The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model
Abstract
:1. Introduction
2. Results
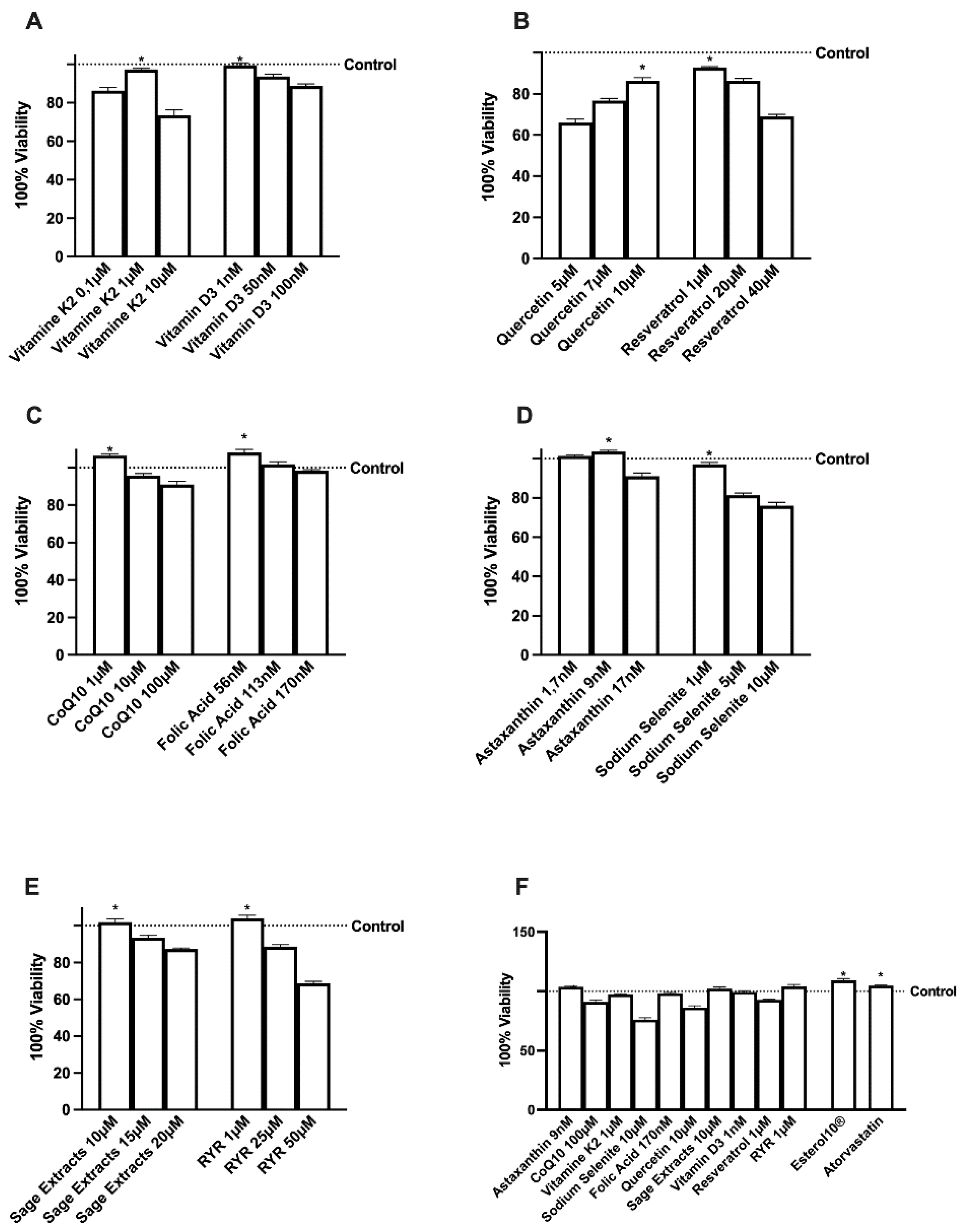
2.1. Effects of Several Natural Extracts, RYR, and Atorvastatin on HepG2 Cell Viability
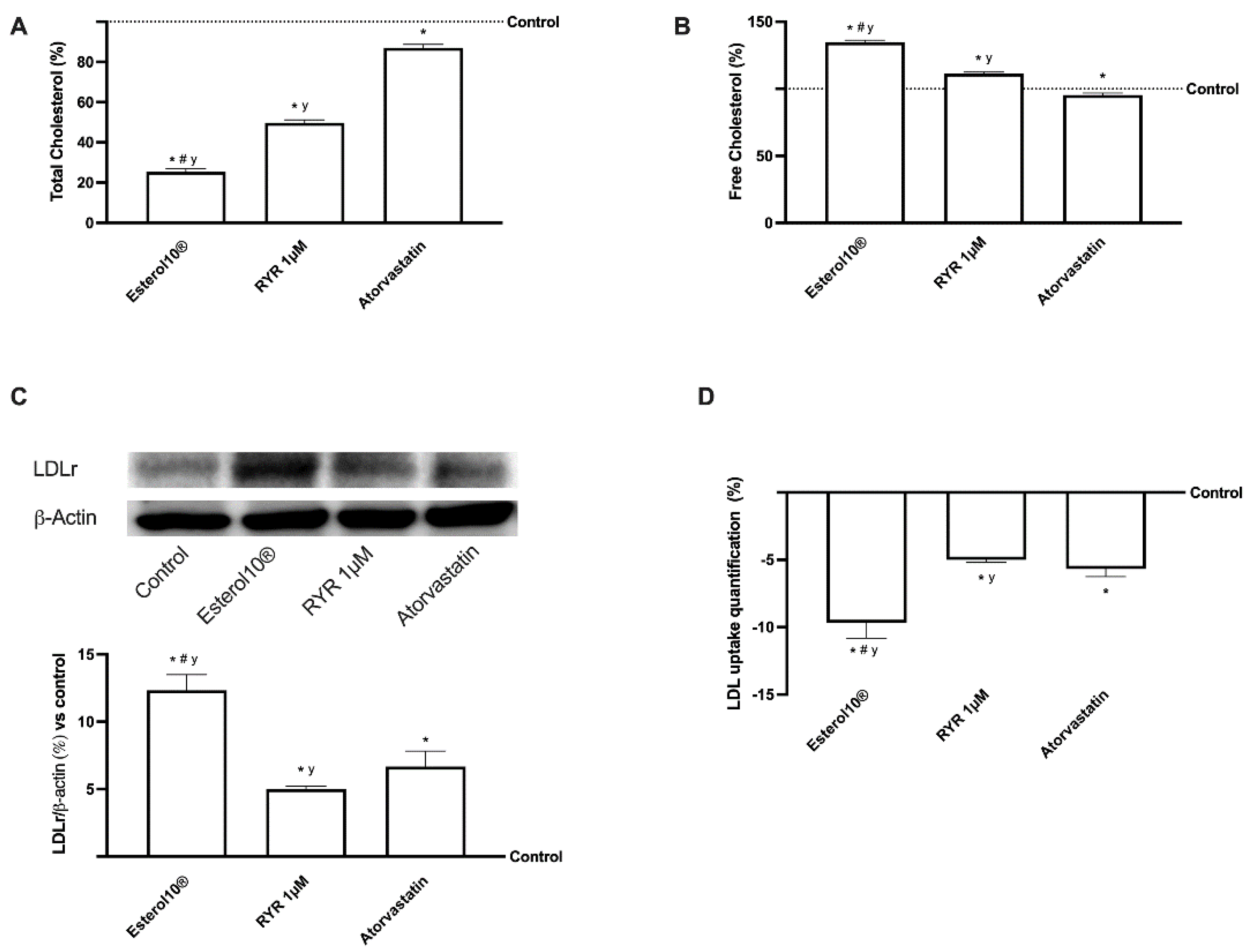
2.2. Effects of Esterol10, RYR, and Atorvastatin on Hepatic Cholesterol Biosynthesis
2.3. Effects of Esterol®, RYR, and Atorvastatin on Cholesterol Metabolism
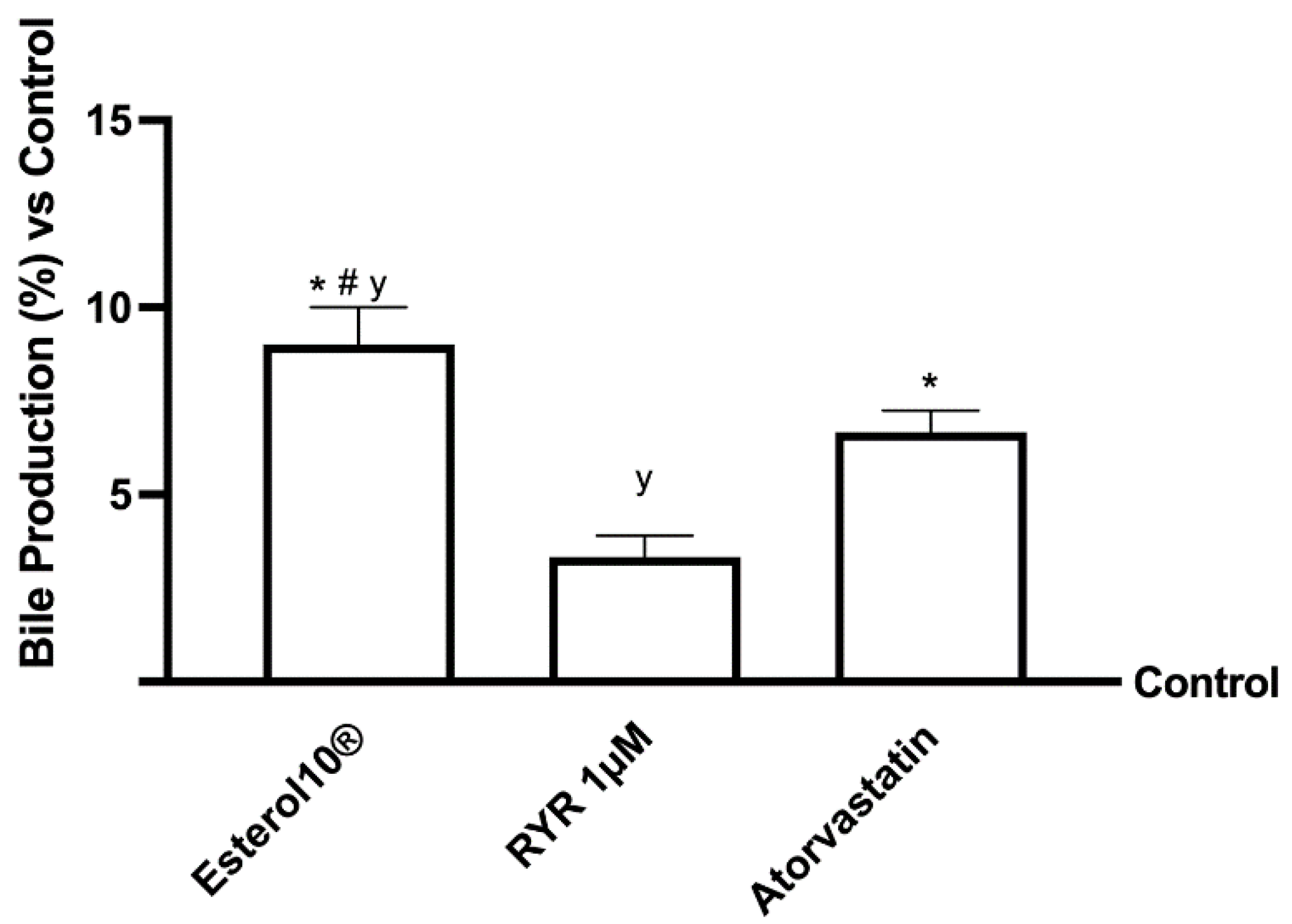
2.4. Effects of Esterol10®, RYR, and Atorvastatin on Choleresis Process
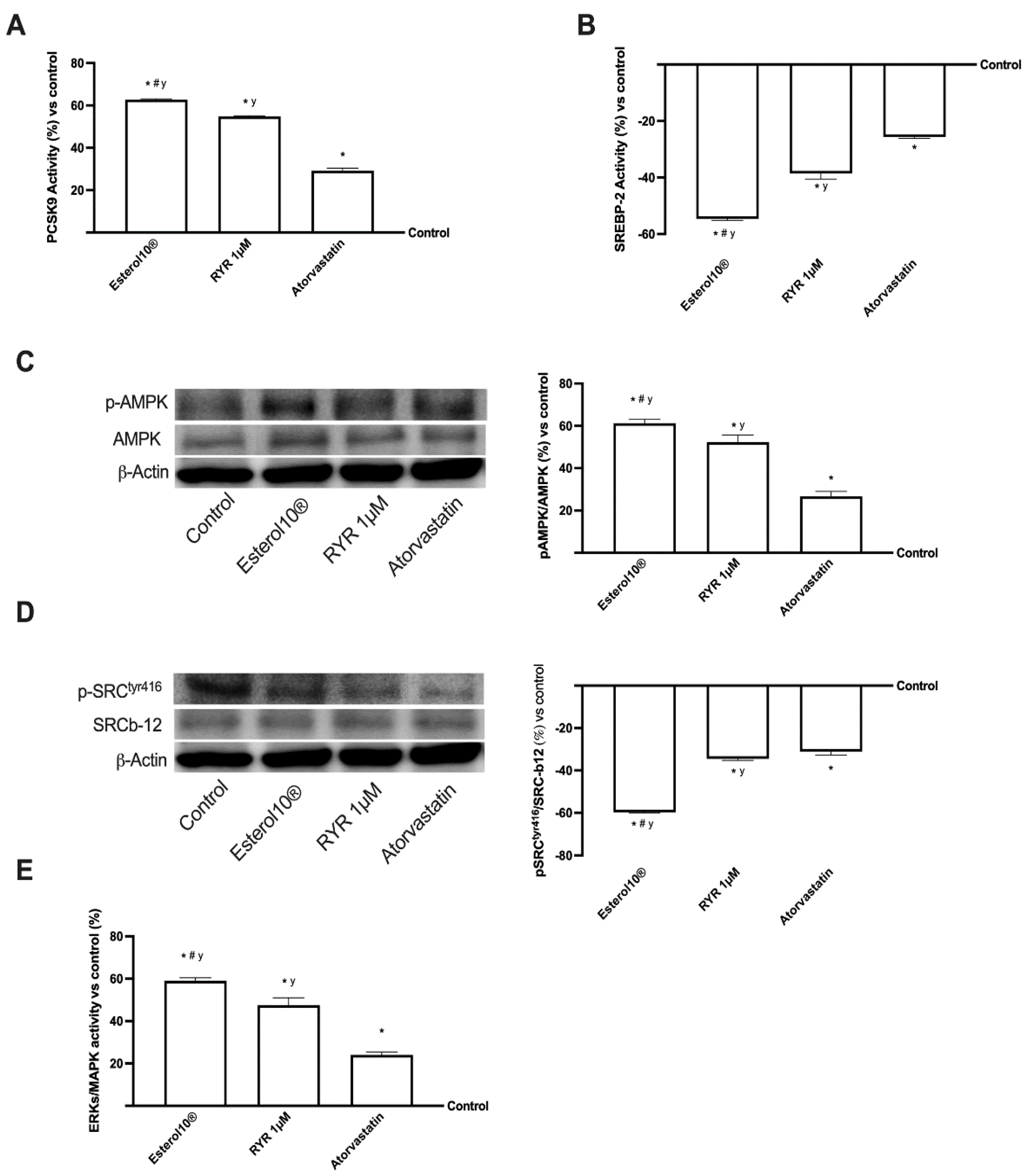
2.5. Effects of Esterol10®, RYR, and Atorvastatin on the Intracellular Pathways Leading to Cholesterol Homeostasis
2.6. Effects of Esterol10®, RYR, and Atorvastatin on Liver Injury Markers
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Experimental Protocol
4.3. MTT Viability
4.4. Measurement of Total and Free Cholesterol
4.5. LDL Uptake Quantification
4.6. The Bile Acids Production
4.7. HMGCoA Reductase ELISA Kit
4.8. Transaminase Analysis
4.9. Lipid Accumulation Assay
4.10. SREBP-2 Detection Assay
4.11. ERK/MAPK Detection Assay
4.12. PCSK9 Detection Assay
4.13. Cell Lysates and Western Blot
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| CHD | coronary heart disease |
| CVD | cardiovascular |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EFSA | European Food Safety Authority |
| ERK | extracellular signal-regulated kinases |
| FBS | fetal bovine serum |
| FDA | US Food and Drug Administration |
| GS/GSSG | reduced glutathione/oxidized glutathione |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein-associated cholesterol |
| HMGCoA | 3-hydroxy-3-methyl-glutaryl-CoA |
| HMGCR | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| LDL-C | low-density lipoprotein-associated cholesterol |
