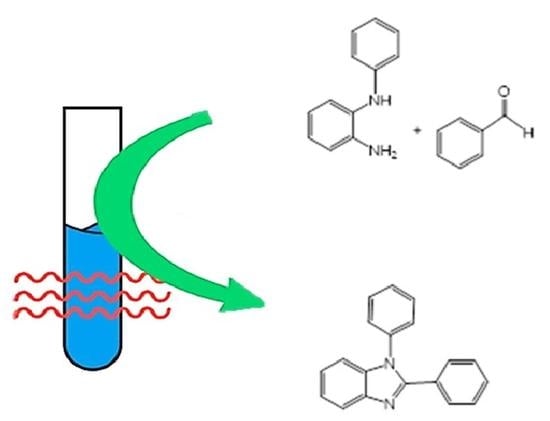
The Highly Efficient Synthesis of 1,2-Disubstituted Benzimidazoles Using Microwave Irradiation
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of 1-phenyl-2-Aryl(alkyl) Benzimidazoles 1a–11a
3.3. General Procedure for the Synthesis of 1-benzyl-2-Aryl-Benzimidazoles 1b–3b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nelso, W.M. Green Solvents for Chemistry Perspectives and Practice. Oxford University Press: Oxford, NY, USA, 2004. [Google Scholar]
- Mikami, K. Green Reaction Media in Organic Synthesis; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Clark, J.H.; Tavener, S.J. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Carloni, L.; Maggi, R.; Sartori, G. Zeolite HSZ-360 as a new reusable catalyst for the direct acetylation of alcohols and phenols under solventless conditions. Tetrahedron Lett. 1998, 39, 6049–6052. [Google Scholar] [CrossRef]
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar] [CrossRef]
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An Eco-Sustainable Erbium(III)-Catalysed Method for Formation/Cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. A Bifuctional Heterogeneous Catalyst Erbium-Based: A Cooperative Route Towards C-C Bond Formation. Molecules 2014, 19, 10218–10229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A Mesoporous Er(III)-MCM-41 Catalyst for the Cyanosilylation of Aldehydes and Ketones under Solvent-free Conditions. ChemSusChem 2008, 1, 916–919. [Google Scholar] [CrossRef]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Lapkin, A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use As solvents. U.S. Patent 7,183,433, 27 February 2007. [Google Scholar]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-Lõpez, D.; Russo, B.; Algieri, V. Efficient organocatalyst supported on a simple ionic liquid as a recoverable system for the asymmetric diels-alder reaction in the presence of water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Cassano, R.; Costanzo, P.; Herrera Cano, N.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules 2019, 24, 2885. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Leitner, W.; Poliakoff, M. Supercritical fluids in green chemistry. Green Chem. 2008, 10, 730. [Google Scholar]
- Carlès, P. A brief review of the thermophysical properties of supercritical fluids. J. Supercr. Fluids 2010, 53, 2–11. [Google Scholar] [CrossRef]
- Lindström, U.M. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 10, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Paonessa, R.; Nardi, M.; Procopio, A. Catalyst-free tosylation of lipophilic alcohols in water. RSC Adv. 2013, 3, 2548–2552. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Olivito, F.; Costanzo, P.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. Efficient synthesis of organic thioacetate in water. Org. Biomol. Chem. 2018, 16, 7753–7759. [Google Scholar] [CrossRef]
- Estevão, M.S.; Afonso, C.A.M. Synthesis of trans-4,5-diaminocyclopent-2-enones from furfural catalyzed by Er(III) immobilized on silica. Tetrahedron Lett. 2017, 58, 302–304. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Maru, M.S.; Somani, R.S.; Bajaj, H.C.; Neogi., S. Unprecedented NH2-MIL-101(Al)/n-Bu4NBr system as solvent-free heterogeneous catalyst for efficient synthesis of cyclic carbonates via CO2 cycloaddition. Dalton Trans. 2018, 47, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Sonochemistry: Current uses and future prospects in the chemical and processing industries. Phil. Trans. R. Soc. Lond. A 1999, 357, 355–369. [Google Scholar]
