Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends
Abstract
1. Introduction
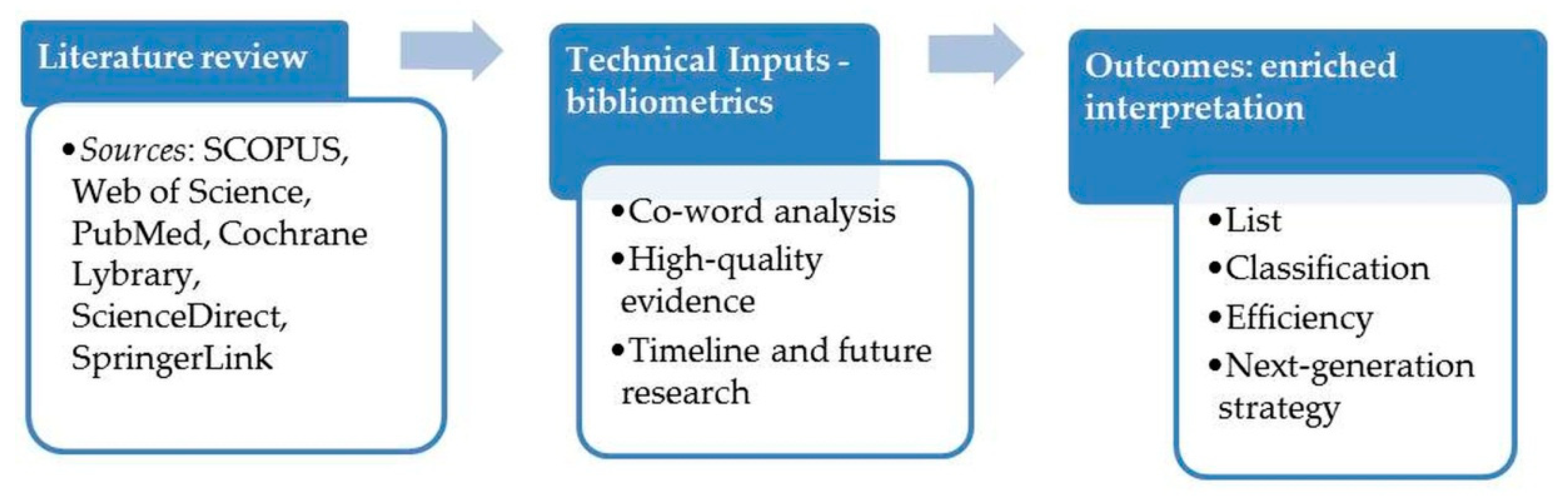
2. Study Design
3. Approaches to Reduce Oxidative Damage
- (1)
- Control of the chain initiation oxidation reactions: (i) preventing O2•− formation through inhibition of xanthine oxidase (allopurinol); (ii) scavenging O2•− (ascorbic acid) or •OH; (iii) chelating metal ions such as Fe2+ (ascorbic acid)—preventive antioxidants;
- (2)
- Control of the chain propagation oxidation reactions: terminating/breaking the auto-oxidative chain reactions (probucol, α-tocopherol and its derivatives)—proper antioxidants/chain-breaking antioxidants.
4. Classes of Antioxidants
5. Synthetic Low-Molecular-Weight Antioxidants: Properties and Pharmacological Effects
6. Effectiveness of Antioxidant Intervention in Human Health
6.1. Dietary Antioxidants
6.2. Medicines in Use (Internal)
6.3. Cosmeceuticals
6.4. Antioxidant Additives
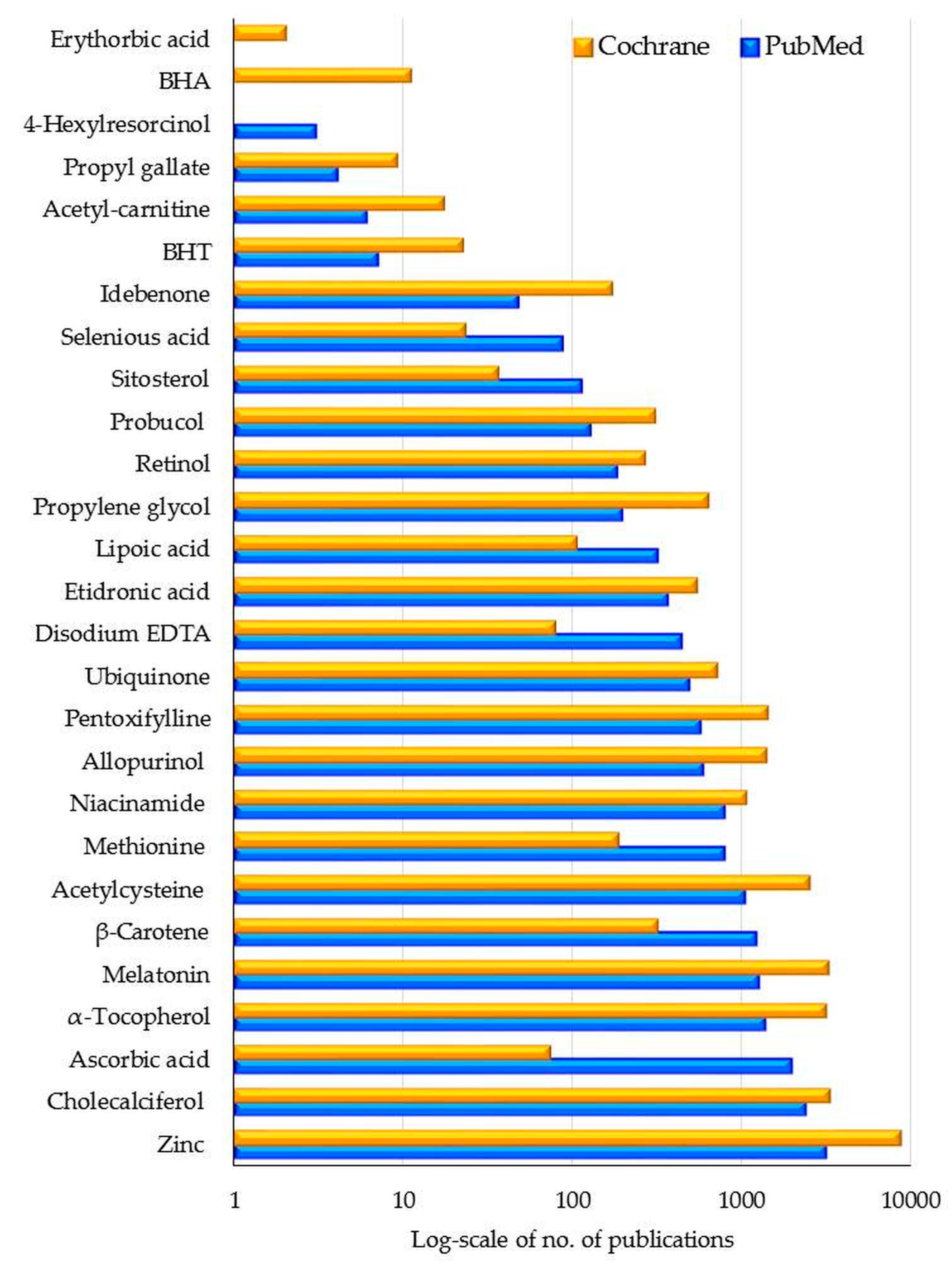
6.5. Summary Bibliometric Data and Future Trends
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATC | Anatomical Therapeutic Chemical (ATC) classification system |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| COVID-19 | Coronavirus disease 2019 |
| DNA | Deoxyribonucleic Acid |
| DSL | Domestic Substance List |
| EDTA | Ethylenediamine tetra acetate |
| EMA | European Medicines Agency |
| FEMA | Flavor and Extract Manufacturers Association |
| FDA | The US Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| HDL | High-density lipoprotein |
| IL-1β | member of the Interleukin-1 family |
| IARC | International Agency for Cancer Research |
| I.P. | Intraperitoneal injection |
| IUPAC | International Union of Pure and Applied Chemistry |
| I.V. | Intravenous medication administration |
| LD50 | 50% Lethal dose |
| LDL | Low-density lipoprotein |
| NAD | Nicotinamide adenine dinucleotide, oxidized form |
| NADH | Nicotinamide adenine dinucleotide, reduced form |
| NRF2 | Nuclear factor erythroid 2-Related Factor 2 |
| PEG | Propylene glycol |
| PG | Propyl gallate |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SOD | Superoxide dismutase |
| TDLo | Toxic Dose Low (the lowest toxic dose) |
| THBQ | t-Butyl hydroquinone |
| TNF | Tumor necrosis factor |
| UNII | Unique ingredient identifiers, generated by the joint FDA/USP Substance Registration System |
| WHO | World Health Organization |
References
- Halliwell, B. How to characterize an antioxidant: An update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Nahler, G. Dictionary of Pharmaceutical Medicine, 4th ed.; Springer: Vienna, Austria, 2017; pp. 18–19. [Google Scholar]
- Daintith, J.; Martin, E. Oxford Dictionary of Science, 6th ed.; Oxford University Press: Oxford, UK, 2010; p. 48. [Google Scholar]
- Rahman, M.S. Handbook of Food Preservation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 264. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better—and safer—than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef] [PubMed]
- Swe, K.M.M.; Abas, A.B.; Bhardwaj, A.; Barua, A.; Nair, N.S. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst. Rev. 2013, 6, CD009415. [Google Scholar] [CrossRef]
- Nagalla, S.; Ballas, S.K. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst. Rev. 2018, 10, CD003426. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterails; Dziubla, T., Butterfield, D.A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E. Handbook of Synthetic Antioxidants; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Naidu, S.D.; Dinkova-Kostova, A.T. KEAP1, a cysteine-based sensor and a drug target for the prevention and treatment of chronic disease. Open Biol. 2020, 10, 200105. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Shvedova, A.; Serbinova, E.; Khan, S.; Swanson, C.; Powell, R.; Packer, L. Dihydrolipoic acid—A universal antioxidant both in the membrane and in the aqueous phase: Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992, 44, 1637–1649. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Martindale, G.J. The Complete Drug Reference, 38th ed.; Pharmaceutical Press: London, UK, 2014. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Prenzler, P.D.; Ryan, D.; Robards, K. Introduction to basic principles of antioxidant activity. In Handbook of Antioxidant Methodology: Approaches to Activity Determination; Prenzler, P.D., Ryan, D., Robards, K., Eds.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–62. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yang, D.-P.; Tang, G.-Y. Multipotent antioxidants: From screening to design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Kasaikina, O.T. Lipid oxidation in homogeneous and micro-heterogeneous media in presence of prooxidants, antioxidants and surfactants. In Lipid Peroxidation; Catala, A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martinez-Pinilla, E. Antioxidants versus food antioxidant additives and food preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Barhwal, K.; Hota, S.; Jain, V.; Prasad, D.; Singh, S.; Ilavazhagan, G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia–induced spatial memory impairment through extracellular related kinase–mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience 2009, 161, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Shivalingappa, P.C.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A. N-acetyl cysteine protects against methamphetamine-induced dopaminergic neurodegeneration via modulation of redox status and autophagy in dopaminergic cells. Parkinsons Dis. 2012, 424285. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Pharmaceutical Additives, 3rd ed.; Synapse Information Resources: New York, NY, USA, 2007. [Google Scholar]
- Ash, M.; Ash, I. Handbook of Cosmetic and Personal Care Additives, 2nd ed.; Synapse Information Resources: New York, NY, USA, 2013. [Google Scholar]
- Dekhuijzen, P. Antioxidant properties of N-acetylcysteine: Their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J. 2004, 23, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Birck, R.; Krzossok, S.; Markowetz, F.; Schnülle, P.; van der Woude, F.J.; Braun, C. Acetylcysteine for prevention of contrast nephropathy: Meta-analysis. Lancet 2003, 362, 598–603. [Google Scholar] [CrossRef]