| LDLr | LDL receptor |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| PBS | phosphate-buffered saline |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PMSF | phenylmethanesulfonyl fluoride |
| PVDF | polyvinylidene difluoride |
| RYR | Red Yeast Rice |
| SREBP | sterol responsive element binding protein |
| TG | triglycerides |
| VLDL | very-low density lipoprotein |
| WHO | World Health Organization |
Appendix A
| HMGCoA Reductase | PCSK9 | SREBP-2 | ERK/MAPK | |
|---|---|---|---|---|
| Control | 1.2 ± 0.27 | 3123 ± 0.38 | 2279 ± 0.24 | 3383 ± 0.28 |
| Esterol 10® | 1.75 ± 0.33 | 5009 ± 0.21 | 1026 ± 0.29 | 5412 ± 0.33 |
| RYR 1 µM | 1.56 ± 0.42 | 4751 ± 0.26 | 1436 ± 0.34 | 4905 ± 0.42 |
| Atorvastatin | 1.43 ± 0.45 | 3840 ± 0.29 | 1687 ± 0.27 | 4228 ± 0.31 |
References
- Gururaja, G.M.; Mundkinajeddu, D.; Kumar, A.S.; Dethe, S.M.; Allan, J.J.; Agarwal, A. Evaluation of Cholesterol-lowering Activity of Standardized Extract of Mangifera indica in Albino Wistar Rats. Pharmacogn. Res. 2017, 9, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercho-lesterolemia. Methodist Debakey. Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [CrossRef]
- Kłosiewicz-Latoszek, L.; Cybulska, B.; Stoś, K.; Tyszko, P. Hypolipaemic nutraceutics: Red yeast rice and Armolipid, berberine and bergamot. Ann. Agric. Environ. Med. 2021, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, M.R.; Ministrini, S.; Pirro, M. Nutraceuticals for the treatment of hypercholesterolemia. Eur. J. Intern. Med. 2014, 25, 592–599. [Google Scholar] [CrossRef]
- Banach, M.; Bruckert, E.; Descamps, O.S.; Ellegård, L.; Ezhov, M.; Föger, B.; Fras, Z.; Kovanen, P.T.; Latkovskis, G.; März, W.; et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: A review and expert opinion. Atheroscler. Suppl. 2019, 39, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. International Lipid Expert Panel (ILEP). The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Ghasi, S.; Nwobodo, E.; Ofili, J.O. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef]