- Loupy Solvent-free microwave organic synthesis as an efficient procedure for green chemistry. C. R. Chim. 2004, 7, 103–112. [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Calandruccio, C.; Salerno, R.; Procopio, A. Tunable microwave-assisted method for the solvent-free and catalyst-free peracetylation of natural products. Beilstein J. Org. Chem. 2016, 12, 2222–2233. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, L.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Delso, I.; Tallarida, M.A.; De Nino, A. Nitrones and nucleobase-containing spiro-isoxazolidines derived from isatin and indanone: Solvent-free microwave-assisted stereoselective synthesis and theoretical calculations. RSC Adv. 2017, 7, 48980–48988. [Google Scholar]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Romeo, R. MW-assisted Er(OTf)3 -catalyzed mild cleavage of isopropylidene acetals in Tricky substrates. Tetrahedron Lett. 2008, 49, 1961–1964. [Google Scholar] [CrossRef]
- Maiuolo, L.; De Nino, A.; Algieri, V.; Nardi, M. Microwave-assisted 1,3-dipolar cyclo-addition: Recent advances in synthesis of isoxazolidines. Mini Rev. Org. Chem. 2017, 14, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Clean. Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Kubo, K.; Oda, K.; Kaneko, T.; Satoh, H.; Nohara, A. Synthesis of 2-(4-Fluoroalkoxy-2-pyridyl) methyl] sulfinyl]-1H-benzimidazoles as Antiulcer Agents. Chem. Pharm. Bull. 1990, 38, 2853–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, M.; Chihiro, M.; Morita, S.; Yamashita, H.; Yamasaki, K.; Kanbe, T.; Yabuuchi, Y.; Nakagawz, K. Synthesis and Antiulcer Activity of 4- Substituted 8-[(2-Benzimidazolyl) sulfinylmethyl]-1, 2, 3, 4-tetrahydroquinolines and Related Compounds. Chem. Pharm. Bull. 1990, 38, 1575–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, A.; Ippen, J.; Bruno, M.; Thomas, G.; Bay, P. A thiazolylamino benzimidazole derivative with gastroprotective properties in the rat. Eur. J. Pharmacol. 1991, 195, 251–259. [Google Scholar] [CrossRef]
- Ozkay, Y.; Tunali, Y.; Karaca, H.; Isikdag, I. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazones moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Algul, O.; Karabulut, A.; Canacankatan, N.; Gorur, A.; Sucu, N.; Vezir, O. Apoptotic and anti-angiogenic effects of benzimidazole compounds: Relationship with oxidative stress mediated ischemia/reperfusion injury in rat hind limb. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 267–275. [Google Scholar] [CrossRef]
- Emerson, G.; Brink, N.G.; Holly, F.W.; Koniuszy, F.; Heyl, D.; Folker, K. Vitamin B12. VIII. Vitamin B12-Like Activity of 5,6-Dimethylbenzimidazole and Tests on related compounds. J. Am. Chem. Soc. 1950, 72, 3084–3085. [Google Scholar] [CrossRef]
- Kommi, D.N.; Jadhavar, P.S.; Kumar, D.; Chakraborti, A.K. “All-water” one-pot diverse synthesis of 1,2-disubstituted benzimidazoles: Hydrogen bond driven synergistic electrophile–nucleophile dual activation by water. Green Chem. 2013, 15, 798–810. [Google Scholar] [CrossRef]
- Shen, M.-G.; Cai, C. Ytterbium perfluorooctanesulfonates catalyzed synthesis of benzimidazole derivatives in fluorous solvents. J. Fluor. Chem. 2007, 128, 232–235. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Jayamoorthy, K. Synthesis of some fluorescent benzimidazole derivatives using cobalt(II) hydroxide as highly efficient catalyst–spectral and physico-chemical studies and ESIPT process. Photochem. Photobiol. Sci. 2013, 12, 1761–1773. [Google Scholar] [CrossRef]
- Srinivasulu, R.; Kumar, K.R.; Satyanarayana, P.V.V. Facile and Efficient Method for Synthesis of Benzimidazole Derivatives Catalyzed by Zinc Triflate. Green Sustain. Chem. 2014, 4, 33–37. [Google Scholar]
