A Study on the Static Magnetic and Electromagnetic Properties of Silica-Coated Carbonyl Iron Powder after Heat Treatment for Improving Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, K.Y.; Han, J.H.; Lee, S.B.; Yi, J.W. Microwave absorbing hybrid composites containing Ni–Fe coated carbon nanofibers prepared by electroless plating. Compos. Part A Appl. Sci. Manuf. 2011, 42, 573–578. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, C.S.; Rhee, K.Y.; Zhao, N.Q. Properties of Co0.5Ni0.5Fe2O4/carbon nanotubes/polyimide nanocomposites for microwave absorption. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2241–2248. [Google Scholar] [CrossRef]
- Niu, D.Y.; Yu, J.K.; Qiao, Q.D.; Wang, D.; Liu, R.H. Improved corrosion resisting property of magnetism iron fiber by SiO2 coating. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 163–166. [Google Scholar]
- Ramo, S.; Whinnery, J.R.; Duzer, T.V. Fields and Waves in Communication, 3rd ed.; Electronics Wiley: New York, NY, USA, 1994; pp. 664–665. [Google Scholar]
- Duan, W.J.; Li, X.D.; Wang, Y.; Qiang, R.; Tian, C.H.; Wang, N.; Han, X.J.; Du, Y.C. Surface functionalization of carbonyl iron with aluminum phosphate coating toward enhanced anti-oxidative ability and microwave absorption properties. Appl. Surf. Sci. 2018, 427, 594–602. [Google Scholar] [CrossRef]