- Shi, Z.; Puyo, C.A. N-acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Sadowska, A.M. N-acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Wypych, A.; Wypych, G. Databook of UV Stabilizers, 2nd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2020. [Google Scholar]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Milani, G.; Macchi, M.; Guz-Mark, A. Vitamin C in the treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Festjens, N.; Kalai, M.; Smet, J.; Meeus, A.; Van Coster, R.; Saelens, X.; Vandenabeele, P. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ. 2006, 13, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Snipes, W.; Person, S.; Keith, A.; Cupp, J. Butylated hydroxytoluene inactivated lipid-containing viruses. Science 1975, 188, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Ooi, N.; Chopra, I.; Eady, A.; Cove, J.; Bojar, R.; O’Neill, A.J. Antibacterial activity and mode of action of tert-butylhydroquinone (TBHQ) and its oxidation product, tert-butylbenzoquinone (TBBQ). J. Antimicrob. Chemother. 2013, 68, 1297–1304. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Zhao, W.; Liu, M.; Liu, L.; Wang, Y. TBHQ-overview of multiple mechanisms against oxidative stress for attenuating methamphetamine-induced neurotoxicity. Oxid. Med. Cell. Longev. 2020, 8874304. [Google Scholar] [CrossRef]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini-Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef]
- Gröber, U.; Holick, M.F. The coronavirus disease (COVID-19)—A supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2021, 92, 13–34. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020, 20, e107–e108. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; McClements, a.D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yazama, F.; Tai, A. Oxidative stress-mediated antitumor activity of erythorbic acid in high doses. Biochem. Biophys. Rep. 2015, 3, 117–122. [Google Scholar] [CrossRef]
- Cilliers, J.J.L.; Singleton, V.L. Caffeic acid autoxidation and the effects of thiols. J. Agric. Food Chem. 1990, 38, 1789–1796. [Google Scholar] [CrossRef]
- Rao, G.S.; Tokuda, H.; Ichiishi, E.; Takasaki, M.; Iida, A.; Suzuki, N.; Konoshima, T.; Kapadia, G.J. Oral chemoprevention of skin cancer in mice by benzophenone sunscreens dioxybenzone and octabenzone in drinking water. Anticancer Res. 2013, 33, 2535–2540. [Google Scholar]
- Cashman, D.P. Why the lower reported prevalence of asthma in patients diagnosed with COVID-19 validates repurposing EDTA solutions to prevent and manage treat COVID-19 disease. Med. Hypotheses 2020, 144, 110027. [Google Scholar] [CrossRef]
- Roussel, A.M.; Hininger-Favier, I.; Waters, R.S.; Osman, M.; Fernholz, K.; Anderson, R.A. EDTA chelation therapy, without added vitamin C, decreases oxidative DNA damage and lipid peroxidation. Altern. Med. Rev. 2009, 14, 56–61. [Google Scholar]
- Evstatiev, R.; Cervenka, A.; Austerlitz, T.; Deim, G.; Baumgartner, M.; Beer, A.; Krnjic, A.; Gmainer, C.; Lang, M.; Frick, A.; et al. The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models. Sci. Rep. 2021, 11, 5188. [Google Scholar] [CrossRef]
- Fidler, M.C.; Davidsson, L.; Zeder, C.; Hurrell, R.F. Erythorbic acid is a potent enhancer of nonheme-iron absorption. Am. J. Clin. Nutr. 2004, 79, 99–102. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int. J. Toxicol. 1999, 18, 1–26. [Google Scholar] [CrossRef]
- Miura, Y.; Honda, S.; Masuda, A.; Masuda, T. Antioxidant activities of cysteine derivatives against lipid oxidation in anhydrous media. Biosci. Biotechnol. Biochem. 2014, 78, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Seifert, M.; Vinholes, J.; Rombaldi, C.; Nora, L.; Cantillano, R. Erythorbic acid and sodium erythorbate effectively prevent pulp browning of minimally processed ‘royal gala’ apples. Ital. J. Food Sci. 2019, 31, 573–590. [Google Scholar] [CrossRef]
- Dunn, C.J.; Fitton, A.; Sorkin, E.M. Etidronic acid. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs Aging 1994, 5, 446–474. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P. Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. Curr. Med. Chem. 2009, 16, 706–733. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, E.; De Tollenaere, C.; Aerts, K.; Cos, P.; Schuerwegh, A.; Bridts, C.; Van Offel, J.; Ebo, D.; Stevens, W.; De Clerck, L. Antioxidant effect of bisphosphonates and simvastatin on chondrocyte lipid peroxidation. Biochem. Biophys. Res. Commun. 2006, 348, 459–464. [Google Scholar] [CrossRef]
- Shephard, A.; Zybeshari, S. Virucidal action of sore throat lozenges against respiratory viruses parainfluenza type 3 and cytomegalovirus. Antivir. Res. 2015, 123, 158–162. [Google Scholar] [CrossRef]
- Fidalgo, J.; Deglesne, P.A.; Arroya, R.; Ranneva, E.; Deprez, P. 4-hexylresorcinol a new molecule for cosmetic application. J. Biomol. Res. Ther. 2019, 8, 2. [Google Scholar] [CrossRef]
- Nagy, I.Z. Chemistry, toxicology, pharmacology and pharmacokinetics if idebenone: A review. Arch. Gerontol. Geriatr. 1990, 11, 177–186. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Eby, H.; Henkel, N.D.; Creeden, J.; Imami, A.; Asah, S.; Zhang, X.; Wu, X.; Alnafisah, R.; Taylor, R.T.; et al. Identification of new drug treatments to combat COVID19: A signature-based approach using iLINCS. Res. Sq. 2020, rs.3.rs-25643. [Google Scholar] [CrossRef]
- Dragomanova, S.; Miteva, S.; Nicoletti, F.; Mangano, K.; Fagone, P.; Pricoco, S.; Staykov, H.; Tancheva, L. Therapeutic potential of alpha-lipoic acid in viral infections, including COVID-19. Antioxidants 2021, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A combination of α-lipoic acid (ALA) and palmitoylethanolamide (PEA) blocks endotoxin-induced oxidative stress and cytokine storm: A possible intervention for COVID-19. J. Diet. Suppl. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cure, E.; Cure, M.C. Alpha-lipoic acid may protect patients with diabetes against COVID-19 infection. Med. Hypotheses 2020, 143, 110185. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.M.M.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Cremer, D.; Rabeler, R.; Roberts, A.; Lynch, B. Safety evaluation of α-lipoic acid (ALA). Regul. Toxicol. Pharmacol. 2006, 46, 29–41. [Google Scholar] [CrossRef]
- Cross, K.M.; Landis, D.M.; Sehgal, L.; Payne, J.D. Melatonin for the early treatment of COVID-19: A narrative review of current evidence and possible efficacy. Endocr. Pract. 2021, 27, 850–855. [Google Scholar] [CrossRef]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef]
- Suzen, S. Melatonin and synthetic analogs as antioxidants. Curr. Drug Deliv. 2013, 10, 71–75. [Google Scholar] [CrossRef]
- Sugden, D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983, 227, 587–591. [Google Scholar]
