Effects of Endogenous Non-Starch Nutrients in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties and In Vitro Digestibility of Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Removal of Nutrients from Acorn Flour
2.2.1. Preparation of Acorn Starch
2.2.2. Preparation of Degreased Acorn Flour
2.2.3. Preparation of Deproteinized Acorn Flour
2.2.4. Preparation of De-β-Glucan Acorn Flour
2.3. Determination of the Proximate Nutritional Components and Amylose Content
2.4. Determination of Molecular Weight of Starch
2.5. Chain Length Distribution (CLD) of Amylopectin
2.6. Scanning Electron Microscopy (SEM)
2.7. The Particle Size Distribution
2.8. X-ray Diffraction Pattern (XRD) Analysis
2.9. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.10. Thermodynamic Characteristic Analysis
2.11. In Vitro Digestibility
2.12. Statistical Analysis
3. Results
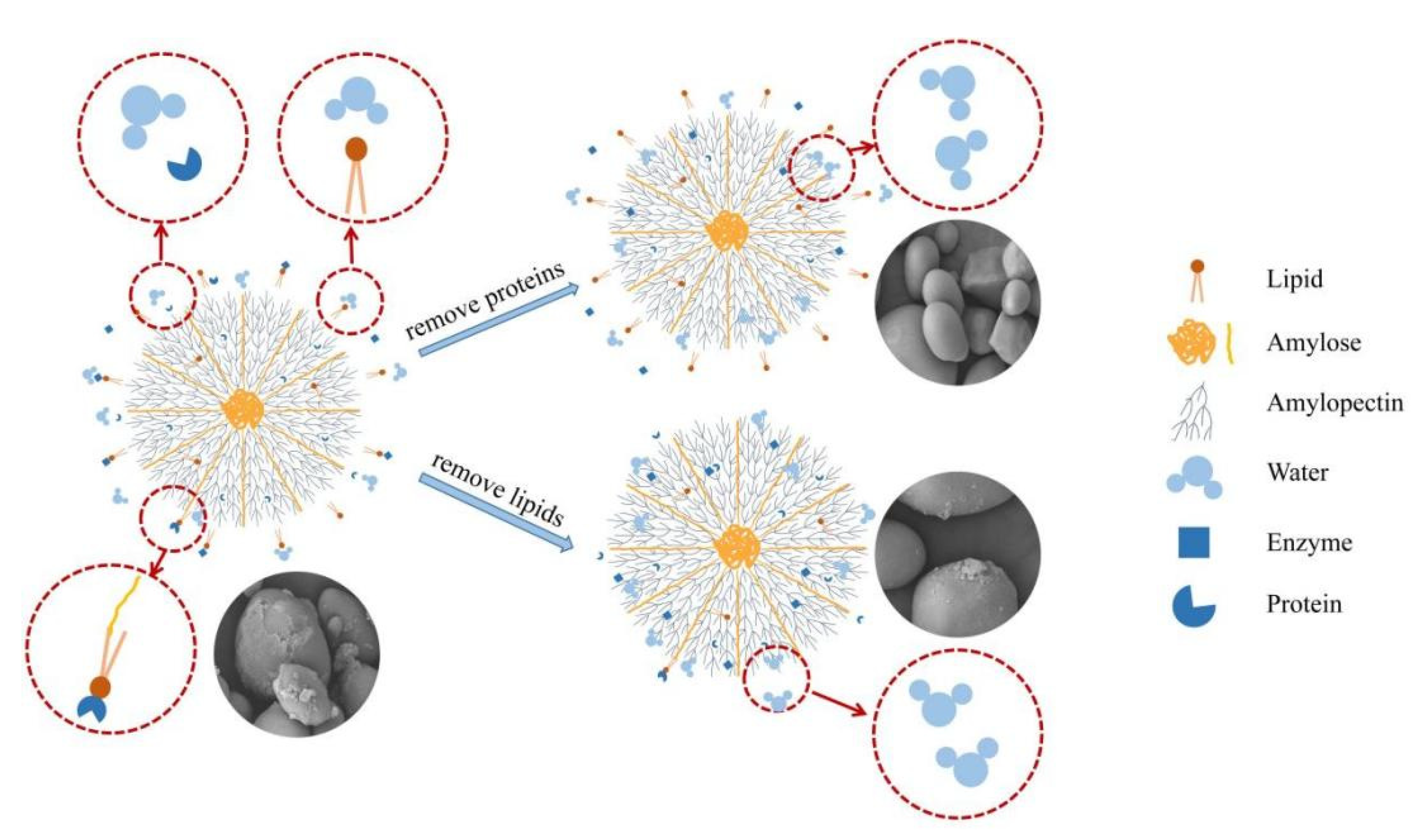
3.1. Proximate Nutritional Components and Starch Molecular Structure
3.2. Proximate Constituents of AF after Removing Non-Starch Nutrients
3.3. Changes of Microstructure and Particle Size
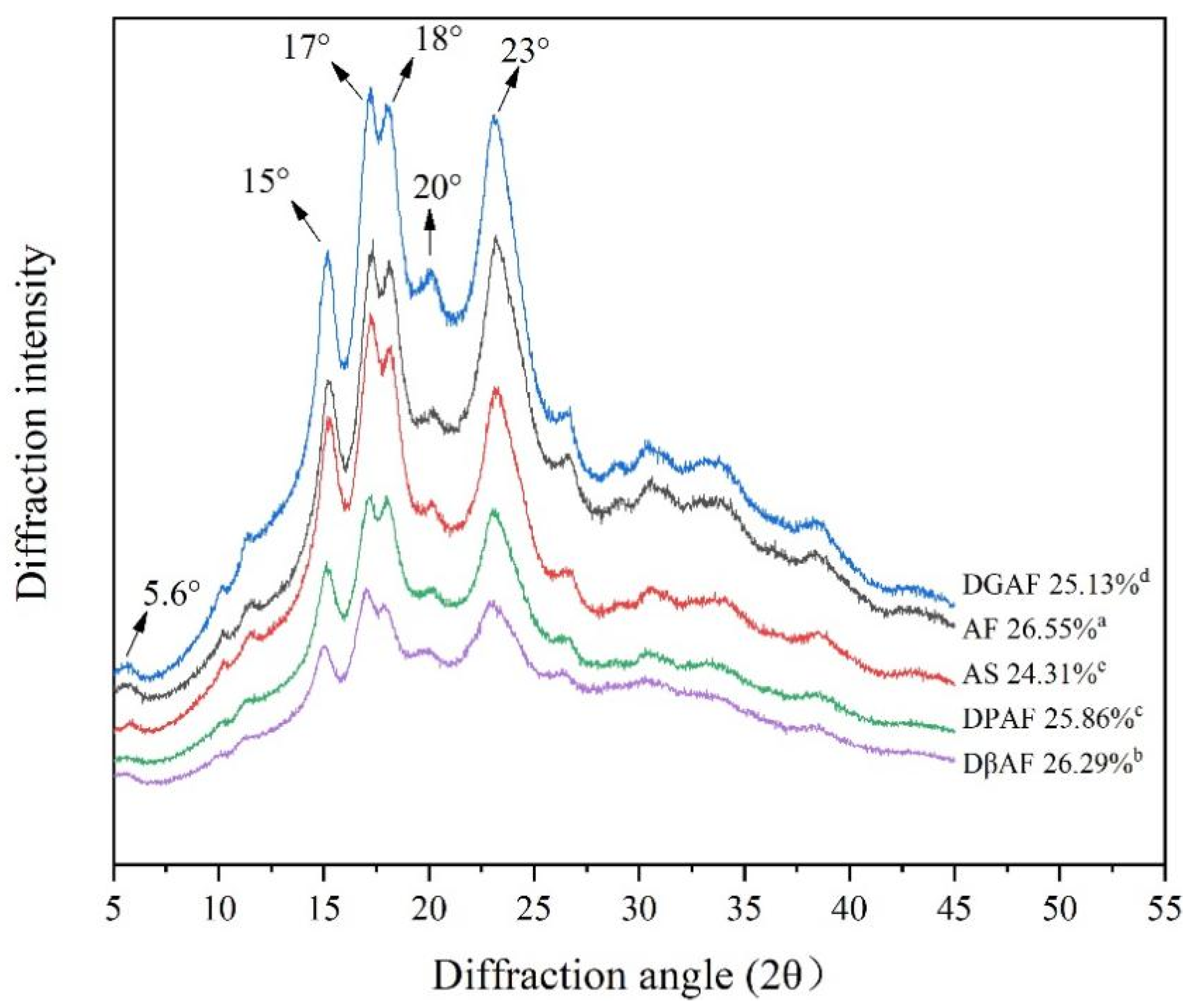
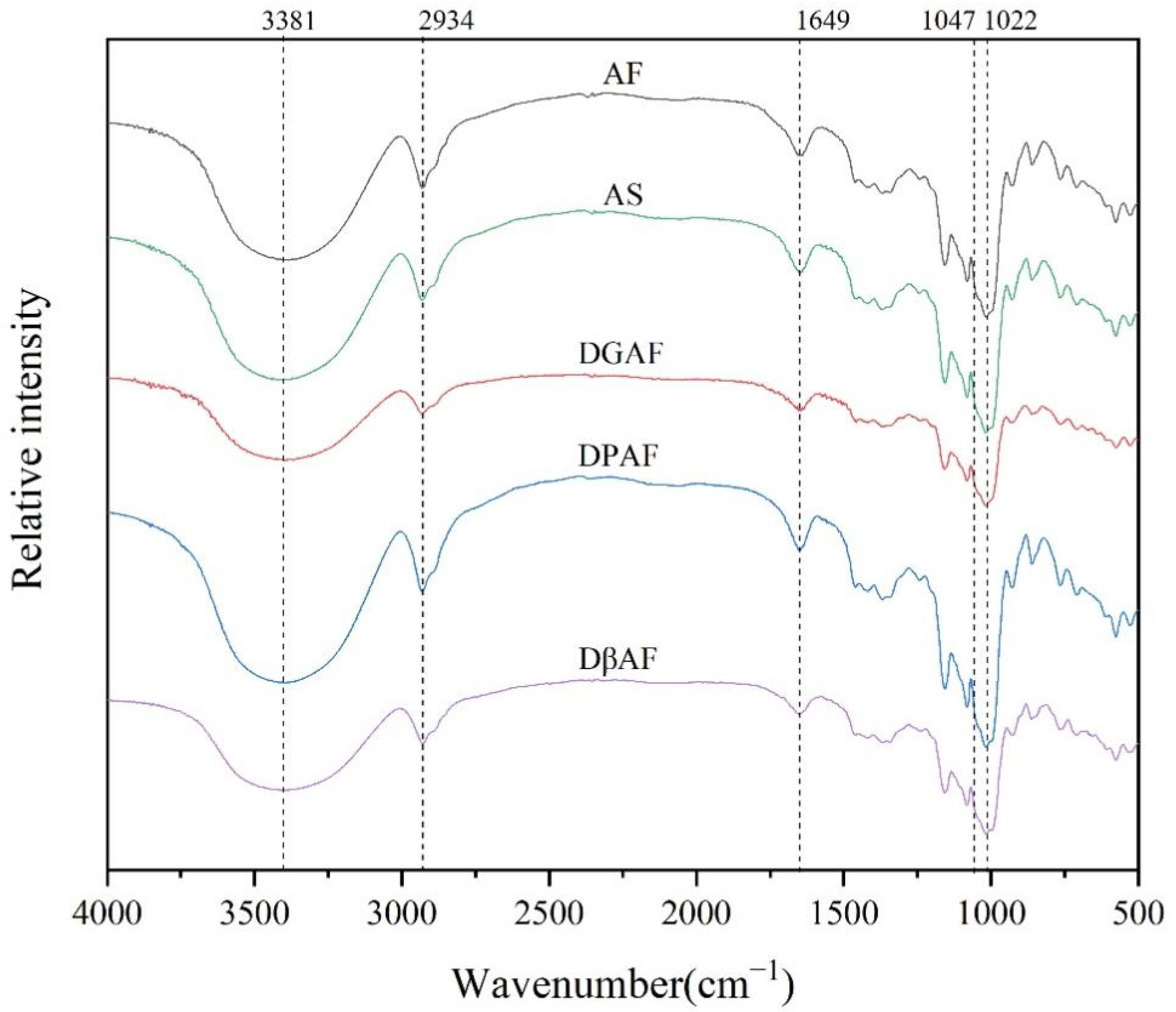
3.4. Long- and Short-Range Molecular Order
3.5. Thermal Properties
3.6. In Vitro Digestibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevenson, D.G.; Jane, J.; Inglett, G.E. Physicochemical properties of pin oak (Quercus palustris Muenchh.) acorn starch. Starch-Stärke 2006, 58, 553–560. [Google Scholar] [CrossRef]
- Correia, P.; Leitão, A.; Beirão-da-Costa, M. Effect of drying temperatures on chemical and morphological properties of dried acorns flours. Int. J. Food Sci. Technol. 2009, 44, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, S.; Sobajic, S.; Rakic, S.; Tomic, A.; Kukic, J. Investigation of kernel oils of Quercus robur and Quercus cerris. Chem. Nat. Compd. 2004, 40, 420–422. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Liu, M.; Zhang, Y.; Cao, S. Nutrient composition and starch characteristics of Quercus glandulifera Bl. seeds from China. Food Chem. 2015, 185, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Molavi, H.; Razavi, S.; Farhoosh, R. Impact of hydrothermal modifications on the physicochemical, morphology, crystallinity, pasting and thermal properties of acorn starch. Food Chem. 2018, 245, 385–393. [Google Scholar] [CrossRef]
