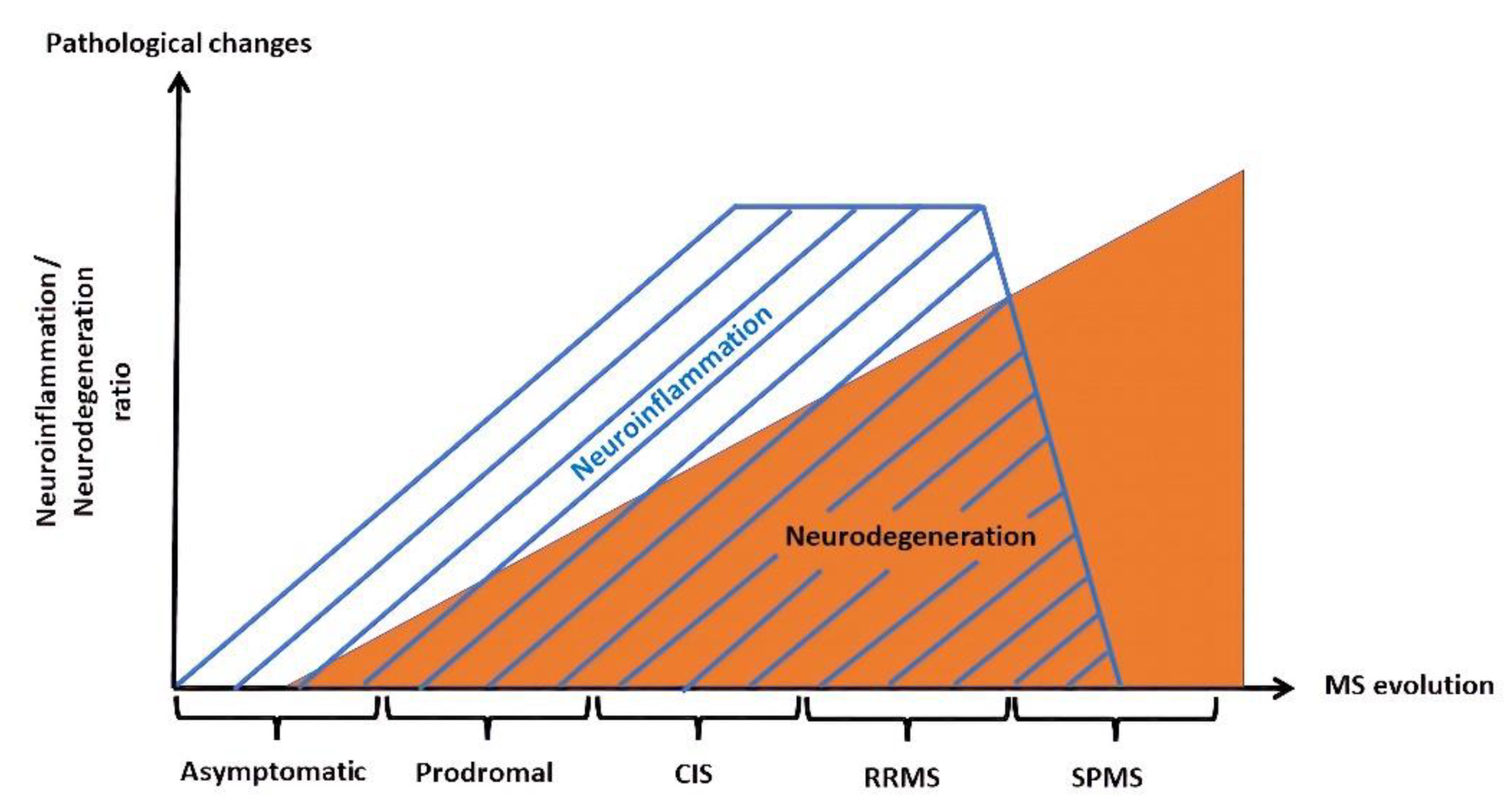
2. The Blood–Brain Barrier in Multiple Sclerosis—From Early Neuroinflammation to Neurodegeneration
2.1. Consequences of Neuroinflammation at the BBB Level
2.1.1. Alteration of ECs during Inflammation
2.1.2. Pericytes Modification in Inflammatory Conditions
2.1.3. Astrocyte Modification in Inflammatory Conditions
2.1.4. Oligodendrocytes and OPCs in Neuroinflammation
2.1.5. Microglia Activation in Inflammation
2.1.6. Immune Cells, BBB, and Inflammation
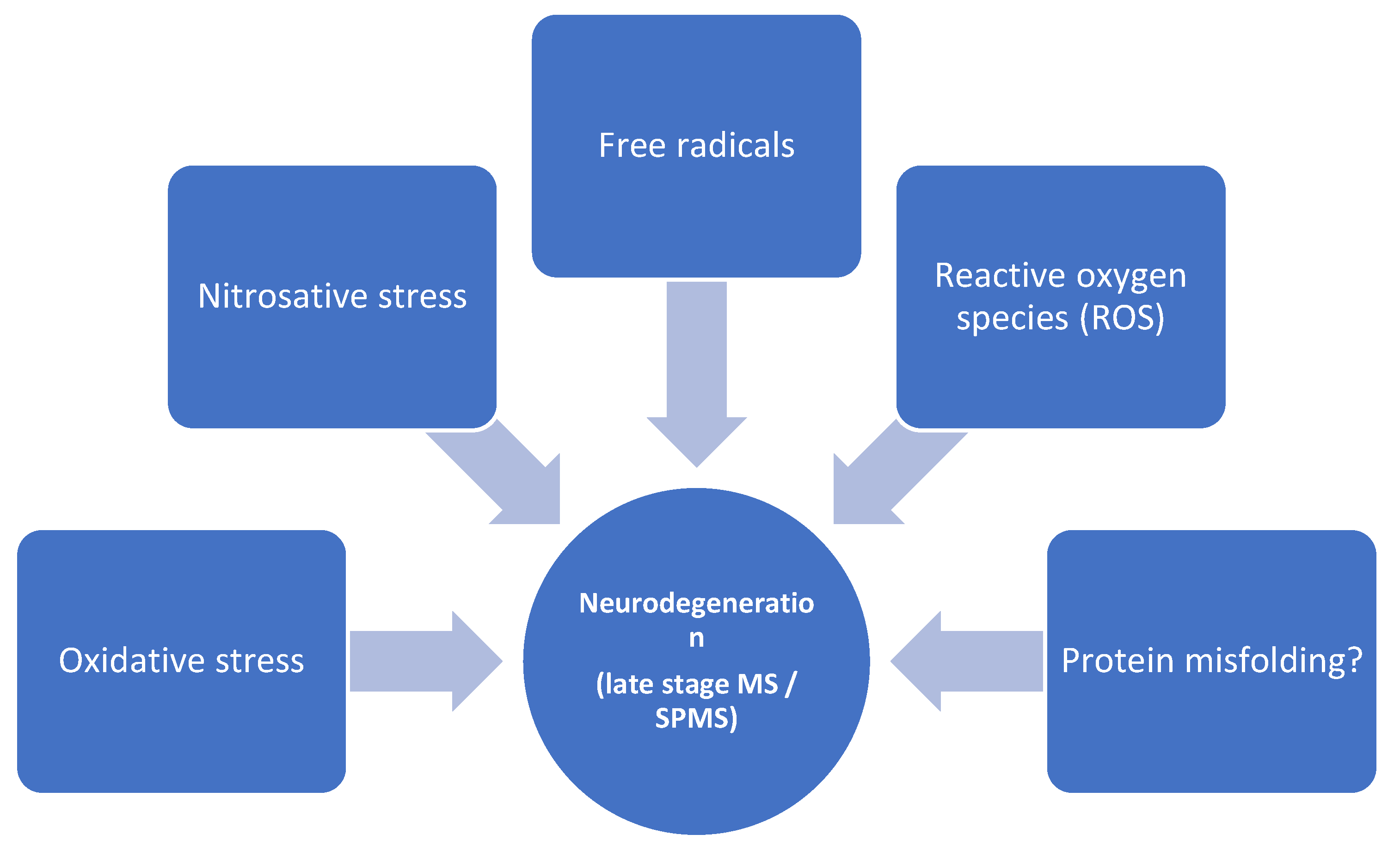
2.2. The BBB in Later Stages of MS—Focus on Neurodegeneration
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solár, P.; Zamani, A.; Kubíčková, L.; Dubový, P.; Joukal, M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H.; Cornford, M.E.; Brown, W.J. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977, 1, 409–417. [Google Scholar] [CrossRef]
- Fenstermacher, J.; Gross, P.; Sposito, N.; Acuff, V.; Pettersen, S.; Gruber, K. Structural and functional variations in capillary systems within the brain. Ann. N. Y. Acad. Sci. 1988, 529, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova, R.; Shivers, R.R.; Del Maestro, R.F. Ultrastructure of the blood-brain barrier in the rabbit. J. Submicrosc. Cytol. Pathol. 1999, 31, 149–161. [Google Scholar] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vascul. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M. Overcoming barriers in the study of tight junction functions: From occludin to claudin. Genes Cells 1998, 3, 569–573. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell. Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Hu, W.; Yang, Z.; Li, T.; Jiang, S.; Ma, Z.; Chen, F.; Yang, Y. Focusing on claudin-5: A promising candidate in the regulation of BBB to treat ischemic stroke. Prog. Neurobiol. 2018, 161, 79–96. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, K.J.; Qi, Z. Occludin regulation of blood-brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020, 6, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 2014, 36, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Martin, T.A.; Zhang, G.; Jiang, W.G. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013, 33, 2353–2359. [Google Scholar] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Stevens, M.Y.; Chen, M.B.; Lee, D.P.; Stähli, D.; Gate, D.; Contrepois, K.; Chen, W.; Iram, T.; Zhang, L.; et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 2020, 583, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Scalisi, J.; Balau, B.; Deneyer, L.; Bouchat, J.; Gilloteaux, J.; Nicaise, C. Blood-brain barrier permeability towards small and large tracers in a mouse model of osmotic demyelination syndrome. Neurosci. Lett. 2021, 746, 135665. [Google Scholar] [CrossRef] [PubMed]
- Bendayan, R.; Ronaldson, P.T.; Gingras, D.; Bendayan, M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 2006, 54, 1159–1167. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Babakhanian, K.; Bendayan, R. Drug transport in the brain. In Drug Transporters: Molecular Characterization and Role in Drug Disposition; You, G., Morris, M.E., Eds.; Wiley: Hokoben, NJ, USA, 2007; pp. 411–461. [Google Scholar]