- Benavides, M.A.; Bosland, M.C.; da Silva, C.P.; Sares, C.T.G.; de Oliveira, A.M.C.; Kemp, R.; Reis, R.; Martins, V.R.; Sampaio, S.V.; Bland, K.I.; et al. L-Methionine inhibits growth of human pancreatic cancer cells. Anti-Cancer Drugs 2014, 25, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Catanesi, M.; Brandolini, L.; D’Angelo, M.; Benedetti, E.; Tupone, M.G.; Alfonsetti, M.; Cabri, E.; Iaconis, D.; Fratelli, M.; Cimini, A.; et al. L-methionine protects against oxidative stress and mitochondrial dysfunction in an in vitro model of Parkinson’s disease. Antioxidants 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. Toxicity of methionine in humans. J. Nutr. 2006, 136, 1722S–1725S. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Lindsay, C.B.; Montecinos-Oliva, C.; Arrazola, M.S.; Retamales, R.M.; Bunout, D.; Hirsch, S.; Inestrosa, N.C. Is L-methionine a trigger factor for Alzheimer’s-like neurodegeneration?: Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol. Neurodegener. 2015, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Kamat, J.P.; Devasagayam, T.P. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kreth, S.; Ledderose, C.; Luchting, B.; Weis, F.; Thiel, M. Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock 2010, 34, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Seirafianpour, F.; Mozafarpoor, S.; Fattahi, N.; Sadeghzadeh-Bazargan, A.; Hanifiha, M.; Goodarzi, A. Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy? Dermatol. Ther. 2020, 33, e13733. [Google Scholar] [CrossRef]
- Zein, C.O.; Lopez, R.; Fu, X.; Kirwan, J.P.; Yerian, L.M.; McCullough, A.J.; Hazen, S.L.; Feldstein, A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology 2012, 56, 1291–1299. [Google Scholar] [CrossRef]
- Lewis, R.J.; Tatken, R.L. Registry of Toxic Effects of Chemical Substances; NIOSH, US Government Printing: Washington, DC, USA, 1978. [Google Scholar]
- Tikhaze, A.K.; Lankin, B.Z.; Konovalova, G.G.; Shumaev, K.B.; Kaminnyi, A.I.; Kozachenko, A.I.; Gurevich, S.M.; Nagler, L.G.; Zaitseva, T.M.; Kukharchuk, V.V. Antioxidant probucol as an effective scavenger of lipid radicals in low density lipoproteinsin vivo and in vitro. Bull. Exp. Biol. Med. 1999, 128, 818–821. [Google Scholar] [CrossRef]
- Lebeau, J.E. Toxicologie animale du probucol [Animal toxicity studies of probucol (author’s translation)]. Nouv. Presse Med. 1980, 9, 3001–3004. [Google Scholar]
- Chen, X.; Lu, X.-Z.; Gao, Y.; Shi, X.-C.; Yu, W.-G. Molecular mechanisms of antioxidant effects of propylene glycol mannate sulfate. Yao Xue Xue Bao 2004, 39, 13–16. [Google Scholar] [PubMed]
- Sax, N.I. Final report on the safety assessment of propylene glycol and polypropylene glycols. J. Am. Coll. Toxicol. 1994, 13, 437–491. [Google Scholar]
- Melo-Cavalcante, A.A.D.C.; Sousa, J.M.C.; Alencar, M.V.O.B.; Santos, J.V.D.O.; da Mata, A.M.O.; Paz, M.F.C.J.; de Carvalho, R.M.; Nunes, N.M.F.; Islam, M.; Mendes, A.N.; et al. Retinol palmitate and ascorbic acid: Role in oncological prevention and therapy. Biomed. Pharmacother. 2019, 109, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Midha, I.K.; Kumar, N.; Kumar, A.; Madan, T. Mega doses of retinol: A possible immunomodulation in COVID-19 illness in resource-limited settings. Rev. Med. Virol. 2021, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic exogenous and endogenous antioxidants. In Free Radical Medicine and Biology; Das, K., Das, S., Biradar, M.S., Bobbarala, V., Tata, S.S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Kamm, J.J. Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J. Am. Acad. Dermatol. 1982, 6, 652–659. [Google Scholar] [CrossRef]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ebert, P.S.; Malinin, G.I. Induction of erythroid differentiation in friend murine erythroleukemic cells by inorganic selenium compounds. Biochem. Biophys. Res. Commun. 1979, 86, 340–349. [Google Scholar] [CrossRef]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar]
- Khan, S.L.; Siddiqui, F.A. Beta-sitosterol: As immunostimulant, antioxidant and inhibitor of SARS-CoV-2 spike glycoprotein. Arch. Pharmacol. Ther. 2020, 2, 12–16. [Google Scholar]
- Baskar, A.A.; Al Numair, K.S.; Paulraj, M.G.; Alsaif, M.A.; Al Muamar, M.; Ignacimuthu, S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef]
- Rieger, M.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1978. [Google Scholar] [CrossRef]
- Armstrong, C.; Husbands, M.; Scott, G. Mechanisms of antioxidant action: Antioxidant-active products formed from the dialkyl thiodipropionate esters. Eur. Polym. J. 1979, 15, 241–248. [Google Scholar] [CrossRef]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; SanGiovanni, J.P.; Kurinij, N.; Davis, M.D. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013, 120, 1604–1611.e4. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Kawase, S.; Yoshimura, I. Comparative oral toxicity of coenzyme Q10 and its (2Z)-isomer in rats: Single and four-week repeated dose toxicity studies. J. Nutr. Sci. Vitaminol. 2006, 52, 9–20. [Google Scholar] [CrossRef][Green Version]
- Prasad, A.S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Cingolani, V. Hypothesis of zinc ascorbate as best zinc ionophore for raising anti-viral resistance against COVID-19. J. Med. Virol. 2021, 93, 5205–5208. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018, 9156285. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gueven, N.; Ravishankar, P.; Eri, R.; Rybalka, E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol. 2021, 38, 101812. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Medini, H.; Zirman, A.; Mishmar, D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience 2021, 24, 103471. [Google Scholar] [CrossRef]
- Cappanera, S.; Palumbo, M.; Kwan, S.; Priante, G.; Martella, L.; Saraca, L.; Sicari, F.; Vernelli, C.; Di Giuli, C.; Andreani, P.; et al. When does the cytokine storm begin in COVID-19 patients? A quick score to recognize it. J. Clin. Med. 2021, 10, 297. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; LA Maestra, S. Antioxidants and COVID-19. J. Prev. Med. Hyg. 2021, 62, E34–E45. (In Italian) [Google Scholar] [CrossRef]
- Jorge-Aarón, R.-M.; Rosa-Ester, M.-P. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiol. 2020, 15, 959–962. [Google Scholar] [CrossRef]
- Peter, A.E.; Sandeep, B.V.; Rao, B.G.; Kalpana, V.L. Calming the storm: Natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19. Front. Pharmacol. 2021, 11, 583777. [Google Scholar] [CrossRef]
- LaForge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516, Correction in Nat. Rev. Immunol. 2020, 20, 579. [Google Scholar] [CrossRef]
- Ramos, E.; López-Muñoz, F.; Gil-Martín, E.; Egea, J.; Álvarez-Merz, I.; Painuli, S.; Semwal, P.; Martins, N.; Hernández-Guijo, J.; Romero, A. The Coronavirus disease 2019 (COVID-19): Key emphasis on melatonin safety and therapeutic efficacy. Antioxidants 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and analogues: Pharmacological properties and synthetic strategies for their preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, Y.; Zhang, J.; Li, Y.; Peng, Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: Study protocol for a multicentre randomised controlled trial. BMJ Open 2020, 10, e039519. [Google Scholar] [CrossRef]