- Martins, G.M.; Puccinelli, T.; Gariani, R.A.; Xavier, F.R.; Silveira, C.C.; Mendes, S.R. Facile and efficient aerobic one-pot synthesis of benzimidazoles using Ce(NO3 )3 ·6H2O as promoter. Tetrahedron Lett. 2017, 58, 1969–1972. [Google Scholar] [CrossRef]
- Peng, X.-C.; Gong, S.-S.; Zeng, D.-Y.; Duo, S.-W.; Sun, Q. Activated carbon supported hafnium(IV) chloride as an efficient, recyclable, and facile removable catalyst for expeditious parallel synthesis of benzimidazoles. Catalysts 2020, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Sontakke, V.A.; Ghosh, S.; Lawande, P.P.; Chopade, B.A.; Shinde, V.S. A simple, efficient synthesis of 2-aryl benzimidazoles using silica supported periodic acid catalyst and evaluation of anticancer activity. ISRN Org. Chem. 2013, 2013, 453682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.R.; Satyanarayana, P.V.V.; Reddy, B.S. NaHSO4-SiO2 promoted synthesis of benzimidazole derivatives. Arch. Appl. Sci. Res. 2012, 4, 1517–1521. [Google Scholar]
- Goswami, M.; Dutta, M.M.; Phukan, P. Sulfonic-acid-functionalized activated carbon made from tea leaves as green catalyst for synthesis of 2-substituted benzimidazole and benzothiazole. Res. Chem. Intermed. 2018, 44, 1597–1615. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Kanagaraj, K.; Pitchumani, K. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011, 52, 69–73. [Google Scholar] [CrossRef]
- Bonacci, S.-; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Procopio, A.; De Nino, A.; Nardi, M.; Oliverio, M.; Paonessa, R.; Pasceri, R. A New Microwave-Assisted Organocatalytic Solvent-Free Synthesis of Optically Enriched Michael Adducts. Synlett 2010, 12, 1849–1853. [Google Scholar] [CrossRef]
- Bonacci, S.; Iriti, G.; Mancuso, S.; Novelli, P.; Paonessa, R.; Tallarico, S.; Nardi, M. Montmorillonite K10: An efficient organheterogeneous catalyst for synthesis of benzimidazole derivatives. Catalysts 2020, 10, 84. [Google Scholar] [CrossRef]
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Romeo, G. Mild and efficient method for the cleavage of benzylidene acetals by using erbium (III) triflate. Org. Biomol. Chem. 2005, 3, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Celia, C.; Nardi, M.; Oliverio, M.; Paolino, D.; Sindona, G. Lipophilic hydroxytyrosol esters: Fatty acid conjugates for potential topical administration. J. Nat. Prod. 2011, 74, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Paonessa, R.; Nardi, M.; Di Gioia, M.L.; Olivito, F.; Oliverio, M.; Procopio, A. Eco-friendly synthesis of lipophilic EGCG derivatives and antitumor and antioxidant evaluation. Nat. Prod. Commun. 2018, 9, 1117–1122. [Google Scholar]
- Herrera Cano, N.; Uranga, J.G.; Nardi, M.; Procopio, A.; Wunderlin, D.A.; Santiago, A.N. Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er(OTf)3 catalyst in the reaction selectivity. Beilstein J. Org. Chem. 2016, 12, 2410–2419. [Google Scholar] [CrossRef] [Green Version]
- Nardi, M.; Cozza, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. 1,5-Benzoheteroazepines through eco-friendly general condensation reactions. Tetrahedron Lett. 2011, 52, 4827–4834. [Google Scholar] [CrossRef]
- Nardi, M.; Cozza, A.; De Nino, A.; Oliverio, M.; Procopio, A. One-pot synthesis of dibenzo[b,e][1,4]diazepin-1-ones. Synthesis 2012, 44, 800–804. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Procopio, A. Cerium(III) triflate versus cerium(III) chloride: Anion dependence of Lewis acid behavior in the deprotection of PMB ethers. Eur. J. Org. Chem. 2004, 10, 2176–2180. [Google Scholar] [CrossRef]
