A Study on the Adsorption Characteristics of Thiourea by Typical Minerals from the Bio-Oxidation Residue of Gold Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipments
2.2. Sample Preparation
2.3. Detection of the Thiourea Concentration
2.4. Adsorption Experiment
2.5. Infrared Spectroscopy Detection
3. Results and Discussion
3.1. Effect of the Adsorption Time
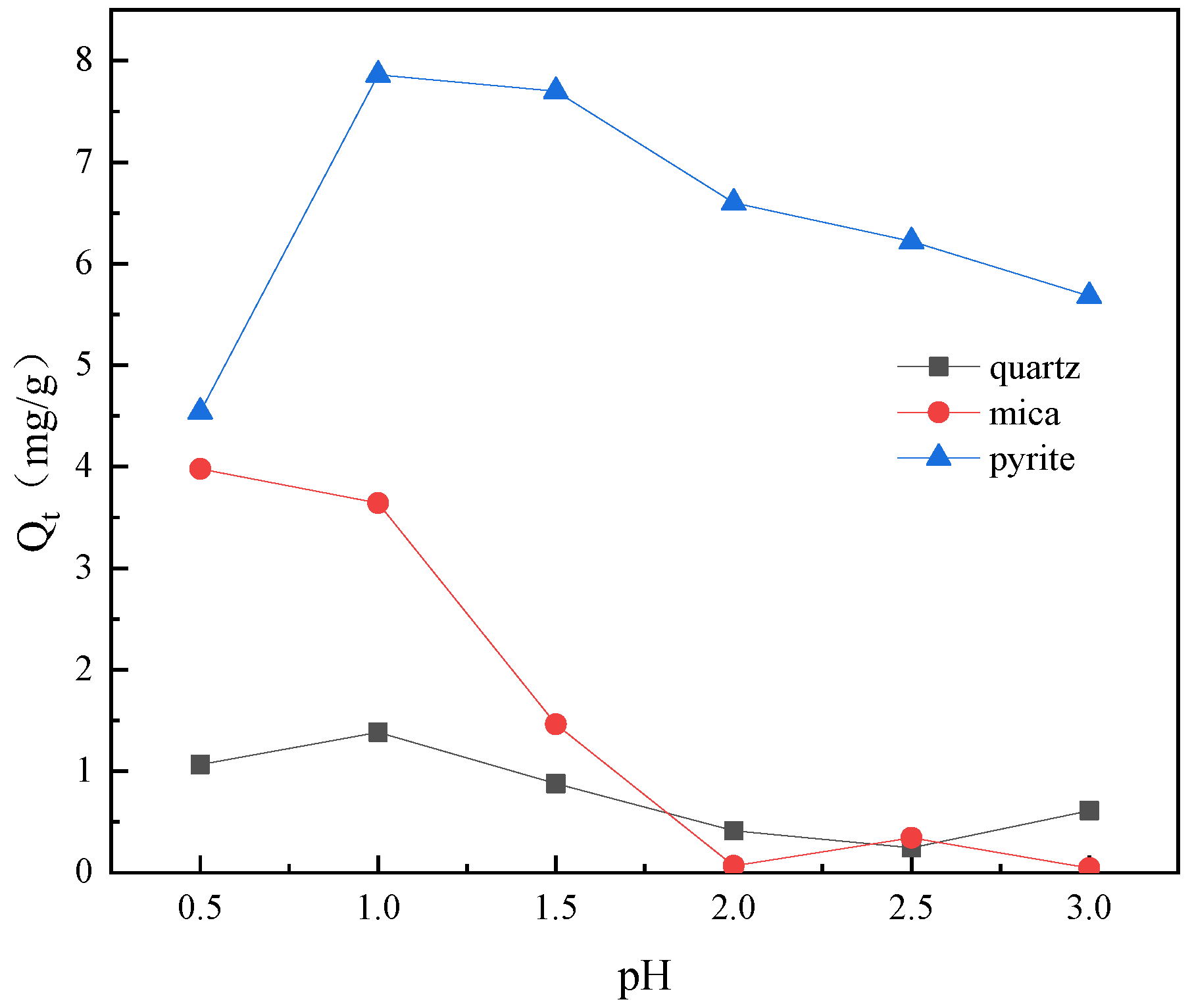
3.2. Effect of pH
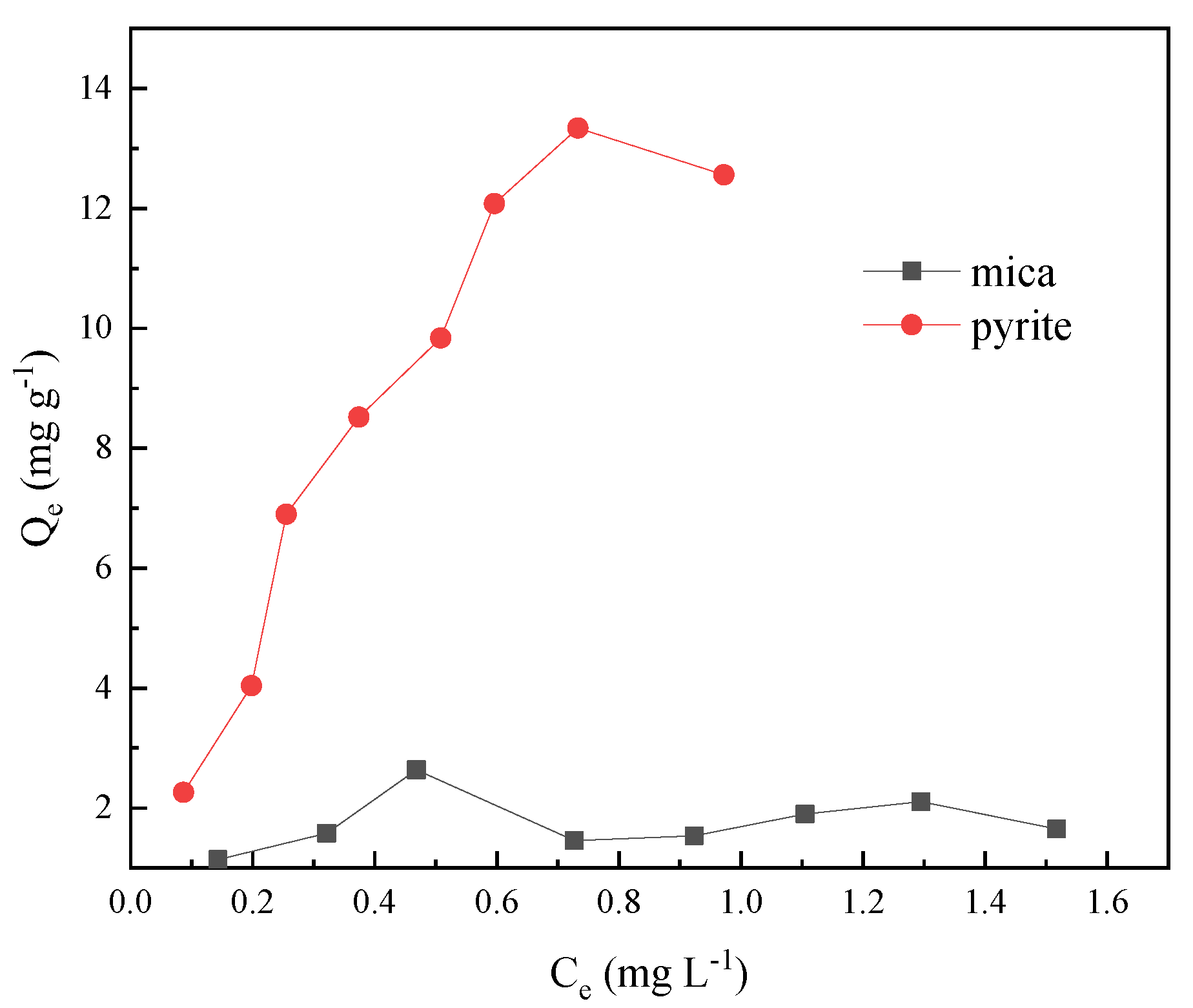
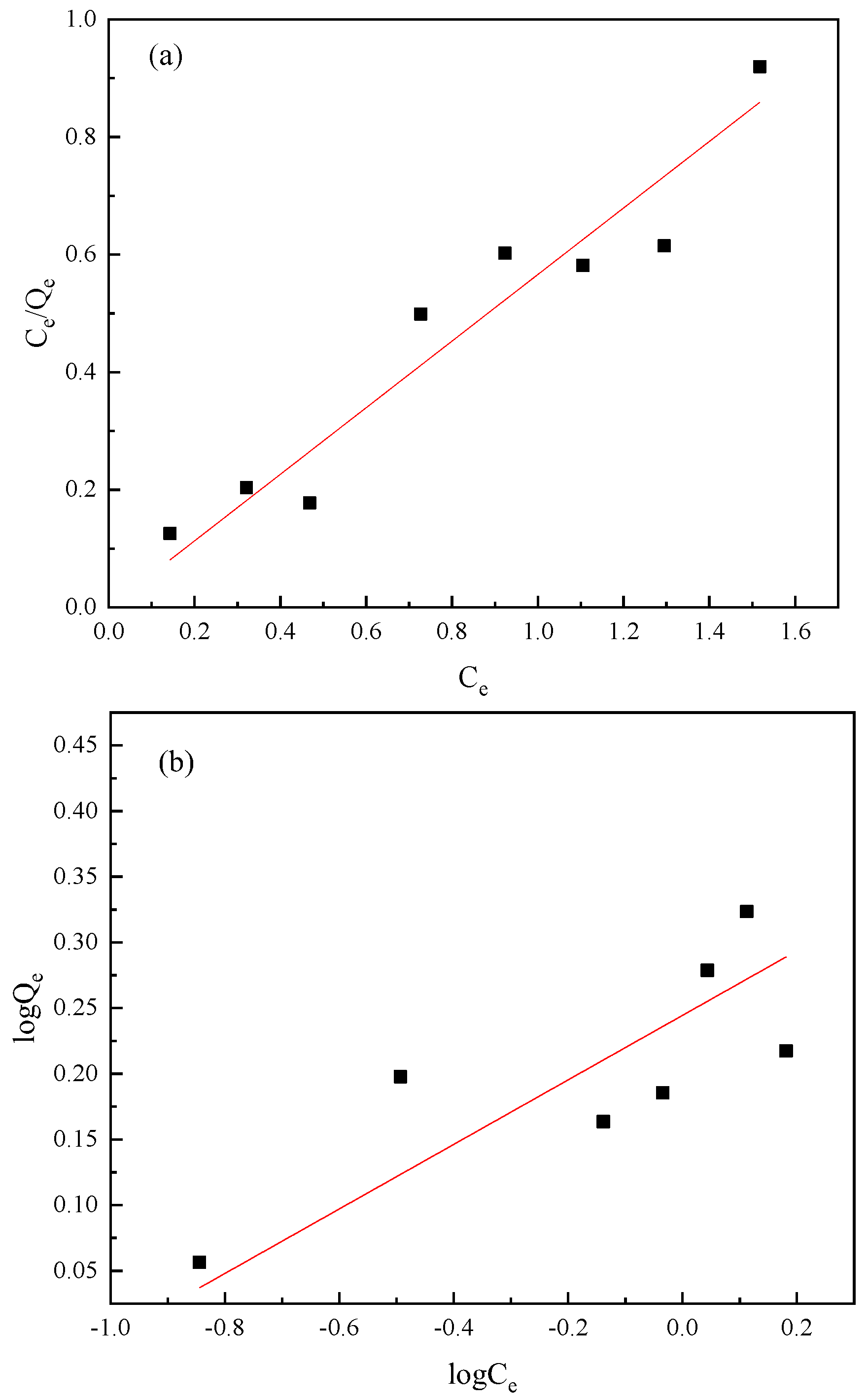
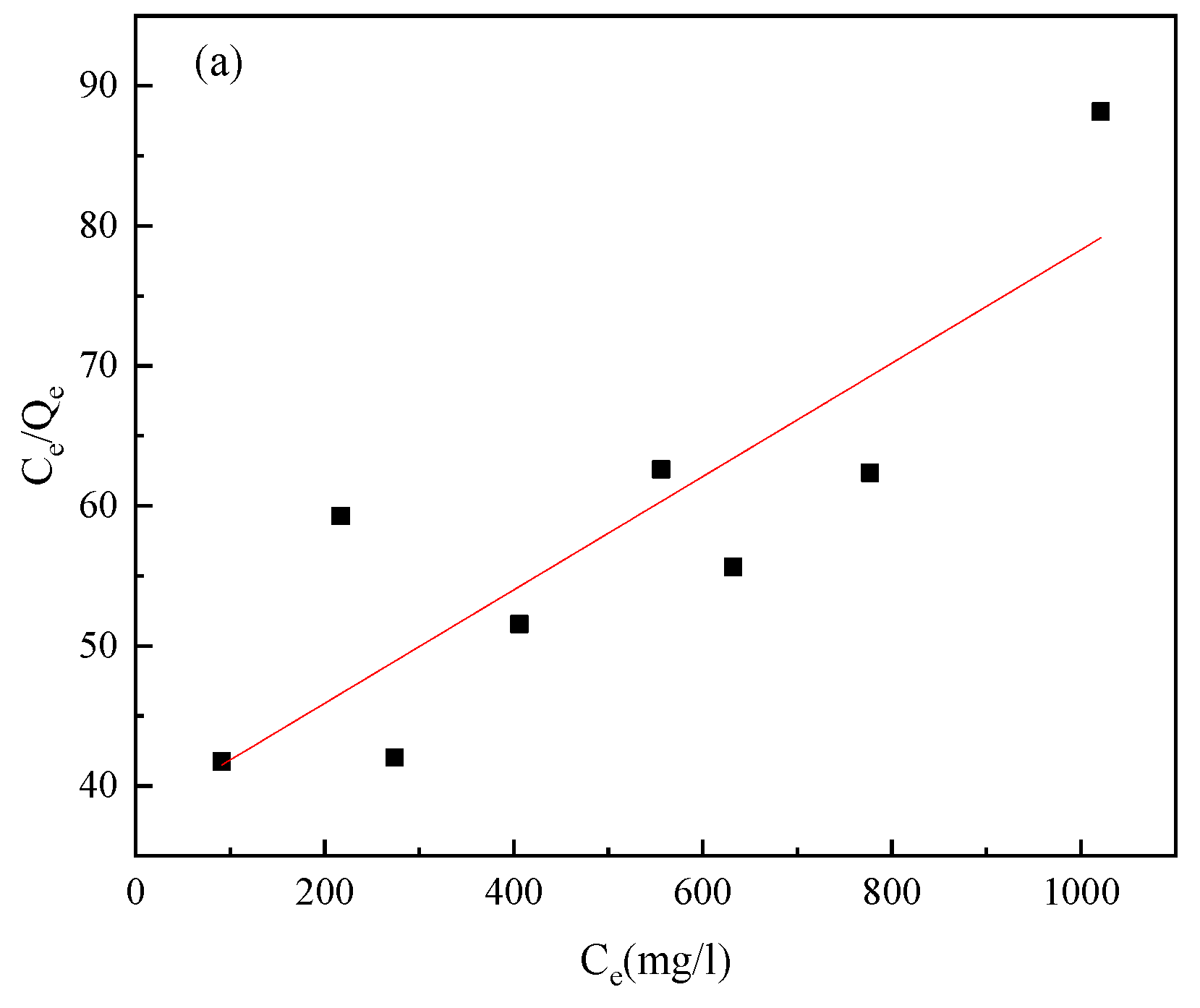
3.3. Adsorption Isotherms
3.4. Kinetic Studies
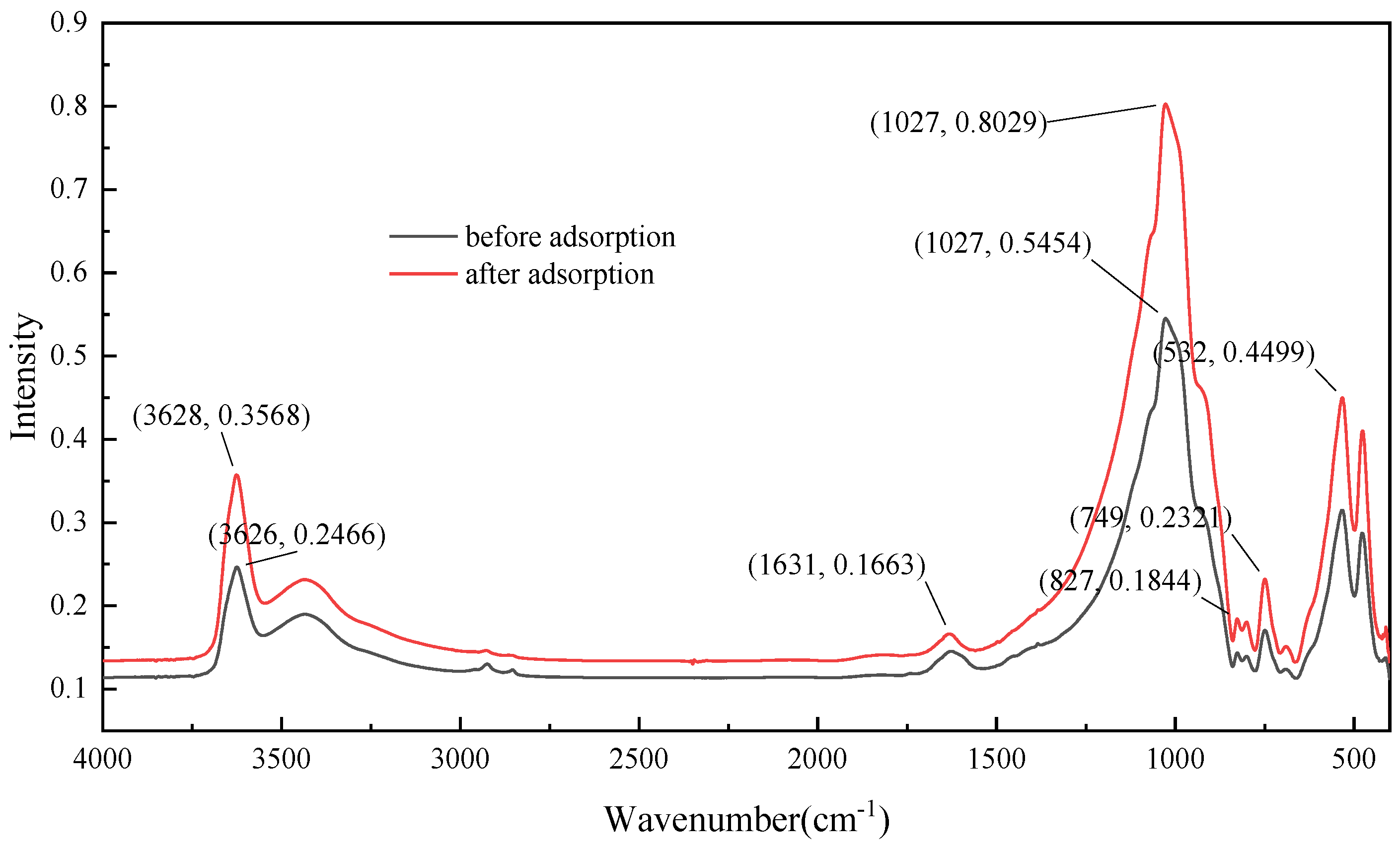
3.5. FT-IR Analysis of Adsorption
4. Conclusions
- The typical minerals in the bio-oxidation residue of refractory gold ore in Xinjiang have a certain adsorption effect on thiourea, and the order, according to the adsorption capacity from large to small, is pyrite, mica, and quartz.
- The adsorption of thiourea on pyrite is an adsorption process that conforms to the Freundlich isotherm equation and pseudo-second-order kinetic equation. The saturated adsorption capacity is 8.93 mg g−1 of pyrite. The adsorption of thiourea on mica is an adsorption process that conforms to the Langmuir isotherm equation and the pseudo-second-order kinetic equation. The saturated adsorption capacity is 2.30 mg g−1 of mica.