- Gönen, M.S.; Alaylıoğlu, M.; Durcan, E.; Özdemir, Y.; Şahin, S.; Konukoğlu, D.; Nohut, O.K.; Ürkmez, S.; Küçükece, B.; Balkan, I.I.; et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Khurana, R.K.; Jain, A.; Jain, A.; Sharma, T.; Singh, B.; Kesharwani, P. Administration of antioxidants in cancer: Debate of the decade. Drug Discov. Today 2018, 23, 763–770. [Google Scholar] [CrossRef]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003, 2010, CD003665. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Stroehlein, J.K.; Wallqvist, J.; Iannizzi, C.; Mikolajewska, A.; Metzendorf, M.-I.; Benstoem, C.; Meybohm, P.; Becker, M.; Skoetz, N.; Stegemann, M.; et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, CD015043. [Google Scholar] [CrossRef]
- Milan, S.J.; Hart, A.; Wilkinson, M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst. Rev. 2013, CD010391. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 2017, CD000254. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.S.; Denton, A.D.; Di Nisio, M.; Chong, L.-Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, A.M.; Vernooij, R.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 2019, CD011906. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Borgstedt, R.; Ebeling, N.; Menzel, L.C.; Jansen, G.; Rehberg, S. Mortality in septic patients treated with vitamin C: A systematic meta-analysis. Crit. Care 2021, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- Curtis, A.J.; Bullen, M.; Piccenna, L.; McNeil, J.J. Vitamin E supplementation and mortality in healthy people: A meta-analysis of randomised controlled trials. Cardiovasc. Drugs Ther. 2014, 28, 563–573. [Google Scholar] [CrossRef]
- Rouhani, P.; Kelishadi, M.R.; Saneei, P. Effect of zinc supplementation on mortality in under 5-year children: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Nutr. 2022, 61, 37–54. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743, Erratum in BMJ 2020, 22, m2329. [Google Scholar] [CrossRef]
- Passerieux, E.; Hayot, M.; Gilles, C.; Pincemail, J.; Mercier, J.; Chenivesse, D. Oxidative stress and dystrophy Facioscapulohumeral: Effects of vitamin C, vitamin E, zinc gluconate and selenomethionine supplementation. Free Radic. Biol. Med. 2014, 75, S14. [Google Scholar] [CrossRef]
- Yamashita, S.; Arai, H.; Bujo, H.; Masuda, D.; Ohama, T.; Ishibashi, T.; Yanagi, K.; Doi, Y.; Nakagawa, S.; Yamashiro, K.; et al. Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (PROSPECTIVE). J. Atheroscler. Thromb. 2021, 28, 103–123. [Google Scholar] [CrossRef]
- Villarruz-Sulit, M.V.; Forster, R.; Dans, A.L.; Tan, F.N.; Sulit, D.V. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD002785. [Google Scholar] [CrossRef]
- Mohammadi, S.; Shokri, J.; Ranjkesh, M.; Hamed, S.A.; Monajjemzadeh, F. Comparative physicochemical stability and clinical anti-wrinkle efficacy of transdermal emulgel preparations of 5% sodium ascorbyl phosphate and or ascorbic acid on human volunteers. J. Cosmet. Dermatol. 2021, 20, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective effects of melatonin on the skin: Future perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.; Nasr, M.; Moftah, N.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin. Drug Deliv. 2018, 15, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, C.; Nepravishta, R.; Bellomaria, A. Antioxidants in food and pharmaceutical research. Alb. J. Pharm. Sci. 2014, 1, 15–25. [Google Scholar]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Chaumont, M.; Van De Borne, P.; Bernard, A.; Van Muylem, A.; Deprez, G.; Ullmo, J.; Starczewska, E.; Briki, R.; De Hemptinne, Q.; Zaher, W.; et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: Results from two randomized clinical trials. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L705–L719. [Google Scholar] [CrossRef]
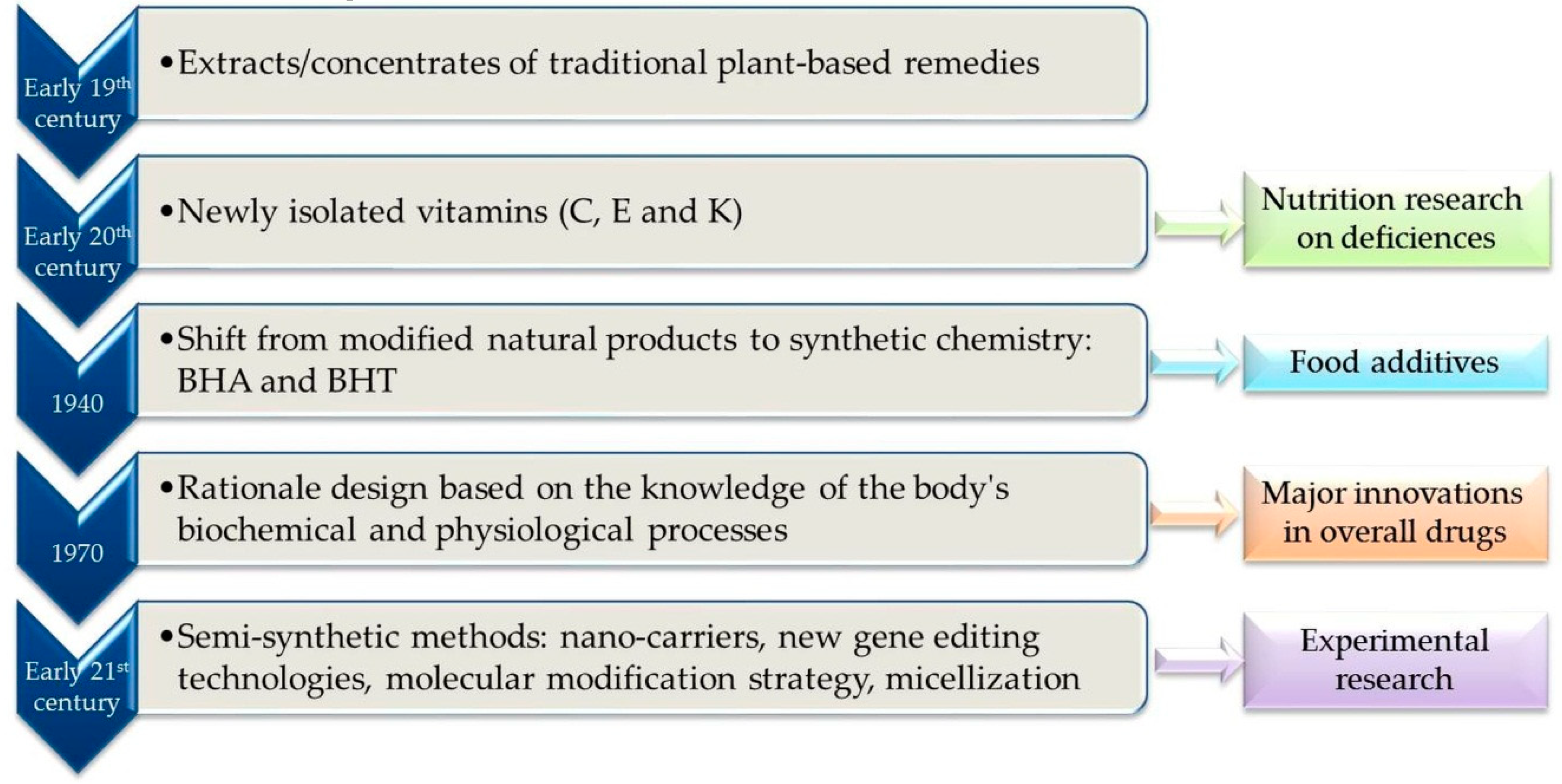
- Semba, R.D. The discovery of the vitamins. Int. J. Vitam. Nutr. Res. 2012, 82, 310–315. [Google Scholar] [CrossRef]
- Bowden, M.E.; Crow, A.B.; Sullivan, T. Pharmaceutical Achievers: The Human Face of Pharmaceutical Research; Chemical Heritage Foundation: Philadelphia, PA, USA, 2003; pp. 32–41. [Google Scholar]
- Delanghe, T.; Huyghe, J.; Lee, S.; Priem, D.; Van Coillie, S.; Gilbert, B.; Choi, S.M.; Vandenabeele, P.; Degterev, A.; Cuny, G.D.; et al. Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor. Cell Death Dis. 2021, 12, 699. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Katayama, T.; Fujisawa, S. Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations. In Vivo 2015, 29, 197–206. [Google Scholar]
- Vo, Q.V.; Van Gon, T.; Van Bay, M.; Mechler, A. Antioxidant activities of monosubstituted indolinonic hydroxylamines: A thermodynamic and kinetic study. J. Phys. Chem. B 2019, 123, 10672–10679. [Google Scholar] [CrossRef] [PubMed]
- Hrizi, A.; Cailler, M.; Romdhani-Younes, M.; Carcenac, Y.; Thibonnet, J. Synthesis of new highly functionalized 1H-indole-2-carbonitriles via cross-coupling reactions. Molecules 2021, 26, 5287. [Google Scholar] [CrossRef] [PubMed]
- Kovacikova, L.; Prnova, M.; Majekova, M.; Bohac, A.; Karasu, C.; Stefek, M. Development of novel indole-based bifunctional aldose reductase inhibitors/antioxidants as promising drugs for the treatment of diabetic complications. Molecules 2021, 26, 2867. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Gahir, S.S.; Lee, C.-Y.I.; Proksch, J.W.; Sakamoto, M.; Ward, K.W. Pharmacokinetics and pharmacodynamics of the novel Nrf2 activator omaveloxolone in primates. Drug Des. Dev. Ther. 2019, 13, 1259–1270. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in Nat. Rev. Drug. Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef]
- Wang, Y.; Bhargava, P. Diroximel fumarate to treat multiple sclerosis. Drugs Today 2020, 56, 431. [Google Scholar] [CrossRef]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front. Neurol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Wundes, A.; Wray, S.; Gold, R.; Singer, B.A.; Jasinska, E.; Ziemssen, T.; de Seze, J.; Repovic, P.; Chen, H.; Hanna, J.; et al. Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: Results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther. Adv. Neurol. Disord. 2021, 14, 1756286421993999. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Xu, X.; Saw, P.E.; Zhang, L. Nanocarrier-mediated antioxidant delivery for liver diseases. Theranostics 2020, 10, 1262–1280. [Google Scholar] [CrossRef]
