Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region
Abstract
1. Introduction
2. Materials and Methods
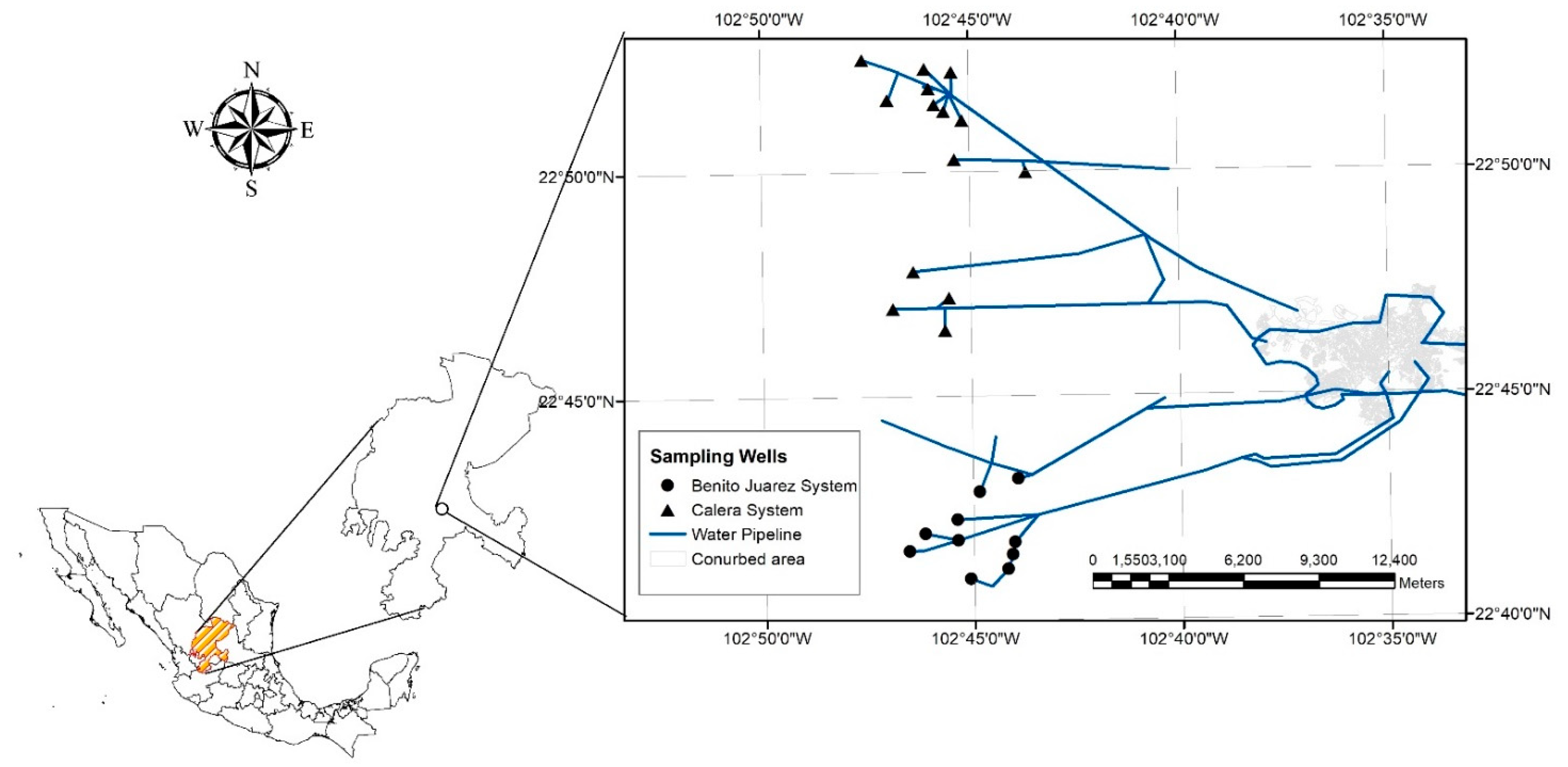
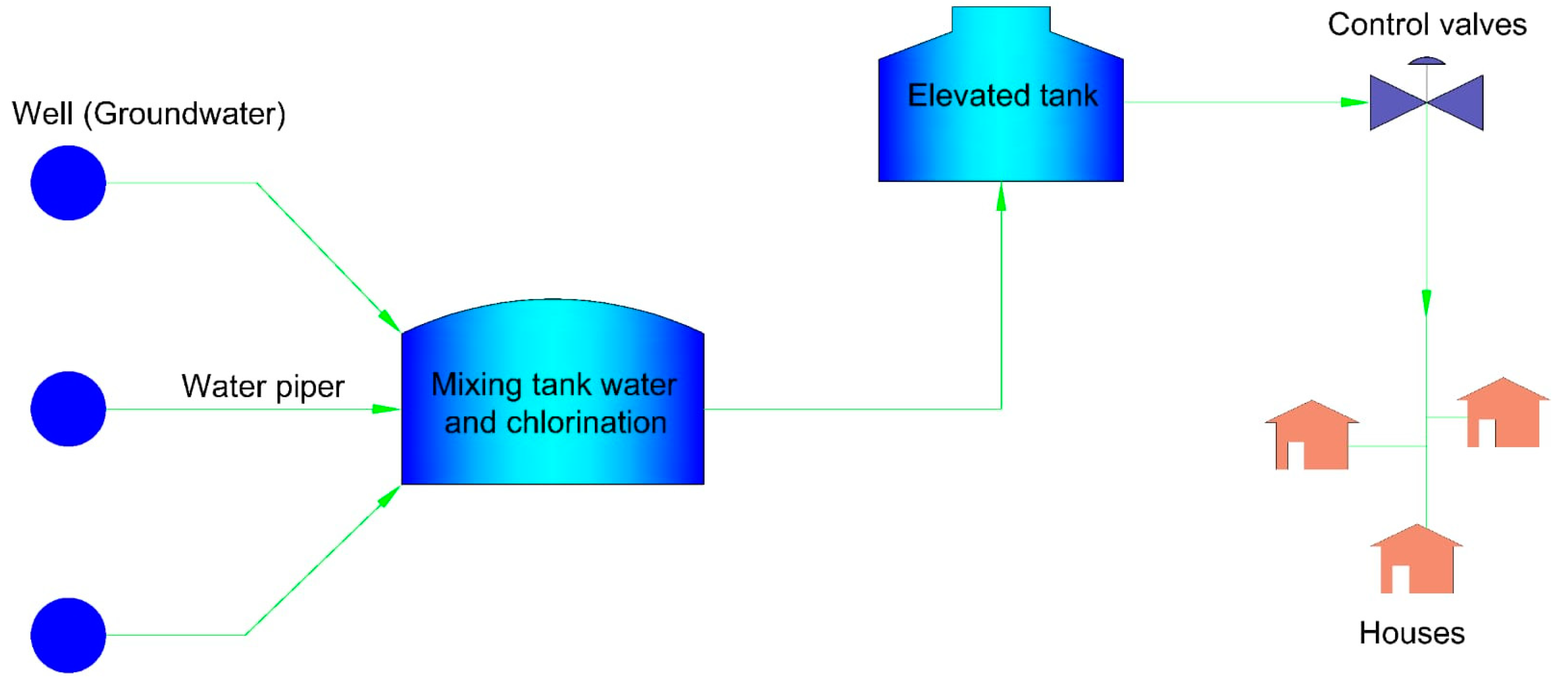
2.1. Study Area
2.2. Drink Water Samples and Saturation Index
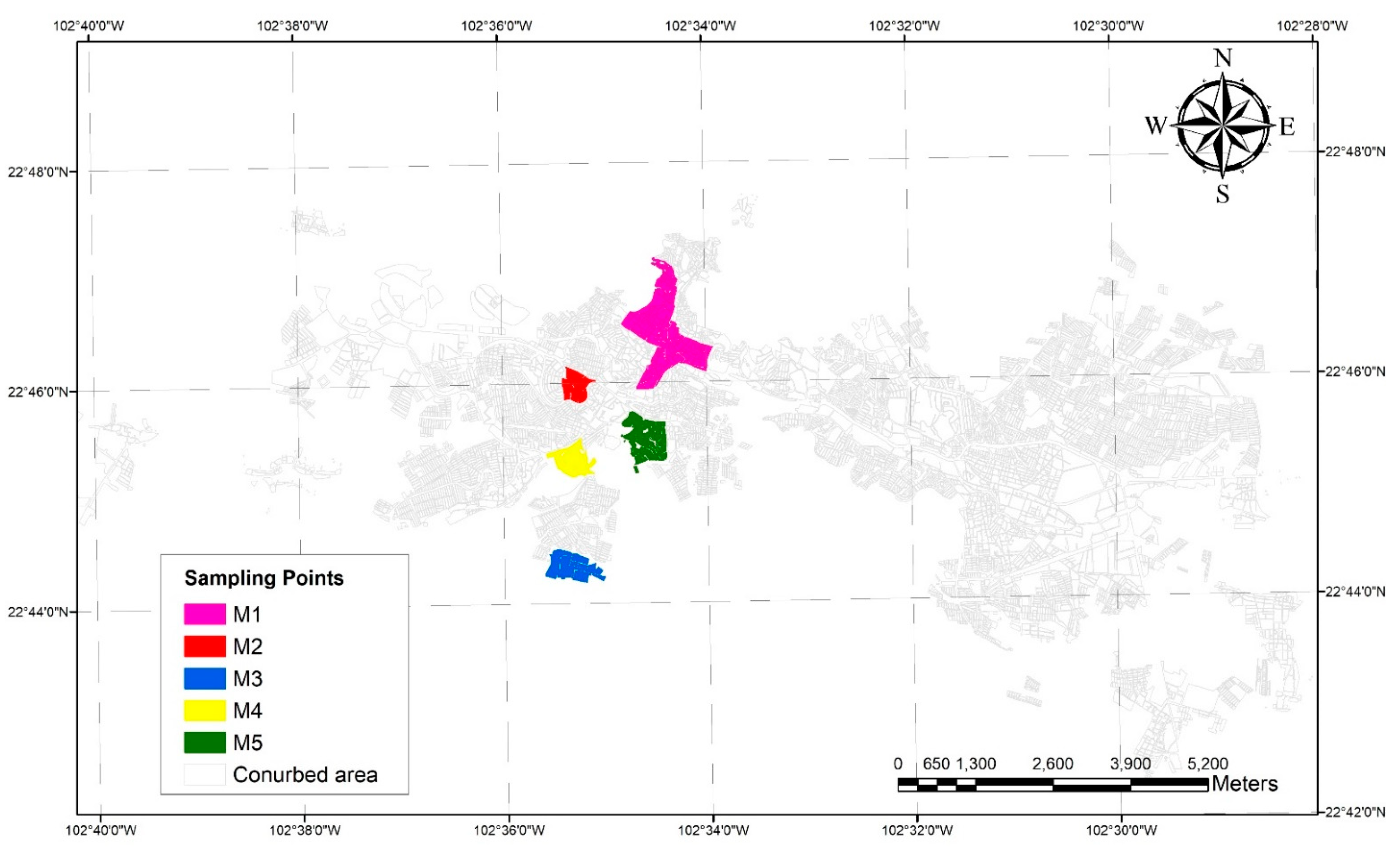
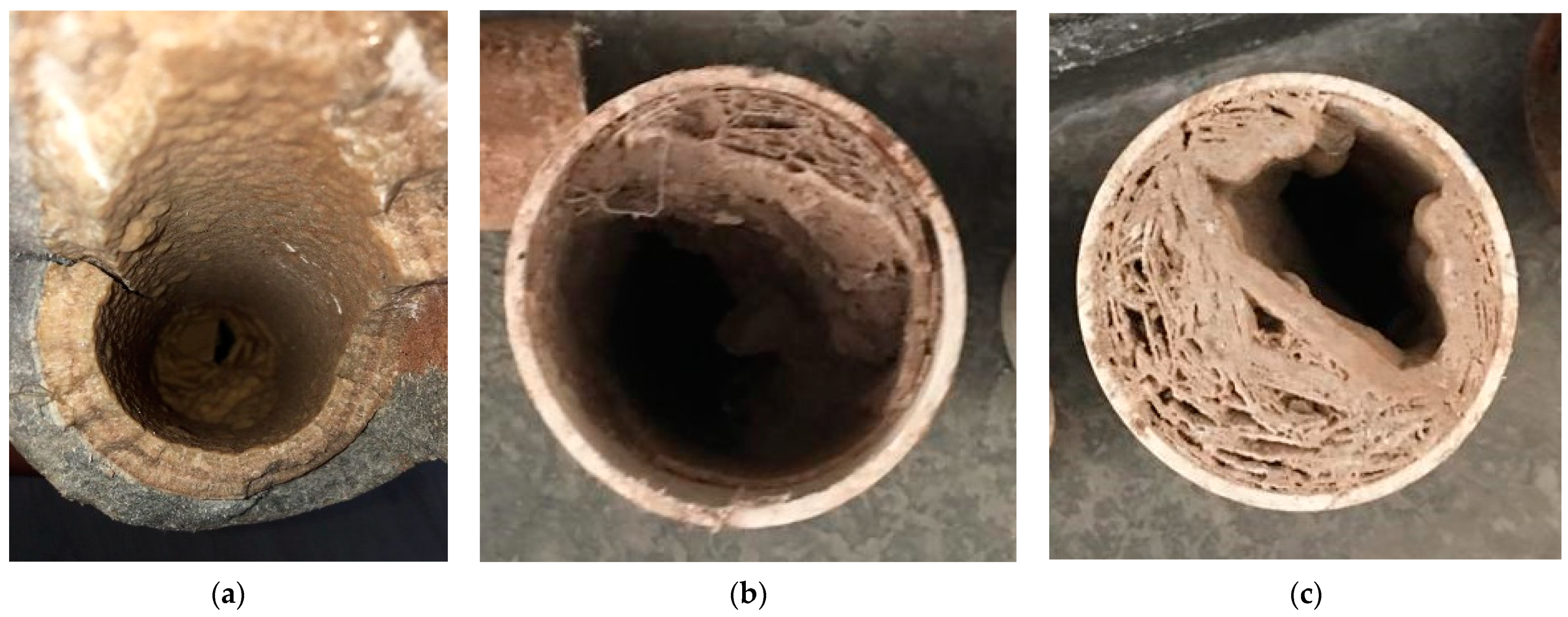
2.3. Pipes Sampling
2.4. Pretreatment Process
2.5. Characterization of Precipitates
2.5.1. X-ray Diffraction (XRD)
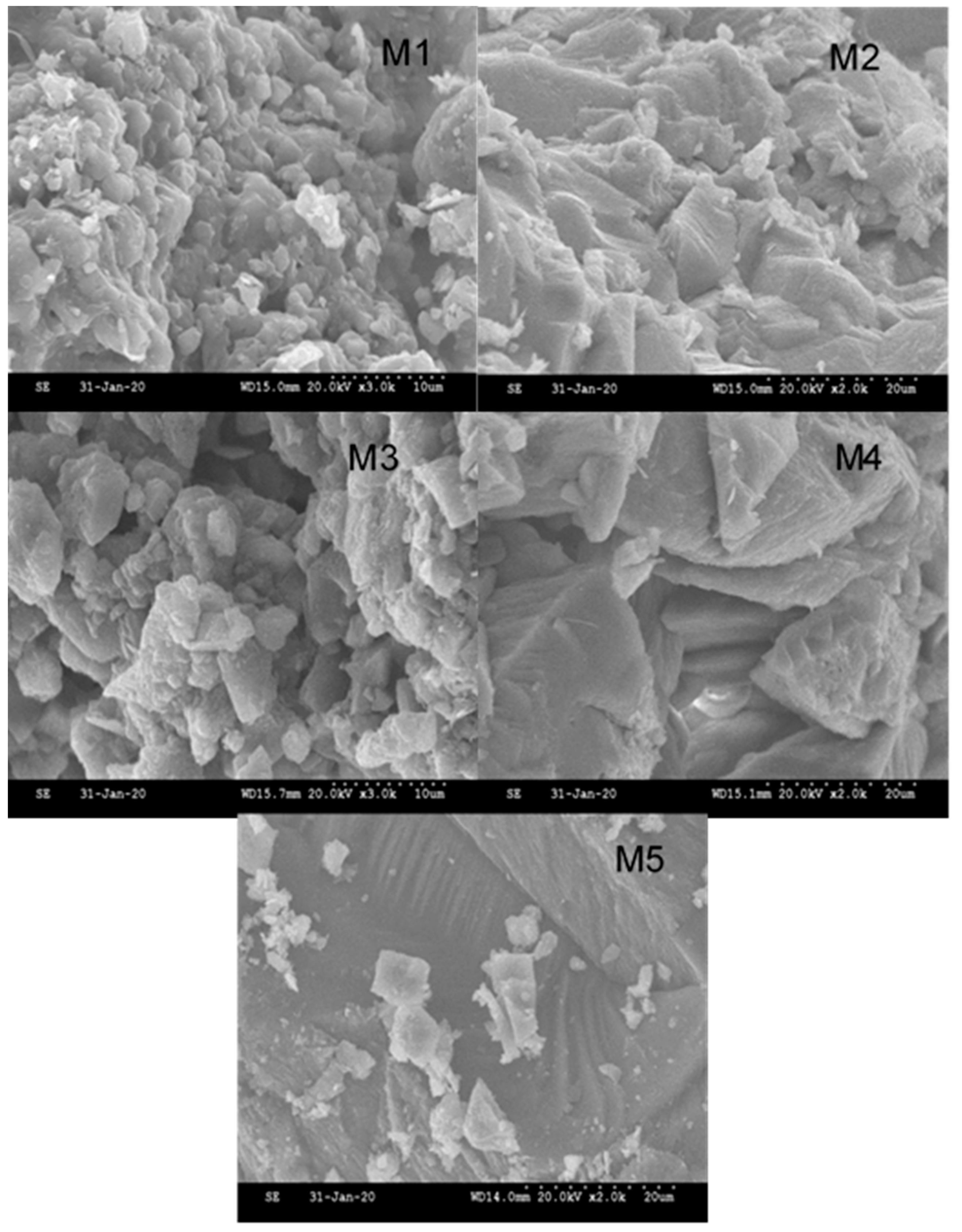
2.5.2. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Water Chemistry Characterization
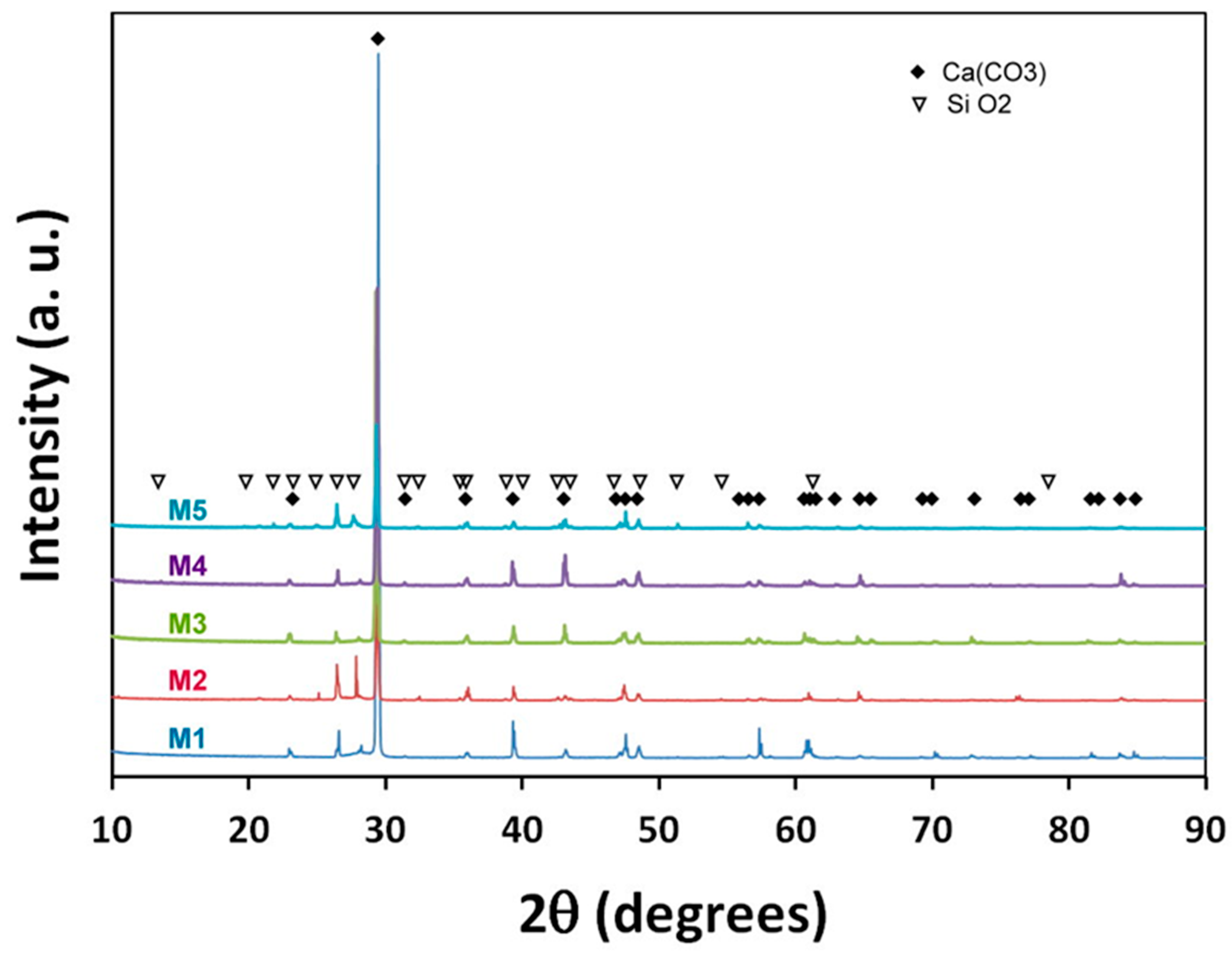
3.2. Scale Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvakumar, S.; Chandrasekar, N.; Kumar, G. Hydrogeochemical Characteristics and Groundwater Contamination in the Rapid Urban Development Areas of Coimbatore, India. Water Resour. Ind. 2017, 17, 26–33. [Google Scholar] [CrossRef]
- Dhanasekarapandian, M.; Chandran, S.; Devi, D.S.; Kumar, V. Spatial and Temporal Variation of Groundwater Quality and Its Suitability for Irrigation and Drinking Purpose Using GIS and WQI in an Urban Fringe. J. Afr. Earth Sci. 2016, 124, 270–288. [Google Scholar] [CrossRef]
- Yousefi, N.; Fatehizedeh, A.; Ghadiri, K.; Mirzaei, N.; Ashrafi, S.D.; Mahvi, A.H. Application of Nanofilter in Removal of Phosphate, Fluoride and Nitrite from Groundwater. Desalin. Water Treat. 2016, 57, 11782–11788. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Wei, L.; Liu, S.; Zhang, B.; Zhou, C. Experimental Study on Prevention of Calcium Carbonate Crystallizing in Drainage Pipe of Tunnel Engineering. Adv. Civ. Eng. 2018, 2018, 9430517. [Google Scholar] [CrossRef]