- Pinto, J.T.; Oliveira, T.T.; Alvarenga, L.F.; Barbosa, A.S.; Pizziolo, V.R.; Costa, M.R. Pharmacological activity of the hydroalcoholic extract from Hovenia dulcis thunberg fruit and the flavonoid dihydromyricetin during hypercholesterolemia induced in rats. Braz. J. Pharm. Sci. 2014, 50, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation. Nutrients. 2017, 16, 621. [Google Scholar] [CrossRef] [Green Version]
- Lupo, M.G.; Macchi, C.; Marchianò, S.; Chen, H.; Sirtori, C.; Corsini, A.; Ruscica, M.; Ferri, N. Differential Effects of Red Yeast Rice, Berberis Aristata and Morus Alba Extracts, Active Components of LopiGLIK®, on PCSK9 in HepG2 Cells. Preprints 2018, 2018100288. [Google Scholar] [CrossRef]
- Emam, M.A.; Khattab, H.I.; Hegazy, M.G. Assessment of anticancer activity of Pulicaria undulata on hepatocellular carcinoma HepG2 cell line. Tumour Biol. 2019, 41, 1010428319880080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, L.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J. Ethnopharmacol. 2005, 97, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wei, L.; Zhao, C.; Li, J.; Liu, Z.; Zhang, M.; Wang, Y. Resveratrol Maintains Lipid Metabolism Homeostasis via One of the Mechanisms Associated with the Key Circadian Regulator Bmal1. Molecules 2019, 24, 2916. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wang, Y.; Song, Y.; Cheng, X.R.; Xia, S.; Rahman, M.R.; Shi, Y.; Le, G. Resveratrol restores the circadian rhythmic disorder of lipid metab-olism induced by high-fat diet in mice. Biochem. Biophys Res. Commun. 2015, 458, 86–91. [Google Scholar] [CrossRef]
