Genetically Modified Crops in Romania before and after the Accession of the European Union
Abstract
:1. Introduction
2. Materials and Methods
3. Results
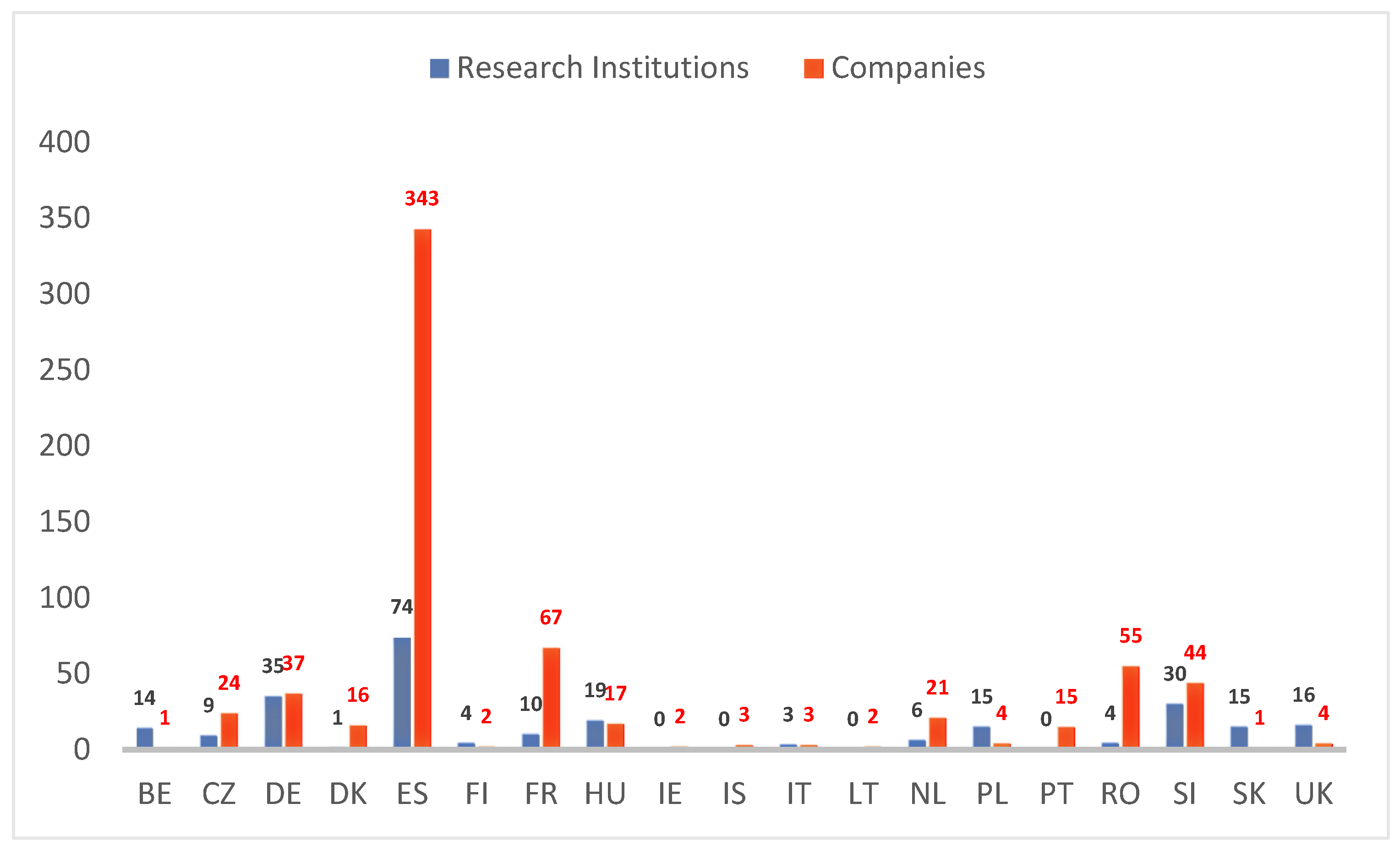
3.1. Modern Biotechnology Research
3.1.1. Containment Facilities for Research
3.1.2. Registering Genetically Modified Microorganisms for Modern Biotechnology Research