- Diogo, A.F.; Resende, R.A.; Oliviera, L.A. Optimised Selection of Water Supply and Irrigation Sources—A Case Study on Surface and Underground Water, Desalination, and Wastewater Reuse in a Sahelian Coastal Arid Region. Sustainability 2021, 13, 12696. [Google Scholar] [CrossRef]
- Duttagupta, S.; Bhanja, S.N.; Dutta, A.; Sarkar, S.; Chakraborty, M.; Ghosh, A.; Mondal, D.; Mukherjee, A. Impact of Covid-19 Lockdown on Availability of Drinking Water in the Arsenic-Affected Ganges River Basin. Int. J. Environ. Res. Public Health 2021, 18, 2832. [Google Scholar] [CrossRef] [PubMed]
- Olivares, R.; Sandoval, R. El Agua Potable en México Historia Reciente, Actores, Procesos y Propuestas; Asociación Nacional de Empresas y Saneamiento A.C.: México City, Mexico, 2008; pp. 1–342. [Google Scholar]
- Alimoradi, J.; Naghipour, D.; Kamani, H.; Asgari, G.; Naimi-Joubani, M.; Ashrafi, S.D. Data on Corrosive Water in the Sources and Distribution Network of Drinking Water in North of Iran. Data Br. 2018, 17, 105–118. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Zheng, X.; Zhao, J.; He, G.; Zhang, T. Effect of Pipe Materials on Chlorine Decay, Trihalomethanes Formation, and Bacterial Communities in Pilot-Scale Water Distribution Systems. Int. J. Environ. Sci. Technol. 2017, 14, 85–94. [Google Scholar] [CrossRef]