- Zhong, R.; Xiong, W.; Zhang, H.; Zeng, T.; Gong, S.; Sun, Q. Highly Efficient and AmbientTemperature Synthesis of Benzimidazoles via Co(III)/Co(II)-Mediated Redox Catalysis. Catalysts 2022, 12, 59. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A. Recent Developments Towards Synthesis of (Het)arylbenzimidazoles. Synthesis 2021, 53, 1849–1878. [Google Scholar] [CrossRef]
- Kumar, T.A.; Devi, B.R.; Dubey, P.K. Green Syntheses of N-Alkyl-2-styrylbenzimidazoles. Asian J. Chem. 2013, 25, 9569–9572. [Google Scholar] [CrossRef]
- Dang, P.; Zeng, W.; Liang, Y. Copper-Catalyzed Three-Component Synthesis of Benzothiazolethiones from o-Iodoanilines, Isocyanide, and Potassium Sulfide. Org. Lett. 2015, 17, 34–37. [Google Scholar] [CrossRef] [PubMed]
| c | Solvent | Temp (°C) | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 | Ethyl lactate | rt | 120 | 0 |
| 2 | Ethyl lactate | 60 | 120 | 3.9 |
| 3 | Ethyl lactate | 100 | 120 | 15.3 |
| 4 | Water | rt | 120 | 10.2 |
| 5 | Water | 60 | 60 | 20.9 |
| 6 | Water | 60 | 120 | 59.6 |
| 7 | Water | 100 | 120 | 89.6 |
| 8 c | Water | 60 | 10 | 71.9 |
| 9 | - | 60 | 60 | 61.4 |
| 10 c | - | 60 | 5 | 89.6 |
| 11 c,d | - | 60 | 5 | 99.9 |
| 12 c,f | - | 60 | 7 | 91.3 |
| 12 c,g | - | 60 | 7 | 99.9 |
| Entry | Aldehyde | Product | Time (min) | Yield (%) b |
|---|---|---|---|---|
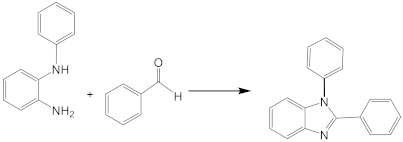
| 1 | 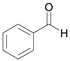 | 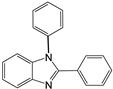 1a | 5 | 99.9 |
| 2 | 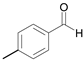 | 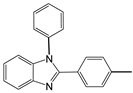 2a | 5 | 98.6 |
| 3 | 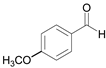 | 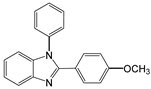 3a | 7 | 99.6 |
| 4 | 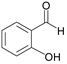 | 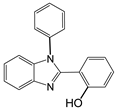 4a | 10 | 96.3 |
| 5 | 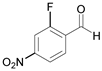 | 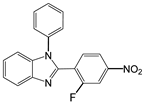 5a | 15 | 96 |
| 6 | 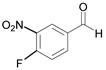 | 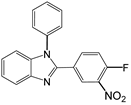 6a | 15 | 97 |
| 7 | 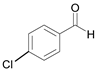 | 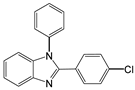 7a | 10 | 97 |
| 8 | 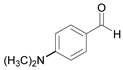 | 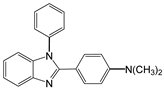 8a | 15 | 91.1 |
| 9 | 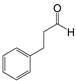 | 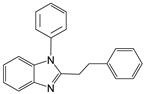 9a | 5 | 98.2 |
| 10 | 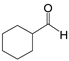 | 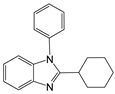 10a | 5 | 98.8 |
| 11 | 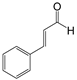 | 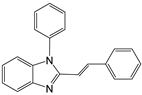 11a | 12 | 85.8 |
| 12 c | 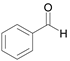 | 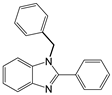 1b | 5 | 99.9 |
| 13 c | 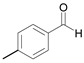 | 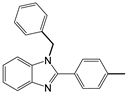 2b | 5 | 98.9 |
| 14 c | 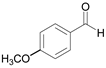 | 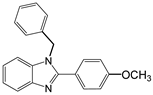 3b | 5 | 99.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardi, M.; Bonacci, S.; Herrera Cano, N.; Oliverio, M.; Procopio, A. The Highly Efficient Synthesis of 1,2-Disubstituted Benzimidazoles Using Microwave Irradiation. Molecules 2022, 27, 1751. https://doi.org/10.3390/molecules27051751
Nardi M, Bonacci S, Herrera Cano N, Oliverio M, Procopio A. The Highly Efficient Synthesis of 1,2-Disubstituted Benzimidazoles Using Microwave Irradiation. Molecules. 2022; 27(5):1751. https://doi.org/10.3390/molecules27051751
Chicago/Turabian StyleNardi, Monica, Sonia Bonacci, Natividad Herrera Cano, Manuela Oliverio, and Antonio Procopio. 2022. "The Highly Efficient Synthesis of 1,2-Disubstituted Benzimidazoles Using Microwave Irradiation" Molecules 27, no. 5: 1751. https://doi.org/10.3390/molecules27051751