- Correia, P.R.; Beirão-da-Costa, M.L. Chestnut and acorn starch properties affected by isolation methods. Starch-Stärke 2010, 62, 421–428. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Tang, M.; Wang, L.; Cheng, X.; Wu, Y.; Ouyang, J. Non-starch constituents influence the in vitro digestibility of naked oat (Avena nuda L.) starch. Food Chem. 2019, 297, 124953. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, A.; Zhao, S.; Liu, Q.; Fu, X.; Jin, Z. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocoll. 2021, 117, 106698. [Google Scholar] [CrossRef]
- Zou, W.; Dhital, S.; Wu, P.; Gidley, M.J.; Fox, G.P.; Gilbert, R.G. The adsorption of α-amylase on barley proteins affects the in vitro digestion of starch in barley flour. Food Chem. 2018, 241, 493–501. [Google Scholar] [CrossRef]
- Zou, W.; Sissons, M.; Gidley, M.J.; Gilbert, R.G.; Warren, F.J. Combined techniques for characterising pasta structure reveals how the gluten network slows enzymic digestion rate. Food Chem. 2015, 188, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Cao, P.; Wu, P.; Yu, W.; Gilbert, R.G.; Li, E. Effects of endogenous proteins on rice digestion during small intestine (in vitro) digestion. Food Chem. 2020, 344, 128687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Maladen, M.; Campanella, O.; Hamaker, B.R. Free fatty acids electronically bridge the self-assembly of a three-component nanocomplex consisting of amylose, protein, and free fatty acids. J. Agric. Food Chem. 2010, 58, 9164–9170. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J. The glycemic index as a measure of health and nutritional quality: An Australian perspective. Cereal Food World 2007, 52, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Debet, M.R.; Gidley, M.J. Three classes of starch granule swelling: Influence of surface proteins and lipids. Carbohyd. Polym. 2006, 64, 452–465. [Google Scholar] [CrossRef]
