Heavy Metal Distribution in Surface Water and Sediment of Megech River, a Tributary of Lake Tana, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
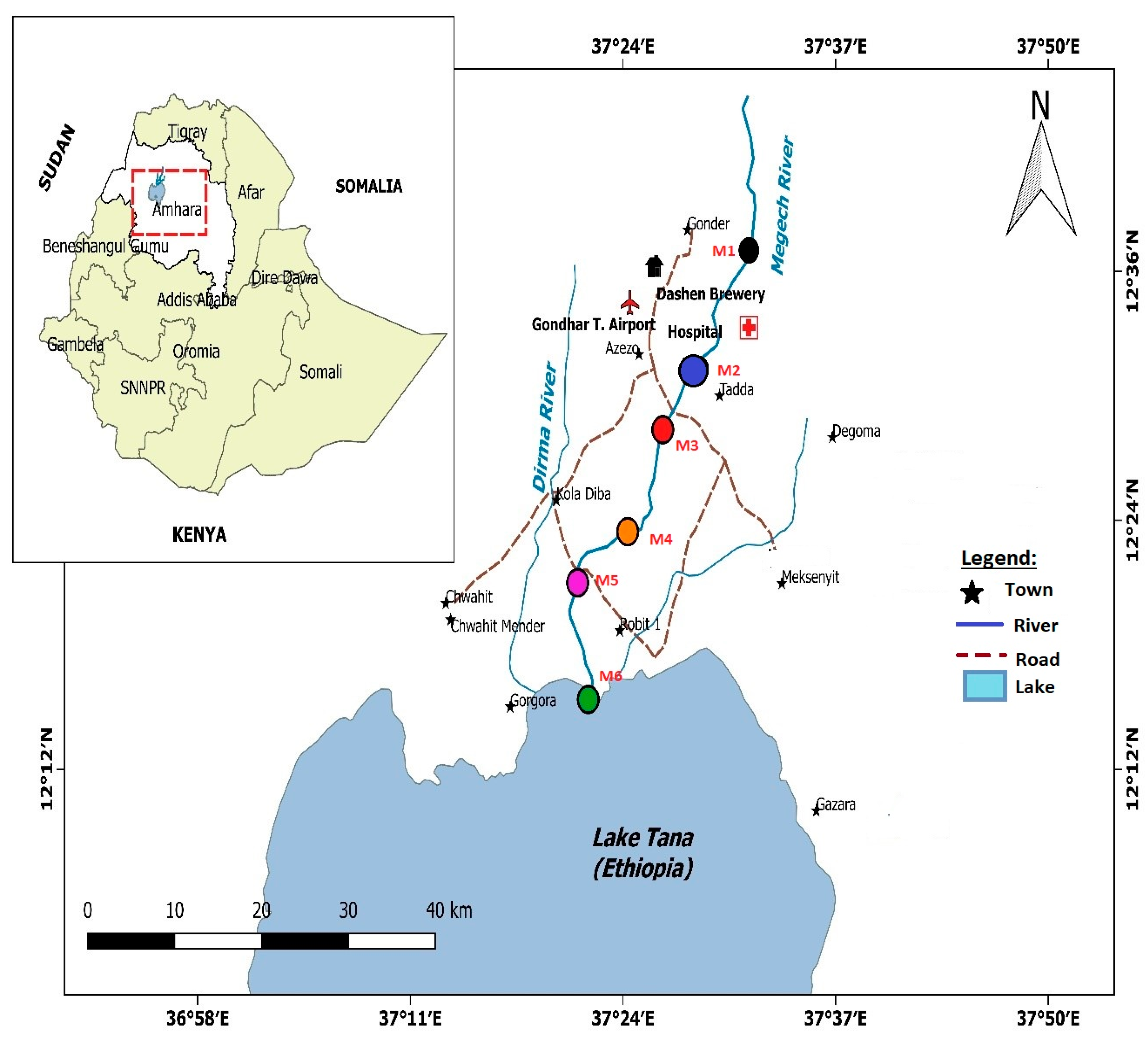
2.1. Description of the Study Area
2.2. Sampling Site Selection
2.3. Sample Collection
2.3.1. Environmental Variables
2.3.2. Sample Digestion and Analysis
2.4. Heavy Metals from Water
2.5. Heavy Metals from Sediment
2.6. Sediment Pollution Indices
2.7. Method Performance and Validation
Instrumental Working Conditions
2.8. Data Analysis
3. Results and Discussion
3.1. Optimization
Method Performance and Validation
3.2. Physico-Chemical Parameters of Megech River
3.3. Dissolved Heavy Metals Concentrations in Megech River Water
3.4. Heavy Metals Concentrations in the Surface Sediment of Megech River
3.5. Correlation Matrix
3.6. Principal Component Analysis
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akele, M.L.; Kelderman, P.; Koning, C.W.; Irvine, K. Trace metal distributions in the sediments of the Little Akaki. River, Addis Ababa, Ethiopia. Environ. Monit. Assess. 2016, 188, 389. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.G.; Schofield, K.A.; Lee, Y.S.; Norton, S.B. Response of chlorophyll a to total nitrogen and total phosphorus concentrations in lotic ecosystems: A systematic review protocol. Environ. Evid. 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Bhuyana, M.S.; Abu-Bakarb, M.; Akhtara, A.; Hossainc, M.B.; Alid, M.M.; Islam, M.S. Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environ. Nano Technol. Monit. Manag. 2017, 8, 273–279. [Google Scholar] [CrossRef]
- Dirbaba, N.B.; Yan, X.; Wu, H.; Colebrooke, L.L.; Wang, J. Occurrences and ecotoxicological risk assessment of heavy metals in surface sediments from Awash River basin, Ethiopia. Water 2018, 10, 535. [Google Scholar] [CrossRef] [Green Version]
- Kassegne, A.B.; Esho, T.B.; Okonkwo, J.O.; Asfaw, S.L. Distribution and ecological risk assessment of trace metals in surface sediments from Akaki River catchment and Aba Samuel reservoir, central Ethiopia. Environ. Syst. Res. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Kerketta, P.; Baxla, S.L.; Gora, R.H.; Kumari, S.; Roushan, R.K. Analysis of physico-chemical properties and heavy metals in drinking water from different sources in and around Ranchi, Jharkhand, India. Vet. World 2013, 6, 370–375. [Google Scholar] [CrossRef]