- De Sousa, C.; Correia, A.; Colmenares, M.C. Corrosión e Incrustaciones En Los Sistemas de Distribución de Agua Potable: Revisión de Las Estrategias de Control. Bol. Malariol. Salud Ambient. 2010, 50, 187–196. [Google Scholar]
- Hajibabaei, M.; Nazif, S.; Tavanaei Sereshgi, F. Life Cycle Assessment of Pipes and Piping Process in Drinking Water Distribution Networks to Reduce Environmental Impact. Sustain. Cities Soc. 2018, 43, 538–549. [Google Scholar] [CrossRef]
- Postigo, C.; Emiliano, P.; Barceló, D.; Valero, F. Chemical Characterization and Relative Toxicity Assessment of Disinfection Byproduct Mixtures in a Large Drinking Water Supply Network. J. Hazard. Mater. 2018, 359, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.A.; Farewell, T.S.; Hallett, S.H.; Acland, T.F. Improving Pipe Failure Predictions: Factors Effecting Pipe Failure in Drinking Water Networks. Water Res. 2019, 164, 114926. [Google Scholar] [CrossRef]
- Walter, R.K.; Lin, P.H.; Edwards, M.; Richardson, R.E. Investigation of Factors Affecting the Accumulation of Vinyl Chloride in Polyvinyl Chloride Piping Used in Drinking Water Distribution Systems. Water Res. 2011, 45, 2607–2615. [Google Scholar] [CrossRef]
- Akujieze, C.N.; Oteze, G.E. Groundwater Quality of Benin City Urban Aquifer of The Pleistocene-Oligocene Benin Formation, Nigeria. Afr. Sci. 2006, 7, 69–85. [Google Scholar]
- Diogo, A.F.; Vilela, F.A. Head Losses and Friction Factors of Steady Turbulent Flows in Plastic Pipes. Urban Water J. 2014, 11, 414–425. [Google Scholar] [CrossRef]
