Plant-Derived Molecule 4-Methylumbelliferone Suppresses FcεRI-Mediated Mast Cell Activation and Allergic Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. β-Hexosaminidase Release Assay
2.5. Reverse Transcriptase (RT)-Quantitative Polymerase Chain Reaction (qPCR)
2.6. Western Blotting
2.7. Toluidine Blue Staining
2.8. F-actin Staining
2.9. IgE-Mediated PCA Mouse Model
2.10. OVA-Induced ASA Mouse Model
2.11. Statistical Analyses
3. Results
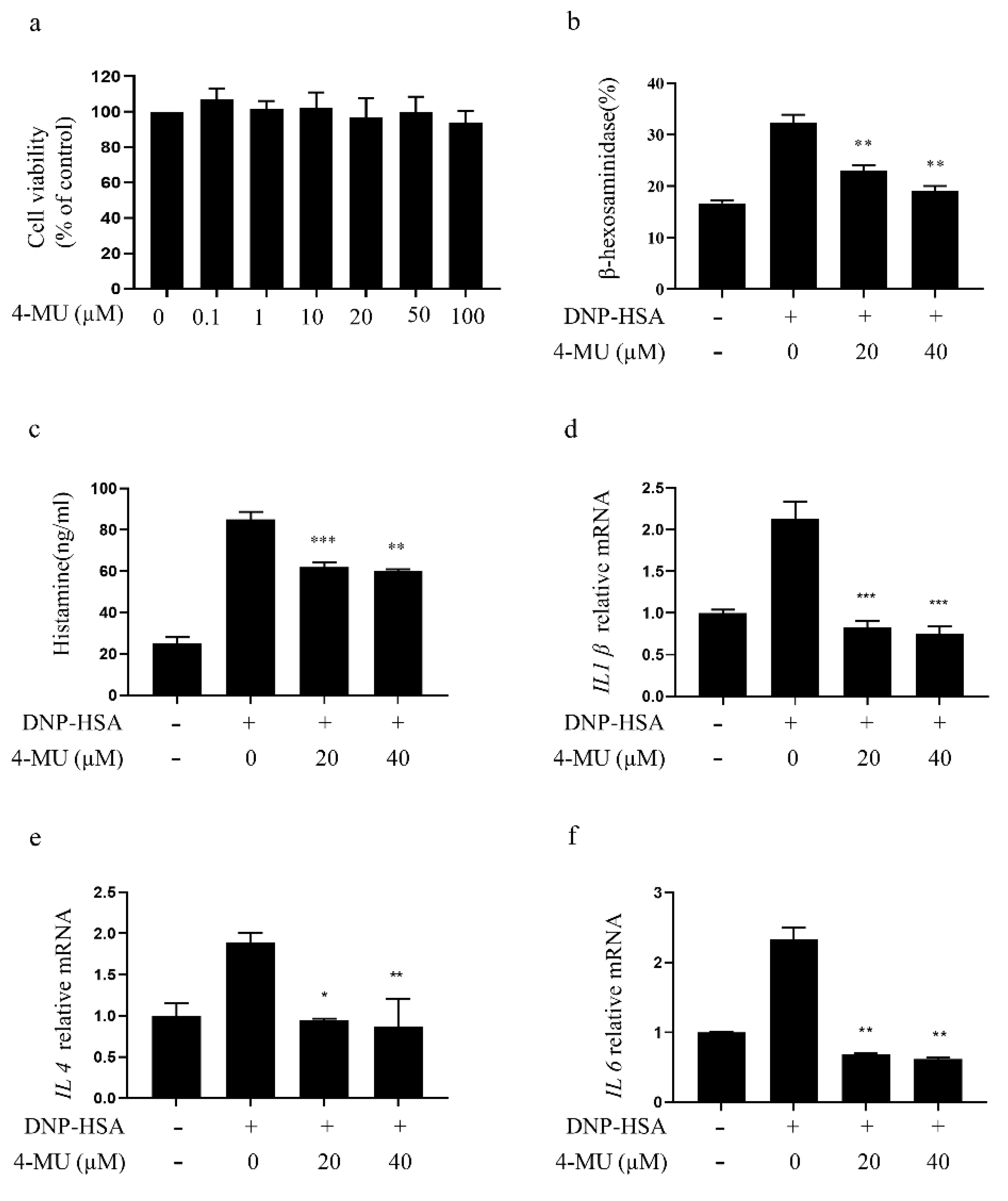
3.1. 4-MU Suppresses FcεRI-Mediated Degranulation in RBLs
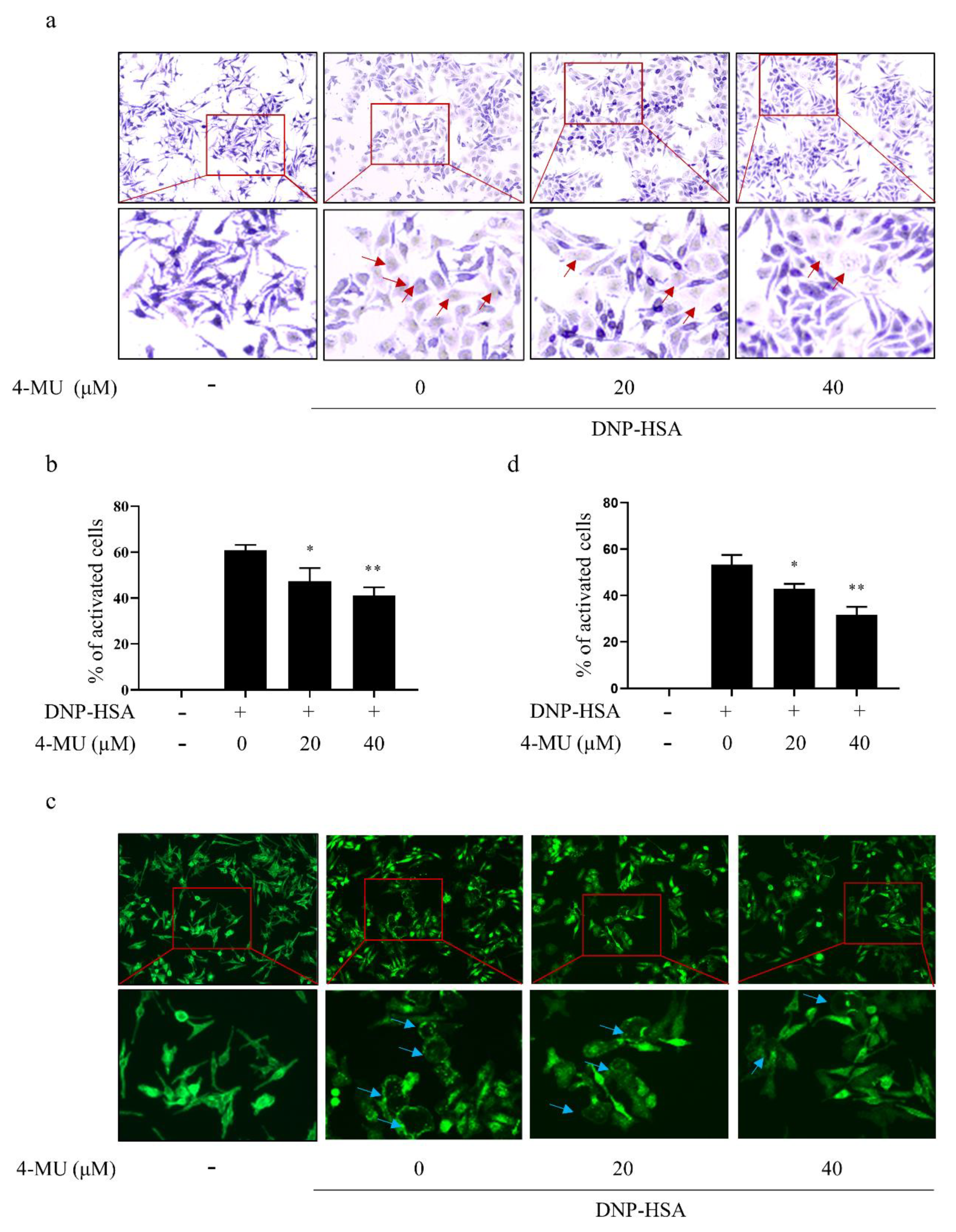
3.2. 4-MU Suppresses FcεRI-Mediated Morphological Changes in RBLs
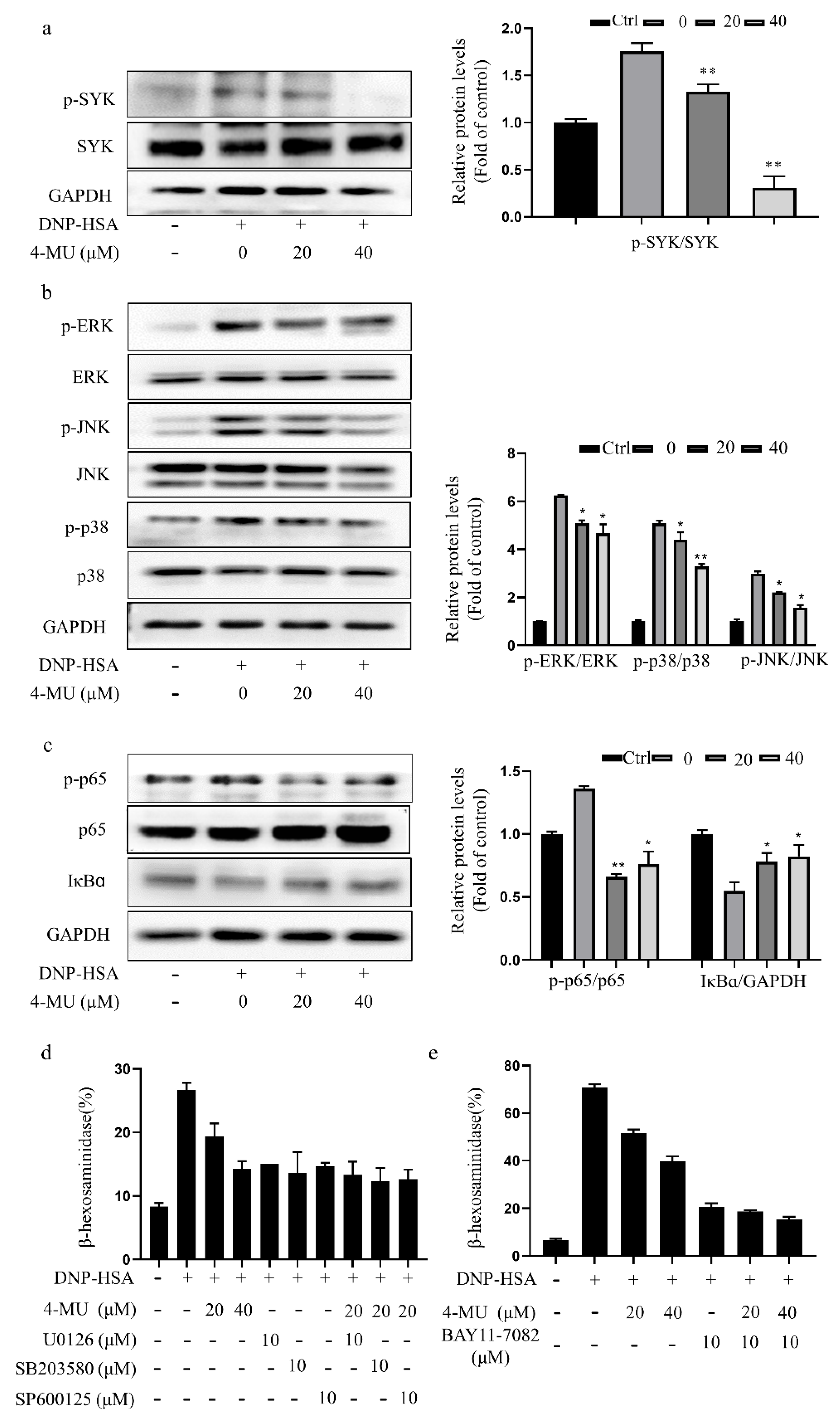
3.3. 4-MU Suppresses Phosphorylation of FcεRI-Linked Signaling Proteins in RBLs
3.4. 4-MU Has an Inhibitory Effect on IgE/Ag-Stimulated Primary BMMCs
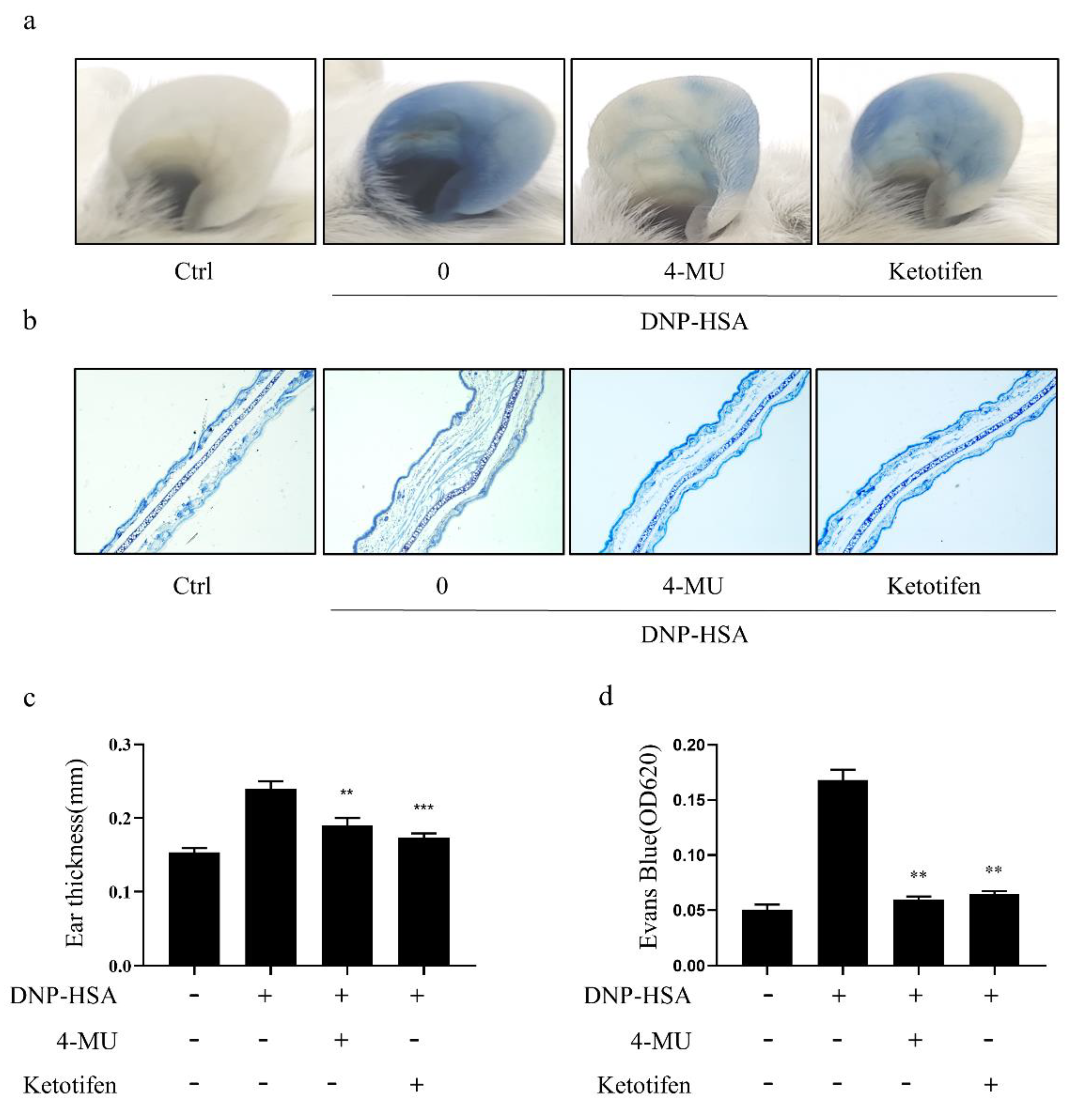
3.5. 4-MU Attenuated IgE-Mediated Allergic Reactions in PCA Mice
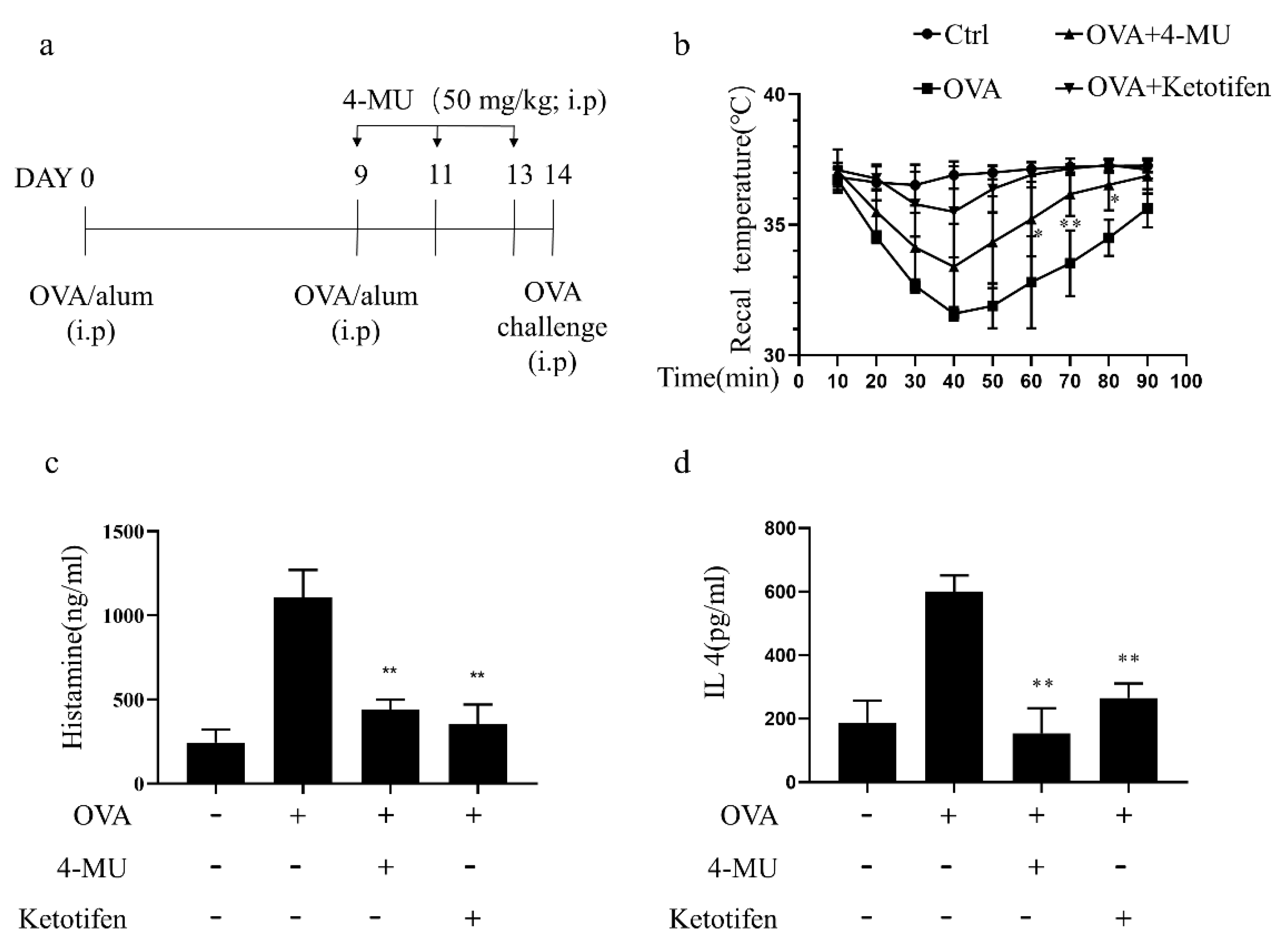
3.6. 4-MU Attenuated Allergic Reactions in OVA-Induced ASA Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 4-MU | 4-Methylumbelliferone |
| MCs | Mast cells |
| BMMCs | Mouse bone marrow-derived mast cells |
| DNP | Anti-dinitrophenyl |
| IgE | immunoglobulin E |
| DNP-HSA | DNP-human serum albumin |
| PCA | Passive cutaneous anaphylaxis |
| ASA | Ovalbumin-induced active systemic anaphylaxis |
| FcεRI | IgE receptor |
| ERK | Extracellular signal-regulated kinase |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| RBLs | Rat basophilic leukemia cells |
| JNK | c-jun and N-terminal kinase |
| FBS | Fetal bovine serum |