- Pigłowski, M. Heavy metals in notifications of rapid alert system for food and feed. Int. J. Environ. Res. Public Health 2018, 15, 365. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defence system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Paul, B. Assessment of heavy metal pollution in water resources and their impacts: A Review. J. Basic Appl. Eng. Res. 2016, 3, 671–675. Available online: http://www.krishisanskriti.org/Publication.html (accessed on 27 July 2021).
- Gebreyohannes, F.; Gebrekidan, A. Health risk assessment of heavy metals via consumption of spinach vegetable grown in Elalla River. Bull. Chem. Soc. Ethiop. 2018, 32, 65–75. [Google Scholar] [CrossRef]
- Showqi, I.; Lone, F.A.; Naikoo, M. Preliminary assessment of heavy metals in water, sediment and macrophyte (Lemna minor) collected from Anchar Lake, Kashmir, India. Appl. Water Sci. 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Njuguna, S.M.; Yan, X.; Gituru, R.W.; Wang, O.; Wang, J. Assessment of macrophyte, heavy metal, and nutrient concentrations in the water of the Nairobi River, Kenya. Environ. Monit. Assess. 2017, 189, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decena, S.C.P.; Arguelles, M.S.; Robel, L.L. Assessing heavy metal contamination in surface sediments in an urban river in the Philippines. Pol. J. Environ. Stud. 2018, 27, 1983–1995. [Google Scholar] [CrossRef]
- Kelderman, P.; Osman, A.A. Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Res. 2007, 41, 4251–4261. [Google Scholar] [CrossRef] [PubMed]
- Fural, S.; Kükrer, S.; Cürebal, I. Geographical information systems based ecological risk analysis of metal accumulation in sediments of İkizcetepeler Dam Lake (Turkey). Ecol. Indic. 2020, 119, 106784. [Google Scholar] [CrossRef]
- Mekonnen, F.H. Liquid waste management: The case of Bahir Dar, Ethiopia. Ethiop. J. Health Dev. 2012, 26, 49–53. Available online: https://www.ajol.info/index.php/ejhd/article/view/83828 (accessed on 24 January 2019).
