Simple Summary
Plants growing at temperate and polar latitudes are exposed to cold stress. With climate change, different durations of low temperatures and sometimes frost are being increasingly observed in regions at low latitudes. This can cause especially great harm to agricultural crops, the mortality of which can pose a serious challenge to the food supply for the global population. One of the factors affecting plant resistance to low and negative temperatures is specific changes in the fatty acid (FA) composition of lipids. It should be noted that most of the crops studied in this regard are angiosperms. It is known that the FA composition of angiosperms has undergone significant evolutionary changes compared to that of nonflowering vascular plants. Studying the FA composition of various taxonomic groups can shed light on and reveal new mechanisms of plant resistance. Therefore, in this paper, we focused on the rare evergreen fern Asplenium scolopendrium, whose fronds can tolerate freezing. A number of specific features of its FA composition were discovered, which, in combination with other resistance mechanisms, determine its ability to grow in temperate climate zones and safely undergo wintering.
Abstract
Ferns are one of the oldest land plants. Among them, there are species that, during the course of evolution, have adapted to living in temperate climates and under winter conditions. Asplenium scolopendrium is one such species whose fronds are able to tolerate low subzero temperatures in winter. It is known that the resistance of ferns to freezing is associated with their prevention of desiccation via unique properties of the xylem and effective photoprotective mechanisms. In this work, the composition of A. scolopendrium lipid fatty acids (FAs) at different times of the year was studied by gas–liquid chromatography with mass spectrometry to determine their role in the resistance of this species to low temperatures. During the growing season, the polyunsaturated FA content increased significantly. This led to increases in the unsaturation and double-bond indices by winter. In addition, after emergence from snow, medium-chain FAs were found in the fronds. Thus, it can be speculated that the FA composition plays an important role in the adaptation of A. scolopendrium to growing conditions and preparation for successful wintering.
1. Introduction
Ferns were one of the first vascular plants that, because of the development of the xylem, successfully settled on Earth’s surface as early as the Palaeozoic era [1]. For many millions of years, they were the dominant plant form on land [2]. However, later, in the Mesozoic, along with a gradual cooling of the climate, gymno- and angiosperms gradually replaced ferns as the most abundant plants [3,4,5]. However, ferns were able to successfully coexist with angiosperms and continued to evolve [6]. They occupy the undergrowth [7], and many ferns have become epiphytes [8]. Thus, ferns now occupy ecological niches with conditions different from those during the period of their dominance. They had to adapt to a lack of moisture and low light levels. In addition, some fern species have successfully adapted to temperate conditions [9], and some species are even truly arctic, subantarctic, and alpine [10,11].
Among temperate ferns, some species retain their fronds in winter [12,13]. It is known that such ferns are able to tolerate negative temperatures, with some species tolerating temperatures as low as −20 °C [14]. Thus, these ferns tolerate repeated freeze-dry cycles [15,16,17,18]. There is a theory that some species of ferns, in particular species of Asplenium, may cope with winter stress via poikilohydric behavior [19]. Plant cells with desiccation tolerance (DT) have anatomical and biochemical mechanisms that facilitate the dehydration process, including metabolic arrest, with successful subsequent restoration of their activity during rehydration [20]. The main features of vegetative DT are photoprotection against redox processes due to low-molecular-weight antioxidants and the ability to preserve the integrity of cells (for example, cell walls and membranes) [21,22]. Vegetative tissue DT is very rare in angiosperms and is also rare but more common (approximately 10 times) among ferns [23,24]. At the same time, it has long been known that for species with DT, resistance to freezing increases significantly under arid conditions [25].
The recent work of Fernández-Marín et al. [26] was focused on the ability of fern fronds to withstand low temperatures and freezing. The authors showed that adaptation for freezing tolerance is associated with DT through complementary xylem properties (which may prevent risk of irreversible cavitation) and effective photoprotection mechanisms. However, the lipid fatty acid (FA) composition of the membrane was not considered by the authors as an important parameter for plant resistance and adaptation.
FAs are considered one of the general plant defense systems against various biotic and abiotic stresses [27]. The protective function is performed mainly by unsaturated FAs, which, in plant cells, act as components and modulators of cellular membrane glycerolipids, stocks of extracellular barrier constituents, precursors of various bioactive molecules, and regulators of stress signaling. Several recent reviews [28,29,30,31] have been devoted to all of these aspects, but in our work, we focused on the role of FAs in plant cold stress.
It is known that the lipid FA composition in plant membranes plays an important role in adaptation to low and subzero temperatures [32,33]. Previous studies reported that the accumulation of unsaturated FAs ensures the safety of winter wheat seedlings in winter [34,35]. The accumulation of unsaturated FAs in cell membranes, especially that of polyunsaturated FAs, promotes plant resistance to damage by low temperatures [36,37]. The synthesis of unsaturated FAs in plant cells occurs with the participation of desaturases [38]. All genes encoding FA desaturases (FADs) exhibit cold-induced and heat-repressed expression, although with distinct regulatory time courses, which indicates the potential roles of FADs in temperature stress resistance [39]. However, the FA composition of the resistance of ferns to winter conditions is practically unstudied. Dehydration leads to damage to cell membranes [40,41,42], and FAs contribute to better adaptation of cells to dehydration due to their role as components of membrane lipids [43]. Based on this current situation, it is obvious that studying the FAs of wintering ferns is a very urgent task. Therefore, we decided to study changes in the total lipid fatty acid composition of ferns with the season.
2. Materials and Methods
2.1. Plant Materials
The object of the study was the fronds of Asplenium scolopendrium (L.) Newman. This fern is 30–80 cm tall and has a short rhizome covered with scales on the top (Figure S1A). The fronds are dark green, leathery, bare, and intact, which is rare among ferns; their length is 10–60 cm, and their width is 3–6 cm. The petioles are shorter than the frond blade [44,45] (Figure S1B). On the abaxial surface of the fronds, along the lateral veins, the sori are arranged parallel to each other and covered with two indusia. Their arrangement is reminiscent of Myriapoda legs, from which the species name scolopendrium was derived; in Latin, it means “centipede” (Figure S1C).