- Lushchak, O.; Zayachkivska, A.; Vaiserman, A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: A hypothetical background and translational perspectives. Oxid. Med. Cell. Longev. 2018, 2018, 3407375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Du, J.; Gu, Z.; Zhao, Y. Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment. Adv. Sci. 2020, 8, 2002797. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Orlando, C.; Muzzalupo, R. New nanomaterials with intrinsic antioxidant activity by surface functionalization of niosomes with natural phenolic acids. Pharmaceutics 2021, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Maldonado, C.J.; Suárez-Rivero, J.M.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10: Novel formulations and medical trends. Int. J. Mol. Sci. 2020, 21, 8432. [Google Scholar] [CrossRef]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef]
- Stamatoski, A.; Fidoski, J.; Vasilev, A.; Todorovska, L.; Petlichkovski, A. New nanoantioxidant approach to improve healing of oral surgery wounds: A randomised, pilot placebo-controlled, double-blind clinical trial. Int. J. Oral Maxillofac. Surg. 2017, 46, 249. [Google Scholar] [CrossRef]

| Mode of Action | Mechanism | Molecular Size | Solubility | Applications | Origin/Occurrence and Others | |||
|---|---|---|---|---|---|---|---|---|
| True antioxidants ascorbic acid, tocopherols, BHA, BHT, PG | Primary/Chain-breaking catalase, glutathione peroxidase, SOD, tocopherols, BHA, BHT, TBHQ, PG | Small ascorbic acid, tocopherols, uric acid, ubiquinol, BHA, BHT, PG, etc. | Hydrophilic ascorbic acid, flavonoids, glutathione transferins, uric acid | Health/Multipotent dietary supplements (vitamins), therapeutic drugs intended for various diseases related to oxidative stress (idebenone, acetylcysteine, allopurinol, etc.) | Natural | Endogenous | Biological defense | Enzymatic catalase, glutathione peroxidase, SOD |
| Non-enzymatic β-carotene, Co-enzyme Q10, glutathione, α-lipoic acid, tocopherols, uric acid | ||||||||
| Location | Intracellular catalase, glutathione peroxidase, SOD1/2 | |||||||
| Extracellular ascorbat, β-carotene, reduced glutathione, SOD3, uric acid | ||||||||
| Membrane-associated α-tocopherol, lutein, zeaxanthin | ||||||||
| Exogenous various chemical structures, e.g., vitamins (A, D3, E, B3, C), pro-vitamins (carotenoids), minerals (Se, Zn), polyphenols, organosulfur compounds (Cys, Met, glutathione) | ||||||||
| Antioxidants synergists improve the effects of true antioxidants, e.g., sodium edetate, preservatives (organic acids, lecithin) | Secondary/Preventive metal chelators, 1O2 quenchers, oxygen scavengers, primary antioxidants regenerators, vit C, EDTA, glutathione reductase, retinol, selenium, thiodipropionate | Large enzymes, proteins | Hydrophobic/Lipophilic ascorbyl palmitate, carotenoids, tocopherols, ubiquinol, BHA, BHT, PG, TBHQ | Additives/Functional agents maintain quality of foods, pharmaceuticals, cosmetics, etc. E306, E310, E311, E312, E319, E320, E321 | Natural-identical pure substances identical to natural ones, but synthetized by industry; ascorbic acid, β-carotene, tocopherols | |||
| Fully synthetic EDTA, BHA, BHT, PG, TBHQ, acetylcysteine | ||||||||
| Nano-antioxidants various antioxidants delivered by nanoparticles such as oxides (magnetite, zinc oxides, copper oxide), mesoporous silica, chitosan, alginate, poly-D,L-lactide, polybutylcyanoacrylate, polycaprolactone, poly (lactic-co-glycolic acid) | ||||||||
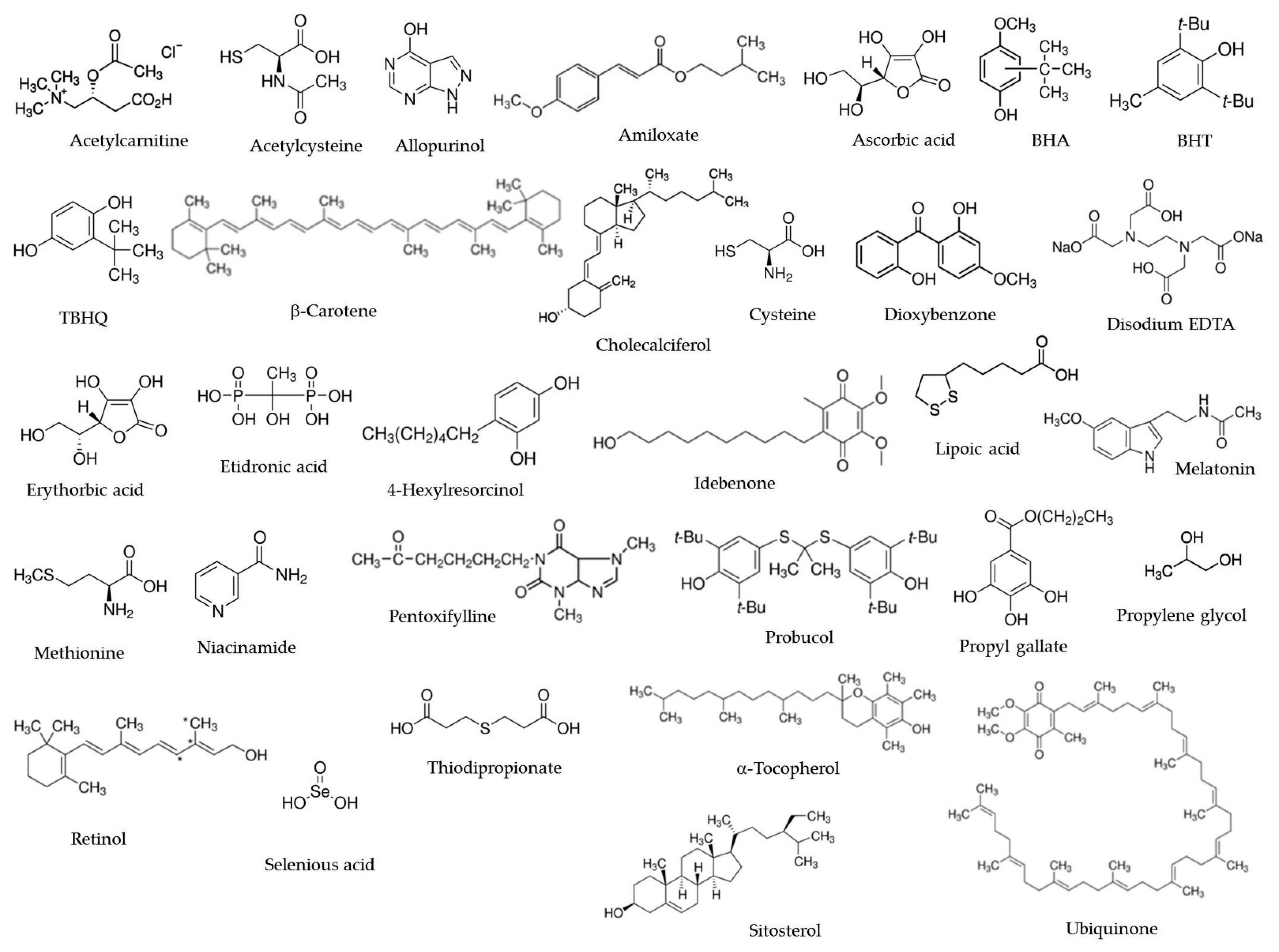
| Common Name/Alternative Names | IUPAC Name (PubChem) | Applications/Regulatory | Description | Ref. |
|---|---|---|---|---|
| Acetylcarnitine | (R)-3-Acetoxy-4-(trimethylammonio)butyrate | Drug Dietary supplements Antioxidant in dietary supplements /ATC N06BX12 UNII NDW10MX58T | Pharmacological properties: reduce oxidative stress in patients with Sickle Cell disease, positive effects on neurological disorders (psychostimulant, nootropic), neuropathies, potential antiviral (supportive and therapeutic option in patients with COVID-19 -clinical trial NCT04623619) | [21,28] |
| Antioxidant mechanism: decreases the generation of free radicals, prevents peroxidation of lipids, and oxidation of proteins through a tyrosine kinase A receptor-mediated mechanism; increases intracellular glutathione levels | [29,30] | |||
| Safety issues: LD50 (oral, rat) >5000 mg/kg; nonirritant to skin; nonmutagenic | [31] | |||
| Acetylcysteine (NAC) | (R)-2-Acetamido-3-sulfanylpropanoic acid | Drug Antioxidant in cosmetics /Eur. Pharmacopoeia | Pharmacological properties: antioxidant, mucolytic therapeutic agent, reduces the effects of acetaminophen overdose, prevents the contrast nephropathy; immune-modulating properties useful for the treatment and prevention of COVID-19 | [32,33,34,35] |
| Antioxidant mechanism: alteration of intracellular redox reactions, deacetylation to cysteine, which participates in the synthesis of the antioxidant glutathione (stimulates glutathione synthetase), scavenging different types of ROS | [36,37] | |||
| Safety issues: hypersensitivity reactions | [21] | |||
| Allopurinol (International Nonproprietary Name by WHO) | 4-Hydroxypyrazolo[3,4-d]pyrimidine | Therapeutic drug /Eur. and US Pharmacopoeia | Pharmacological properties: antioxidant, antigout, anticancer (leukemia, lymphoma) | [12,21] |
| Antioxidant mechanism: inhibitor of xanthine oxidase, prevents O2∙− | [12] | |||
| Safety issues: rushes, lymphadenopathy, leucopenia or leukocytosis, eosinophilia, arthralgia, and vasculitis, hepatotoxic | [21] | |||
| Amiloxate (International Nonproprietary Name by WHO) | 3-Methylbutyl (E)-3-(4-methoxyphenyl)prop-2-enoate | Cosmetics /US Pharmacopeia | Pharmacological properties: antioxidant, UV light absorber, anti-inflammatory, antimicrobial, antiedematous | [21] |
| Antioxidant mechanism of action: free radical scavenger | [38] | |||
| Safety issues: contact and photocontact allergen | [39] | |||
| Ascorbic acid (Vitamin C) | (2R)-2-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one | Dietary supplements Antioxidant in foods (E300; sodium ascorbate E301; calcium ascorbate E302), pharmaceuticals and cosmetics (ascorbyl palmitate) /FDA GRAS | Pharmacological properties: antioxidant, scavenger of free radicals, protection of DNA damage, involved in collagen synthesis, increases the intestinal absorption of iron, antiviral/immune-modulating properties useful for treatment and prevention of COVID-19, positive effects in age-related macular degeneration, neurological disorders, atherosclerosis, cancer | [21,40] |
| Antioxidant mechanism: reducing agent, hydrogen donor forming a relatively stable ascorbyl-free radical Asc−∙ (efficient electron donor in biological redox reactions) and dehydroascorbic acid; efficiently recycles other antioxidants (e.g., α-tocopherol, glutathione); ascorbic acid regenerates itself from Asc−∙ with NADH or NADPH-reductases; in the presence of copper and iron becomes pro-oxidant | [41] | |||