3.1.3. Registering Genetically Modified Microorganisms for Modern Biotechnology Research after 2007
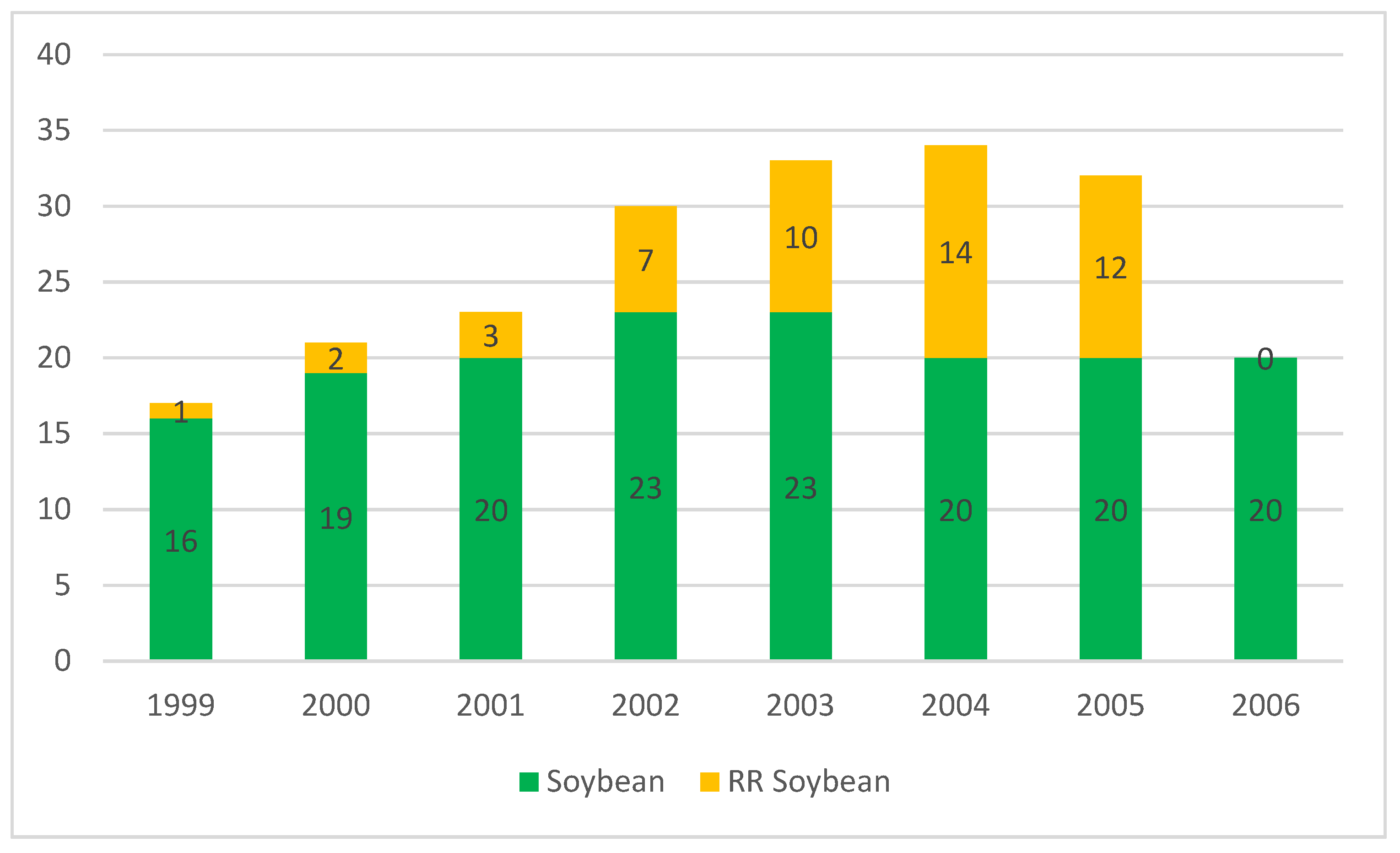
3.2. Genetically Modified Crops for Field Testing
3.2.1. Notification System Regarding GM Crops for Field Testing before the EU Accession
3.2.2. Notification System Regarding GM Crops for Field Testing after the EU Accession
- Genetically modified potato for field testing.