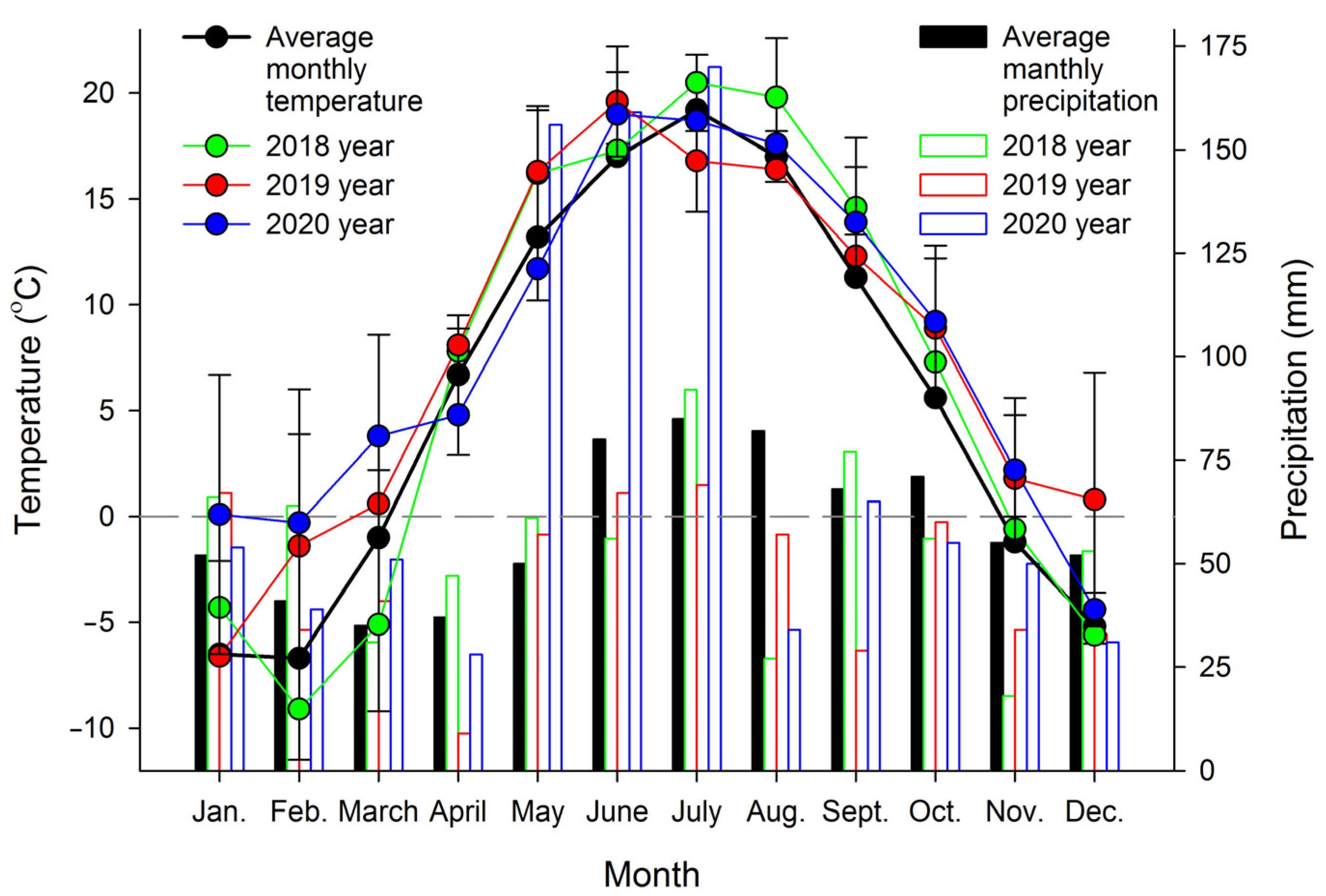
Plant specimens were cultivated in open ground (55°69′85′′ N 38°91′16′′ E) under similar semishaded conditions. In temperate climates, A. scolopendrium fronds are evergreen, i.e., they do not die off in winter but remain under snow cover and vegetate the next year. For this study, 3–5 fronds were taken from three different plants three times per year: in spring (April 1–10, after the snow had completely melted and the average daily temperatures were stable above 0 °C), in summer (July 20–30, the period with the longest days) and in autumn (November 1–10, in the absence of permanent snow cover but with temporary snow cover possible; the average daily temperature was stable at below 0 °C). The average monthly temperatures during the experiment (2018–2020) were recorded by using an EClerk-M-T-a automatic thermometer (Relsib, Russia), and the average monthly precipitation was calculated according to http://www.pogodaiklimat.ru (accessed on 6 February 2022) (Figure 1).
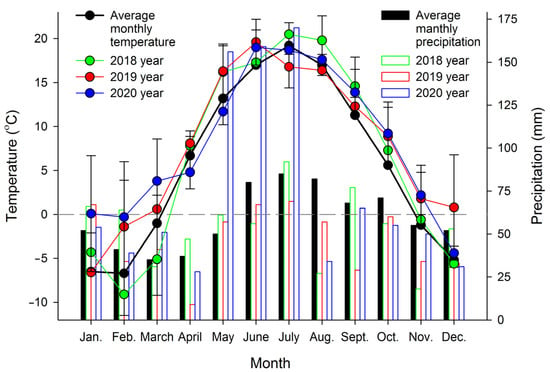
Figure 1.
Monthly averages of temperature and precipitation at the Asplenium scolopendrium collection site during 2018–2020. Values are presented as the means ± SEMs.
2.2. Lipid Extraction
Freshly collected fern leaves were weighed and fixed in boiling isopropyl alcohol (CH3CH(OH)CH3) for 1 h to preserve the native lipid composition. At a preliminary stage, surface waxes were removed with chloroform (CHCl3). The samples were stored at +4 °C until extraction. To extract total lipids, the CH3CH(OH)CH3 was separated through a Schott glass filter into a 250 mL volumetric flask, and the plant tissue was homogenized in a porcelain mortar and extracted. Lipids were extracted three times sequentially in CHCl3/methyl alcohol (CH3-OH)/water (H2O) (30:20:1.7, by vol.) and CHCl3/CH3-OH/nitric acid (HNO3) (20:10:0.1, by vol.). All the extracts were added to the volumetric flask, and the total volume was adjusted to 250 mL with CH3CH(OH)CH3 containing 0.001% butylated hydroxytoluene (Sigma-Aldrich, Burlington, MA, USA, 34750) as an antioxidant. The extracts were stored in a refrigerator [46].
2.3. Preparation of Fatty Acid Methyl Esters
FA methyl esters (FAMEs) were prepared according to a previously described method [47]. The sample was evaporated to dryness using a rotary evaporator under standard conditions (at not higher 50 °C and 9.5 × 10−1 kgf/cm2). After saponification was conducted in a boiling solution of 4% sodium hydroxide (NaOH) in CH3OH/H2O (1:1, by vol.). Then, the sample was evaporated to dryness. Approximately 2 mL of water was added to the dried sample, and unsaponifiable FAs were washed several times with n-hexane (C6H14) until they became clear. Then, FAs were extracted six times with 2 mL of C6H14 in acidic conditions (acidifier 20% sulphuric acid (H2SO4)). The collected C6H14 was evaporated, 3 mL of CH3OH and a 750 μL of acetyl chloride (CH3COCl) were added to the sample, and the sample was boiled for 1 h. Then, the sample was again evaporated, 2 mL of H2O was added, and FAMEs were extracted six times with C6H14 under acidic conditions (acidifier 20% H2SO4). After that, the hexane was evaporated, and 500 μL of benzene (C6H6) was added [48].
The extract was pipetted onto a chromatographic plate, and a mixture of C6H14/diethyl ether (C2H5-O-C2H5)/glacial acetic acid (CH3COOH) (8:2:0.1, by vol.) was used as the mobile phase. When the front reached the top of the plate, the plate was removed and air-dried for 1–2 min. Then, the plate was treated with a 0.001% solution of 2′,7′-dichlorofluorescein in ethanol (C2H5OH) and air-dried for 5–7 min. The zones with FAMEs were visualized in UV light (λ = 365 nm). Then, the silica gel from the FAME zone of the chromatographic plate was transferred to a Schott glass filter, and the FAMEs were eluted from the sorbent by washing with C6H14 six times [49].