- Abshinova, M.A.; Kazantseva, N.E.; Sáha, P.; Sapurina, I.; Kovářová, J.; Stejskal, J. The enhancement of the oxidation resistance of carbonyl iron by polyaniline coating and consequent changes in electromagnetic properties. Polym. Degrad. Stab. 2008, 93, 1826–1831. [Google Scholar] [CrossRef]
- Bruncková, H.; Kabátová, M.; Dudrová, E. The effect of iron phosphate, alumina and silica coatings on the morphology of carbonyl iron particles. Surf. Interface Anal. 2010, 42, 13–20. [Google Scholar] [CrossRef]
- Jaya, F.; Gauthier-Brunet, V.; Pailloux, F.; Mimault, J.; Bucher, S.; Dubois, S. Al-coated iron particles: Synthesis, characterization and improvement of oxidation resistance. Surf. Coat. Technol. 2008, 202, 4302–4306. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Ma, L.Y.; Li, R.; Chen, D.; Lu, Y.Y.; Cheng, Y.Y.; Luo, X.X.; Xie, H.; Zhou, W.C. Enhanced heat-resistance property of aluminum-coated carbonyl iron particles as microwave absorption materials. J. Magn. Magn. Mater. 2021, 524, 167681. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, Y.Y.; Ren, Z.W.; Wei, X.; Tao, S.P.; Yang, C.Q. Enhancement of electromagnetic interference shielding and heat-resistance properties of silver-coated carbonyl iron powders composite material. J. Magn. Magn. Mater. 2020, 499, 166244. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, W.C.; Li, R.; Mu, Y.; Qing, Y.C. Enhanced antioxidation and electromagnetic properties of Co-coated flaky carbonyl iron particles prepared by electroless plating. J. Alloy. Compd. 2015, 637, 10–15. [Google Scholar] [CrossRef]