- Beyene, H.; Redaie, G. Assessment of Waste Stabilization Ponds for the Treatment of Hospital Wastewater: The Case of Hawassa University Referral Hospital. World Appl. Sci. J. 2011, 15, 142–150. Available online: https://www.idosi.org/wasj/wasj15(1)11/21.pdf (accessed on 24 January 2019).
- Engdaw, F.; Dadebo, E.; Naggapan, R. Morphometric Relationships and Feeding Habits of Nile Tilapia Oreochromis niloticus (L.) (Pisces: Cichlidae) from Lake Koka, Ethiopia. Int. J. Fish. Aqua. Sci. 2013, 2, 65–71. Available online: https://maxwellsci.com/print/ijfas/v2-65-71.pdf (accessed on 17 November 2021).
- FAO. Food and Agriculture Organization of the United Nations Rome and the International Water Management Institute on Behalf of the Water Land and Ecosystems Research Program. Colombo. 2017. Available online: http://www.fao.org/3/a-i7754e.pdf (accessed on 24 January 2019).
- Gebremedhin, S.; Getahun, A.; Anteneh, A.; Bruneel, S.; Goethals, P.A. Drivers-Pressure-State Impact Responses Framework to support the sustainability of fish and Fisheries in Lake Tana, Ethiopia. Sustainability 2018, 10, 2957. [Google Scholar] [CrossRef] [Green Version]
- Lanckriet, S.; Nyssen, J.; Adgo, E. Environmental change in lake catchments of Ethiopia. Land Degrad. Dev. 2017, 28, 1793–1794. [Google Scholar] [CrossRef]
- Alemu, M.L.; Geset, M.; Mosa, H.M.; Zemale, F.A.; Moges, M.A.; Giri, S.K.; Tillahun, S.A.; Melesse, A.M.; Ayana, E.K.; Steenhuis, T.S. Spatial and temporal trends of recent dissolved phosphorus concentrations in Lake Tana and its four main tributaries. Land Degrad. Dev. 2017, 28, 1742–1751. [Google Scholar] [CrossRef]
- Wondim, Y.K. Water quality status of Lake Tana, Ethiopia. Civil. Environ. Res. 2016, 8, 3–51. Available online: https://core.ac.uk/download/pdf/234678437.pdf (accessed on 17 December 2018).
- Goshu, G.; Aynalem, S. Problem overview of the Lake Tana basin. Soc. Ecol. Syst. Dyn. 2017, 9–23. [Google Scholar] [CrossRef]
- Aghoghovwia, O.A.; Miri, F.A.; Izah, S.C. Impacts of anthropogenic activities on heavy metal levels in surface water of Nun River around Gbarantoru and Tombia Towns, Bayelsa State, Nigeria. Ann. Ecol. Environ. Sci. 2018, 2, 1–8. Available online: http://www.sryahwapublications.com/annals-of-ecology-and-environmental-science/pdf/v2-i2/1.pdf (accessed on 5 January 2022).