The FAMEs were analyzed by gas chromatography-mass spectrometry (GC-MS) on an Agilent 7890A GC with a quadrupole mass detector Agilent 5975C fitted with a 60-m capillary column DB-23 (inner diameter 0.25 mm, thickness of stationary phase-(50%-cyanopropyl)-methylpolysyloxane—250 µm). The prepared FAMEs were separated under the following conditions: carrier gas, helium at 1 mL/min; sample volume, 1 µL; split ratio, 4:1 (in numerous analyses, splitless injection was used); evaporator temperature, 260 °C. The oven temperature program was as follows: from 130 to 170 °C at 6.5 °C/min, to 215 °C at 2.75 °C/min (25 min hold at this temperature), to 240 °C at 40 °C/min (30 min hold at 240 °C). The operational temperature of the mass detector was set to 240 °C, and the ionization energy was set to 70 eV. To identify individual FAME species (Figure S2), NIST and Wiley search libraries and ChemStation software were used, and the relative retention time and equal chain length (ECL) value were calculated for each peak [50].
2.4. Data and Statistical Analyses
To characterize the unsaturation level of lipid FAs, the unsaturation index (UI) [51] and double-bond index (DBI = (2 × %18:2 + 3 × %18:3)/(%16:0 + %18:0 + %18:1) [52,53] were calculated. The activity efficiencies of the Δ9-, Δ6-, and Δ3-desaturases were calculated as the palmitic desaturation ratio (PDR = %16:1/(%16:0 + %16:1)), palmitoleic desaturation ratio (PlDR = (%16:2 + %16:3)/(%16:1 + %16:2 + %16:3)), palmitolinolenic desaturation ratio (PlnDR = %16:3/(%16:2 + %16:3)), stearic desaturation ratio (SDR), oleic desaturation ratio (ODR), and linoleic desaturation ratio (LDR) [8]. Desaturation and elongation partitioning ratios were calculated based on the ratios of the following FAs: C16 desaturation— %16:1/(%16:0 + %18:0 + %18:1 + %18:2 + %18:3 + %20:0 + %20:1 + %22:0 + %22:1); C18 desaturation—(%18:1 + %18:2 + %18:3 + %20:1 + %22:1)/(%18:0 + %20:0 + %22:0); C16 elongation—(%18:0 + %18:1 + %18:2 + %18:3 + %20:0 + %20:1 + %22:0 + %22:1)/(%16:0 + %16:1) [54].
All experiments were performed in 3–5 replicates with at least three independent executions. The data are presented in tables and a graph as the means ± SEMs. Statistical analysis was performed as described by us previously [8]. Different symbols or letters indicate significantly different values. Mean values were considered significantly different at p ≤ 0.05.
3. Results and Discussion
Throughout the period of material collection, the major FAs in the total lipids of A. scolopendrium were palmitic (16:0), linoleic (9,12-18:2), and α-linolenic (9,12,15-18:3) acids; moreover, the amount of 16:0 decreased significantly during the year, and the amounts of 9,12-18:2 and 9,12,15-18:3 increased in summer and autumn (Table 1). Cold-resistant genotypes of rice are characterized by the accumulation of 9,12-18:2 acids after such exposure when the content of 16:0 decreases and the opposite changes occur in thermophilic genotypes [33]. Thus, the A. scolopendrium FA composition changes