- Miller, D.S. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 2010, 31, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, J.A.; Katneni, K. Drug transport across the blood-brain barrier and the impact of breast cancer resistance protein (ABCG2). Curr. Top. Med. Chem. 2009, 9, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Revest, P.A.; Romero, I.A. Astrocyte-endothelial inter-action: Physiology and pathology. Neuropathol. Appl. Neurobiol. 1992, 18, 424–433. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Taylor, H. Review Article: Capturing the physiological complexity of the brain′s neuro-vascular unit in vitro. Biomicrofluidics 2018, 12, 051502. [Google Scholar] [CrossRef]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019, 13, 405. [Google Scholar] [CrossRef]
- Allt, G.; Lawrenson, J.G. Pericytes: Cell biology and pathology. Cells Tissues Organs 2001, 169, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Kuo, K.H. The critical component to establish in vitro BBB model: Pericyte. Brain Res. Rev. 2005, 50, 258–265. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, M.; Kunz, J.; Krause, D.; Dermietzel, R. Regulation of a blood-brain barrier-specific enzyme expressed by cerebral pericytes (pericytic aminopeptidase N/pAPN) under cell culture conditions. J. Cereb. Blood Flow Metab. 1998, 18, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Berezowski, V.; Landry, C.; Dehouck, M.P.; Cecchelli, R.; Fenart, L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004, 1018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M. Pericyte signaling in the neurovascular unit. Stroke 2009, 40 (Suppl. 1), S13–S15. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Badaut, J.; Dargazanli, C.; De Maudave, A.F.; Klement, W.; Costalat, V.; Marchi, N. The pericyte-glia interface at the blood-brain barrier. Clin. Sci. 2018, 132, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Naik, U.P. Pericyte-endothelial cell interaction: A survival mechanism for the tumor vasculature. Cell Adhes. Migr. 2012, 6, 157–159. [Google Scholar] [CrossRef]
- Fabry, Z.; Fitzsimmons, K.M.; Herlein, J.A.; Moninger, T.O.; Dobbs, M.B.; Hart, M.N. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 1993, 47, 23–34. [Google Scholar] [CrossRef]
- Kovac, A.; Erickson, M.A.; Banks, W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflamm. 2011, 8, 139. [Google Scholar] [CrossRef]
- Balabanov, R.; Beaumont, T.; Dore-Duffy, P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 1999, 55, 578–587. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kılıç, K.; Villafranca-Baughman, D.; Can, A.; Di Polo, A.; Dalkara, T. Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 2019, 7, 134. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Tait, M.J.; Saadoun, S.; Bell, B.A.; Papadopoulos, M.C. Water movements in the brain: Role of aquaporins. Trends Neurosci. 2008, 31, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ikeshima-Kataoka, H. Neuroimmunological Implications of AQP4 in Astrocytes. Int. J. Mol. Sci. 2016, 17, 1306. [Google Scholar] [CrossRef] [PubMed]
- Raub, T.J.; Kuentzel, S.L.; Sawada, G.A. Permeability of bovine brain microvessel endothelial cells in vitro: Barrier tightening by a factor released from astroglioma cells. Exp. Cell Res. 1992, 199, 330–340. [Google Scholar] [CrossRef]
- Estrada, C.; Bready, J.V.; Berliner, J.A.; Pardridge, W.M.; Cancilla, P.A. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J. Neuropathol. Exp. Neurol. 1990, 49, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Berezowski, V.; Fukuda, A.M.; Cecchelli, R.; Badaut, J. Endothelial Cells and Astrocytes: A Concerto en Duo in Ischemic Pathophysiology. Int. J. Cell Biol. 2012, 2012, 176287. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, J.; Qu, M.; Yuan, F.; Wang, Y.; Pan, J.; Li, Y.; Ma, Y.; Zhou, P.; Zhang, Z.; et al. Oligodendrocyte precursor cells transplantation protects blood-brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, C.; Marneffe, C.; Bouchat, J.; Gilloteaux, J. Osmotic Demyelination: From an Oligodendrocyte to an Astrocyte Perspective. Int. J. Mol. Sci. 2019, 20, 1124. [Google Scholar] [CrossRef] [PubMed]