- Combining the adsorption mechanism of the two minerals, it can be seen that the adsorption of thiourea by the pre-oxidation residue is a mixed process of physical adsorption and chemical adsorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boboev, I.R.; Selnitsin, R.S.; Kholikov, T.A.; Sharipov, B.K. Technology of Gold Extraction from Mature Dumps by Thiourea Leaching. Russ. J. NonFerrous Met. 2020, 61, 257–264. [Google Scholar] [CrossRef]
- Boboev, I.R.; Kurbonov, S.K.; Sel’Nitsyn, R.S. Use of Thiourea Leaching During Gold-Containing Dump Treatment. Metallurgist 2019, 63, 633–641. [Google Scholar] [CrossRef]
- Deng, T.; Liao, M. Gold recovery enhancement from a refractory flotation concentrate by sequential bioleaching and thiourea leach. Hydrometallurgy 2002, 63, 249–255. [Google Scholar] [CrossRef]
- Vargas, C.; Navarro, P.; Espinoza, D.; Manríquez, J.; Mejía, E. Dissolution Behavior of Gold in Alkaline Media Using Thiourea. Int. J. Nonferrous Met. 2019, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Intan, N.R.; Yu, T.; Naoko, O. Thiourea Bioleaching for Gold Recycling from E-waste. Waste Manag. 2019, 84, 158–165. [Google Scholar]
- Schulze, R.G. New Aspects in Thiourea Leaching of Precious Metals. JOM 1984, 36, 62–65. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Zhang, L.; Tian, Q.-H.; Qin, H. Stepwise extraction of gold and silver from refractory gold concentrate calcine by thiourea. Hydrometallurgy 2020, 194, 105330. [Google Scholar] [CrossRef]
- Le, G.; Li, W.J.; Song, K.; Song, Y.S.; Chen, Y.; Bai, A.P.; Cheng, Y. Electrochemical Dissolution Behavior of Gold and Its Main Coexistent Sulfide Minerals in Acid Thiocyanate Solutions. Rare Met. 2022, 41, 254–261. [Google Scholar] [CrossRef]
- Li, W.J.; Zhou, H.; Bai, A.P.; Song, Y.S.; Cai, L.L.; Zheng, S.L.; Zhang, Q.D.; Cao, S. Electrochemical adsorption and pas-sivation on gold surface in alkaline thiourea solutions. Rare Met. 2020, 39, 951–958. [Google Scholar] [CrossRef]
- Bolzán, A.; Piatti, R.; Arvia, A. Electrochemical processes at gold|thiourea-containing aqueous acid solution interfaces. J. Electroanal. Chem. 2003, 552, 19–34. [Google Scholar] [CrossRef]