in autumn (Table 1), as in cold-resistant species. However, a decrease in the level of 9,12,15-18:3 after wintering may indicate dehydration of the plant, since for species that are less resistant to drought, a decrease in the level of this acid during tissue dehydration has been shown [55,56]. In addition, the decrease in the amount of 9,12,15-18:3 may be because this FA, released from membrane lipids by means of regulated lipase activity, is the precursor molecule for phyto-oxylipin biosynthesis [57]. Oxylipins can play an important role in the resistance of plants to pathogens [58,59], which is especially important when plants are emerging from a wintering state.

Table 1.
Lipid fatty acid composition of Asplenium scolopendrium fronds in spring, summer, and autumn (mass % of the amount of FAMEs).
In spring, oleic acid (9-18:1) was also a major FA (Table 1). It is known that 9-18:1 can stimulate plasma membrane phospholipase D, which generates phosphatidic acid to attenuate H2O2-induced cell death [60,61]. At the same time, peroxide is one of the main reactive oxygen species (ROS) that plants encounter when exposed to lows temperatures [29,62], and larger amounts of 9-18:1 in A. scolopendrium in the spring may indicate its role in the antioxidant defense of the fern. In autumn, the major FAs in A. scolopendrium include arachidonic acid (5,8,11,14-20:4), which is not found in the tissues of flowering plants [63,64]. However, this FA is characteristic of lipids in algae [65], mosses [66,67], and other ferns [8,68,69].
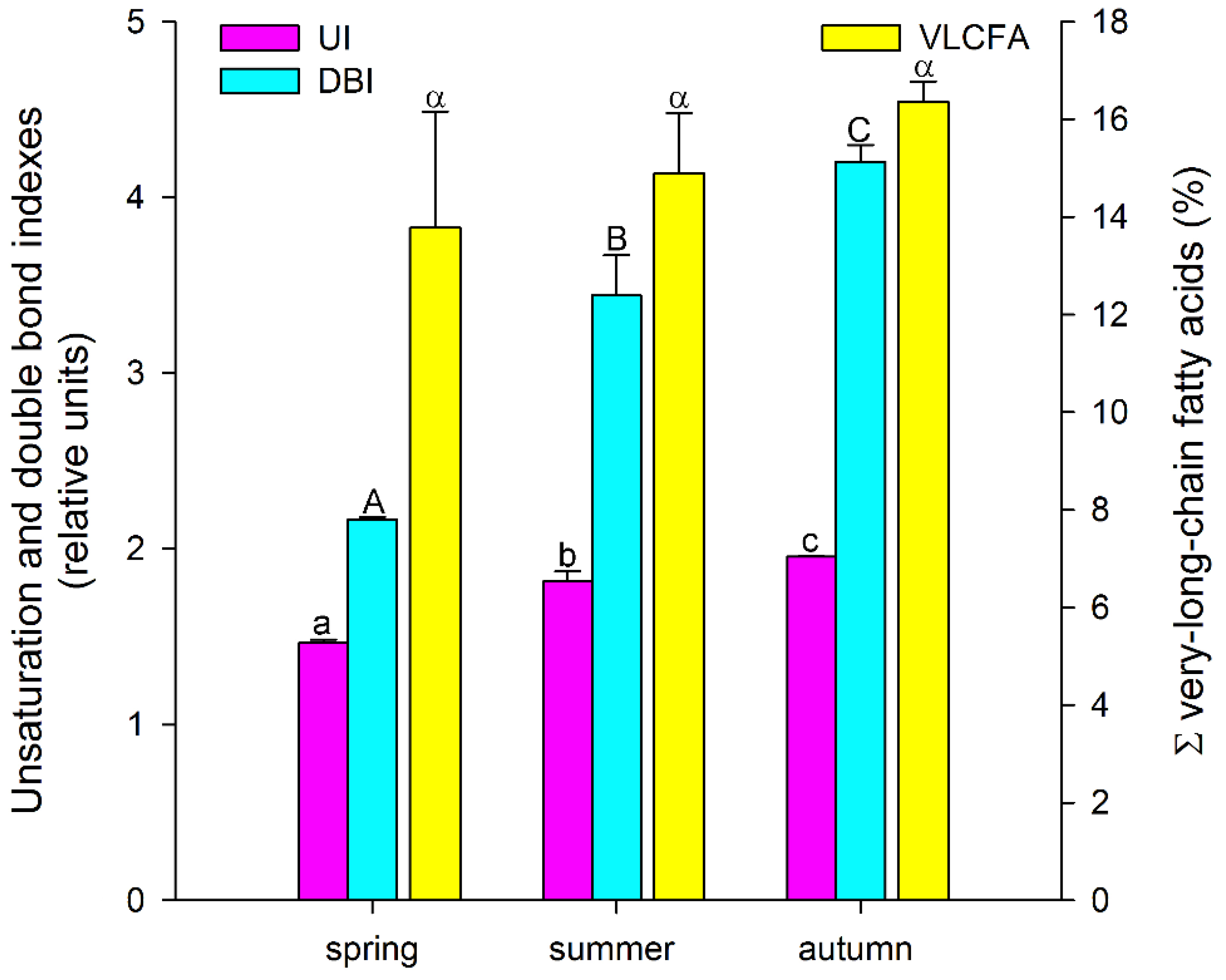
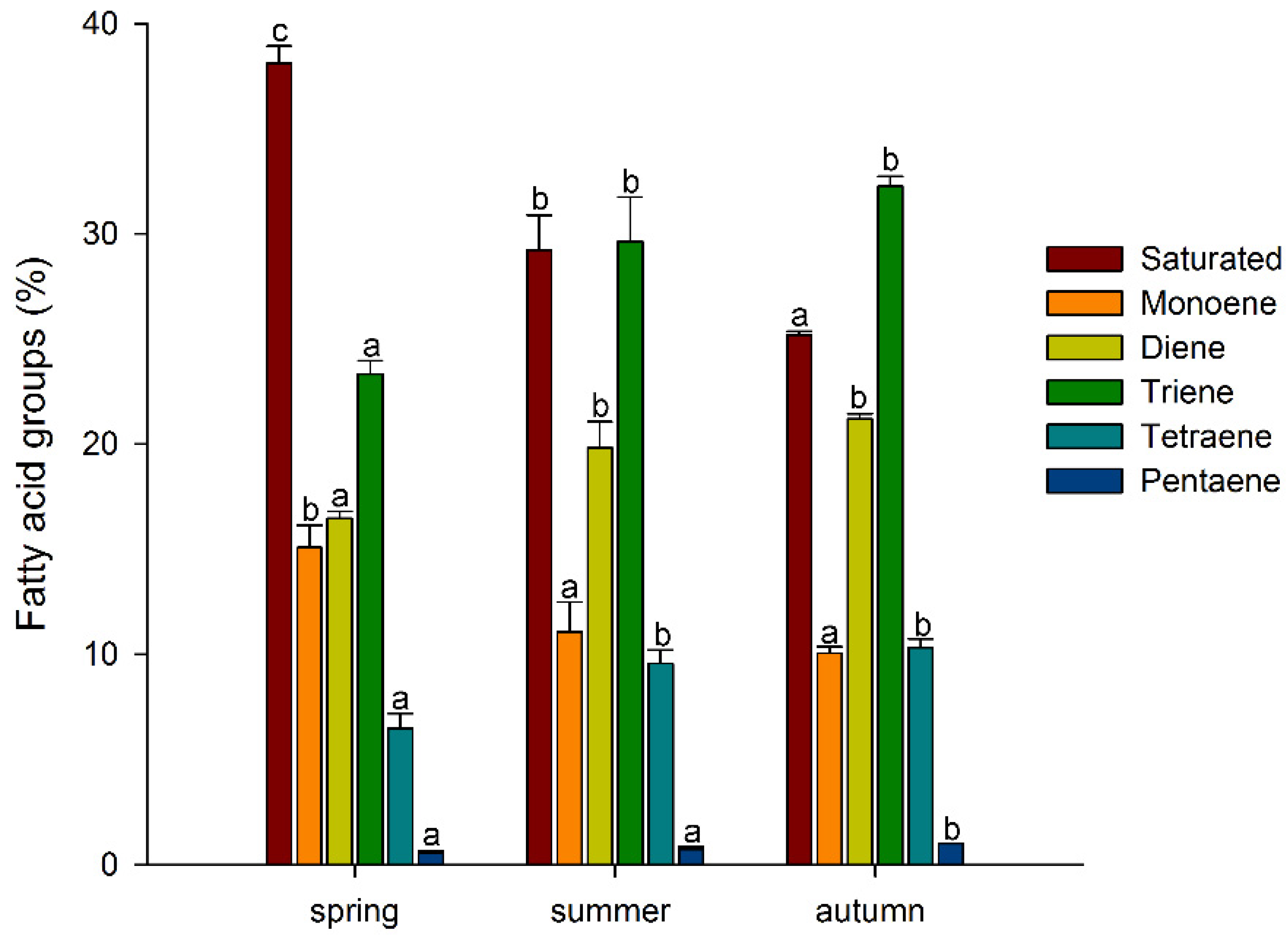
Thus, among the major FAs, there was a tendency for the accumulation of unsaturated FAs, especially polyunsaturated FAs, during the growing season, with a decrease in the level of saturated FAs. A similar trend was also observed for minor FAs: the levels of myristic (14:0), pentadecanoic (15:0), and stearic (18:0) acids decreased significantly, while the levels of 7,10-hexadecadienoic (7,10-16:2) and 7,10,13-hexadecatrienoic (7,10,13-16:3) acids increased in a stepwise manner (Table 1). Such tendencies in the A. scolopendrium FA composition could affect the level of total lipid saturation. In fact, we observed that two integral indicators of the level of lipid unsaturation and the number of double bonds (the UI and DBI, respectively) increased significantly during the growing season (Figure 2). The ratio of the levels of saturated and unsaturated fatty acids underlies the regulation of the phase transition point of biological membranes [57]. The presence of a large number of unsaturated FAs in the membrane, especially of polyunsaturated FAs, the proportion of which in A. scolopendrium increased as the cold season approached (Figure 3), leads to increased membrane fluidity. Therefore, the higher the UI and DBI values are, the more resistant the cell is to low temperatures [70,71]. Thus, changes in the levels of FAs systematically prepare A. scolopendrium for successful wintering.