- Gankam Kengne, F.; Nicaise, C.; Soupart, A.; Boom, A.; Schiettecatte, J.; Pochet, R.; Brion, J.P.; Decaux, G. Astrocytes are an early target in osmotic demyelination syndrome. J. Am. Soc. Nephrol. 2011, 22, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Gankam-Kengne, F.; Couturier, B.S.; Soupart, A.; Brion, J.P.; Decaux, G. Osmotic Stress-Induced Defective Glial Proteostasis Contributes to Brain Demyelination after Hyponatremia Treatment. J. Am. Soc. Nephrol. 2017, 28, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Bunyan, R.F.; Guo, Y.; Parisi, J.E.; Lennon, V.A.; Lucchinetti, C.F. Evidence of aquaporin involvement in human central pontine myelinolysis. Acta Neuropathol. Commun. 2013, 1, 40. [Google Scholar] [CrossRef]
- Gankam Kengne, F.; Soupart, A.; Pochet, R.; Brion, J.P.; Decaux, G. Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int. 2009, 76, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Ginhoux, F.; Garel, S. Microglia and early brain development: An intimate journey. Science 2018, 362, 185–189. [Google Scholar] [CrossRef]
- Eyo, U.B.; Wu, L.J. Microglia: Lifelong patrolling immune cells of the brain. Prog. Neurobiol. 2019, 179, 101614. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Hübel, N.; Schöll, E.; Dahlem, M.A. Bistable dynamics underlying excitability of ion homeostasis in neuron models. PLoS Comput. Biol. 2014, 10, e1003551. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.S.; Munji, R.N.; Chan, T.C.; Quirk, C.R.; Weiner, G.A.; Weger, B.D.; Rossi, M.J.; Elmsaouri, S.; Malfavon, M.; Deng, A.; et al. Neuronal Activity Regulates Blood-Brain Barrier Efflux Transport through Endothelial Circadian Genes. Neuron 2020, 108, 937–952.e7. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Damodarasamy, M.; Banks, W.A. The extracellular matrix of the blood-brain barrier: Structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 2019, 7, 1651157. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82. [Google Scholar] [CrossRef]
- Aumailley, M. The laminin family. Cell Adhes. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef]
- Louboutin, J.P.; Strayer, D.S. Blood-brain barrier abnormalities caused by HIV-1 gp120: Mechanistic and therapeutic implications. Sci. World J. 2012, 2012, 482575. [Google Scholar] [CrossRef]
- Del Zoppo, G.J. Bleeding in the brain: Amyloid-beta may keep clots away. Nat. Med. 2009, 15, 1132–1133. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases in neuroinflammation. Glia 2002, 39, 279–291, Erratum in Glia 2002, 40, 130. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, R. Transport of Amino Acids across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.L.; Cacaccio, J.; Wrabel, E.; Schwartz, M.E.; Coleman, T.P.; Sirianni, R.W. Pathogen-inspired drug delivery to the central nervous system. Tissue Barriers 2014, 2, e944449. [Google Scholar] [CrossRef][Green Version]
- Yarlagadda, A.; Kaushik, S.; Clayton, A.H. Blood brain barrier: The role of calcium homeostasis. Psychiatry 2007, 4, 55–59. [Google Scholar]
- Jukkola, P.; Gu, C. Regulation of neurovascular coupling in autoimmunity to water and ion channels. Autoimmun. Rev. 2015, 14, 258–267. [Google Scholar] [CrossRef]