- Jia, S.; Luo, F.; Qing, Y.C.; Zhou, W.C.; Zhu, D.M. Electroless plating preparation and microwave electromagnetic properties of Ni-coated carbonyl iron particle/epoxy coatings. Physica B 2010, 405, 3611–3615. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Xie, H.; Zhou, W.C.; Ren, Z.W. Enhanced antioxidation and microwave absorbing properties of SiO2-coated flaky carbonyl iron particles. J. Magn. Magn. Mater. 2018, 446, 143–149. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wei, S.Y.; Lee, I.Y.; Park, S.; Willis, J.; Haldolaarachchige, N.; Young, D.P.; Luo, Z.P.; Guo, Z.H. Silica stabilized iron particles toward anti-corrosion magnetic polyurethane nanocomposites. RSC Adv. 2012, 2, 1136–1143. [Google Scholar] [CrossRef]
- Qing, Y.C.; Zhou, W.C.; Jia, S.; Luo, F.; Zhu, D.M. Microwave electromagnetic property of SiO2-coated carbonyl iron particles with higher oxidation resistance. Physica B 2011, 406, 777–780. [Google Scholar]
- Maklakov, S.S.; Lagarkov, A.N.; Maklakov, S.A.; Adamovich, Y.A.; Petrov, D.A.; Rozanov, K.N.; Ryzhikov, I.A.; Zarubina, A.Y.; Pokholok, K.V.; Filimonov, D.S. Corrosion-resistive magnetic powder Fe@SiO2 for microwave applications. J. Alloy. Compd. 2017, 706, 267–273. [Google Scholar] [CrossRef]