- Zhou, R.; Yi, L.; Ye, X.; Zeng, X.; Liu, K.; Qin, Y.; Zhang, Q.; Mi, M. Resveratrol Ameliorates Lipid Droplet Accumulation in Liver through a SIRT1/ ATF6-Dependent Mechanism. Cell Physiol. Biochem. 2018, 51, 2397–2420. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Nazarian, B.; Pourmirzaei Olyaei, H.; Rezaei Kelishadi, M.; Nordvall, M.; Wong, A.; Dutheil, F.; Naeini, A.A. Beneficial effects of folic acid supplementation on lipid markers in adults: A GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 18, 1–19. [Google Scholar] [CrossRef]
- Yin, K.; You, Y.; Swier, V.; Tang, L.; Radwan, M.M.; Pandya, A.N.; Agrawal, D.K. Vitamin D Protects Against Atherosclerosis via Regulation of Cholesterol Efflux and Macrophage Polarization in Hypercholesterolemic Swine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2432–2442. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Li, Y.M.; He, Z.; Hao, W.; Zhao, Y.; Liu, J.; Zhu, H.; Kwek, E.; Ma, K.Y.; He, W.S.; et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules 2021, 26, 3766. [Google Scholar] [CrossRef]
- Tsai, M.C.; Huang, S.C.; Chang, W.T.; Chen, S.C.; Hsu, C.L. Effect of Astaxanthin on the Inhibition of Lipid Accumulation in 3T3-L1 Adi-pocytes via Modulation of Lipogenesis and Fatty Acid Transport Pathways. Molecules 2020, 25, 3598. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X.; Xue, H.; Zhang, P.; Fang, W.; Chen, X.; Ling, W. Coenzyme Q10 attenuates high-fat diet-induced non-alcoholic fatty liver disease through activation of the AMPK pathway. Food Funct. 2019, 10, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Kołakowski, A.; Kurzyna, P.F.; Bzdęga, W.; Żywno, H.; Harasim-Symbor, E.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of vitamin K2 on lipid precursors of inflammation and fatty acids pathway activities in HepG2 cells. Eur. J. Cell Biol. 2021, 100, 151188. [Google Scholar] [CrossRef] [PubMed]
- Janaina, S.; Rosa, A.F.; Moncau, C.T.; Silva-Vignato, B.; Pugine, S.M.P.; de Melo, M.P.; Sanchez, J.M.D.; Zanetti, M.A. Effect of different selenium sources and concentrations on glutathione peroxidase activity and cholesterol metabolism of beef cattle. J. Anim. Sci. 2021, 99. [Google Scholar] [CrossRef]