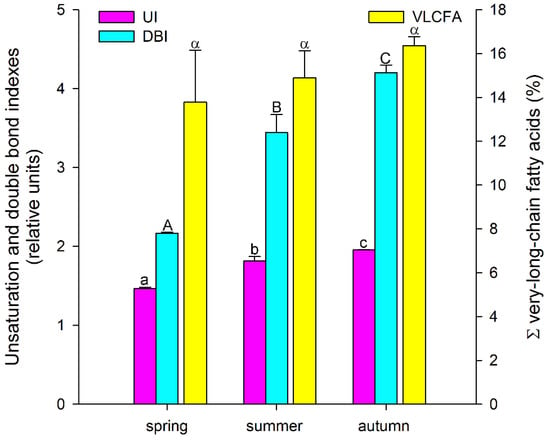
Figure 2.
Unsaturation index (UI) and double-bond index (DBI), as well as the total amount of very-long-chain fatty acids (ΣVLCFAs) in Asplenium scolopendrium fronds in spring, summer, and autumn. Values are presented as the means ± SEMs. Different letters indicate a significant difference between the means (p ≤ 0.05). One-way ANOVA, followed by Tukey’s HSD test, was performed separately for each parameter.
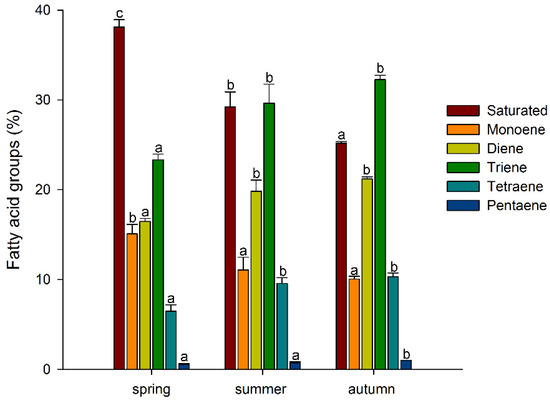
Figure 3.
Percentage of saturated and unsaturated (monoene, diene, triene, tetraene, and pentaene) fatty acid components in Asplenium scolopendrium fronds in spring, summer, and autumn. Values are presented as the means ± SEMs. Different letters indicate a significant difference between the means (p ≤ 0.05). One-way ANOVA, followed by Tukey’s HSD test, was performed separately for FA groups.
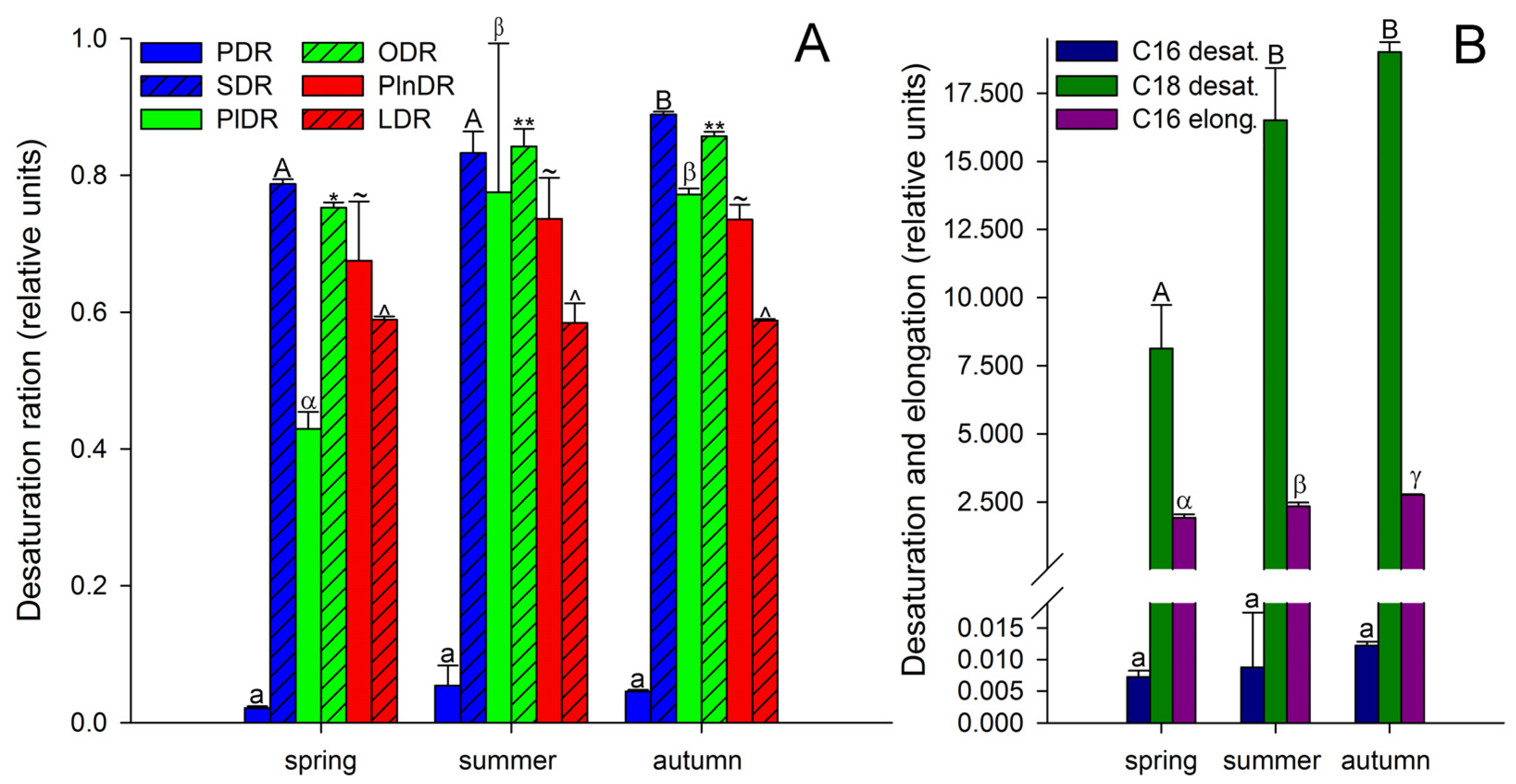
It is known that unsaturated FAs are formed from saturated FAs with the help of enzymes desaturases [38]. The activity of all enzymes, as protein molecules, depends on temperature [72,73]. Our research showed that the activities of only Δ6-desaturases (PlDR, ODR) and Δ3-desaturases (PlnDR, LDR) were significantly higher in summer and autumn (Figure 4A). At the same time, the desaturation process was significantly intensified only for 18-FAs, against the background of a monotonous increase in the level of 16-FA elongation (Figure 4B). Thus, in summer and spring, active synthesis of 9,12-18:2 and 6,9,12-18:3, which are precursors of 5,8,11,14-20:4 and eicosapentaenoic acid (5,8,11,14,17-20:5), respectively, occurs in the tissues of A. scolopendrium fronds [74,75]. Therefore, the presence of 5,8,11,14,17-20:5 in the tissues of A. scolopendrium was notable, the absence of which we previously noted in some thermophilic ferns [8]. The abundance of this FA was not very high, but it increased significantly in autumn (Table 1). Thus, significant contributions to the increase in UI and DBI values in autumn (Figure 2) were observed for tetra- and pentaenic very-long-chain FAs (VLCFAs), particularly 5,8,11,14-20:4 and 5,8,11,14,17-20:5 (Figure 3). It is obvious that preparation for the winter period via the accumulation of unsaturated FAs begins in the summer, and it is believed that the accumulation of polyunsaturated VLCFAs can prevent membranes from undergoing overfluidization in the warm period [76].