- Hu, P.; Fan, X.; Lin, L.; Wang, J.; Zhang, L.; Wei, C. Effects of surface proteins and lipids on molecular structure, thermal properties, and enzymatic hydrolysis of rice starch. Food Sci. Technol. (Camp.) 2017, 38, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jiao, A.; Liu, Q.; Ren, X.; Zhu, K.; Jin, Z. The effects of removing endogenous proteins, β-glucan and lipids on the surface microstructure, water migration and glucose diffusion in vitro of starch in highland barley flour. Food Hydrocoll. 2021, 127, 107457. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, K.; Zhang, G. Impact of native form oat β-glucan on starch digestion and postprandial glycemia. J. Cereal Sci. 2017, 73, 84–90. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Vilaplana, F.; Gilbert, R.G. Characterization of branched polysaccharides using multiple-detection size separation techniques. J. Sep. Sci. 2010, 33, 3537–3554. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Xu, J.; Wang, K.; Zhou, S.; Liu, X. Structure and digestion of hybrid indica rice starch and its biosynthesis. Int. J. Biol. Macromol. 2016, 93, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Jan, K.; Singh, S.; Saxena, D.C. Physicochemical, pasting, rheological, thermal and morphological properties of horse chestnut starch. J. Food Sci. Technol. 2015, 52, 5651–5660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Liu, G.; Ouyang, J. Inhibitory effect of chestnut (Castanea mollissima Blume) inner skin extract on the activity of α-amylase, α-glucosidase, dipeptidyl peptidase IV and in vitro digestibility of starches. Food Chem. 2020, 324, 126847. [Google Scholar] [CrossRef]
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical properties and starch digestibility of whole grain sorghums, millet, quinoa and amaranth flours, as affected by starch and non-starch constituents. Food Chem. 2017, 233, 1–10. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Curvelo, A.A.S.; Corrêa, A.C.; Marconcini, J.M.; Glenn, G.M.; Mattoso, L.H.C. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid). Ind. Crops Prod. 2012, 37, 61–68. [Google Scholar] [CrossRef]