- Tumani, H.; Huss, A.; Bachhuber, F. The cerebrospinal fluid and barriers—Anatomic and physiologic considerations. Handb. Clin. Neurol. 2017, 146, 21–32. [Google Scholar]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Huang, L.; Li, B.; Li, X.; Liu, G.; Liu, R.; Guo, J.; Xu, B.; Li, Y.; Fang, W. Significance and Mechanisms of P-glycoprotein in Central Nervous System Diseases. Curr. Drug Targets 2019, 20, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Piehl, F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J. Intern. Med. 2021, 289, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.Z.; Church, J.S.; Hesp, Z.C.; Popovich, P.G.; McTigue, D.M. A silver lining of neuroinflammation: Beneficial effects on myelination. Exp. Neurol. 2016, 283 Pt B, 550–559. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Španić, E.; Langer Horvat, L.; Hof, P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer′s disease. Prog. Mol. Biol. Transl. Sci. 2019, 168, 99–145. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Gavard, J. Endothelial permeability and VE-cadherin: A wacky comradeship. Cell Adhes. Migr. 2014, 8, 158–164. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Phosphorylation of tight junction transmembrane proteins: Many sites, much to do. Tissue Barriers 2018, 6, e1382671. [Google Scholar] [CrossRef]
- Nagyoszi, P.; Wilhelm, I.; Farkas, A.E.; Fazakas, C.; Dung, N.T.; Haskó, J.; Krizbai, I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010, 57, 556–564. [Google Scholar] [CrossRef]
- Chodobski, A.; Chung, I.; Koz´niewska, E.; Koz´niewska, K.; Ivanenko, T.; Chang, W.; Harrington, J.F.; Duncan, J.A.; Szmydynger-Chodobska, J. Early Neutrophilic Expression of Vascular Endothelial Growth Factor after Traumatic Brain Injury. Neuroscience 2003, 122, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Takenaga, F.; Yamori, Y.; Itoh, S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 2004, 17, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, G.; Mohácsik, P.; Balkhi, M.Y.; Gereben, B.; Lechan, R.M. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS 2015, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Goralski, K.B. A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Meuwese, M.C.; Mooij, H.L.; van Lieshout, M.H.; Hayden, A.; Levi, M.; Meijers, J.C.; Ince, C.; Kastelein, J.J.; Vink, H.; et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 2009, 202, 296–303. [Google Scholar] [CrossRef]
- Pieper, C.; Marek, J.J.; Unterberg, M.; Schwerdtle, T.; Galla, H.J. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014, 1550, 1–8. [Google Scholar] [CrossRef]
- Nakamura, K.; Ikeuchi, T.; Nara, K.; Rhodes, C.S.; Zhang, P.; Chiba, Y.; Kazuno, S.; Miura, Y.; Ago, T.; Arikawa-Hirasawa, E.; et al. Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J. Cell Biol. 2019, 218, 3506–3525. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Brew, B.J. Microglia, macrophages, perivascular macrophages, and pericytes: A review of function and identification. J. Leukoc. Biol. 2004, 75, 388–397. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Viña, J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef]
- Yu, H.; Wang, P.; An, P.; Xue, Y. Recombinant human angiopoietin-1 ameliorates the expressions of ZO-1, occludin, VE-cadherin, and PKCα signaling after focal cerebral ischemia/reperfusion in rats. J. Mol. Neurosci. 2012, 46, 236–247. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Psenicka, M.W.; Smith, B.C.; Tinkey, R.A.; Williams, J.L. Connecting Neuroinflammation and Neurodegeneration in Multiple Sclerosis: Are Oligodendrocyte Precursor Cells a Nexus of Disease? Front. Cell. Neurosci. 2021, 15, 654284. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Limatola, C.; Ransohoff, R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017, 14, 204. [Google Scholar] [CrossRef]