- Hartinger, S.M.; Medina-Pizzali, M.L.; Salmon-Mulanovich, G.; Larson, A.J.; Pinedo-Bardales, M.; Verastegui, H.; Riberos, M.; Mäusezahl, D. Antimicrobial Resistance in Humans, Animals, Water and Household Environs in Rural Andean Peru: Exploring Dissemination Pathways through the One Health Lens. Int. J. Environ. Res. Public Health 2021, 18, 4604. [Google Scholar] [CrossRef] [PubMed]
- Zulu, G.; Toyota, M.; Misawa, S.I. Characteristics of Water Reuse and Its Effects on Paddy Irrigation System Water Balance and the Riceland Ecosystem. Agric. Water Manag. 1996, 31, 269–283. [Google Scholar] [CrossRef]
- Hafid, N.; Belaatar, M.; Ben-Aazza, S.; Hadfi, A.; Ezahri, M.; Driouiche, A. Characterization of Scale Formed in Drinking Water and Hot Water Pipes in the Taliouine Downtown—Morocco. Am. J. Anal. Chem. 2015, 6, 677–686. [Google Scholar] [CrossRef]
- Peng, C.Y.; Korshin, G.V.; Valentine, R.L.; Hill, A.S.; Friedman, M.J.; Reiber, S.H. Characterization of Elemental and Structural Composition of Corrosion Scales and Deposits Formed in Drinking Water Distribution Systems. Water Res. 2010, 44, 4570–4580. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.; Johnson, A. La Lucha Contra Las Incrustaciones—Remoción y Prevención. Oilf. Rev. 2009, 11, 20. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; Routledge: Oxfordshire, UK, 1993. [Google Scholar] [CrossRef]
- Dobersek, D.; Goricanec, D. An Experimentally Evaluated Magnetic Device’s Efficiency for Water-Scale Reduction on Electric Heaters. Energy 2014, 77, 271–278. [Google Scholar] [CrossRef]