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating Cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Sambito, M.; Freni, G. Strategies for Improving Optimal Positioning of Quality Sensors in Urban Drainage Systems for Non-Conservative Contaminants. Water 2021, 13, 934. [Google Scholar] [CrossRef]
- Abebe, E.; Kebede, A. Assessment of Climate Change Impacts on the Water Resources of Megech River Catchment, Abbay Basin, Ethiopia. Open J. Mod. Hydrol. 2017, 7, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Anteneh, W.; Mengist, M.; Wondie, A.; Tewabe, D.; WeldeKidan, W.; Assefa, A.; Engida, W. Water Hyacinth Coverage Survey Report on Lake Tana; Technical Report Series 1; Bahir Dar University: Bahir Dar, Ethiopia, 2014; pp. 1–29. [Google Scholar]
- American Public Health Association (APHA); American Water Works Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Asefa, W.; Beranu, T. Levels of Some Trace Metals in Fishes Tissues, Water and Sediment at Tendaho Water Reservoir, Afar Region, Ethiopia. J. Environ. Anal. Toxicol. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Müller, G. The Heavy Metal Pollution of the Sediments of Neckars and its Tributary: A Stocktaking. Chem. Ztg. 1981, 105, 157–164. [Google Scholar]
- Ladigbolu, L.A.; Balogun, K.J. Distribution of heavy metals in sediments of selected streams in Ibadan me tropolis, Nigeria. Int. J. Environ. Sci. 2011, 1, 1186–1191. [Google Scholar]
- Arti Yadav, A.; Yadav, P.K. Pollution Load Index (PLI) of Field Irrigated with Wastewater of Mawaiya Drain in Naini Suburbs of Allahabad District. Curr. World Environ. 2018, 13, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Zahra, A.; Hashmi, M.Z.; Malik, R.N.; Ahmed, Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-Feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014, 470, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Akele, M.L.; Abebe, D.Z.; Alemu, A.K.; Assefa, A.G.; Madhusudhan, A.; de Oliveira, R.R. Analysis of trace metal concentrations in raw cow’s milk from three dairy farms in North Gondar, Ethiopia: Chemometric approach. Environ. Monit. Assess. 2017, 189, 499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, A.; Li, M.; Zhang, X. Effect of pH, Temperature, Dissolved Oxygen, and Flow Rate of Overlying Water on Heavy Metals Release from Storm Sewer Sediments. J. Chem. 2013, 2013, 434012. [Google Scholar] [CrossRef]
- Egun, N.K.; Ogiesoba-Eguakun, C.U. Physico-chemical and Water Quality Index analysis of the Okhuaihe River, Edo State, Nigeria. Afr. J. Aquat. Sci. 2018, 43, 345–351. [Google Scholar] [CrossRef]
- Oboh, I.P.; Agbala, C.S. Water quality assessment of the Siluko River, southern Nigeria. Afr. J. Aquat. Sci. 2017, 42, 279–286. [Google Scholar] [CrossRef]
- DWAF. South African Water Quality Guidelines. 1: Domestic Uses, 2nd ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996. Available online: https://www.iwanetwork.org/filemanager-uploads/WQ_Compendium/Database/Selected_guidelines/041.pdf (accessed on 15 February 2022).
- USEPA. The Incidence and Severity of Sediment Contamination in Surface Waters of the United States, 2nd ed.; National Sediment Quality Survey; USEPA: Washington, DC, USA, 2004; EPA823-R-04-007.
- WHO. Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf (accessed on 13 March 2019).
- Fernández-Luqueño, F.; Valdez, F.L.; Melo, P.G.; Suárez, S.L.; Gonzále, E.N.A.; Martínez, A.I.; Guillermo, M.S.G.; Martínez, G.H.; Mendoza, R.H.; Garza, M.A.A.; et al. Heavy metal pollution in drinking water a global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar] [CrossRef]
- Kassa, Y. Macrophyte Ecology, Nutrient Dynamics and Water Quality of the Littoral Zone and Yitamot Wetland, Lake Tana, Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2016; p. 157. [Google Scholar]
- Mengesha, A.; Mekuria, A.; Husen, A. Algal biomass and nutrient enrichment in the Angereb reservoir, Gondar, Ethiopia. Environ. Exp. Biol. 2013, 11, 91–98. Available online: http://eeb.lu.lv/EEB/201306/EEB_11_Mengesha.pdf (accessed on 15 February 2019).
- Walle, H.; Zewde, S.; Heldal, T. Building stone of central and southern Ethiopia: Deposits and resource potential. NGU Bull. 2000, 436, 175–182. Available online: https://www.ngu.no/FileArchive/102/Bulletin436_17.pdf (accessed on 21 March 2019).