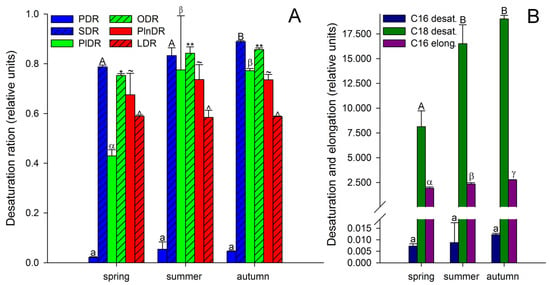
Figure 4.
Desaturation ratios (A) and desaturation and elongation (B) of Asplenium scolopendrium fronds in spring, summer, and autumn. Abbreviations: PDR—palmitic desaturation ratio, and SDR—stearic desaturation ratio (the activity efficiencies of the Δ9 desaturases); PlDR—palmitoleic desaturation ratio, and ODR—oleic desaturation ratio (the activity efficiencies of the Δ6 desaturases); PlnDR—palmitolinolenic desaturation ratio, and LDR—linoleic desaturation ratio (the activity efficiencies of the Δ3 desaturases); C16 desat.—C16 desaturation; C18 desat.—C18 desaturation; C16 elong.—C16 elongation ratio. Values are presented as the means ± SEMs. Different letters or symbols indicate a significant difference between the means (p ≤ 0.05). One-way ANOVA, followed by Tukey’s HSD test, was performed separately for each parameter.
The presence of capric (10:0), lauric (12:0), and tridecanoic (13:0) acids, also referred to as medium-chain FAs (MCFAs) [77,78,79], can be considered a distinctive feature of the FA composition of lipids in A. scolopendrium fronds after wintering (Table 1). MCFAs are formed de novo during synthesis via the participation of two enzyme systems, acetyl-CoA carboxylase and fatty acid synthase, the end products of which are 16:0 and 18:0 [80,81]. Thus, the appearance of MCFAs among A. scolopendrium lipids indicates the existence of active FA synthesis processes in spring. This is likely due to the restoration of the photosynthetic apparatus of the thylakoids after wintering, as it is known that de novo biosynthesis of FAs plays a critical role in the response of the photosynthetic apparatus to low temperature [82].
In addition, MCFAs are agonists of peroxisome proliferator-activated receptors [83]. Unfortunately, there is no such information available for plants. However, it is known that wintering can lead to damage to lipids [84,85] during oxidative stress [86,87,88], with ROS representing one type of factor involved in this process [89,90]. Based on these data, it can be generally stated that A. scolopendrium MCFAs can participate in the modulation of peroxisome activity, which is an integral part of cellular protection against oxidative damage caused by ROS [91,92].
In contrast, in the summer and autumn, MCFAs were absent from the A. scolopendrium lipids. However, new species of VLCFAs, namely, pentacosylic (25:0), cerotic (26:0), and montanic (28:0) acids, which were not observed in spring, appeared. Their presence did not lead to any notable changes in the total number of VLCFAs (Figure 2). This likely supports the hypothesis that the ΣVLCFA levels remain constant even as new species of FAs emerge, which may be because an increase in their level would lead to the suppression of cell proliferation [93] and accordingly could slow the growth of A. scolopendrium. It can be assumed that new types of VLCFAs appear as a consequence of activation of the synthesis of epicuticular waxes, which are the substrate for the biosynthesis of such FAs [94,95]. It is logical to assume that the impregnation of cuticle waxes can protect fern fronds from critical water loss in the winter.
4. Conclusions
In summary, the results suggest that in addition to other factors [26] associated with stability mechanisms, FAs play a very important role in the resistance of A. scolopendrium to low and subzero temperatures. Because of the presence of polyunsaturated VLCFAs, which are not characteristic of flowering plants (5,8,11,14-20:4 and 5,8,11,14,17-20:5 [96]), ferns adapt their membrane composition to frost, and as a result, they are likely able to grow at moderate latitudes and even closer to the poles, although they initially inhabited only places with a warm climate. At the same time, the FAs probably not only prepare A. scolopendrium for winter but also aid in the emergence of the fronds from snow. Since MCFAs were detected at the beginning of the spring, they are likely actively involved in the antioxidant protection of photosystems (along with 9-18:1) and in the repair of photosystems after exposure to cold stress.
All of the above findings suggest that the FA composition in ferns, which previously could be considered archaic in evolutionary terms, provides many hidden opportunities for the adaptation of Polypodiophyta and is a fundamental component of the ecological plasticity of these plants.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology11040507/s1, Figure S1: Asplenium scolopendrium: A—botanical illustration (Lindman, C.A.M. Bilder ur Nordens Flora. 1922; Volume 3, pp. 506); B—plant appearance, bar = 10 cm; C—abaxial surface of the fronds bearing sori, bar = 2 cm. Figure S2: Chromatogram of Asplenium scolopendrium fatty acids.