- Mahmoud, B.; Yosra, M.; Nadia, A. Effects of Magnetic Treatment on Scaling Power of Hard Waters. Sep. Purif. Technol. 2016, 171, 88–92. [Google Scholar] [CrossRef]
- Putranto, W.A.; Umardhani, Y.; Sulistyo; Yurianto; Bayuseno, A.P. Analysis of Calcium Carbonate Polymorphs Deposited in Water Piping System and the Effect of Tartaric Acid Additive. MATEC Web Conf. 2018, 159, 01054. [Google Scholar] [CrossRef][Green Version]
- Clavijo, J. Caracterización de Materiales a Través de Medidas de Microscopía Electrónica de Barrido (SEM). Elementos 2013, 3, 133–146. [Google Scholar] [CrossRef]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.F.; Cardona, A. Correlation of Arsenic and Fluoride in the Groundwater for Human Consumption in a Semiarid Region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Hernández-Ayón, J.M.; Chapa-Balcorta, C.; Delgadillo-Hinojosa, F.; Camacho-Ibar, V.F.; Huerta-Díaz, M.A.; Santamaría-del-Angel, E.; Galindo-Bect, S.; Segovia-Zavala, J.A. Dynamics of Dissolved Inorganic Carbon in the Midriff Islands of the Gulf of California: Influence of Water Masses. Ciencias Mar. 2013, 39, 183–201. [Google Scholar] [CrossRef]
- Richards, C.S. Calcium Carbonate Formation in Water Distribution Systems and Autogenous Repair of Leaks by Inert Particle Clogging; Virginia Polytechnic Institute: Blacksburg, VA, USA, 2016. [Google Scholar]
- EPA. Toxic and Priority Pollutants. Available online: http://water.epa.gov/scitech/methods/cwa/pollutants-background.cfm (accessed on 14 September 2021).