- Yan, M.; Liu, K.; Jiang, Z. Electrochemical oxidation of thiourea studied by use of in situ FTIR spectroscopy. J. Electroanal. Chem. 1996, 408, 225–229. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.Y.; Feng, J.M.; Lu, Y.C. Reaction Dynamics in the Oxidation of Thiourea. Chem. Res. Appl. 2006, 18, 758–764. [Google Scholar]
- Lan, X.Z.; Zhang, J.; Yuan, Z.C.; Jia, Y.P.; Cheng, W.Q. A Study of Reagents Consumption During Extracting Gold and Silver by Thiourea, J. Xi’an Inst. Metall. Cons. Eng. 1994, 25, 167–171. [Google Scholar]
- Yang, B.; Tong, X.; Xie, X.; Wang, X. Study on the Gold Recovery from Flotation Tailings of a Refractory Gold Ores in Gansu Province by a Process Combing Mineral Processing and Metallurgy. Gold Sci. Technol. 2020, 28, 123–131. [Google Scholar]
- Wu, B. Current Status and Progress of the Research on Complex Refractory Gold Ore Pretreatment Technology. Gold 2020, 5, 69–78. [Google Scholar]
- Ren, C.G.; Zhou, S.J.; Wang, K.R.; Zhou, Y.Q. A Study on Existing State of Superfine Golden Minerals and Inclusions with Scanning Proton Microprobe. Nucl. Technol. 1993, 16, 479–482. [Google Scholar]
- Zheng, C.J.; Xiong, Y.; Hu, J.P. Analysis of the Occurrence in Microarea for WRAP Type Gold Mine by SEM, PTCA. Part A Phys. Test. 2006, 42, 184–186. [Google Scholar]
- Qiu, Z.M.; Rang, M.; Qiu, L. W. Discovery of Gold with Negative Oxidation Number in Pyrite and Arsenopyrite. J. Chang. Univ. Earth Sci. 1994, 24, 168–173. [Google Scholar]
- Zhang, S.; Yang, H.; Ma, P.; Luan, Z.; Tong, L.; Jin, Z.; Sand, W. Column bio-oxidation of low-grade refractory gold ore containing high-arsenic and high-sulfur: Insight on change in microbial community structure and sulfide surface corrosion. Miner. Eng. 2020, 175, 107201. [Google Scholar] [CrossRef]
- Monachon, M.; Berenguer, M.A.; Joseph, E. Chapter One-Biological Oxidation of Iron Sulfides. Adv. Appl. Microbiol. 2019, 107, 1–27. [Google Scholar]
- Zhao, H.-F.; Yang, H.-Y.; Tong, L.-L.; Zhang, Q.; Kong, Y. Biooxidation-thiosulfate leaching of refractory gold concentrate. Int. J. Miner. Met. Mater. 2020, 27, 1075–1082. [Google Scholar] [CrossRef]