| SD | Standard deviation |
| PBS | Phosphate buffered saline |
| ANOVAs | One-way analyses of variance |
| HRP | Horse radish peroxidase |
| MAPK | Mitogen-activated protein kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Ag | antigen |
| i.v. | Tail intravenous injection |
| i.p. | Intraperitoneal injection |
References
- Zheng, P.Y.; Geng, X.R.; Hong, J.Y.; Yang, G.; Liu, J.Q.; Mo, L.H.; Feng, Y.; Zhang, Y.Y.; Liu, T.; Ran, P.; et al. Regulating Bcl2L12 expression in mast cells inhibits food allergy. Theranostics 2019, 9, 4982–4992. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, X.; Deng, Y.; Timilshina, M.; Huang, B.; Kim, D.Y.; Chang, J.H.; Ichinose, H.; Baek, S.H.; Murakami, M.; et al. The orphan nuclear receptor NR4A1 promotes FcepsilonRI-stimulated mast cell activation and anaphylaxis by counteracting the inhibitory LKB1/AMPK axis. Allergy 2019, 74, 1145–1156. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Kondo, H.; Hasegawa, M.; Kurimoto, C.; Ishii, Y.; Kato, C.; Botei, T.; Shinya, M.; Murate, T.; Ueno, Y.; et al. Inhibition of mast cell degranulation by melanin. Biochem. Pharm. 2019, 163, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Triggiani, M.; de Paulis, A. Mast cells and basophils: Friends as well as foes in bronchial asthma? Trends Immunol 2005, 26, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Vitale, D.L.; Icardi, A.; Rosales, P.; Spinelli, F.M.; Sevic, I.; Alaniz, L.D. Targeting the Tumor Extracellular Matrix by the Natural Molecule 4-Methylumbelliferone: A Complementary and Alternative Cancer Therapeutic Strategy. Front. Oncol. 2021, 11, 710061. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Edward, M.; Quinn, J.A.; Pasonen-Seppänen, S.M.; McCann, B.A.; Tammi, R.H. 4-Methylumbelliferone inhibits tumour cell growth and the activation of stromal hyaluronan synthesis by melanoma cell-derived factors. Br. J. Derm. 2010, 162, 1224–1232. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M. 4-methylumbelliferone inhibits angiogenesis in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 4063–4071. [Google Scholar] [CrossRef]
- Nagy, N.; Freudenberger, T.; Melchior-Becker, A.; Röck, K.; Ter Braak, M.; Jastrow, H.; Kinzig, M.; Lucke, S.; Suvorava, T.; Kojda, G.; et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis. Circulation 2010, 122, 2313–2322. [Google Scholar] [CrossRef] [Green Version]
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 2009, 315, 1914–1923. [Google Scholar] [CrossRef]
- Kakizaki, I.; Kojima, K.; Takagaki, K.; Endo, M.; Kannagi, R.; Ito, M.; Maruo, Y.; Sato, H.; Yasuda, T.; Mita, S.; et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J. Biol. Chem. 2004, 279, 33281–33289. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Hagele, H.F.; Uchakina, O.N. Treatment with the hyaluronic acid synthesis inhibitor 4-methylumbelliferone suppresses SEB-induced lung inflammation. Toxins 2013, 5, 1814–1826. [Google Scholar] [CrossRef]
- McKallip, R.J.; Ban, H.; Uchakina, O.N. Treatment with the hyaluronic Acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation 2015, 38, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kozawa, E.; Urakawa, H.; Arai, E.; Futamura, N.; Zhuo, L.; Kimata, K.; Ishiguro, N.; Nishida, Y. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2013, 65, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.F.; Nagy, N.; Ruppert, S.M.; Sunkari, V.G.; Marshall, P.L.; Gebe, J.A.; Ishak, H.D.; Keswani, S.G.; Bollyky, J.; Frymoyer, A.R.; et al. The pharmacokinetics and dosing of oral 4-methylumbelliferone for inhibition of hyaluronan synthesis in mice. Clin. Exp. Immunol. 2016, 185, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Colombaro, V.; Declèves, A.E.; Jadot, I.; Voisin, V.; Giordano, L.; Habsch, I.; Nonclercq, D.; Flamion, B.; Caron, N. Inhibition of hyaluronan is protective against renal ischaemia-reperfusion injury. Nephrol. Dial. Transpl. 2013, 28, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forteza, R.M.; Casalino-Matsuda, S.M.; Falcon, N.S.; Valencia Gattas, M.; Monzon, M.E. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J. Biol. Chem. 2012, 287, 42288–42298. [Google Scholar] [CrossRef] [Green Version]
- Swindle, E.J. Generation of mast cells from murine stem cell progenitors. Methods Mol. Biol. 2014, 1192, 63–67. [Google Scholar]
- Kim, M.J.; Kim, Y.Y.; Choi, Y.A.; Baek, M.C.; Lee, B.; Park, P.H.; Shin, T.Y.; Kwon, T.K.; Khang, D.; Kim, S.H. Elaeocarpusin Inhibits Mast Cell-Mediated Allergic Inflammation. Front Pharm. 2018, 9, 591. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflamm. 2019, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.B.; Zhang, L.N.; Wang, H.N.; Zhao, Z.F.; Sun, Y.T.; Ji, K.; Chen, J.J. The antipsychotic drug pimozide inhibits IgE-mediated mast cell degranulation and migration. Int. Immunopharmacol. 2020, 84, 106500. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.R.; Park, H.J. Antiallergic effect of fisetin on IgE-mediated mast cell activation in vitro and on passive cutaneous anaphylaxis (PCA). J. Nutr. Biochem. 2017, 48, 103–111. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ji, K.; Sun, Y.T.; Hou, Y.B.; Chen, J.J. Aurora kinase inhibitor tozasertib suppresses mast cell activation in vitro and in vivo. Br. J. Pharm. 2020, 177, 2848–2859. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, Y.; Hirasawa, N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. Allergy 2012, 67, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, T.; Azevedo, C.T.; Pmr, E.S.; Martins, M.A.; Carvalho, V.F. Glucocorticoids decrease the numbers and activation of mast cells by inducing the transactivation receptors of AGEs. J. Leukoc. Biol. 2019, 105, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, P.Y.; O’Sullivan, K.M.; Ooi, J.D.; Alikhan, M.A.; Odobasic, D.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Mast Cell Stabilization Ameliorates Autoimmune Anti-Myeloperoxidase Glomerulonephritis. J. Am. Soc. Nephrol. 2016, 27, 1321–1333. [Google Scholar] [CrossRef] [Green Version]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Derm. 2019, 181, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Bachert, C.; Maspero, J. Efficacy of second-generation antihistamines in patients with allergic rhinitis and comorbid asthma. J Asthma 2011, 48, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, C.K.; Galappatthy, P.; Seneviratne, S.L. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cave, A.; Arlett, P.; Lee, E. Inhaled and nasal corticosteroids: Factors affecting the risks of systemic adverse effects. Pharmacol. Ther. 1999, 83, 153–179. [Google Scholar] [CrossRef]