| Safety issues: LD50 (oral, rat) 11,900 mg/kg, (IV, mouse) 518 mg/kg; large doses may result in hyperoxaluria and the formation of renal calcium oxalate calculi | [21,31] | |||
| Butylated hydroxyanisole (BHA) | mixture of 2-t-Butyl-4-methoxyphenol and 3-t-Butyl-4-methoxyphenol | Antioxidant in foods (E320), food packages, animal feed, pharmaceuticals and cosmetics /FDA (0.02% max. of fat/oil), GRAS | Properties: high antioxidant activity; increases the levels of liver glutathione and glutathione-S-transferase; not preferred for pharmacological use, due to safety concerns. It acts synergistically with BHT, PG | [42] |
| Antioxidant mechanism: prevents lipid peroxidation acting as hydrogen donor and interrupting the free radical autoxidative chain reactions (the resulting oxidized phenolic ion is stabilized by the inherent resonance of the benzene ring) | [43] | |||
| Safety issues: LD50 (oral, mouse) 2000 mg/kg; may cause rashes, hyperactivity; confirmed carcinogen (IARC group 2B) | [31,44] | |||
| Butylated hydroxytoluene (BHT) | 2,6-Di-t-butyl-4-methylphenol | Antioxidant in foods (E321), food packages, animal feed, pharmaceuticals and cosmetics /FDA (0.02% max. of fat/oil), GRAS | Properties: high antioxidant activity; antiviral (inactivates lipid-containing viruses); not preferred for pharmacological use, due to safety concerns. It acts synergistically with BHA | [42,45] |
| Antioxidant mechanism: prevents lipid peroxidation acting as hydrogen donor and interrupting the free radical autoxidative chain reactions (the resulting oxidized phenolic ion is stabilized by the inherent resonance of the benzene ring) | [43] | |||
| Safety issues: LD50 (oral, rat) 890 mg/kg, (IP, mouse) 138 mg/kg, (IV, mouse) 180 mg/kg; suspected carcinogen (IARC group 3); human skin irritant; eye irritant; may cause rashes, hyperactivity | [31,44] | |||
| t-Butyl hydroquinone (TBHQ) | 2-t-Butylbenzene-1,4-diol | Antioxidant in foods (E319), pet foods, animal feed, pharmaceuticals and cosmetics /FDA (limitation 0.02% of oil, 0.003% in dry sausage, 0.01% in rendered animal fat, 0.02% in margarine, 0.01% on fat in poultry) | Properties: antioxidant, antibacterial; not preferred for pharmacological use, due to safety concerns | [46] |
| Antioxidant mechanism: is a nuclear factor E2-related factor 2 (Nrf2) agonist, increases the levels of glutathione | [47] | |||
| Safety issues: LD50 (oral, rat) 700 mg/kg, (IP, rat) 300 mg/kg; tumorigenic and mutagen in experimental animals | [31,44] | |||
| β-Carotene (Provitamin A) | 1,3,3-Trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene | Antioxidant in foods [E160a(i)], animal feed and cosmetics Dietary supplements /FDA GRAS | Pharmacological properties: antioxidant, precursor of vitamin A, prevents age-related macular degeneration and ischemic heart disease, protects against cancer, antiviral; potential treatment option for COVID-19 with vitamin A | [21,48,49] |
| Antioxidant mechanism: quenching of singlet oxygen 1O2, prevents lipid peroxidation by scavenging peroxide radicals | [50] | |||
| Safety issues: nontoxic on skin; massive doses may cause yellowing of the skin; increase in cancer incidence by administration of high doses; LD50 >5000 mg/kg | [21,31] | |||
| Cholecalciferol (Vitamin D3) | (3S,5Z,7E)-9,10-Secocholesta-5,7,10-trien-3-ol | Dietary supplements Drug Pharmaceuticals /FDA GRAS | Pharmacological properties: antioxidant (despite some studies showing controversy), therapeutic potential in diseases related to oxidative stress, anti-ricket, enhances absorption of calcium and phosphorus along the small intestine, anti-inflammatory, cardioprotective, potential in the treatment of COVID-19 (clinical trial phase 1, 2019, NCT04407286) | [51,52,53] |
| Antioxidant mechanism: reduces lipid peroxidation, induces antioxidant enzymes (SOD), stimulates the enzyme sirtuin 1 involved in reduction in oxidative stress and inflammatory response | [51] | |||
| Safety issues: LD50 (oral, rat) 42 mg/kg; experimental teratogen | [31] | |||
| Cysteine | (2R)-2-Amino-3-sulfanylpropanoic acid | Antioxidant in foods (E920), pharmaceuticals and cosmetics Dietary supplements /FDA GRAS, Eur. and US Pharmacopoeia | Pharmacological properties: antioxidant, prevention of corneal ulceration after chemical burn, skin-whitening | [54] |
| Antioxidant mechanism of action: reducing agent, precursor of reduced glutathione (GSH), chain-breaking antioxidant mechanism; in foods (fruits), L-Cys inhibits the activity of polyphenol oxidase and reduces browning by combination with reactive electrophilic quinones | [55,56] | |||
| Safety issues: LD50 (IP, mouse) 1250 mg/kg, (IV, mouse) 771 mg/kg | [31] | |||
| Dioxybenzone | (2-Hydroxy-4-methoxyphenyl)-(2-hydroxyphenyl)methanone | Dermatological drug (sunscreen in cosmetics) /US Pharmacopeia | Pharmacological properties: antioxidant, UV light absorber, skin cancer chemopreventive | [57] |
| Antioxidant mechanism: scavenging free radicals and relieving of oxidative stress related to cancer from UV exposure | [57] | |||
| Safety issues: LD50 (oral, rat) >10 g/kg; nontoxic in single oral doses; nonirritating to rabbit eye or skin; | [32] | |||
| Disodium EDTA (disodium edetate) | Disodium 2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetate | Foods (calcium disodium EDTA E385; Disodium EDTA E386) Drug (Calcium disodium EDTA) Pharmaceuticals Cosmetics /FDA, Eur. and US Pharmacopoeia | Pharmacological properties: antioxidant, metal chelating, for treatment of lead poisoning (Calcium disodium EDTA) or hypercalcemia (disodium EDTA), potential use in COVID-19 | [31,58] |
| Antioxidant mechanism: reduces metal-induced free radical production, protects against DNA damage and lipid oxidation | [59] | |||
| Safety issues: LD50 (oral, rat) 2 g/kg, (IV, mouse) 56 mg/kg, (IP, mouse) 260 mg/kg; experimental teratogen, reproductive effects; mutagenic data | [32,60] | |||
| Erythorbic acid (isoascorbic acid, isovitamin C) | (2R)-2-[(1R)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one | Antioxidant in foods (E315; sodium erythorbate E316) and pharmaceuticals /FDA GRAS for E315 | Pharmacological properties: antioxidant, antimicrobial, enhance iron bioavailability, antitumor | [31,61,62,63] |
| Antioxidant mechanism: sodium erythorbate is a reducing agent acting similar to ascorbic acid despite it lacking vitamin C activity, and inhibits nitrite reaction in meat curing | [64] | |||