- Ren, C.Y. Study on the Mechanism and Process of Gold Leaching by Thiourea in the Acid System of Biological Pre-Oxidation Residue of Refractory Gold Ore Containing Arsenic; General Research Institute for Nonferrous Metals: Beijing, China, 2020; p. 18. [Google Scholar]
- Zhong, X.; Jiang, E.C.; Lu, L.Y.; Gao, Z.N.; Wang, M.F. Preparation of rice husk biochar and adsorption characteristics of urea nitrogen. J. Agro-Environ. Sci. 2021, 40, 2150–2158. [Google Scholar]
- Balram, S.Y.; Sudip, D. Effect of Time, pH, and Temperature on Kinetics for Adsorption of Methyl-orange Dye into the Modified Nitrate Intercalated MgAl LDH Adsorbent. Inorg. Chem. Commun. 2022, 137, 109203. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xu, N.; Wu, Y.; Zhang, G.Y.; Zhou, Y.J.; Niu, Y.N. Study on the adsorption of methylene blue by the glass pumice. Anhui Chem. Ind. 2021, 47, 31–33. [Google Scholar]
- Liu, H.W. Study on the Preparation and Adsorption Properties of Modified Clay for Heavy Metal Ion; Northwest Normal University: Lanzhou, China, 2011. [Google Scholar]
- Chen, H.; Wang, A.Q. Adsorption characteristics of Cu(II) from aqueous solution onto poly(acrylamide)/attapulgite composite. J. Hazard. Mater. 2009, 165, 223–231. [Google Scholar] [CrossRef]
- Shi, S.; Wu, Q.Y.; Li, X.Z.; Huang, M.H. Adsorption of Sb(V) in Water by Natural Pyrite: Performance and Mechanism. Environ. Sci. 2020, 41, 4124–4132. [Google Scholar]
- Tu, Y.B.; Han, P.W.; Zhang, X.M.; Du, Y.C.; Wang, Y.L.; Ye, S.F. Adsorption of Cyanide by Typical Minerals of Cyanide Slag. CIESS J. 2019, 70, 2684–2690. [Google Scholar]
- Liang, L.; Wang, Y.B.; Lin, X.T.; Wang, W.W.; Wen, K. Effect Mechanism of Electrochemical Modification on Calcium-activated Muscovite. Conserv. Util. Miner. Resour. 2021, 41, 107–113. [Google Scholar]
- Jin, X.T.; Li, G.G.; Liao, B.B.; Li, X.M.; Long, C. Gemological and Vibrational Spectrum Characteristics of Micaceous Quartzose Jade ‘Strawberry Crystal’. J. Gems Gemmol. 2021, 23, 19–28. [Google Scholar]
- Guo, G.W. Preparation of Ultra-fine Powder of Muscovite and Research of Aid-grinding Mechanism; Xi’an University of Architecture and Technology: Xi’an, China, 2015. [Google Scholar]