- Butler, R.F.; Banerjee, S.K. Single-domain grain size limits for metallic iron. J. Geophys. Res. 1975, 80, 252. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.C.; Zhou, W.C.; Luo, F.; Zhu, D.M. Epoxy-silicone filled with multi-walled carbon nanotubes and carbonyl iron particles as a microwave absorber. Carbon 2010, 48, 4074. [Google Scholar] [CrossRef]
- Naito, Y.; Suetake, K. Application of Ferrite to Electromagnetic Wave Absorber and its Characteristics. IEEE Trans. Microw. Theory 1971, 19, 65–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.; Zhang, L.; Lu, H.; Guo, Y.; Ma, X.; Zhang, M.; Yin, J.; Dai, L.; Jian, X.; et al. High antioxidant lamellar structure Cr2AlC: Dielectric and microwave absorption properties in X band. J. Alloy. Compd. 2021, 860, 157896. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Qiang, R.; Tian, C.; Xu, P.; Han, X. Interfacially engineered sandwich-like rGO/carbon microspheres/rGO composite as an efficient and durable microwave absorber. Adv. Mater. Interfaces 2016, 3, 1500684. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wan, W.H.; Wang, B.; Zhang, L.B.; Yin, L.J.; Bui, H.V.; Xie, J.L.; Zhang, L.; Lu, H.P.; Deng, L.J. Enhanced anti-corrosion and microwave absorption performance with carbonyl iron modified by organic fluorinated chemicals. Appl. Surf. Sci. 2022, 572, 151320. [Google Scholar] [CrossRef]