- Salou, M.; Nicol, B.; Garcia, A.; Laplaud, D.A. Involvement of CD8(+) T Cells in Multiple Sclerosis. Front. Immunol. 2015, 6, 604. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Iwanowski, P. Role of B cells and antibodies in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101416. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Gałuszka, A.; Stec, M.; Węglarczyk, K.; Kluczewska, A.; Siedlar, M.; Baran, J. Transition Metal Containing Particulate Matter Promotes Th1 and Th17 Inflammatory Response by Monocyte Activation in Organic and Inorganic Compounds Dependent Manner. Int. J. Environ. Res. Public Health 2020, 17, 1227. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Ren, H. Recent advances in our understanding of mast cell activation—or should it be mast cell mediator disorders? Expert Rev. Clin. Immunol. 2019, 15, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Winder, A.A.; Wohlford-Lenane, C.; Scheetz, T.E.; Nardy, B.N.; Manzel, L.J.; Look, D.C.; McCray, P.B., Jr. Differential effects of cytokines and corticosteroids on toll-like receptor 2 expression and activity in human airway epithelia. Respir. Res. 2009, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.O.; Cho, M.L.; Lee, S.Y.; Oh, H.J.; Park, J.S.; Park, M.K.; Park, M.J.; Ju, J.H.; Kim, S.I.; Park, S.H.; et al. Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNFα) in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol. Lett. 2009, 123, 138–143. [Google Scholar] [CrossRef]
- Balabanov, R.; Goldman, H.; Murphy, S.; Pellizon, G.; Owen, C.; Rafols, J.; Dore-Duffy, P. Endothelial Cell Activation Following Moderate Traumatic Brain Injury. Neurol. Res. 2001, 23, 175–182. [Google Scholar] [CrossRef]
- Morgan, R.; Kreipke, C.W.; Roberts, G.; Bagchi, M.; Rafols, J.A. Neovascularization Following Traumatic Brain Injury: Possible Evidence for Both Angiogenesis and Vasculogenesis. Neurol. Res. 2013, 29, 375–381. [Google Scholar] [CrossRef]
- Winn, R.K.; Harlan, J.M. The role of endothelial cell apoptosis in inflammatory and immune diseases. J. Thromb. Haemost. 2005, 3, 1815–1824. [Google Scholar] [CrossRef]
- Cidad, P.; Garcia-Nogales, P.; Almeida, A.; Bolaños, J.P. Expression of glucose transporter GLUT3 by endotoxin in cultured rat astrocytes: The role of nitric oxide. J. Neurochem. 2001, 79, 17–24. [Google Scholar] [CrossRef]
- Gyawali, A.; Kang, Y.S. Pretreatment Effect of Inflammatory Stimuli and Characteristics of Tryptophan Transport on Brain Capillary Endothelial (TR-BBB) and Motor Neuron Like (NSC-34) Cell Lines. Biomedicines 2020, 9, 9. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, E.; Passov, A.; Andersson, S.; Suojaranta, R.; Niemi, T.; Raivio, P.; Salmenperä, M.; Schramko, A. Glycocalyx Degradation and Inflammation in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Mahmoud, A.M.; Le Master, E.; Levitan, I.; Phillips, S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H647–H663. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Berkestedt, I.; Bodelsson, M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol. Scand. 2014, 58, 36–43. [Google Scholar] [CrossRef]
- Geranmayeh, M.H.; Rahbarghazi, R.; Farhoudi, M. Targeting pericytes for neurovascular regeneration. Cell Commun. Signal. 2019, 17, 26. [Google Scholar] [CrossRef]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef]