- Instituto de Altos Estudios “Dr. Arnoldo Gabaldon”; Correia, A.C.; Colmenares, M.C. Boletín de Malariología y Salud Ambiental; Instituto de Altos Estudios en Salud Pública Dr. Arnoldo Gabaldon: Maracay, Venezuela, 2010; Volume 50. [Google Scholar]
- World Health Organization. Asbestos in Drinking Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Health Criteria Other Support Information; World Health Organization: Geneva, Switzerland, 1996; Volume 2, pp. 81–84. [Google Scholar]
- Fish, K.E.; Reeves-McLaren, N.; Husband, S.; Boxall, J. Unchartered Waters: The Unintended Impacts of Residual Chlorine on Water Quality and Biofilms. npj Biofilms Microbiomes 2020, 6, 34. [Google Scholar] [CrossRef]
- Padhi, R.K.; Satpathy, K.K.; Subramanian, S. Impact of Groundwater Surface Storage on Chlorination and Disinfection By-Product Formation. J. Water Health 2015, 13, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Gorchev, H.G.; Ozolins, G. Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2004; Volume 1, p. 564. [Google Scholar]
- De Battisti, A.; Formaglio, P.; Ferro, S.; Al Aukidy, M.; Verlicchi, P. Electrochemical Disinfection of Groundwater for Civil Use—An Example of an Effective Endogenous Advanced Oxidation Process. Chemosphere 2018, 207, 101–109. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency; Office of Water. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; US EPA: Washington, DC, USA, 2018.
- Modificación a la Norma Oficial Mexicana: NOM-127-SSA1-1994. Salud ambiental, agua para uso y consumo humano-Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Diario Oficial de la Federacion, 22 November 2000.