- Frigerio, J.; Tedesco, E.; Benetti, F.; Insolia, V.; Nicotra, G.; Mezzasalma, V.; Pagliari, S.; Labra, M.; Campone, L. Anticholesterolemic Activity of Three Vegetal Extracts (Artichoke, Caigua, and Fenugreek) and Their Unique Blend. Front. Pharmacol. 2021, 12, 726199. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Chean, J.; Chen, C.J.; Gugiu, G.; Wong, P.; Cha, S.; Li, H.; Nguyen, T.; Bhatticharya, S.; Shively, J.E. Human CEACAM1-LF regulates lipid storage in HepG2 cells via fatty acid transporter CD36. J. Biol. Chem. 2021, 297, 101311. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Yan, J.; Liu, H.; Liu, L.; Cheng, L.; Qiu, F.; Kang, N. Berberrubine and its analog, hydroxypropyl-berberrubine, regulate LDLR and PCSK9 expression via the ERK signal pathway to exert cholesterol-lowering effects in human hepatoma HepG2 cells. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [Green Version]
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A.; et al. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar] [CrossRef]
- Sartore, G.; Burlina, S.; Ragazzi, E.; Ferraresso, S.; Valentini, R.; Lapolla, A. Mediterranean Diet and Red Yeast Rice Supplementation for the Management of Hyperlipidemia in Statin-Intolerant Patients with or without Type 2 Diabetes. Evid. Based Complementary Altern. Med. 2013, 2013, 743473. [Google Scholar] [CrossRef]
- Soy, G.; Forns, X. Red yeast rice extract: The risky trend of natural products. Gastroenterol. Hepatol. 2021, 44, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qi, F.; Wu, J. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef] [Green Version]