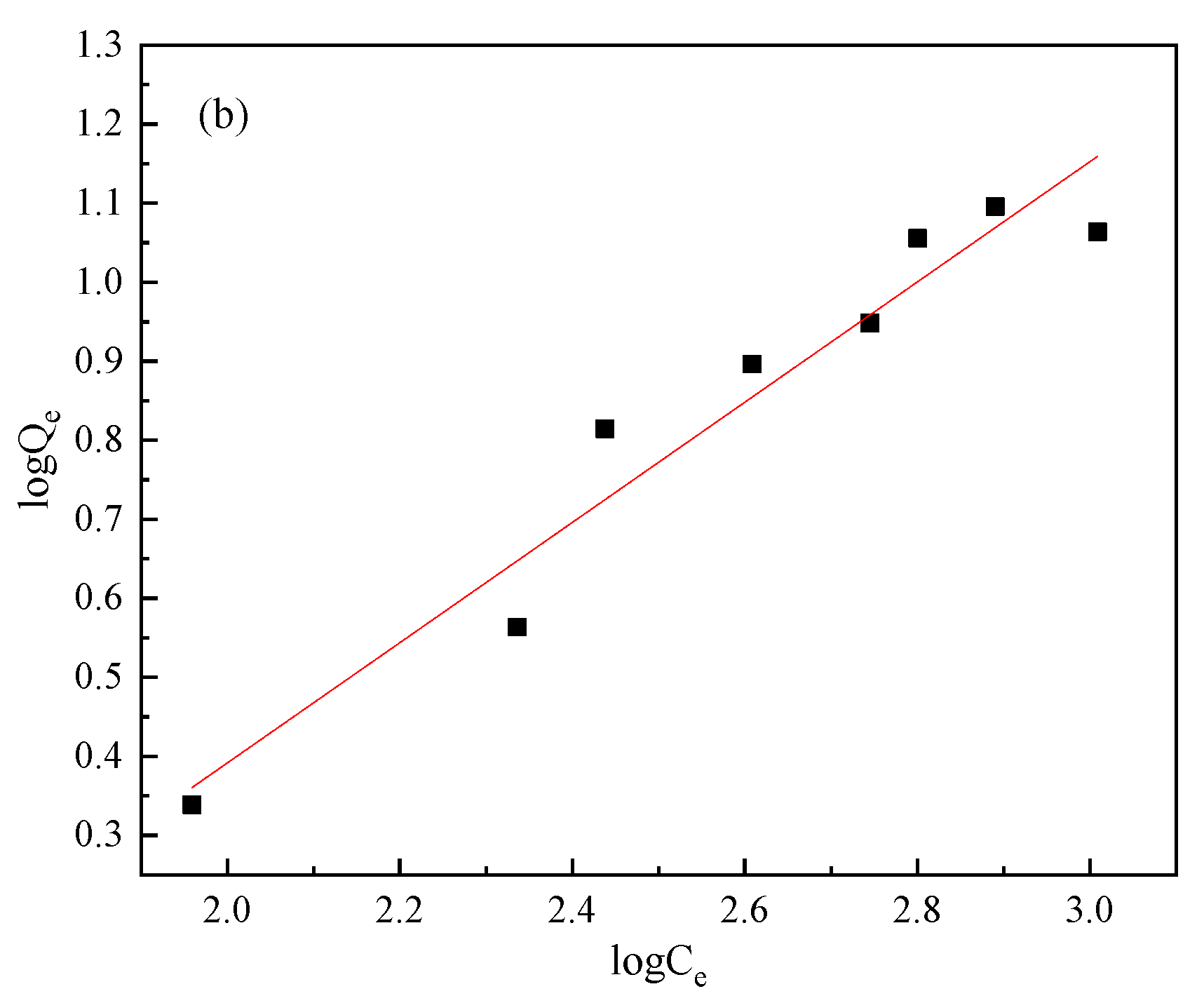
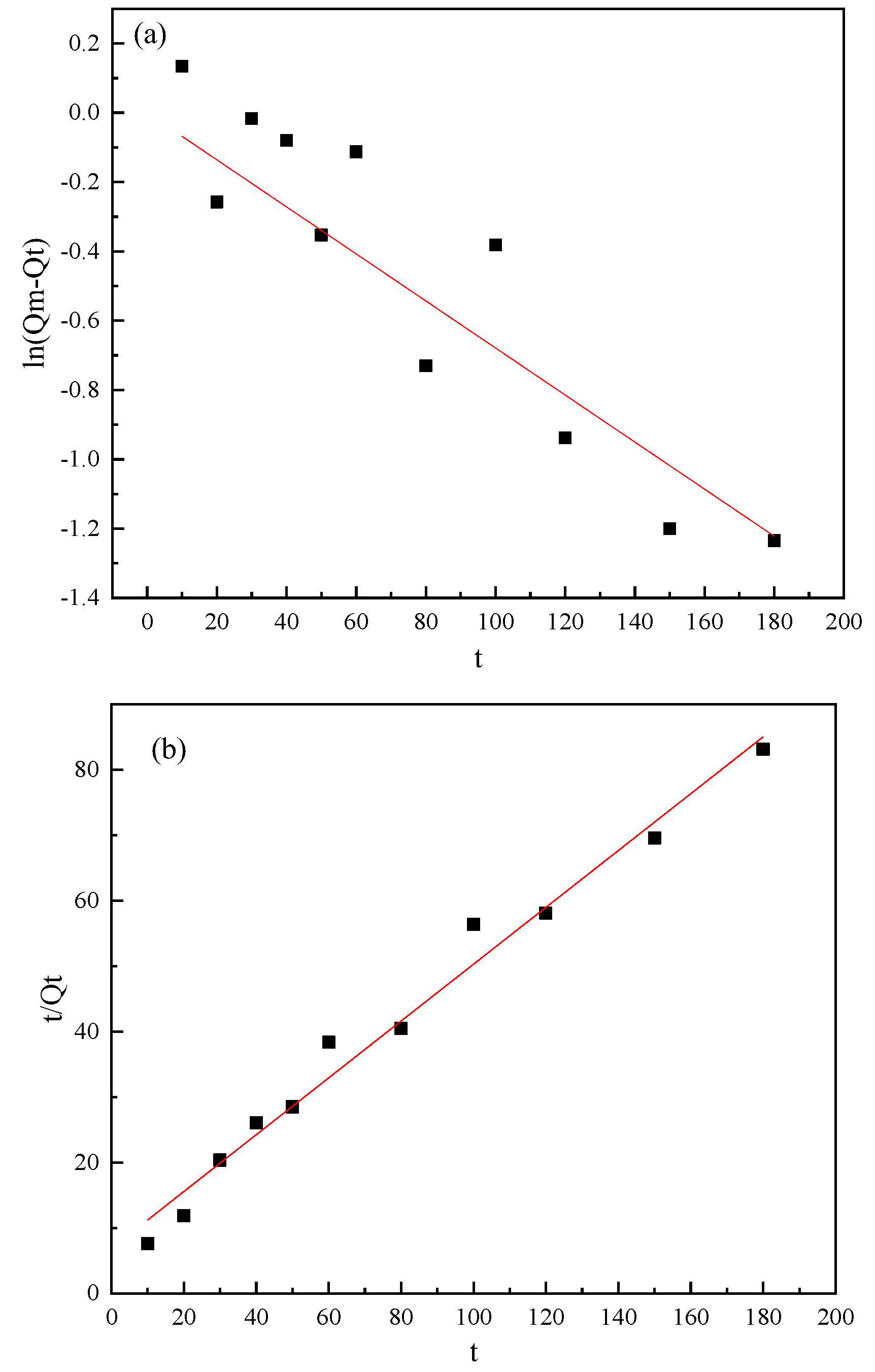
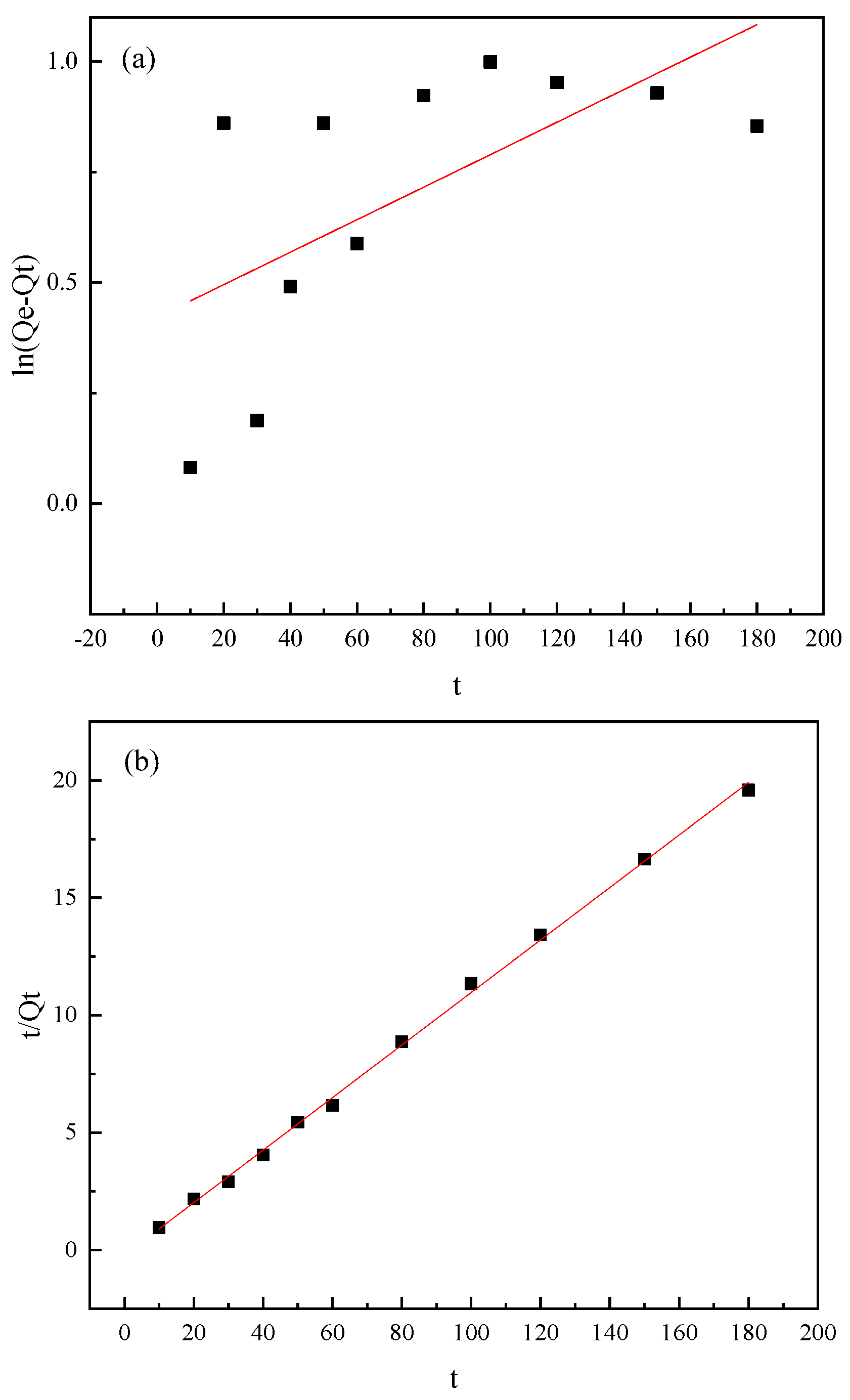

| Minerals | Items | Fitting Results | Parameters | ||
|---|---|---|---|---|---|
| Mica | Langmuir equation | Ce/Qe = 0.5561Ce + 0.0001 | b | Qm | R2 |
| 5.59 × 103 | 1.80 | 0.98 | |||
| Freundlich equation | logQe = 0.2456logCe − 0.244 | K | n | R2 | |
| - | - | 0.57 | |||
| Pyrite | Langmuir equation | Ce/Qe = 0.0405Ce + 37.808 | B | Qm | R2 |
| 1.07 × 10−3 | 24.69 | 0.73 | |||
| Freundlich equation | logQe = 0.761logCe − 1.130 | K | n | R2 | |
| 0.07 | 1.32 | 0.94 | |||
| Minerals | Items | Fitting Results | Parameters | ||
|---|---|---|---|---|---|
| Mica | Pseudo-first-order model | ln(Qe − Qt) = −0.00679t | Qe | k1 | R2 |
| 1.01 | 0.0068 | 0.91 | |||
| Pseudo-second-order model | t/Qt = 0.434t + 6.902 | Qe | k2 | R2 | |
| 2.30 | 0.027 | 0.98 | |||
| Pyrite | Pseudo-first-order model | ln(Qe − Qt) = 0.0367t + 0.422 | Qe | k1 | R2 |
| 1.53 | −0.037 | 0.34 | |||
| Pseudo-second-order model | t/Qt = 0.112t − 0.09 | Qe | k2 | R2 | |
| 8.93 | −0.14 | 0.99 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wu, B.; Shang, H.; Wang, M.; Wen, J. A Study on the Adsorption Characteristics of Thiourea by Typical Minerals from the Bio-Oxidation Residue of Gold Ore. Minerals 2022, 12, 421. https://doi.org/10.3390/min12040421
Chen S, Wu B, Shang H, Wang M, Wen J. A Study on the Adsorption Characteristics of Thiourea by Typical Minerals from the Bio-Oxidation Residue of Gold Ore. Minerals. 2022; 12(4):421. https://doi.org/10.3390/min12040421
Chicago/Turabian StyleChen, Shaoda, Biao Wu, He Shang, Miao Wang, and Jiankang Wen. 2022. "A Study on the Adsorption Characteristics of Thiourea by Typical Minerals from the Bio-Oxidation Residue of Gold Ore" Minerals 12, no. 4: 421. https://doi.org/10.3390/min12040421
APA StyleChen, S., Wu, B., Shang, H., Wang, M., & Wen, J. (2022). A Study on the Adsorption Characteristics of Thiourea by Typical Minerals from the Bio-Oxidation Residue of Gold Ore. Minerals, 12(4), 421. https://doi.org/10.3390/min12040421