- Genetically modified maize for field testing.
3.3. Genetically Modified Crops for Authorizing the Cultivation for Commercial Purpose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beeckman, D.S.; Rüdelsheim, P. Biosafety and Biosecurity in Containment: A Regulatory Overview. Front. Bioeng. Biotechnol. 2020, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Berg, P. The dual-use conundrum. Science 2012, 337, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, S.O. How extreme is the precautionary principle? NanoEthics 2020, 14, 245–257. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.; Boyer, H.W.; Helling, R.B. Construction of biologically functional bacterial plasmids In Vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240–3244. [Google Scholar] [CrossRef] [Green Version]
- Berg, P.; Baltimore, D.; Brenner, S.; Roblin, R.O., III; Singer, M.F. Asilomar conference on recombinant DNA molecules. Science 1975, 188, 991–994. [Google Scholar] [CrossRef]
- Gilbert, S.G. Precautionary principle. In Information Resources in Toxicology, 3rd ed.; Hakkinen, B.P.J., Kennedy, G., Stoss, F.W., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 489–494. [Google Scholar]
- Chargaff, E. On the dangers of genetic meddling. Science 1976, 192, 938. [Google Scholar] [CrossRef] [Green Version]
- Vasil, I.K. A history of plant biotechnology: From the cell theory of Schleiden and Schwann to biotech crops. Plant Cell Rep. 2008, 27, 1423–1440. [Google Scholar] [CrossRef]
- Ammann, K.; Ammann, B.P. Factors Influencing Public Policy Development in Agricultural Biotechnology. In Handbook of Plant Biotechnology, 1st ed.; Christou, P., Klee, H., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2004; pp. 1005–1017. [Google Scholar]
- Kinderlerer, J. Risk and the law: Scientists do not necessarily like the restrictions and regulations of biotechnology, but they need to accept that lawmakers must reflect the wishes and demands of the majority of the population, who are increasingly aware of the potential risks. EMBO Rep. 2004, 5 (Suppl. S1), S66–S70. [Google Scholar]
- Brans, E.H. The environmental liability directive: Legal background and requirements. In Equivalency Methods for Environmental Liability, 1st ed.; Lipton, J., Özdemiroğlu, E., Chapman, D., Peers, J., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 3–20. [Google Scholar]
- Boja, E.S.; Kinsinger, C.R.; Rodriguez, H.; Srinivas, P. Integration of omics sciences to advance biology and medicine. Clin. Proteom. 2014, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Venter, J.C.; et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 2010, 329, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Voigt, C.A. Synthetic biology 2020–2030: Six commercially-available products that are changing our world. Nat. Commun. 2010, 11, 6379. [Google Scholar] [CrossRef] [PubMed]
- French, K.E. Harnessing synthetic biology for sustainable development. Nat. Sustain. 2019, 2, 250–252. [Google Scholar] [CrossRef]
- Callaway, E. CRISPR plants now subject to tough GM laws in European Union. Nature 2018, 560, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Okoli, A.S.; Blix, T.; Myhr, A.I.; Xu, W.; Xu, X. Sustainable use of CRISPR/Cas in fish aquaculture: The biosafety perspective. Transgenic Res. 2021, 31, 1–21. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2021, 2021, 15. [Google Scholar] [CrossRef]
- Parvathy, S.T. Engineering plants as platforms for production of vaccines. Am. J. Plant Sci. 2020, 11, 707–735. [Google Scholar] [CrossRef]
- Deliberate Release of GMOs into the Environment-Register of Directive 2001/18/EC. Available online: https://webgate.ec.europa.eu/fip/GMO_Registers/ (accessed on 25 February 2022).
- Biosafety Clearing-House. Available online: https://bch.cbd.int/en/ (accessed on 25 February 2022).