- Gui, Y.; Zou, F.; Zhu, Y.; Li, J.; Wang, N.; Guo, L.; Cui, B. The structural, thermal, pasting and gel properties of the mixtures of enzyme-treated potato protein and potato starch. LWT-Food Sci. Technol. 2022, 154, 112882. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef]
- Yoo, S.H.; Jane, J.L. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angle laser-light scattering and refractive index detectors. Carbohyd. Polym. 2002, 49, 307–314. [Google Scholar] [CrossRef]
- Hizukuri, S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohyd. Res. 1986, 147, 342–347. [Google Scholar] [CrossRef]
- Källman, A.; Vamadevan, V.; Bertoft, E.; Koch, K.; Seetharaman, K.; Åman, P.; Andersson, R. Thermal properties of barley starch and its relation to starch characteristics. Int. J. Biol. Macromol. 2015, 81, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Annor, G.A.; Marcone, M.; Bertoft, E.; Seetharaman, K. In vitro starch digestibility and expected glycemic index of kodo millet (Paspalum scrobiculatum) as affected by starch-protein-lipid interactions. Cereal Chem. 2013, 90, 211–217. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Shahidi, F.; Perera, C.; Jane, J. Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem. 2001, 74, 189–202. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Z.; Zhu, L.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Buckwheat digestibility affected by the chemical and structural features of its main components. Food Hydrocoll. 2019, 96, 596–603. [Google Scholar] [CrossRef]
- Chen, W.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effect of protein-fatty acid interactions on the formation of starch-lipid-protein complexes. Food Chem. 2021, 364, 130390. [Google Scholar] [CrossRef]
- Li, W.; Gao, J.; Wu, G.; Zheng, J.; Ouyang, S.; Luo, Q.; Zhang, G. Physicochemical and structural properties of A- and B-starch isolated from normal and waxy wheat: Effects of lipids removal. Food Hydrocoll. 2016, 60, 364–373. [Google Scholar] [CrossRef]