- Wan, E.S.; Qiu, W.; Baccarelli, A.; Carey, V.J.; Bacherman, H.; Rennard, S.I.; Agustí, A.; Anderson, W.H.; Lomas, D.A.; DeMeo, D.L. Systemic steroid exposure is associated with differential methylation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Han, E.J.; Kim, H.S.; Sanjeewa, K.K.A.; Herath, K.; Jeon, Y.J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Suzuki, Y.; Ra, C. Epigallocatechin-3-gallate induces cytokine production in mast cells by stimulating an extracellular superoxide-mediated calcium influx. Biochem. Pharm. 2011, 82, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hao, P.; Liu, G.; Wang, W.; Han, R.; Jiang, Z.; Li, X. Effects of 4-methylumbelliferone and high molecular weight hyaluronic acid on the inflammation of corneal stromal cells induced by LPS. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 559–566. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Nikolskaya, A.I.; Goriainov, S.V.; Astakhova, A.A.; Sergeeva, M.G. Inhibitor of Hyaluronic Acid Synthesis 4-Methylumbelliferone as an Anti-Inflammatory Modulator of LPS-Mediated Astrocyte Responses. Int. J. Mol. Sci. 2020, 21, 8203. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, L.N.; Wang, H.N.; Xie, C.C.; Li, W.Y.; Gao, P.; Hu, W.Z.; Zhao, Z.F.; Ji, K. FAK inhibitor PF-431396 suppresses IgE-mediated mast cell activation and allergic inflammation in mice. Biochem. Pharm. 2021, 192, 114722. [Google Scholar] [CrossRef]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcepsilonRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Sibilano, R.; Frossi, B.; Pucillo, C.E. Mast cell activation: A complex interplay of positive and negative signaling pathways. Eur. J. Immunol. 