- Antofie, M.M.; Baz, A. The Evaluation of Modern Biotechnology Research in Romania (Original in Romanian: Evaluarea Capacității De cercetare în Domeniul Biotehnologiilor Moderne din România); Antofie, M.M., Baz, A., Eds.; Editura Cartea Universitară: București, Romania, 2005; p. 90. ISBN 973-731-137-X. [Google Scholar]
- Antofie, M.M.; Baz, A. (Eds.) Draft National Biosafety Framework for Romania; Europrint Publishing House: Petrosani, Romania, 2006; p. 80. ISBN 973-87565-2-9. [Google Scholar]
- National Official Catalogues on Crop Varieties and Hybrids. Available online: https://istis.ro/ (accessed on 25 February 2022).
- Calin, A.; Cucu, N.; Tessio, C. Stability of a transgene in potato depends on endogenous plant tissue factors. Biotechnol. Biotechnol. Equip. 1996, 10, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Tenea, G.; Cucu, N. The influence of T-DNA copy numbers on gene expression in primary transformants Atropa belladonna plants. Rom. Biotechnol. Lett. 2006, 11, 2661–2667. [Google Scholar]
- Romania, Interim National Report on the Cartagena Protocol on Biosafety. 2015. Available online: https://www.cbd.int/doc/world/ro/ro-nr-cpbi-en.pdf (accessed on 20 February 2022).
- Antofie, M.M.; Sand, C.; Brezeanu, A.; Doroftei, E. Key elements related to GMOs and novel food. Ann. Rom. Soc. Cell Biol. 2010, 15, 148–154. [Google Scholar]
- Wessels, S.; Axelsson, L.; Hansen, E.B.; De Vuyst, L.; Laulund, S.; Lähteenmäki, L.; Lindgren, S.; Mollet, B.; Salminen, S.; von Wright, A. The lactic acid bacteria, the food chain, and their regulation. Trends Food Sci. Technol. 2004, 15, 498–505. [Google Scholar] [CrossRef]
- Antofie, M.M.; Sand, C. Insights into the biotech policy and Europeans tendency. Res. J. Agric. Sci. 2010, 42, 376–381. [Google Scholar]
- The Romanian Microorganisms Register. Available online: https://www.anpm.ro/registre?p_p_id=122_INSTANCE_LBVGi1NmYGK8&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-2&p_p_col_count=2&p_r_p_564233524_resetCur=true&p_r_p_564233524_categoryId=66964840 (accessed on 14 February 2022).
- Clive, J. A global overview of biotech (GM) crops: Adoption, impact and future prospects. GM Crops 2010, 1, 8–12. [Google Scholar] [CrossRef]
- Park, J.; McFarlane, I.; Phipps, R.; Ceddia, G. The impact of the EU regulatory constraint of transgenic crops on farm income. New Biotechnol. 2011, 28, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Paarlberg, R.L. The real threat to GM crops in poor countries: Consumer and policy resistance to GM foods in rich countries. Food Policy 2002, 27, 247–250. [Google Scholar] [CrossRef]
- Maxim, P.; Belc, N. Biosafety framework of transgenic organisms and related issues –reviews. Rom. Biotechnol. Lett. 2008, 13, 3539–3550. [Google Scholar]
- Antofie, A.M. Potato resistance to cyst nematodes-peculiarities for Romania. Oltenia. Stud. Şi Comunicări. Ştiinţele Nat. 2016, 32, 181–186. [Google Scholar]
- Antofie, M.M.; Sava Sand, C. Crops varieties under conservation: Study case cultivated Triticum ssp. Sci. Pap. Ser.-Manag. Econ. Eng. Agric. Rural. Dev. 2018, 18, 61–66. [Google Scholar]
- Maxim, A.; Străjeru, S.; Albu, C.; Sandor, M.; Mihalescu, L.; Pauliuc, S.E. Conservation of vegetable genetic diversity in Transylvania-Romania. Sci. Rep. 2020, 10, 18416. [Google Scholar] [CrossRef]
- Otiman, I.P.; Badea, E.M.; Buzdugan, L. Roundup Ready soybean, a Romanian story. Bull. UASVM Anim. Sci. Biotechnol. 2008, 65, 352–357. [Google Scholar]
- Zambryski, P.; Joos, H.; Genetello, C.; Leemans, J.; Van Montagu, M.; Schell, J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983, 2, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.C.; Klein, T.M.; Wolf, E.D.; Allen, N. Delivery of substances into cells and tissues using a particle bombardment process. Part. Sci. Technol. 1987, 5, 27–37. [Google Scholar] [CrossRef]