- Qin, R.; Yu, J.; Li, Y.; Copeland, L.; Wang, S.; Wang, S. Structural changes of starch-lipid complexes during postprocessing and their effect on in vitro enzymatic digestibility. J. Agric. Food Chem. 2019, 67, 1530–1536. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying amylose content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wu, Y.; Liu, L.; Ouyang, J. Physicochemical properties and in vitro digestibility of starch from naturally air-dried chestnut. Int. J. Biol. Macromol. 2018, 117, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The impact of heatmoisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohyd. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. In-vitro digestibility, rheology, structure, and functionality of RS3 from oat starch. Food Chem. 2016, 212, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Bae, I.Y.; Lee, H.G. In vitro starch digestion and cake quality: Impact of the ratio of soluble and insoluble dietary fiber. Int. J. Biol. Macromol. 2014, 63, 98–103. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, C.; Wang, S. Drying methods used in starch isolation change properties of C-type chestnut (Castanea mollissima) starches. LWT-Food Sci. Technol. 2016, 73, 663–669. [Google Scholar] [CrossRef]
- Htoon, A.; Shrestha, A.K.; Flanagan, B.M.; Lopez-Rubio, A.; Bird, A.R.; Gilbert, E.P.; Gidley, M.J. Effects of processing high amylose maize starches under controlled conditions on structural organisation and amylase digestibility. Carbohyd. Polym. 2009, 75, 236–245. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Zhang, B.; Fu, X.; Jane, J.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef]

| Sample | Total Starch (g/100 g) | Amylose (% of Starch) | Lipid (g/100 g) | Protein (g/100 g) | β-Glucan (g/100 g) |
|---|---|---|---|---|---|
| AF | 58.3 ± 2.9 d | 33.1 ± 2.3 a | 5.9 ± 0.1 a | 8.4 ± 0.2 ab | 6.8 ± 0.5 a |
| AS | 87.6 ± 2.2 a | 31.4 ± 2.1 ab | 0.9 ± 0.4 c | 1.6 ± 0.3 c | 2.4 ± 0.5 b |
| DGAF | 64.2 ± 3.1 bc | 32.6 ± 1.1 a | 1.1 ± 0.3 c | 10.2 ± 1.2 a | 6.4 ± 0.6 a |
| DPAF | 64.9 ± 1.9 c | 33.7 ± 3.2 a | 3.9 ± 0.2 b | 2.0 ± 0.5 c | 6.7 ± 0.9 a |
| DβAF | 67.7 ± 1.3 b | 29.8 ± 3.7 b | 6.5 ± 0.6 a | 10.7 ± 1.7 a | 1.1 ± 0.8 c |