| Safety issues: Nontoxic; mutagenic; causes DNA damage; LD50 (oral, mouse) 8300 mg/kg and LD50 (oral, rats) 18 g/kg (erythorbic acid); LD50 (oral, rats) >5 g/kg (sodium erythorbate) | [31,62] | |||
| Etidronic acid | (1-Hydroxy-1-phosphonoethyl)phosphonic acid | Drug (etidronate disodium) Antioxidant in pharmaceuticals and cosmetics /FDA, Eur. and US Pharmacopeia | Pharmacological properties: antioxidant, chelating agent for heavy metal ions, reduces osteoclastic activity (treatment of Paget’s disease, osteoporosis), increase the bone mineral density, inhibition of protein tyrosine phosphatase | [65,66] |
| Antioxidant mechanism: calcium, iron chelator inhibiting the chondrocyte lipid peroxidation, suppress radical formation | [67] | |||
| Safety issues: LD50 (oral, mouse) 1800 mg/kg; impairment of bone mineralization, hypercalcemia, esophageal cancer | [21,32] | |||
| 4-Hexylresorcinol | 4-Hexylbenzene-1,3-diol | Flavoring agent in food (E586) Topical antiseptic Cosmetics /Eur. and US Pharmacopeia | Pharmacological properties: antioxidant, antiseptic, antihelmintic, local anesthetic, antiviral (against parainfluenza virus type 3) | [21,68] |
| Antioxidant mechanism: inhibits tyrosinase, increases glutathione levels preventing DNA damage, scavenging of peroxyl radicals and oxygen superoxide, reduces lipid and protein peroxidation | [69] | |||
| Safety issues: LD50 (oral, rat) 550 mg/kg; LDLo (IP, mouse) 50 mg/kg, (subcut., mouse) 750 mg/kg; may irritate eyes, skin, respiratory tract; experimental reproductive effects | [31] | |||
| Idebenone | 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | Drug (nootropic, antioxidant therapy) /EMA | Pharmacological properties: antioxidant, stimulates ATP production, neuroprotective (dementia, Alzheimer’s disease), treatment of visual impairment in patients with Leber’s Hereditary Optic Neuropathy; adjuvant for secondary effects of viral infection | [21,70,71] |
| Antioxidant mechanism: scavenger of free radicals (superoxide) being recycled by mitochondrial and cytosolic reductase), inhibits lipid peroxidation in mitochondrial membrane; electron donor to mitochondrial electron transport chain | [70] | |||
| Safety issues: LD50 (oral, rat) 10 g/kg, LD50 (IP, rat) 757–886 mg/kg | [70] | |||
| Lipoic acid | 5-[(3R)-1,2-Dithiolan-3-yl]pentanoic acid | Drug (antioxidant therapy of diabetic neuropathy) Nutraceutical /Eur. and US Pharmacopeia | Pharmacological properties: antioxidant, analgesic, treatment of diabetic neuropathy, detoxification of mercury in brain cells, treatment of multiple sclerosis (clinical trial 2021) and schizophrenia (clinical trial 2021), potential antiviral including COVID-19 | [21,72,73,74] |
| Antioxidant mechanism: iron, copper chelation, scavenging ROS, the reduced form (dihydrolipoic acid) regenerates endogenous antioxidants (vitamin C, vitamin E, glutathione) repairing oxidative damage | [75] | |||
| Safety issues: cholestatic hepatitis; LD50 (oral, rats) >2000 mg/kg; no mutagenic (Ames assay), not genotoxic (mouse micronucleus assay) | [21,76] | |||
| Melatonin | N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide | Drug /US Pharmacopeia | Pharmacological properties: antioxidant, increases GABA and serotonin, efficient in sleep and autistic disorders, anticancer, sunburn prevention, potential positive effect on COVID-19 (clinical trial 2021) | [21,77,78] |
| Antioxidant mechanism: direct free radical scavenging, stimulation of antioxidant enzymes, lowering of free radical generation by increasing oxidative phosphorylation in mitochondria and reducing electron leakage, protects against DNA damage | [79,80] | |||
| Safety issues: LD50 (oral, mouse) 1250 mg/kg | [81] | |||
| Methionine | (2S)-2-Amino-4-methylsulfanylbutanoic acid | Dietary supplements Food and pharmaceutical flavoring agent Feed additive /FDA, Eur. and US, Pharmacopeia, FEMA GRAS | Pharmacological properties: antioxidant, antihepatotoxic, alternative to acetylcysteine in the treatment of acetaminophen overdose, metal chelating, precursor of cysteine; anticancer (clinical trial phase 2, 2021) | [21,82] |
| Antioxidant mechanism: reduces ROS levels due to Met-sulfoxide reductase, activation of endogenous antioxidant enzymes, stimulating glutathione synthesis, heavy metal chelator | [83] | |||
| Safety issues: LD50 (oral, rat) 36 g/kg, (IP, rat) 4328 mg/kg; as precursor of homocysteine, high doses may result in susceptibility of cardiovascular disease, type-2 diabetes, brain alterations | [31,84,85] | |||
| Niacinamide (Nicotinamide) | Pyridine-3-carboxylic acid amide | Dietary supplements Food fortification Feed additive (nutritional) Pharmaceutical intermediate Cosmetics /FDA GRAS, Eur., US Pharmacopeia | Pharmacological properties: antioxidant, prevents pellagra, precursor of coenzymes (NAD) involved in electron transfer reactions in the respiratory chain, skin stimulant, anticancer, reduces LDL, improves HDL, early Alzheimer’s disease treatment (clinical trial phase 2, 2021) | [21,31,32,86] |
| Antioxidant mechanism: scavenging •OH, 1O2 and superoxide O2•−, inhibits the initiation step of lipid peroxidation, increases glutathione levels, protects against both lipid and protein oxidation in brain | [86] | |||
| Safety issues: LD50 (oral, rat) 3500 mg/kg, (IP, mouse) 2050 mg/kg, (subcut., rat) 1680 mg/kg | [31] | |||
| Pentoxifylline (International Nonproprietary Name by WHO) | 3,7-Dimethyl-1-(5-oxohexyl)purine-2,6-dione | Drug /FDA, Eur. and US Pharmacopeia | Pharmacological properties: antioxidant, anti-inflammatory, immunomodulatory, treatment of peripheral vascular disorders, inhibits the production of the cytokine TNFα, treatment of Diabetic Kidney Disease (clinical trial phase 4, 2021); antiviral potential adjuvant in treatment of COVID-19 (clinical trial) | [21,87,88] |
| Antioxidant mechanism: scavenges free radicals (∙OH), decreases lipid peroxidation | [89] | |||
| Safety issues: LD50 (oral, rat) 1385 mg/kg | [90] | |||
| Probucol (International Nonproprietary Name by WHO) | 2,6-Di-t-butyl-4-[2-(3,5-ditert-butyl-4-hydroxyphenyl)sulfanylpropan-2-ylsulfanyl]phenol | Drug US Pharmacopeia | Pharmacological properties: antioxidant, anticholesterolemic | [21] |
| Antioxidant mechanism: inhibits lipid peroxidation | [91] | |||
| Safety issues: LD50 (oral, mouse, rat) >5000 mg/kg | [92] | |||
| Propyl gallate (PG) | Propyl 3,4,5-trihydroxybenzoate | Antioxidant in food (E310), food packages, food-contact coatings, feed, pharmaceuticals and cosmetics /FDA (0.005% migrating from food pkg., 0.02% max. of fat or oil, GRAS, Eur. Pharmacopeia | Pharmacological properties: antioxidant, hepatoprotector, limited antibacterial and antifungal activity | [21,31] |
| Antioxidant mechanism: hydrogen donor interrupting the free radical autoxidative chain reactions | [43] | |||