- Dujovne, C.A. Red yeast rice preparations: Are they suitable substitutions for statins? Am. J. Med. 2017, 130, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to monacolin K in SYLVAN BIO red yeast rice and maintenance of normal blood LDL-cholesterol concentrations pursuant to Article 13 (5) of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3084. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Świątkiewicz, M.; Grela, E.R. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. 2015, 108, 61–66. [Google Scholar] [CrossRef]
- Huang, C.H.; Shiu, S.M.; Wu, M.T.; Chen, W.L.; Wang, S.G.; Lee, H.M. Monacolin K affects lipid metabolism through SIRT1/AMPK pathway in HepG2 cells. Arch. Pharm. Res. 2013, 36, 1541–1551. [Google Scholar] [CrossRef]
- Huang, J.; Yang, G.; Huang, Y.; Zhang, S. Inhibitory effects of 1,25(OH)2D3 on the proliferation of hepatocellular carcinoma cells through the downregulation of HDAC2. Oncol. Rep. 2017, 38, 1845–1850. [Google Scholar] [CrossRef]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Pi, J.; Li, B.; Tu, L.; Zhu, H.; Jin, H.; Yang, F.; Bai, H.; Cai, H.; Cai, J. Investigation of quercetin-induced HepG2 cell apoptosis-associated cellular biophysical alterations by atomic force microscopy. Scanning 2016, 38, 100–112. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Rimbach, G.; Jungblut, A.; Frank, J. Comparison of tetrahydrofu-ran, fetal calf serum, and Tween 40 for the delivery of astaxanthin and canthaxanthin to HepG2 cells. Cytotechnology 2011, 63, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Mine, Y.; Okamoto, T. Extracellular coenzyme Q10 (CoQ10) is reduced to ubiquinol-10 by intact Hep G2 cells independent of intracellular CoQ10 reduction. Arch. Biochem. Biophys. 2019, 672, 108067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Zhang, A.; Zhao, Y.; Zhao, J.; Liu, J.; Gao, J.; Fang, D.; Rao, Z. Synergistic growth inhibition by sorafenib and vitamin K2 in human hepatocellular carcinoma cells. Clinics 2012, 67, 1093–1099. [Google Scholar] [CrossRef]