| Sample | ˂5 μm | 5–10 μm | 10–25 μm | ˃25 μm | IR Ratio of 1047 cm−1/1022 cm−1 |
|---|---|---|---|---|---|
| AF | 10.18 | 36.87 | 45.38 | 7.60 | 1.025 ± 0.023 d |
| AS | 10.56 | 34.50 | 40.84 | 14.14 | 1.049 ± 0.035 c |
| DGAF | 12.21 | 42.58 | 38.91 | 6.32 | 1.073 ± 0.004 b |
| DPAF | 10.89 | 39.33 | 35.45 | 14.32 | 1.077 ± 0.022 ab |
| DβAF | 9.27 | 34.36 | 40.18 | 16.20 | 1.081 ± 0.006 a |
| Sample | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|---|---|---|---|
| AF | 66.3 ± 0.1 a | 72.7 ± 0.1 b | 81.6 ± 0.3 a | 3.3 ± 0.2 b | 4.9 ± 1.6 d | 25.9 ± 1.5 a | 69.2 ± 1.2 a |
| AS | 60.1 ± 0.4 d | 70.5 ± 0.2 a | 79.3 ± 0.2 b | 4.3 ± 0.2 a | 17.2 ± 2.0 a | 23.8 ± 2.1 b | 59.0 ± 2.4 c |
| DGAF | 65.1 ± 1.1 ab | 72.1 ± 0.3 b | 79.2 ± 0.1 b | 3.5 ± 0.3 ab | 11.9 ± 1.6 b | 28.4 ± 2.8 a | 59.7 ± 1.4 c |
| DPAF | 62.8 ± 0.4 c | 72.8 ± 0.9 b | 80.7 ± 0.4 a | 3.7 ± 0.1 ab | 9.2 ± 2.3 bc | 26.7 ± 1.5 ab | 64.0 ± 1.6 b |
| DβAF | 63.8 ± 0.2 bc | 72.0 ± 0.5 b | 80.8 ± 0.3 a | 3.3 ± 0.2 b | 6.5 ± 1.9 cd | 26.6 ± 2.6 ab | 66.9 ± 0.9 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Ding, T.; Wu, Y.; Ouyang, J. Effects of Endogenous Non-Starch Nutrients in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties and In Vitro Digestibility of Starch. Foods 2022, 11, 825. https://doi.org/10.3390/foods11060825
He M, Ding T, Wu Y, Ouyang J. Effects of Endogenous Non-Starch Nutrients in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties and In Vitro Digestibility of Starch. Foods. 2022; 11(6):825. https://doi.org/10.3390/foods11060825
Chicago/Turabian StyleHe, Mohe, Tianyi Ding, Yanwen Wu, and Jie Ouyang. 2022. "Effects of Endogenous Non-Starch Nutrients in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties and In Vitro Digestibility of Starch" Foods 11, no. 6: 825. https://doi.org/10.3390/foods11060825
APA StyleHe, M., Ding, T., Wu, Y., & Ouyang, J. (2022). Effects of Endogenous Non-Starch Nutrients in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties and In Vitro Digestibility of Starch. Foods, 11(6), 825. https://doi.org/10.3390/foods11060825