- Crespo, P.V.; Campos, F.; Leal, M.; Maraver, F. Effects of Sodium Chloride-Rich Mineral Water on Intestinal Epithelium. Experimental Study. Int. J. Environ. Res. Public Health 2021, 18, 3261. [Google Scholar] [CrossRef]
- Betancourth, D.G.; Fernando Gomez, J.C.; Mosquera, J.C.; Tirado-Mejía, L. Análisis Por Difracción De Rayos X De Rocas Provenientes De Región Esmeraldífera X-Ray Diffraction Analysis on Rocks from Emerald Mining Region. Sciencia Technica Año XVI 2010, 44, 257–260. [Google Scholar]
- Echeverría, F.; Castaño, J.G.; Arroyave, C.; Peñuela, G.; Ramírez, A.; Morató, J. Characterization of Deposits Formed in a Water Distribution System. Ingeniare. Rev. Chil. Ing. 2009, 17, 275–281. [Google Scholar] [CrossRef]
- Woessner, W.W.; Poeter, E.P. Hydrogeologic Properties of Earth Materials and Principles of Groundwater Flow; Cherry, J., Eileen, P., Eds.; The Groundwater Project: Guelph, ON, Canada, 2020; Volume 59. [Google Scholar] [CrossRef]
- Ketrane, R.; Leleyter, L.; Baraud, F.; Jeannin, M.; Gil, O.; Saidani, B. Characterization of Natural Scale Deposits Formed in Southern Algeria Groundwater. Effect of Its Major Ions on Calcium Carbonate Precipitation. Desalination 2010, 262, 21–30. [Google Scholar] [CrossRef]
- EPA. Wastewater Technology Fact Sheet Chemical Precipitation; EPA 832-F-99-062; EPA: Washington, DC, USA, 2000.
- Freeze, R.A.; Cherry, J.A. Água Subterânea; Instituto Água Sustentável: Sao Paulo, Brazil, 2017. [Google Scholar]
- Larsen, T.; Randhol, P.; Lioliou, M.; Jøsang, L.O.; Østvold, T. Kinetics of CaCO3 Scale Formation during Core Flooding. In Proceedings of the 9th International Conference on Oilfield Scale “Managing Scale Through F. Lifetime”, Aberdeen, UK, 28 May 2008; pp. 162–173. [Google Scholar] [CrossRef]
- Rosales-Rivera, M.; Díaz-González, L.; Verma, S.P. A New Online Computer Program (Bidasys) for Ordinary and Uncertainty Weighted Least-Squares Linear Regressions: Case Studies from Food Chemistry. Rev. Mex. Ing. Química 2018, 17, 507–522. [Google Scholar] [CrossRef]
- Armendáriz, S.; Porta-Gándara, M.Á.; Foster, R.; Koutsoukos, P.; Bautista-Margulis, R.; Alonso, G. Comportamiento Isotérmico de La Precipitación Salina En Un Destilador Solar Experimental. Rev. Soc. Química México 2003, 47, 287–294. [Google Scholar]
- Zhou, Y.; Xiao, W.; Wang, J.; Zhao, Y.; Huang, Y.; Tian, J.; Chen, Y. Evaluating Spatiotemporal Variation of Groundwater Depth/Level in Beijing Plain, a Groundwater-Fed Area from 2001 to 2010. Adv. Meteorol. 2016, 2016, 8714209. [Google Scholar] [CrossRef]
- Moulessehoul, A.; Gallart-Mateu, D.; Harrache, D.; Djaroud, S.; de la Guardia, M.; Kameche, M. Conductimetric Study of Struvite Crystallization in Water as a Function of PH. J. Cryst. Growth 2017, 471, 42–52. [Google Scholar] [CrossRef]
- Bouropoulos, N.C.; Koutsoukos, P.G. Spontaneous Precipitation of Struvite from Aqueous Solutions. J. Cryst. Growth 2000, 213, 381–388. [Google Scholar] [CrossRef]
- Natsi, P.D.; Rokidi, S.G.; Koutsoukos, P.G. Precipitation of Calcium Carbonate (CaCO3) in Water-Monoethylene Glycol Solutions. Ind. Eng. Chem. Res. 2019, 58, 4732–4743. [Google Scholar] [CrossRef]
| Index | Equation | Index Value | Water Condition |
|---|---|---|---|
| Langelier saturation (LSI) | If the then use Else, then use | LSI > 0 LSI = 0 LSI < 0 | Super saturated, tends to precipitate CaCO3 Saturated, CaCO3 is in equilibrium Undersaturated, tends to dissolve solid CaCO3 |
| Ryznar stability (RSI) | RSI < 6 6 < RSI < 7 RSI > 7 | Super saturated, tends to precipitate CaCO3 Saturated, CaCO3 is in equilibrium Undersaturated, tends to dissolve solid CaCO3 | |
| Puckorius scaling (PSI) | PSI < 6 PSI > 7 | Scaling is unlikely to occur Likely to dissolve scale | |
| Larson–Skold (LS) | LS < 0.8 0.8 < LS < 1.2 LS > 1.2 | Chloride and sulfate are unlikely to interfere with the formation of protecting film Corrosion rates may be higher than expected High rates of localized corrosion may be expected | |
| Aggressive (AI) | AI > 12 10 < AI < 12 AI < 10 | Non-aggressive Moderately aggressive Very aggressive |