2014, 44, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Ji, K.; Zhang, L.N.; Xie, C.C.; Li, W.Y.; Zhao, Z.F.; Chen, J.J. Inhibition of c-Fos expression attenuates IgE-mediated mast cell activation and allergic inflammation by counteracting an inhibitory AP1/Egr1/IL-4 axis. J. Transl. Med. 2021, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, S.; Askew, E.B.; Ishizuka, N.; Knudson, C.B.; Knudson, W. 4-Methylumbelliferone Diminishes Catabolically Activated Articular Chondrocytes and Cartilage Explants via a Mechanism Independent of Hyaluronan Inhibition. J. Biol. Chem. 2016, 291, 12087–12104. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Matsumoto, S.; Fujita, Y.; Kuroda, A.; Menju, T.; Sonobe, M.; Kondo, N.; Torii, I.; Nakano, T.; Lara, P.N.; et al. Trametinib plus 4-Methylumbelliferone Exhibits Antitumor Effects by ERK Blockade and CD44 Downregulation and Affects PD-1 and PD-L1 in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2017, 12, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, I.; Freudenberger, T.; Twarock, S.; Fischer, J.W. Synergistic effect of targeting the epidermal growth factor receptor and hyaluronan synthesis in oesophageal squamous cell carcinoma cells. Br. J. Pharm. 2015, 172, 4560–4574. [Google Scholar] [CrossRef] [Green Version]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, H.; Uchakina, O.; McKallip, R.J. Hyaluronic Acid Inhibitor 4-Methylumbelliferone Activates the Intrinsic Apoptosis Pathway in K562 Chronic Myelogenous Leukemia Cells. Anticancer Res. 2015, 35, 5231–5240. [Google Scholar] [PubMed]