Author Contributions
A.V. and T.I. contributed equally to this work. Conceptualization, A.V. and T.I.; software, T.I.; formal analysis, A.V.; writing—original draft preparation, A.V.; writing—review and editing, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme no. 121033000137–1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Vladimir Kuznetsov and Elena Voronkova for taking care of the experimental plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DiMichele, W.A.; Phillips, T.L. The ecology of Paleozoic ferns. Rev. Palaeobot. Palynol. 2002, 119, 143–159. [Google Scholar] [CrossRef]
- Pryer, K.M.; Schuettpelz, E.; Wolf, P.G.; Schneider, H.; Smith, A.R.; Cranfill, R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am. J. Bot. 2004, 91, 1582–1598. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, S.; Crane, P.R. Angiosperm diversification and Cretaceous floristic trends: A comparison of palynofloras and leaf macrofloras. Paleobiology 1990, 16, 77–93. [Google Scholar] [CrossRef]
- Crane, P.R.; Friis, E.M.; Pedersen, K.R. The origin and early diversification of angiosperms. Nature 1995, 374, 27–33. [Google Scholar] [CrossRef]
- Nagalingum, N.; Drinnan, A.; Lupia, R.; McLoughlin, S. Fern spore diversity and abundance in Australia during the Cretaceous. Rev. Palaeobot. Palynol. 2002, 119, 69–92. [Google Scholar] [CrossRef]
- Schneider, H.; Schuettpelz, E.; Pryer, K.M.; Cranfill, R.; Magallón, S.; Lupia, R. Ferns diversified in the shadow of angiosperms. Nature 2004, 428, 553–557. [Google Scholar] [CrossRef]
- Schuettpelz, E.; Pryer, K.M. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc. Natl. Acad. Sci. USA 2009, 106, 11200–11205. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V. Fatty Acids Composition of the Epiphytic Ferns, Platycerium bifurcatum and Asplenium nidus, and the Terrestrial Fern, Asplenium trichomanes. Am. Fern J. 2021, 111, 117–128. [Google Scholar] [CrossRef]
- Rickard, M. Ferns for a Cool Temperate Climate; Pemberley Books: Iver, UK, 2021; ISBN 9781785008917. [Google Scholar]
- Peterson, K.M. Plants in Arctic Environments. In Ecology and the Environment; Springer: New York, NY, USA, 2014; pp. 363–388. [Google Scholar]
- McKenzie, E.H.C. Rust fungi in the subantarctic islands of New Zealand. Mycoscience 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Bannister, P. A note on some observations on frost damage in the field, with particular reference to various ferns. Trans. Bot. Soc. Edinb. 1973, 42, 111–113. [Google Scholar] [CrossRef]
- Tessier, J.T. Reduced winter snowfall damages the structure and function of wintergreen ferns. Am. J. Bot. 2014, 101, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sakai, A. Cold tolerance of gametophytes and sporophytes of some cool temperate ferns native to Hokkaido. Can. J. Bot. 1981, 59, 604–608. [Google Scholar] [CrossRef]
- Buchner, O.; Neuner, G. Freezing cytorrhysis and critical temperature thresholds for photosystem II in the peat moss Sphagnum capillifolium. Protoplasma 2010, 243, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Schott, R.T.; Voigt, D.; Roth-Nebelsick, A. Extracellular ice management in the frost hardy horsetail Equisetum hyemale L. Flora 2017, 234, 207–214. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Konrad, W.; Schott, R.; Roth-Nebelsick, A. A model for extracellular freezing based on observations on Equisetum hyemale. J. Theor. Biol. 2019, 478, 161–168. [Google Scholar] [CrossRef]
- Loesch, R.; Biron, U.; Patrias, T.; Hoeptner, B. Gas exchange and water relations of Asplenium ferns growing on limestone rocks. Nov. Hedwig. 2007, 131, 221–236. [Google Scholar]
- Rascio, N.; Rocca, N. La Resurrection Plants: The Puzzle of Surviving Extreme Vegetative Desiccation. CRC Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Van der Vyver, C.; Peters, S. How Do Plants Deal with Dry Days? Front. Young Minds 2017, 5, 58. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315. [Google Scholar] [CrossRef] [PubMed]
- López-Pozo, M.; Ballesteros, D.; Laza, J.M.; García-Plazaola, J.I.; Fernández-Marín, B. Desiccation Tolerance in Chlorophyllous Fern Spores: Are Ecophysiological Features Related to Environmental Conditions? Front. Plant Sci. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Kappen, L. Der Einfluß des Wassergehaltes auf die Widerstandsfähigkeit von Pflanzen gegenüber hohen und tiefen Temperaturen, untersucht an Blättern einiger Farne und von Ramonda myconi. Flora Allg. Bot. Ztg. Abt. A Physiol. Biochem. 1966, 156, 427–445. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Arzac, M.I.; López-Pozo, M.; Laza, J.M.; Roach, T.; Stegner, M.; Neuner, G.; García-Plazaola, J.I. Frozen in the dark: Interplay of night-time activity of xanthophyll cycle, xylem attributes, and desiccation tolerance in fern resistance to winter. J. Exp. Bot. 2021, 72, 3168–3184. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Kachroo, P.; Shanklin, J.; Shah, J.; Whittle, E.J.; Klessig, D.F. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 9448–9453. [Google Scholar] [CrossRef]
- Lim, G.-H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty Acid– and Lipid-Mediated Signaling in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Mironov, K.S.; Sidorov, R.A.; Trofimova, M.S.; Bedbenov, V.S.; Tsydendambaev, V.D.; Allakhverdiev, S.I.; Los, D.A. Light-dependent cold-induced fatty acid unsaturation, changes in membrane fluidity, and alterations in gene expression in Synechocystis. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 1352–1359. [Google Scholar] [CrossRef]
- Da Cruz, R.P.; Golombieski, J.I.; Bazana, M.T.; Cabreira, C.; Silveira, T.F.; da Silva, L.P. Alterations in fatty acid composition due to cold exposure at the vegetative stage in rice. Braz. J. Plant Physiol. 2010, 22, 199–207. [Google Scholar] [CrossRef]
- Willemot, C.; Hope, H.J.; Williams, R.J.; Michaud, R. Changes in fatty acid composition of winter wheat during frost hardening. Cryobiology 1977, 14, 87–93. [Google Scholar] [CrossRef]
- Skoczowski, A.; Filek, M.; Dubert, F. The long-term effect of cold on the metabolism of winter wheat seedlings. II. composition of fatty acids of phospholipids. J. Therm. Biol. 1994, 19, 171–176. [Google Scholar] [CrossRef]
- Nejadsadeghi, L.; Maali-Amiri, R.; Zeinali, H.; Ramezanpour, S.; Sadeghzade, B. Membrane fatty acid compositions and cold-induced responses in tetraploid and hexaploid wheats. Mol. Biol. Rep. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta—Lipids Lipid Metab. 1998, 1394, 3–15. [Google Scholar] [CrossRef]
- Dong, C.-J.; Cao, N.; Zhang, Z.-G.; Shang, Q.-M. Characterization of the Fatty Acid Desaturase Genes in Cucumber: Structure, Phylogeny, and Expression Patterns. PLoS ONE 2016, 11, e0149917. [Google Scholar] [CrossRef]
- Gupta, D.B.; Rai, Y.; Gayali, S.; Chakraborty, S.; Chakraborty, N. Plant Organellar Proteomics in Response to Dehydration: Turning Protein Repertoire into Insights. Front. Plant Sci. 2016, 7, 460. [Google Scholar] [CrossRef]
- De Barajas-Lopez, J.D.; Tiwari, A.; Zarza, X.; Shaw, M.W.; Pascual, J.; Punkkinen, M.; Bakowska, J.C.; Munnik, T.; Fujii, H. Early Response to Dehydration 7 Remodels Cell Membrane Lipid Composition during Cold Stress in Arabidopsis. Plant Cell Physiol. 2021, 62, 80–91. [Google Scholar] [CrossRef]
- Quartacci, M.F. Plasma membrane lipids in the resurrection plant Ramonda serbica following dehydration and rehydration. J. Exp. Bot. 2002, 53, 2159–2166. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Vasheka, O.; Puglielli, G.; Crescente, M.F.; Varone, L.; Gratani, L. Anatomical and Morphological Leaf Traits of Three Evergreen Ferns (Polystichum setiferum, Polypodium interjectum and Asplenium scolopendrium). Am. Fern J. 2016, 106, 258–268. [Google Scholar] [CrossRef]