- Hofman, R.; Westheim, J.G.F.; Pouwel, I.; Fransen, T.; Gellings, P.J. FTIR and XPS Studies on Corrosion-resistant SiO2 Coatings as a Function of the Humidity during Deposition. Surf. Interface Anal. 1996, 24, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, T.; Wen, B.; Lu, M.; Xu, Z.; Zhu, C.; Chen, Y.; Xue, X.; Sun, C.; Cao, M. Graphene/polyaniline nanorod arrays: Synthesis and excellent electromagnetic absorption properties. J. Mater. Chem. 2012, 22, 21679–21685. [Google Scholar] [CrossRef]
- Meng, F.B.; Wang, H.G.; Huang, F.; Guo, Y.F.; Wang, Z.Y.; Hui, D.; Zhou, Z.W. Graphene-based microwave absorbing composites: A review and prospective. Compos. B Eng. 2018, 137, 260–277. [Google Scholar] [CrossRef]
- Li, Q.S.; Zhu, J.J.; Wang, S.N.; Huang, F.; Liu, Q.C.; Kong, X.K. Microwave absorption on a bare biomass derived holey silica-hybridized carbon absorbent. Carbon 2020, 161, 639–646. [Google Scholar] [CrossRef]
- Wang, X.; Pan, F.; Xiang, Z.; Zeng, Q.W.; Pei, K.; Che, R.C.; Lu, W. Magnetic vortex coreshell Fe3O4@C nanorings with enhanced microwave absorption performance. Carbon 2020, 157, 130–139. [Google Scholar] [CrossRef]
- Li, Q.S.; Li, S.T.; Liu, Q.G.; Liu, X.F.; Shui, J.L.; Kong, X.K. Iodine cation bridged graphene sheets with strengthened interface combination for electromagnetic wave absorption. Carbon 2021, 183, 100–107. [Google Scholar] [CrossRef]

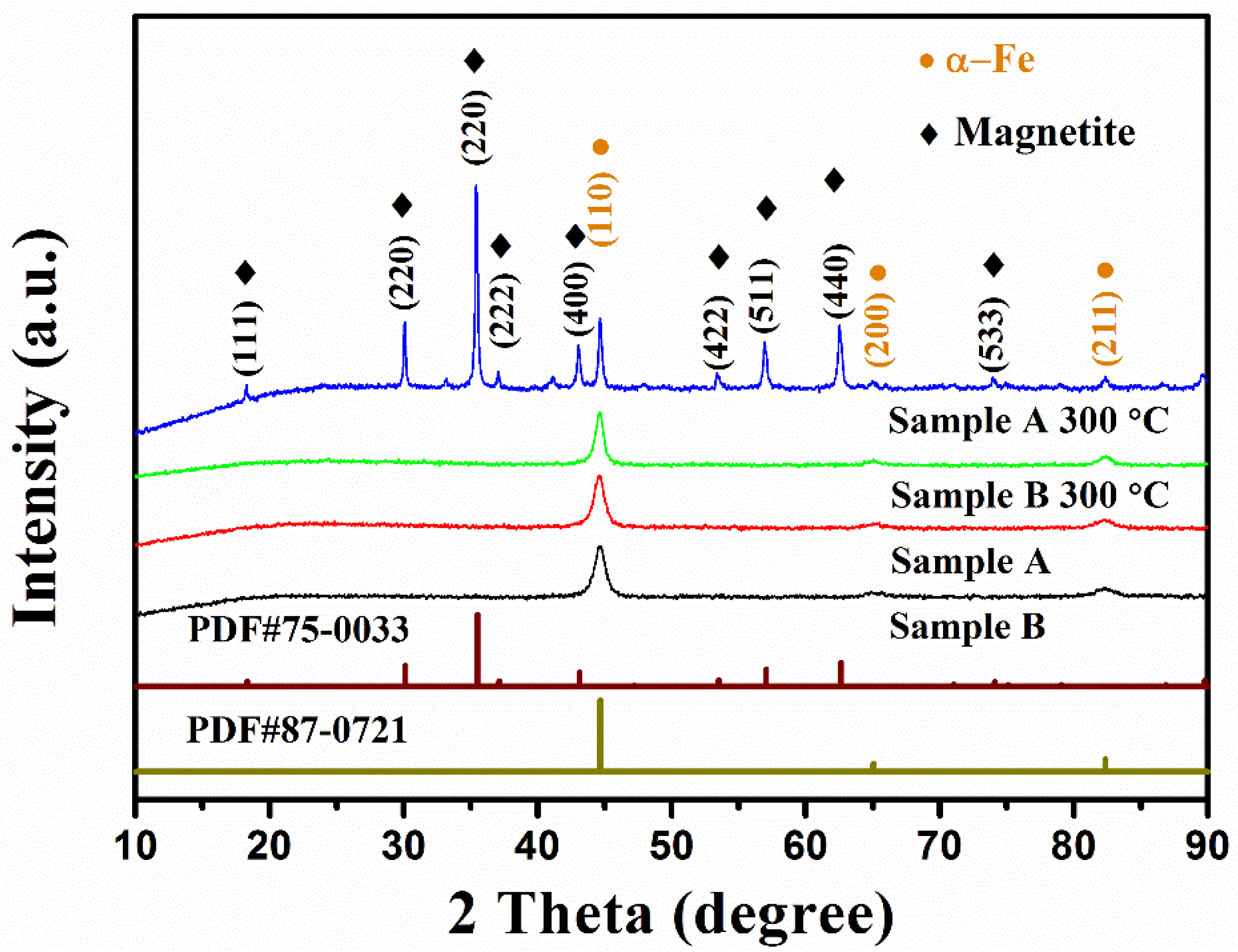
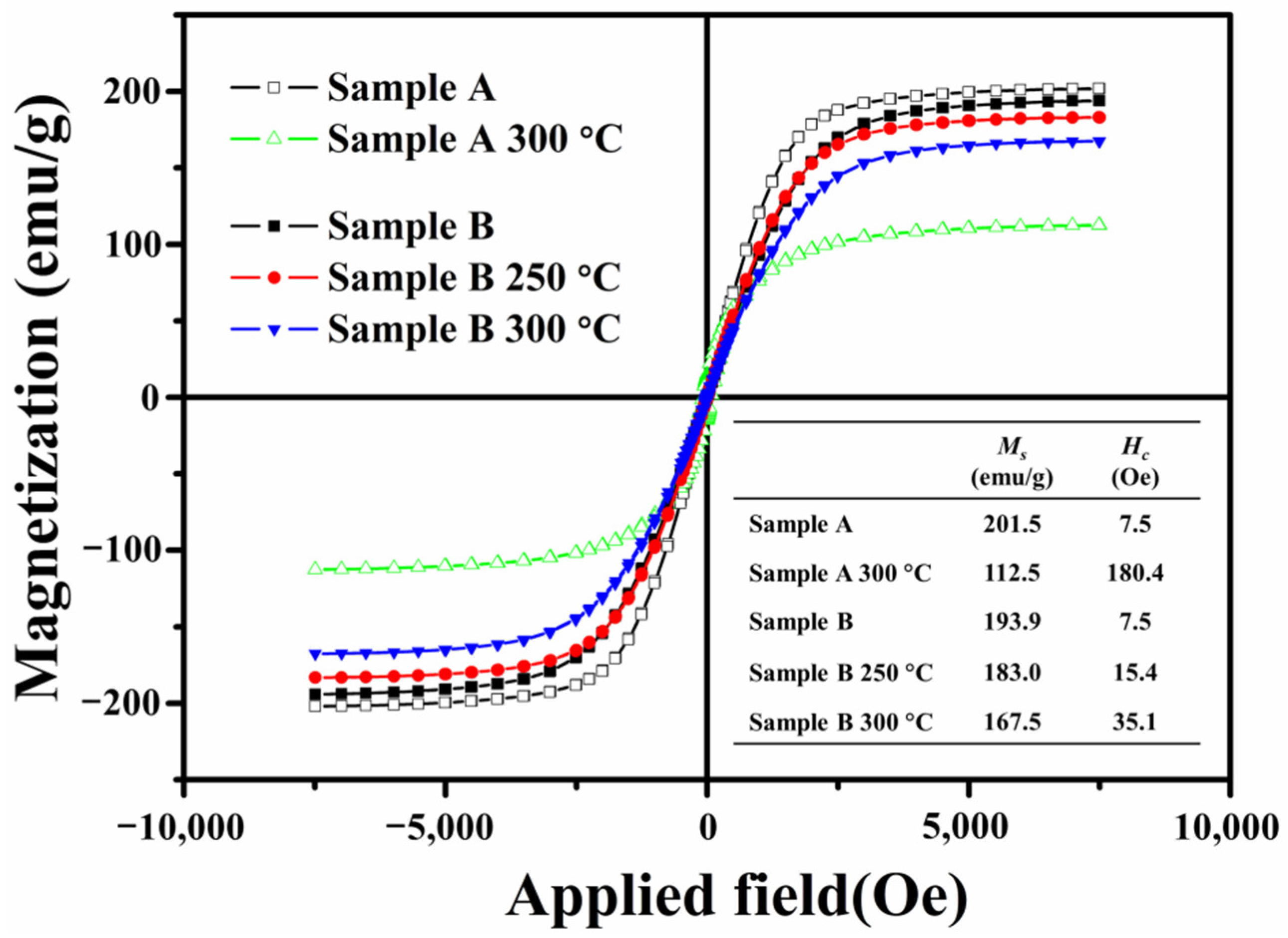

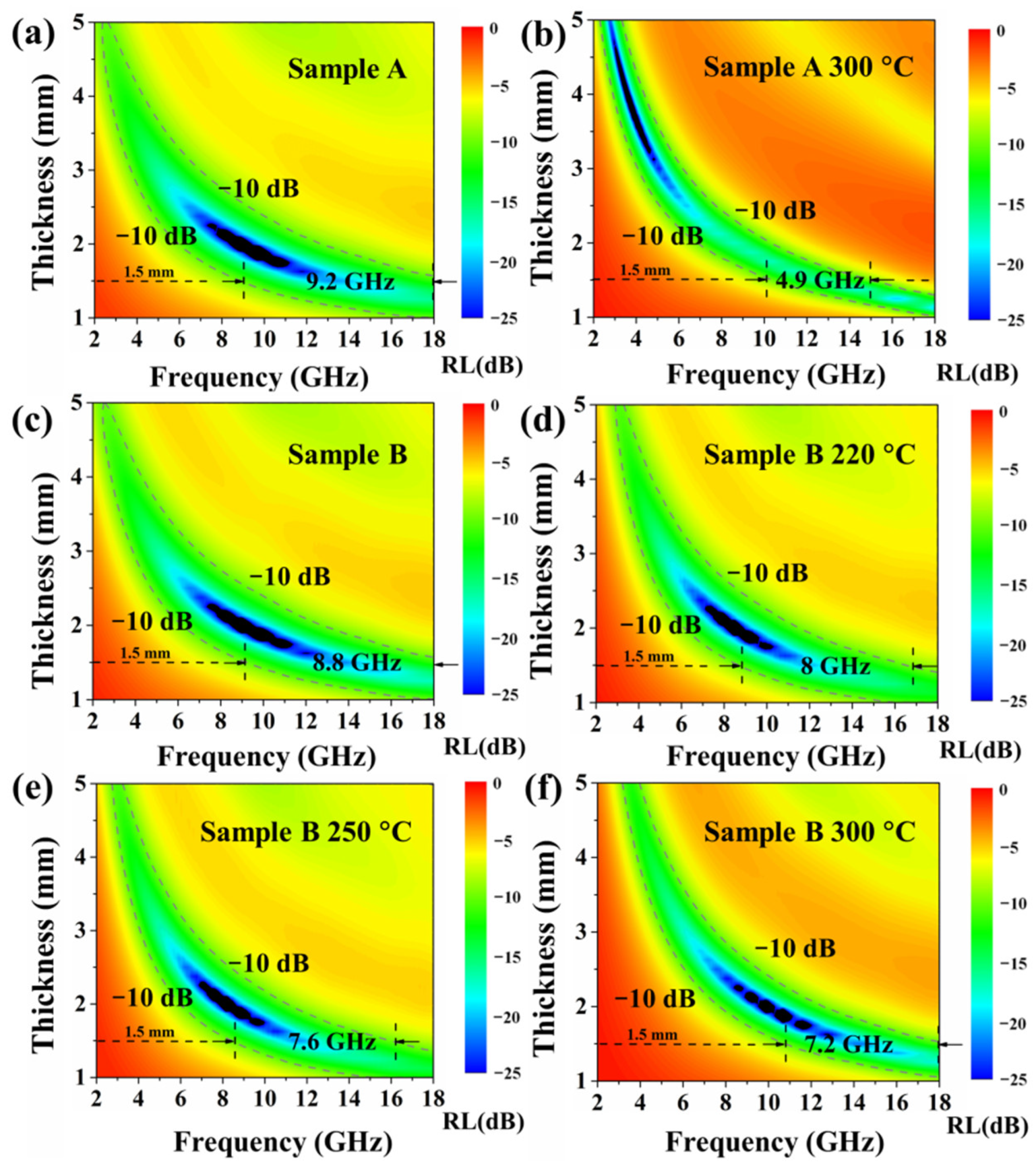
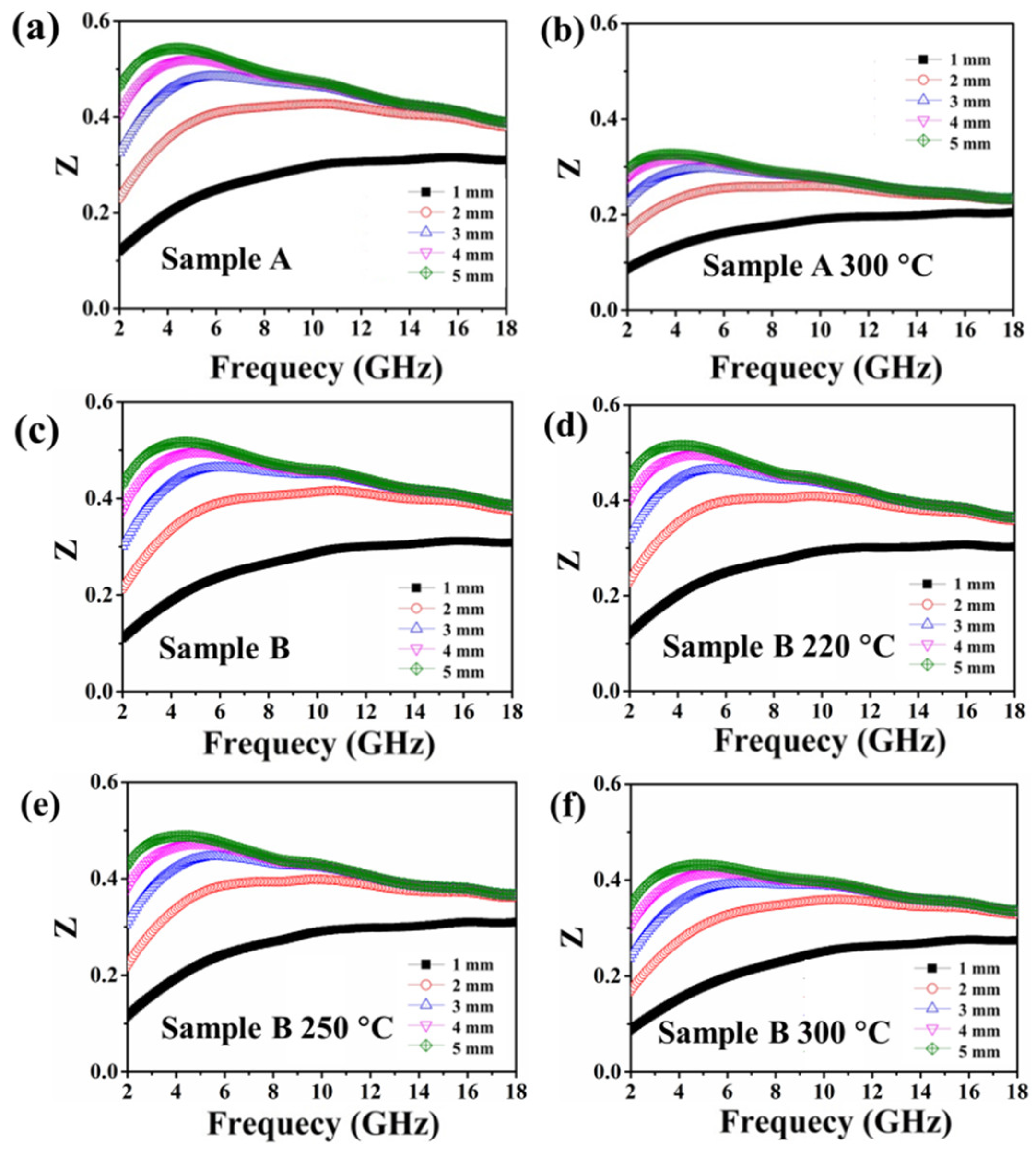