- Ozen, I.; Boix, J.; Paul, G. Perivascular mesenchymal stem cells in the adult human brain: A future target for neuroregeneration? Clin. Transl. Med. 2012, 1, 30. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef]
- Chidiac, R.; Zhang, Y.; Tessier, S.; Faubert, D.; Delisle, C.; Gratton, J.P. Comparative Phosphoproteomics Analysis of VEGF and Angiopoietin-1 Signaling Reveals ZO-1 as a Critical Regulator of Endothelial Cell Proliferation. Mol. Cell. Proteom. 2016, 15, 1511–1525. [Google Scholar] [CrossRef]
- Zemva, J.; Schubert, M. The role of neuronal insulin/insulin-like growth factor-1 signaling for the pathogenesis of Alzheimer′s disease: Possible therapeutic implications. CNS Neurol. Disord. Drug Targets 2014, 13, 322–337. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef] [PubMed]
- Panayi, G.S.; Corrigall, V.M.; Henderson, B. Stress cytokines: Pivotal proteins in immune regulatory networks. Curr. Opin. Immunol. 2004, 16, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195, Erratum in Nature 2010, 467, 622. [Google Scholar] [CrossRef] [PubMed]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774254. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Fu, Y.; Hsiao, J.H.; Paxinos, G.; Halliday, G.M.; Kim, W.S. ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J. Alzheimers Dis. 2016, 54, 569–584. [Google Scholar] [CrossRef]
- Zettergren, A.; Höglund, K.; Kern, S.; Thorvaldsson, V.; Johan Skoog, M.; Hansson, O.; Andreasen, N.; Bogdanovic, N.; Blennow, K.; Skoog, I.; et al. Association of IL1RAP-related genetic variation with cerebrospinal fluid concentration of Alzheimer-associated tau protein. Sci. Rep. 2019, 9, 2460. [Google Scholar] [CrossRef]
- Casali, B.T.; Reed-Geaghan, E.G. Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease. Cells 2021, 10, 957. [Google Scholar] [CrossRef]
- Liu, X.; Quan, N. Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front. Neurol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef]
- Kowal, K.; Silver, R.; Slawinska, E.; Bielecki, M.; Chyczewski, L.; Kowal-Bielecka, O. CD163 and its role in inflammation. Folia Histochem. Cytobiol. 2011, 49, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Takahashi, K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J. Neuroimmunol. 2007, 184, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Raes, G.; Van den Bergh, R.; De Baetselier, P.; Ghassabeh, G.H.; Scotton, C.; Locati, M.; Mantovani, A.; Sozzani, S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 2005, 174, 6561. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.; Bahbah, E.I.; Kamel, S.; Barreto, G.E.; Ashraf, G.M.; Elfil, M. The neutrophil-to-lymphocyte ratio in Alzheimer′s disease: Current understanding and potential applications. J. Neuroimmunol. 2020, 349, 577398. [Google Scholar] [CrossRef]
- Al-Yassin, G.A.; Bretscher, P.A. Does T Cell Activation Require a Quorum of Lymphocytes? J. Immunol. 2018, 201, 2855–2861. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Ferrari, C.C.; Rezaei, N. Deciphering variability in the role of interleukin-1β in Parkinson’s disease. Rev. Neurosci. 2016, 27, 635–650. [Google Scholar] [CrossRef]
- Morgello, S. HIV neuropathology. Handb. Clin. Neurol. 2018, 152, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Misra, S.; Kumar, A.; Pandit, A.K.; Chakravarty, K.; Prasad, K. Association between Tumor Necrosis Factor-α (−238G/A and −308G/A) Gene Polymorphisms and Risk of Ischemic Stroke: A Meta-Analysis. Pulse 2016, 3, 217–228. [Google Scholar] [CrossRef]