- Zou, Y.; Niu, P.; Gong, Z.; Yang, J.; Yuan, J.; Wu, T.; Chen, X. Relationship between reactive oxygen species and sodium-selenite-induced DNA damage in HepG2 cells. Front. Med. China 2007, 1, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bagherieh, M.; Kheirollahi, A.; Zamani-Garmsiri, F.; Emamgholipour, S.; Meshkani, R. Folic acid ameliorates palmitate-induced inflammation through decreasing homocysteine and inhibiting NF-κB pathway in HepG2 cells. Arch. Physiol. Biochem. 2021, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.G.; Macchi, C.; Marchianò, S.; Cristofani, R.; Greco, M.F.; Dall’Acqua, S.; Chen, H.; Sirtori, C.R.; Corsini, A.; Ruscica, M.; et al. Differential effects of red yeast rice, Berberis aristata and Morus alba extracts on PCSK9 and LDL uptake. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1245–1253. [Google Scholar] [CrossRef]
- Lim, D.W.; Jeon, H.; Kim, M.; Yoon, M.; Jung, J.; Kwon, S.; Cho, S.; Um, M.Y. Standardized rice bran extract improves hepatic steatosis in HepG2 cells and ovariectomized rats. Nutr. Res. Pract. 2020, 14, 568–579. [Google Scholar] [CrossRef]
- Molinari, C.; Ruga, S.; Farghali, M.; Galla, R.; Bassiouny, A.; Uberti, F. Preventing c2c12 muscular cells damage combining magnesium and potassium with vitamin D3 and curcumin. J. Tradit. Complementary Med. 2021, 11, 532–544. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Chen, Y.; Cheng, Y.; Li, J.; Zheng, L.; Zeng, X.; Luo, T. Diet-induced obesity is associated with altered expression of sperm motility-related genes and testicular post-translational modifications in a mouse model. Theriogenology 2020, 158, 233–238. [Google Scholar] [CrossRef]
- Shao, D.; Wang, Y.; Huang, Q.; Shi, J.; Yang, H.; Pan, Z.; Jin, M.; Zhao, H.; Xu, X. Cholesterol-Lowering Effects and Mechanisms in View of Bile Acid Pathway of Resveratrol and Resveratrol Glucuronides. J. Food Sci. 2016, 81, H2841–H2848. [Google Scholar] [CrossRef]