| Annealing Temperature | Sample A Incremental Mass Ratio | Sample A Ratio of Oxidized Fe Atoms | Sample B Incremental Mass Ratio | Sample B Ratio of Oxidized Fe Atoms |
|---|---|---|---|---|
| 220 °C | 0.5% | 1.3% | ||
| 250 °C | 2.0% | 5.0% | ||
| 300 °C | 23% | 60.8% | 3.8% | 10.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Mu, X.; Zhang, Q.; Ma, Z.; Song, C.; Hu, B. A Study on the Static Magnetic and Electromagnetic Properties of Silica-Coated Carbonyl Iron Powder after Heat Treatment for Improving Thermal Stability. Materials 2022, 15, 2499. https://doi.org/10.3390/ma15072499
Yan X, Mu X, Zhang Q, Ma Z, Song C, Hu B. A Study on the Static Magnetic and Electromagnetic Properties of Silica-Coated Carbonyl Iron Powder after Heat Treatment for Improving Thermal Stability. Materials. 2022; 15(7):2499. https://doi.org/10.3390/ma15072499
Chicago/Turabian StyleYan, Xu, Xinyuan Mu, Qinsheng Zhang, Zhanwei Ma, Chengli Song, and Bin Hu. 2022. "A Study on the Static Magnetic and Electromagnetic Properties of Silica-Coated Carbonyl Iron Powder after Heat Treatment for Improving Thermal Stability" Materials 15, no. 7: 2499. https://doi.org/10.3390/ma15072499
APA StyleYan, X., Mu, X., Zhang, Q., Ma, Z., Song, C., & Hu, B. (2022). A Study on the Static Magnetic and Electromagnetic Properties of Silica-Coated Carbonyl Iron Powder after Heat Treatment for Improving Thermal Stability. Materials, 15(7), 2499. https://doi.org/10.3390/ma15072499