- Weiner, H. A 21 Point Unifying Hypothesis on the Etiology and Treatment of Multiple Sclerosis. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 1988, 25, 93–101. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Kalra, S.; Lowndes, C.; Durant, L.; Strange, R.C.; Al-Araji, A.; Hawkins, C.P.; Curnow, S.J. Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217319899695. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos, G.R.; Sato, D.K.; Becker, J.; Fujihara, K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediat. Inflamm. 2016, 2016, 5314541. [Google Scholar] [CrossRef] [PubMed]
- Mockus, T.E.; Munie, A.; Atkinson, J.R.; Segal, B.M. Encephalitogenic and Regulatory CD8 T Cells in Multiple Sclerosis and Its Animal Models. J. Immunol. 2021, 206, 3–10. [Google Scholar] [CrossRef]
- Denic, A.; Wootla, B.; Rodriguez, M. CD8(+) T cells in multiple sclerosis. Expert Opin. Ther. Targets 2013, 17, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Chisari, C.G.; Sgarlata, E.; Arena, S.; Toscano, S.; Luca, M.; Patti, F. Rituximab for the treatment of multiple sclerosis: A review. J. Neurol. 2022, 269, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Florou, D.; Katsara, M.; Feehan, J.; Dardiotis, E.; Apostolopoulos, V. Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab. Brain Sci. 2020, 10, 758. [Google Scholar] [CrossRef]
- Petrova, N.; Nutma, E.; Carassiti, D.; Rs Newman, J.; Amor, S.; Altmann, D.R.; Baker, D.; Schmierer, K. Synaptic Loss in Multiple Sclerosis Spinal Cord. Ann. Neurol. 2020, 88, 619–625. [Google Scholar] [CrossRef]
- Dutta, R.; Trapp, B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011, 93, 1–12. [Google Scholar] [CrossRef]
- Banks, W.A.; Rhea, E.M. The Blood–Brain Barrier, Oxidative Stress, and Insulin Resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef]
- Sanz, A.; Stefanatos, R.K. The mitochondrial free radical theory of aging: A critical view. Curr. Aging. Sci. 2008, 1, 10–21. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R. Sources and triggers of oxidative damage in neurodegeneration. Free Radic. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef]
- Adiutori, R.; Puentes, F.; Bremang, M.; Lombardi, V.; Zubiri, I.; Leoni, E.; Aarum, J.; Sheer, D.; McArthur, S.; Pike, I.; et al. Analysis of circulating protein aggregates as a route of investigation into neurodegenerative disorders. Brain Commun. 2021, 3, fcab148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef] [PubMed]
- Valis, M.; Talab, R.; Stourac, P.; Andrys, C.; Masopust, J. Tau protein, phosphorylated tau protein and beta-amyloid42 in the cerebrospinal fluid of multiple sclerosis patients. Neuroendocrinol. Lett. 2008, 29, 971–976. [Google Scholar] [PubMed]
- David, M.A.; Tayebi, M. Detection of protein aggregates in brain and cerebrospinal fluid derived from multiple sclerosis patients. Front. Neurol. 2014, 5, 251. [Google Scholar] [CrossRef] [PubMed]
- Szalardy, L.; Zadori, D.; Simu, M.; Bencsik, K.; Vecsei, L.; Klivenyi, P. Evaluating biomarkers of neuronal degeneration and neuroinflammation in CSF of patients with multiple sclerosis-osteopontin as a potential marker of clinical severity. J. Neurol. Sci. 2013, 331, 38–42. [Google Scholar] [CrossRef]
- Zeydan, B.; Lowe, V.J.; Reichard, R.R.; Przybelski, S.A.; Lesnick, T.G.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Parisi, J.E.; Machulda, M.M.; et al. Multiple sclerosis is associated with lower amyloid but normal tau burden on PET. Alzheimer’s Dement. 2020, 16, e039179. [Google Scholar] [CrossRef]