| Well Identifier | pH | Temp (°C) | CE (μmhos/cm) | Na+ | Ca2+ | HCO3− | SO42− | Cl− | Alkalinity | TDS | Hardness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | |||||||||||
| C1 | 7.7 | 23 | 375 | 32 | 58 | 256 | 30 | 8 | 210 | 185 | 170 |
| C2 | 7.7 | 26 | 375 | 35 | 58 | 276 | 19 | 8 | 226 | 193 | 129 |
| C3 | 8.4 | 26 | 435 | 51 | 37 | 217 | 32 | 12 | 210 | 220 | 155 |
| C4 | 7.8 | 26 | 380 | 34 | 35 | 167 | 10 | 14 | 138 | 186 | 105 |
| C5 | 8.1 | 26 | 470 | 70 | 18 | 235 | 26 | 20 | 193 | 230 | 56 |
| B1 | 6.7 | 23 | 439 | 45 | 48 | 252 | 12 | 19 | 207 | 215 | 126 |
| B2 | 6.8 | 24 | 441 | 53 | 40 | 239 | 4 | 17 | 196 | 216 | 139 |
| B3 | 7.9 | 25 | 447 | 35 | 48 | 200 | 24 | 20 | 194 | 219 | 143 |
| B4 | 8.0 | 28 | 498 | 54 | 40 | 258 | 26 | 21 | 211 | 244 | 144 |
| B5 | 7.9 | 30 | 508 | 75 | 33 | 224 | 30 | 20 | 184 | 248 | 170 |
| Parameter | Mean Values Measured from the Groundwater Wells | Standard Values According to the NOM-SSA-1997 | Water Quality Classification |
|---|---|---|---|
| Hardness (CaCO3) * | 130.0 | 500 | In range |
| Chloride (Cl−) * | 15.0 | 250.0 | In range |
| Fluoride (F−) * | 1.2 | 1.5 | In range |
| pH | 7.7 | 6.5–8.5 | In range |
| Sodium * | 46.0 | 200.0 | In range |
| Total dissolved solids * | 212.0 | 1000.0 | In range |
| Sulfate (SO42−) * | 20.0 | 400.0 | In range |
| Well Identifier | LSI | RSI | PSI | LS | AI |
|---|---|---|---|---|---|
| C1 | 0.31 | 7.3 | 7.1 | 0.15 | 10.0 |
| C2 | 0.39 | 7.1 | 6.9 | 0.10 | 10.0 |
| C3 | 0.76 | 7.0 | 6.9 | 0.10 | 11.0 |
| C4 | −0.005 | 7.8 | 7.0 | 0.15 | 10.0 |
| C5 | 0.016 | 7.9 | 7.8 | 0.15 | 10.5 |
| B1 | −0.67 | 8.4 | 7.2 | 0.12 | 9.0 |
| B2 | −0.66 | 8.5 | 7.4 | 0.10 | 9.0 |
| B3 | 0.63 | 7.0 | 7.1 | 0.22 | 10.0 |
| B4 | 0.62 | 7.1 | 7.1 | 0.18 | 10.0 |
| B5 | 0.40 | 7.4 | 7.4 | 0.22 | 10.0 |
| Well Identifier | pH | Temp (°C) | Alkalinity (mg/L) | DIC (mg/L) | Saturation pH | Ca2+ (mg/L) | Mg2+ (mg/L) | SiO (mg/L) |
|---|---|---|---|---|---|---|---|---|
| C1 | 7.7 | 23.3 | 210 | 49 | 7.6 | 58 | 11.98 | 54 |
| C2 | 7.7 | 26 | 226 | 55 | 8.0 | 58 | 2.70 | 52 |
| C3 | 8.4 | 26 | 210 | 48 | 7.8 | 37 | 4.5 | 66 |
| C4 | 7.8 | 26 | 138 | 37 | 8.0 | 35 | 4.2 | 38 |
| C5 | 8.1 | 26.4 | 193 | 49 | 8.2 | 18 | 3.6 | 58 |
| B1 | 6.7 | 23 | 207 | 64 | 7.8 | 48 | 5.51 | 64 |
| B2 | 6.8 | 24 | 196 | 64 | 7.8 | 40 | 2.47 | 29 |
| B3 | 7.9 | 25 | 194 | 49 | 7.6 | 48 | 5.93 | 43 |
| B4 | 8.0 | 28 | 211 | 55 | 7.8 | 40 | 3.47 | 38 |
| B5 | 7.9 | 30 | 184 | 35 | 8.0 | 33 | 7.57 | 49 |
| Sample | |||||
|---|---|---|---|---|---|
| Phase | M1 | M2 | M3 | M4 | M5 |
| CaCO3 | 93.6 | 66.6 | 94.9 | 91.4 | 69.5 |
| SiO2 | 6.4 | 33.4 | 5.1 | 8.6 | 30.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla González, P.; Bautista-Capetillo, C.; Ruiz-Canales, A.; González-Trinidad, J.; Júnez-Ferreira, H.E.; Contreras Rodríguez, A.R.; Robles Rovelo, C.O. Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region. Int. J. Environ. Res. Public Health 2022, 19, 3257. https://doi.org/10.3390/ijerph19063257
Padilla González P, Bautista-Capetillo C, Ruiz-Canales A, González-Trinidad J, Júnez-Ferreira HE, Contreras Rodríguez AR, Robles Rovelo CO. Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region. International Journal of Environmental Research and Public Health. 2022; 19(6):3257. https://doi.org/10.3390/ijerph19063257
Chicago/Turabian StylePadilla González, Pedro, Carlos Bautista-Capetillo, Antonio Ruiz-Canales, Julián González-Trinidad, Hugo Enrique Júnez-Ferreira, Ada Rebeca Contreras Rodríguez, and Cruz Octavio Robles Rovelo. 2022. "Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region" International Journal of Environmental Research and Public Health 19, no. 6: 3257. https://doi.org/10.3390/ijerph19063257
APA StylePadilla González, P., Bautista-Capetillo, C., Ruiz-Canales, A., González-Trinidad, J., Júnez-Ferreira, H. E., Contreras Rodríguez, A. R., & Robles Rovelo, C. O. (2022). Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region. International Journal of Environmental Research and Public Health, 19(6), 3257. https://doi.org/10.3390/ijerph19063257