- Scorza, R.; Callahan, A.; Dardick, C.; Ravelonandro, M.; Polak, J.; Malinowski, T.; Zagrai, I.; Cambra, M.; Kamenova, I. Genetic engineering of Plum pox virus resistance: ‘HoneySweet’plum—From concept to product. Plant Cell Tiss Org. 2013, 115, 1–12. [Google Scholar] [CrossRef]
- Scorza, R.; Hily, J.M.; Callahan, A.; Malinowski, T.; Cambra, M.; Capote, N.; Zagrai, I.; Damsteegt, V.; Briard, P.; Ravelonandro, M. Deregulation of Plum Pox resistant transgenic plum‘HoneySweet’. In Proceedings of the International Symposium on Biotechnology of Temperate Fruit Crops and Tropical Species: Daytona Beach, Florida, FL, USA, 10–14 October 2005; Volume 738, pp. 669–673. [Google Scholar]
- Scorza, R.; Ravelonandro, M.; Callahan, A.M.; Cordts, J.M.; Fuchs, M.; Dunez, J.; Gonsalves, D. Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep. 1994, 14, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Scorza, R.; Ravelonandro, M.; Callahan, A.; Zagrai, I.; Polak, J.; Malinowski, T.; Cambra, M.; Levy, L.; Damsteegt, V.; Krška, B.; et al. ‘HoneySweet’(C5), the first genetically engineered plum pox virus–resistant plum (Prunus domestica L.) cultivar. HortScience 2016, 51, 601–603. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Palmqvist, S.; Olsson, H.; Borén, M.; Ahlandsberg, S.; Jansson, C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 2003, 15, 2076–2092. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Zhang, F.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Dănilă, D.M.; Gaceu, L.; Thierheimer, W.W.; Ola, D.; Ţane, N.; Hodirnau, M. Management of the potato crops for minimizing environmental and human health impacts. Env. Eng Manag. J. 2010, 9, 1685–1691. [Google Scholar] [CrossRef]
- Perju, N.; Chiran, A.; Ungureanu, G. The economic aspects of potato produce in Romania in the context of European integration and globalization. Lucr. Științifice Univ. Stiinte Agric. Și Med. Vet. Ion Ionescu Brad Iași Ser. Agron. 2009, 52, 538–545. [Google Scholar]
- Jones, H.D. Future of breeding by genome editing is in the hands of regulators. GM Crops Food 2015, 6, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Podevin, N.; Devos, Y.; Davies, H.V.; Nielsen, K.M. Transgenic or not? No simple answer! New biotechnology-based plant breeding techniques and the regulatory landscape. EMBO Rep. 2012, 13, 1057–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decaestecker, W.; Buono, R.A.; Pfeiffer, M.L.; Vangheluwe, N.; Jourquin, J.; Karimi, M.; Van Isterdael, G.; Beeckman, T.; Nowack, M.K.; Jacobs, T.B. CRISPR-TSKO: A technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell 2019, 31, 2868–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Zhong, Y.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Exploration of susceptible genes with clustered regularly interspaced short palindromic repeats–tissue-specific knockout (CRISPR-TSKO) to enhance host resistance. Crit. Rev. Plant Sci. 2020, 39, 387–417. [Google Scholar] [CrossRef]
- Clive, J. Global Status of Commercialized Biotech/GM Crops: 2007; No. 37; ISAAA Brief: Ithaca, NY, USA, 2007. [Google Scholar]
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) crop use 1996–2016: Impacts on pesticide use and carbon emissions. GM Crops Food 2018, 9, 109–139. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.; Gul, N. Impact of Genetically Modified Crops on Environment. In Environmental Processes and Management; Singh, R.M., Shukla, P., Singh, P., Eds.; Publishing House Springer Nature: Cham, Switzerland, 2020; pp. 237–248. [Google Scholar]
- Khush, G.S. Genetically modified crops: The fastest adopted crop technology in the history of modern agriculture. Agric. Food Secur. 2012, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Gereffi, G. Capitalism, development and global commodity chains. In Capitalism and Development; Sklair, L., Ed.; Publishing House Routledge: London, UK, 2002; pp. 225–245. [Google Scholar]