- Schäfer, H.; Rasbach, H. Asplenium × rouyi Viane (A. onopteris L. × A. scolopendrium L.) in the Azores (Aspleniaceae, Pteridophyta). Willdenowia 2000, 30, 219–227. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K. The features of the fatty acid composition of Pyrus L. total lipids are determined by mountain ecosystem conditions. Plant Physiol. Biochem. 2022, 170, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.V.; Voronkov, A.S.; Kumakhova, T.K.; Tsydendambaev, V.D. Distinguishing Features of Fatty Acid Content and Composition in Total Lipids of Malus orientalis Uglitzk. Pericarp. Russ. J. Plant Physiol. 2020, 67, 463–471. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T.; Kumachova, T. Micromorphological and biochemical features of Malus fruit: Malus domestica Borkh. and its parent species—Malus orientalis Uglitzk. Braz. J. Bot. 2020, 43, 21–28. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K.; Kozhevnikova, A.D.; Tsydendambaev, V.D. Polyunsaturated and Very-Long-Chain Fatty Acids Are Involved in the Adaptation of Maloideae (Rosaceae) to Combined Stress in the Mountains. Chem. Biodivers. 2020, 17, e1900588. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.S.; Ivanova, T.V.; Kuznetsova, E.I.; Kumachova, T.K. Adaptations of Malus domestica Borkh. (Rosaceae) Fruits Grown at Different Altitudes. Russ. J. Plant Physiol. 2019, 66, 922–931. [Google Scholar] [CrossRef]
- Lyons, J.M.; Wheaton, T.A.; Pratt, H.K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef]
- Wismer, W.V.; Worthing, W.M.; Yada, R.Y.; Marangoni, A.G. Membrane lipid dynamics and lipid peroxidation in the early stages of low-temperature sweetening in tubers of Solanum tuberosum. Physiol. Plant. 1998, 102, 396–410. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, S.J.; Im, Y.J.; Cho, K.; Chung, G.-C.; Cho, B.-H.; Han, O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in figleaf gourd and cucumber roots. Biochem. Biophys. Res. Commun. 2005, 330, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.J.; Trieu, H.H.; Topp, B.; Russell, D.; Pun, S.; Torrisi, C.; Liu, D. Assessing Fatty Acid Profiles of Macadamia Nuts. HortScience 2019, 54, 633–637. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Hamrouni, I.; Salah, H.B.; Marzouk, B. Effects of water-deficit on lipids of safflower aerial parts. Phytochemistry 2001, 58, 277–280. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Blée, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Jasmonic acid signalling and the plant holobiont. Curr. Opin. Microbiol. 2017, 37, 42–47. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Qin, C.; Wood, T.; Olafsdottir, G.; Welti, R.; Wang, X. The oleate-stimulated phospholipase D, PLD, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 2003, 15, 2285–2295. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X. A Novel Phospholipase D of Arabidopsis That Is Activated by Oleic Acid and Associated with the Plasma Membrane. Plant Physiol. 2001, 127, 1102–1112. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Maiorova, O.V.; Orlova, Y.V.; Kuznetsova, E.I.; Khalilova, L.A.; Myasoedov, N.A.; Balnokin, Y.V.; Tsydendambaev, V.D. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Voronkov, A.S.; Kuznetsova, E.I.; Kumachova, T.K.; Zhirov, V.K.; Tsydendambaev, V.D. Lipid Fatty Acids from the Pericarp of Cydonia oblonga Mill. and Mespilus germanica L. are Involved in Plant Adaptation to Altitudinal Zonality. Dokl. Biochem. Biophys. 2019, 486, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Beike, A.K.; Jaeger, C.; Zink, F.; Decker, E.L.; Reski, R. High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 2014, 33, 245–254. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, A.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Fatty acids of some moss species from Germany. Asian J. Chem. 2011, 23, 5187–5188. [Google Scholar]
- Nekrasov, E.V.; Shelikhan, L.A.; Svetashev, V.I. Fatty acid composition of gametophytes of Matteuccia struthiopteris (L.) Tod. (Onocleaceae, Polypodiophyta). Bot. Pac. 2019, 8, 63–66. [Google Scholar] [CrossRef][Green Version]
- Nekrasov, E.V.; Svetashev, V.I.; Khrapko, O.V.; Vyssotski, M.V. Variability of fatty acid profiles in ferns: Relation to fern taxonomy and seasonal development. Phytochemistry 2019, 162, 47–55. [Google Scholar] [CrossRef]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Murray, P.A.; Gracey, A.Y.; Cossins, A.R. Beyond the Lipid Hypothesis. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks; Springer: New York, NY, USA, 2007; pp. 132–142. [Google Scholar]
- Ferrante, G.; Ohno, Y.; Kates, M. Influence of temperature and growth phase on desaturase activity of the mesophilic yeast Candida lipolytica. Can. J. Biochem. Cell Biol. 1983, 61, 171–177. [Google Scholar] [CrossRef]
- Jones, A.L.; Harwood, J.L.; Lloyd, D. Induction of Δ12-desaturase activity during temperature adaptation in Acanthamoeba castellanii. Biochem. Soc. Trans. 1992, 20, 170S. [Google Scholar] [CrossRef]
- Sayanova, O.V.; Napier, J.A. Eicosapentaenoic acid: Biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 2004, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, A.V.; Shumskaya, M. Very-long-chain fatty acids (VLCFAs) in plant response to stress. Funct. Plant Biol. 2020, 47, 695. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Reynolds, K.B.; Taylor, M.C.; Zhou, X.; Vanhercke, T.; Wood, C.C.; Blanchard, C.L.; Singh, S.P.; Petrie, J.R. Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids. Front. Plant Sci. 2015, 6, 164. [Google Scholar] [CrossRef]
- Gu, H.; Jinkerson, R.E.; Davies, F.K.; Sisson, L.A.; Schneider, P.E.; Posewitz, M.C. Modulation of Medium-Chain Fatty Acid Synthesis in Synechococcus sp. PCC 7002 by Replacing FabH with a Chaetoceros Ketoacyl-ACP Synthase. Front. Plant Sci. 2016, 7, 690. [Google Scholar] [CrossRef]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef]
- Rawsthorne, S. Carbon flux and fatty acid synthesis in plants. Prog. Lipid Res. 2002, 41, 182–196. [Google Scholar] [CrossRef]
- Takami, T.; Shibata, M.; Kobayashi, Y.; Shikanai, T. De Novo Biosynthesis of Fatty Acids Plays Critical Roles in the Response of the Photosynthetic Machinery to Low Temperature in Arabidopsis. Plant Cell Physiol. 2010, 51, 1265–1275. [Google Scholar] [CrossRef]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, L.; Li, W. Glycerolipidome responses to freezing- and chilling-induced injuries: Examples in Arabidopsis and rice. BMC Plant Biol. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-H.; Skinner, D.Z. Production of reactive oxygen species by freezing stress and the protective roles of antioxidant enzymes in plants. J. Agric. Chem. Environ. 2012, 1, 34–40. [Google Scholar] [CrossRef]
- Benson, E.; Bremner, D. Oxidative Stress in the Frozen Plant. In Life in the Frozen State; CRC Press: Boca Raton, FL, USA, 2004; pp. 205–241. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Migdal, C.; Serres, M. Espèces réactives de l’oxygène et stress oxydant. Méd. Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Nobusawa, T.; Okushima, Y.; Nagata, N.; Kojima, M.; Sakakibara, H.; Umeda, M. Synthesis of Very-Long-Chain Fatty Acids in the Epidermis Controls Plant Organ Growth by Restricting Cell Proliferation. PLoS Biol. 2013, 11, e1001531. [Google Scholar] [CrossRef]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Kunst, L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Svetashev, V.I. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).