- Sim, M.O.; Ham, J.R.; Lee, H.I.; Seo, K.I.; Lee, M.K. Long-term supplementation of umbelliferone and 4-methylumbelliferone alleviates high-fat diet induced hypertriglyceridemia and hyperglycemia in mice. Chem. Biol. Interact. 2014, 216, 9–16. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-N.; Xiang, Q.-A.; Lin, H.-H.; Chen, J.-N.; Guo, W.-J.; Guo, W.-M.; Yue, X.-N.; Zhao, Z.-F.; Ji, K.; Chen, J.-J. Plant-Derived Molecule 4-Methylumbelliferone Suppresses FcεRI-Mediated Mast Cell Activation and Allergic Inflammation. Molecules 2022, 27, 1577. https://doi.org/10.3390/molecules27051577
Wang H-N, Xiang Q-A, Lin H-H, Chen J-N, Guo W-J, Guo W-M, Yue X-N, Zhao Z-F, Ji K, Chen J-J. Plant-Derived Molecule 4-Methylumbelliferone Suppresses FcεRI-Mediated Mast Cell Activation and Allergic Inflammation. Molecules. 2022; 27(5):1577. https://doi.org/10.3390/molecules27051577
Chicago/Turabian StyleWang, Hui-Na, Qiu-An Xiang, Hao-Hui Lin, Jie-Ning Chen, Wen-Jie Guo, Wan-Meng Guo, Xiang-Ning Yue, Zhen-Fu Zhao, Kunmei Ji, and Jia-Jie Chen. 2022. "Plant-Derived Molecule 4-Methylumbelliferone Suppresses FcεRI-Mediated Mast Cell Activation and Allergic Inflammation" Molecules 27, no. 5: 1577. https://doi.org/10.3390/molecules27051577
APA StyleWang, H.-N., Xiang, Q.-A., Lin, H.-H., Chen, J.-N., Guo, W.-J., Guo, W.-M., Yue, X.-N., Zhao, Z.-F., Ji, K., & Chen, J.-J. (2022). Plant-Derived Molecule 4-Methylumbelliferone Suppresses FcεRI-Mediated Mast Cell Activation and Allergic Inflammation. Molecules, 27(5), 1577. https://doi.org/10.3390/molecules27051577