| Physiological Roles of the BBB | BBB Dysfunctions in Pathological Conditions (including MS) |
|---|---|
| Maintaining of ionic metastasis | Ionic imbalance (neuronal hyperpolarization) |
| Facilitating brain nutrition | Impaired brain nutrition |
| Regulating levels of neurotransmitters | Neurotransmitter imbalance (pathologic inhibition/stimulation) |
| Limiting plasma macromolecules penetrating the brain | Macromolecule leakage |
| Protecting the brain against neurotoxins | Penetration of neurotoxins in the brain |
| Facilitating molecules elimination from the brain | Impaired residual products elimination |
| NVU Components and Other Related Cells Encountered in MS Pathogenesis | Behavior in the Neuroinflammation Phase of MS | Most Relevant References | |
|---|---|---|---|
| Brain microvascular endothelial cells | Reduction in the expression of TJ proteins Upregulation of different membrane receptors (i.e., Toll-like receptors) Increased production of adhesion molecules Increased pinocytosis Upregulation of glucose transporters (GLUT1) Downregulation of amino acid transporters (LAT1) Alterations of P-glycoprotein expression Degradation of the glycocalyx | [88,89,90,91,92,93,94,95,96] | |
| Pericytes | Increased production of proinflammatory cytokines and chemokines Differentiation into fibroblasts or phagocytes Secretion of endothelial-disrupting factors | [97,98,99] | |
| Astrocytes | Increased glutamate production Decreased production of protective factors (angiopoietin-1, Shh, IGF-1) | [100,101,102] | |
| Oligodendrocytes/oligodendrocyte progenitor cells (OPCs) | Increased expression of inflammatory genes Increased phagocytic capacity Inhibition of OPC recruitment and maturation Increased apoptosis of oligodendrocytes Regional CNS demyelination | [53,103] | |
| Microglia | Activation of microglia (M1 and/or M2 phenotype) Increased production of inflammatory mediators (IL-1, IL-6, TNF-α), chemokines (CCL2, CX3CL1, MIP-1), matrix metalloproteinases (MMPs), and oxygen free radicals | [104,105,106] | |
| Neurons | Dendritic transection Axonal transection Apoptosis | [107] | |
| Immune cells | Th1 CD4 cells | Secretion of IFN-γ and TNF-α | [108] |
| Th17 CD4 cells | Production of IL-17, IL-21, IL-22 | ||
| CD8 T cells | Direct cytotoxic effect Production of IFN-γ and IL-17 | [109] | |
| B cells | Antibody-dependent mechanisms (secretion of intrathecal IgG) Antibody-independent mechanisms (secretion of cytokines, neurotoxic factors, formation of tertiary lymphoid organs) | [110] | |
| Neutrophils | Secrete pro-inflammatory cytokines (IL-1β, IL-6, IL-12, TNF-α, and IFN-γ) | [111] | |
| Monocytes | Change in phenotype (CD83 +, CD209 +) Promote the differentiation of Th1 and Th17 cells | [112] | |
| Mast-cells | Production of TNF-α | [113] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. https://doi.org/10.3390/biom12040538
Schreiner TG, Romanescu C, Popescu BO. The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules. 2022; 12(4):538. https://doi.org/10.3390/biom12040538
Chicago/Turabian StyleSchreiner, Thomas Gabriel, Constantin Romanescu, and Bogdan Ovidiu Popescu. 2022. "The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms" Biomolecules 12, no. 4: 538. https://doi.org/10.3390/biom12040538
APA StyleSchreiner, T. G., Romanescu, C., & Popescu, B. O. (2022). The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules, 12(4), 538. https://doi.org/10.3390/biom12040538