- Griffith, F. The significance of pneumococcal types. Epidemiol. Infect. 1928, 27, 113–159. [Google Scholar] [CrossRef] [Green Version]
- Avery, O.T.; MacLeod, C.M.; McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef]
- Haberlandt, G. Culturversuche mit isolierten Pflanzenzellen. Sitzungsberichte 1902, 111, 69–91. [Google Scholar]
- Shull, G.H. The genotypes of maize. Am. Nat. 1911, 45, 234–252. [Google Scholar] [CrossRef]
- Borlaug, N.E. Contributions of conventional plant breeding to food production. Science 1983, 219, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Kozak, M.; Bornmann, L.; Leydesdorff, L. How have the Eastern European countries of the former Warsaw Pact developed since 1990? A bibliometric study. Scientometrics 2015, 102, 1101–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ion, G.; Iucu, R. Does research influence educational policy? The perspective of researchers and policy-makers in Romania. In The European Higher Education Area; Curaj, A., Matei, L., Pricopie, R., Salmi, J., Scott, P., Eds.; Publishing House Springer: Cham, Switzerland, 2015; pp. 865–880. [Google Scholar]
- European Research Infrastructures System. Available online: https://eeris.eu/ (accessed on 14 February 2022).
- Taiex, Report Activity 2006. 2006. Available online: https://www.ab.gov.tr/files/TAIEX/TAIEX2006act.report/taiexactivityreport_2006.pdf (accessed on 14 February 2022).
- European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/ENGLabs (accessed on 25 February 2022).
- McMichael, P. Biotechnology and Food Security. In Global Tensions: Challenges and Opportunities in the World Economy; Beneria, L., Bisnath, S., Eds.; Routledge Publishing House: New York, NY, USA, 2004; pp. 113–123. [Google Scholar]
- Clive, J. Global Status of Commercialized Biotech/GM Crops: 2008; No. 39; ISAAA Brief: Ithaca, NY, USA, 2008. [Google Scholar]
- Haslberger, A. GMO contamination of seeds. Nat. Biotechnol. 2001, 19, 613. [Google Scholar] [CrossRef] [PubMed]
- Rostoks, N.; Grantiņa-Ieviņa, L.; Ieviņa, B.; Evelone, V.; Valciņa, O.; Aleksejeva, I. Genetically modified seeds and plant propagating material in Europe: Potential routes of entrance and current status. Heliyon 2019, 5, e01242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antofie, M.M. Red lists for crop species and their role in adaptation strategies. In On Farm Conservation of Neglected and Underutilized Species: Status, Trends and Novel Approaches to Cope with Climate Change, Proceedings of an International Conference, Frankfurt, Germany, 14–16 June 2011; Padulosi, S., Bergamini, N., Lawrence, T., Eds.; Publishing House Bioversity International: Rome, Italy, 2011; pp. 143–169. [Google Scholar]
- The Suceava Gene Bank. Available online: https://svgenebank.ro/index_en.htm (accessed on 1 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antofie, M.-M.; Sand-Sava, C. Genetically Modified Crops in Romania before and after the Accession of the European Union. Agriculture 2022, 12, 458. https://doi.org/10.3390/agriculture12040458
Antofie M-M, Sand-Sava C. Genetically Modified Crops in Romania before and after the Accession of the European Union. Agriculture. 2022; 12(4):458. https://doi.org/10.3390/agriculture12040458
Chicago/Turabian StyleAntofie, Maria-Mihaela, and Camelia Sand-Sava. 2022. "Genetically Modified Crops in Romania before and after the Accession of the European Union" Agriculture 12, no. 4: 458. https://doi.org/10.3390/agriculture12040458
APA StyleAntofie, M.-M., & Sand-Sava, C. (2022). Genetically Modified Crops in Romania before and after the Accession of the European Union. Agriculture, 12(4), 458. https://doi.org/10.3390/agriculture12040458