| Safety issues: LD50 (oral, rat) 3.8 g/kg, (IP, rat) 0.38 g/kg; skin irritant; questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects | [21,31,44] | |||
| Propylene glycol (PEG) | Propane-1,2-diol | Food emulsifier (E1520) Pet foods Agent for pharmaceuticals and cosmetics (viscosity control) /FDA GRAS, US and Eur. Pharmacopeia | Properties: antiseptic, humectant, efficient solvent and extractant of active ingredients including antioxidants | [31] |
| Antioxidant mechanism: propylene glycol mannate sulfate induces the antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase) eliminating the oxygen free radicals (study on hyperlipidemic rats) | [93] | |||
| Safety issues: LD50 (oral, rat) 21 g/kg; ocular and skin irritant; no reproductive toxicity; not mutagenic, not carcinogenic | [31,94] | |||
| Retinol (vitamin A) | (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraen-1-ol | Dietary supplements Cosmetics /FDA GRAS, Eur. and US Pharmacopeia | Pharmacological properties: antioxidant, role in vision, epithelial differentiation, growth, bone development, immunity, anticancer, anti-inflammatory, potential immunomodulator in COVID-19 | [95,96] |
| Antioxidant mechanism: scavenges lipid peroxyl radicals (LOO∙) by forming a stable trans-retinol radical intermediate, reduces DNA damage (studies in cancer therapy), stimulates endogenous antioxidant enzymes | [97] | |||
| Safety issues: daily intakes of Vitamin A >50,000 IU in adults and 20,000 IU in infants and young children may cause toxic manifestations; LD50 (oral, rat, 10 day) 7910 mg/kg, (oral, mouse) 6060 mg/kg; experimental reproductive effects; hepatomegaly, visual disturbances | [98,99] | |||
| Selenious acid | Selenous acid | Pharmaceuticals Supplements /US Pharmacopeia | Pharmacological properties: antioxidant, enzymatic cofactor (glutathione peroxidase), anticancer, stimulates hemoglobin synthesis in erythroleukemia cell lines, immunomodulatory, prevention of atherosclerosis | [21,100] |
| Antioxidant mechanism: selenium is a Cu+ chelator, inhibits DNA damage from •OH radical, maintains the enzymatic activity of glutathione peroxidase | [16] | |||
| Safety issues: LD50 7 mg Se/kg for sodium selenite, 138 mg Se/kg for selenium sulfides (as formulated for anti-dandruff shampoos), and 6700 mg Se/kg for elemental selenium | [101] | |||
| Sitosterol | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-Ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | Dietary supplements Stabilizer in pharmaceuticals, cosmetics /Canadian Provisional DSL | Pharmacological properties: antioxidant, hypolipidemic, treatment of diaper rash, anti-inflammatory, antiapoptotic, anticancer, potential role as immunostimulant and inhibitor of SARS-CoV-2 spike glycoprotein | [21,31,32,102] |
| Antioxidant mechanism: reduces liver lipid peroxidation in induced cancer colon, maintains the level of antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione S-transferase, reduced glutathione) | [103] | |||
| Safety issues: LD50 (oral, mouse) >25,000 mg/kg; skin and eye irritant; nontoxic | [31] | |||
| Thiodipropionate | 3-(2-Carboxylatoethylsulfanyl)propanoate | Foods (0.02% of fat or oil content of food) Food packages Pharmaceuticals Cosmetics (0.1% and rarely exceed 0.2%) /FDA | Properties: antioxidant, skin lightening. Acts synergistically with phenols | [104] |
| Antioxidant mechanism: chain-breaking, decomposes hydrogen peroxide | [105] | |||
| Safety issues: Dilauryl thiodipropionate: LD50 (oral, rat) >10.3 g/kg; no known toxicity; eye irritant Distearyl thiodipropionate: LD50 (oral, rat) >2500 mg/kg, (IP, rat) >2 g/kg; | [31] | |||
| α-Tocopherol (vitamin E) | (2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol | Dietary supplements Antioxidant in food (E306; α-tocopherol E307; γ-tocopherol E308; δ-tocopherol E309), food packages, animal feed, pharmaceuticals and cosmetics /FDA GRAS | Pharmacological properties: high antioxidant activity, anticancer, prevents atherosclerosis, cardiovascular diseases and age-related macular degeneration | [106,107] |
| Antioxidant mechanism: directly reacts and neutralizes •OH, alkoxyl and lipid peroxyl (ROO∙) radicals stopping the ROS-induced damage; may be regenerated with vitamin C | [16] | |||
| Safety issues: TDLo (oral, rat) 7500 mg/kg | [31] | |||
| Ubiquinone (ubidecarenona, coenzyme Q 10) | 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | Dietary supplements Antioxidant in pharmaceuticals and cosmetics /US Pharmacopoeia | Pharmacological properties: antioxidant, cofactor in the mitochondrial electron transport chain, useful in the treatment of cardiovascular diseases, Parkinson’s, fibromyalgia, migraine, diabetes, adjuvant in COVID-19 (clinical trial phase 2, 2021, NCT04960215, 2020-005961-16) | [31,108,109] |
| Antioxidant mechanism: reduces lipid peroxidation, increases antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase) | [110] | |||
| Safety issues: lethal dose >2000 mg/kg (oral, rat) | [111] | |||
| Zinc | Zinc | Dietary supplements Antioxidant in pharmaceuticals (zinc glycinate) Cosmetics /FDA FDA GRAS (zinc gluconate) | Pharmacological properties: antioxidant, enzyme activator, antimicrobial, antidiarrheic, anti-inflammatory, antitumor (clinical trial NCT04488783), antiviral including protection against COVID-19 | [31,112,113] |
| Antioxidant mechanism: induces antioxidant glutathione and enzymes (SOD, glutathione S-transferase, hemeoxygenase-1); protection of protein –SH groups; gene regulation (p53, NF-kB, AP-1) | [114] | |||
| Safety issues: Zinc acetate: LD50 (oral, rat) 2510 mg/kg Zinc carbonate: TDLo (oral, mouse) 2800 mg/kg; experimental teratogen Zinc chloride: LD50 (oral, rat) 350 mg/kg; irritant, questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects Zinc citrate: LD50 (oral, rat) >5000 mg/kg; experimental reproductive effects Zinc gluconate: LD50 (oral, mouse) 1290 mg/kg; experimental reproductive effects Zinc sulfate: LD50 (oral, rat) 2949 mg/kg; irritant, questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoia, M.; Oancea, S. Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends. Antioxidants 2022, 11, 638. https://doi.org/10.3390/antiox11040638
Stoia M, Oancea S. Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends. Antioxidants. 2022; 11(4):638. https://doi.org/10.3390/antiox11040638
Chicago/Turabian StyleStoia, Mihaela, and Simona Oancea. 2022. "Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends" Antioxidants 11, no. 4: 638. https://doi.org/10.3390/antiox11040638
APA StyleStoia, M., & Oancea, S. (2022). Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends. Antioxidants, 11(4), 638. https://doi.org/10.3390/antiox11040638