- Mullen, P.J.; Lüscher, B.; Scharnagl, H.; Krähenbühl, S.; Brecht, K. Effect of simvastatin on cholesterol metabolism in C2C12 myotubes and HepG2 cells, and consequences for statin-induced myopathy. Biochem. Pharmacol. 2010, 79, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Brereton, M.F.; Rohm, M.; Shimomura, K.; Holland, C.; Tornovsky-Babeay, S.; Dadon, D.; Iberl, M.; Chibalina, M.V.; Lee, S.; Glaser, B.; et al. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat. Commun. 2016, 24, 13496. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell. Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, H.S.; You, B.H.; Kim, D.Y.; Lee, H.; Ko, H.W.; Ko, H.J.; Choi, Y.H.; Choi, S.S.; Chin, Y.W. Sauchinone controls hepatic cholesterol homeostasis by the negative regulation of PCSK9 transcriptional network. Sci. Rep. 2018, 30, 6737. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruga, S.; Galla, R.; Penna, C.; Molinari, C.; Uberti, F. The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model. Int. J. Mol. Sci. 2022, 23, 3805. https://doi.org/10.3390/ijms23073805
Ruga S, Galla R, Penna C, Molinari C, Uberti F. The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model. International Journal of Molecular Sciences. 2022; 23(7):3805. https://doi.org/10.3390/ijms23073805
Chicago/Turabian StyleRuga, Sara, Rebecca Galla, Claudia Penna, Claudio Molinari, and Francesca Uberti. 2022. "The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model" International Journal of Molecular Sciences 23, no. 7: 3805. https://doi.org/10.3390/ijms23073805
APA StyleRuga, S., Galla, R., Penna, C., Molinari, C., & Uberti, F. (2022). The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model. International Journal of Molecular Sciences, 23(7), 3805. https://doi.org/10.3390/ijms23073805