- Rameshkumar, S.; Radhakrishnan, K.; Aanand, S.; Rajaram, R. Influence of physicochemical water quality on aquatic macrophyte diversity in seasonal wetlands. Appl. Water Sci. 2019, 9, 12. [Google Scholar] [CrossRef]
- Wondie, A.; Mengistou, S.; Vijverberg, J.; Dejen, E. Seasonal variation in primary production of a high-altitude tropical lake (Lake Tana, Ethiopia): Effects of nutrient availability and water transparency. Aquat. Ecol. 2007, 41, 207. [Google Scholar] [CrossRef]
- Ratan, A.; Verma, V.N. Photochemical studies of Eichornia crassipes (Water Hyacinth). Int. Lett. Chem. Phys. Astron. 2014, 11, 214–222. [Google Scholar]
- Admas, A.; Sahle, S.; Belete, E.; Agidie, A.; Alebachew, M. Controlling Water Hyacinth in Lake Tana Using Biological Method at Green House and Pond Level. Eur. Exp. Biol. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Ugonna, C.N.; Precious, O.O.; Nneka, I.O. A Review of the Health Implications of Heavy Metals in Food Chain in Nigeria. Sci. World J. 2020, 2020, 6594109. [Google Scholar] [CrossRef]
- Okunowo, W.O.; Ogunkanmi, L.A. Phytoremediation potential of some heavy metals by water hyacinth. Int. J. Biol. Chem. Sci. 2010, 4, 347–353. Available online: https://www.ajol.info/index.php/ijbcs/article/view/58121 (accessed on 21 February 2019). [CrossRef] [Green Version]
- Sun, H.; Alexander, J.; Gove, B.; Pezzi, E.; Chakowski, N.; Husch, J. Mineralogical and anthropogenic controls of stream water chemistry in salted watersheds. Appl. Geochem. 2014, 48, 141–154. [Google Scholar] [CrossRef]
- Tessema, K.; Lemma, B.; Fetahi, T.; Kebede, E. Accumulation of heavy metals in the physical and biological systems of Lake Koka, Ethiopia: Implications for potential health risks. Lakes Reserv. 2020, 25, 314–325. [Google Scholar] [CrossRef]
- Hou, D.; He, J.; Lü, C.; Sun, Y.; Zhang, F.; Otgonbayar, K. Effects of environmental factors on nutrients release at sediment-water interface and assessment of trophic status for a typical shallow Lake, Northwest China. Sci. World J. 2013, 2013, 630654. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.; Zhao, X.; Dong, J.; Zheng, B. Risk assessment of heavy metals in the surface sediment at the drinking water source of the Xiangjiang River in South China. Environ. Sci. Eur. 2020, 32, 23. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Li, F.; Liu, C.; Qiu, Z.; Xiao, M.; Cai, Y. Comparison of toxic metal distribution characteristics and health risk between cultured and wild fish captured from Honghu city, China. Int. J. Environ. Res. Public Health 2018, 15, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dsikowitzky, L.; Mengesha, M.; Dadebo, E.; Carvalho, C.E.V.; Sindern, S. Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environ. Monit. Assess. 2012, 185, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Filho, J.L.; Abe, D.S.; Gatti-Junior, P.; Medeiros, G.R.; Degani, R.M.; Blanco, F.P.; Faria, C.R.L.; Campanelli, L.; Soares, F.S.; Sidagis-Galli, C.V.; et al. Spatial patterns of water quality in Xingu River Basin (Amazonia) prior to the Belo Monte dam impoundment. Braz. J. Biol. 2015, 75, S34–S46. [Google Scholar] [CrossRef] [Green Version]
- Ogundiran, M.A.; Fawole, O.O. Toxic Effects of Water Pollution on Two Bio-indicators of Aquatic Resources of Asa River, Nigeria. J. Fish. Sci. 2018, 12, 20–27. [Google Scholar] [CrossRef]



| Element | Wave Length | Temperature (°C) Ramp Drying and Payrolysis | Slit Width | % of Recovery | MDL | |
|---|---|---|---|---|---|---|
| Water | Sediment | |||||
| Fe | 248.3 | 1500/12 s | 0.7 | 94.47 | 98.1 | 0.02 |
| Mn | 279.5 | 1600/10 s | 0.7 | 97.14 | 104.2 | 0.018 |
| Cu | 324.8 | 900/8 s | 0.7 | 95.85 | 103.4 | 0.024 |
| Zn | 213.8 | 1000/5 s | 0.7 | 105.17 | 93.76 | 0.024 |
| Cr | 357.9 | 1100/12 s | 0.7 | 92.70 | 106.2 | 0.018 |
| Cd | 228.8 | 700/22 s | 0.7 | 97.19 | 101.4 | 0.018 |
| Site | Temp (°C) | pH | DO (mg L−1) | EC (µS/cm) | Tur (NTU) |
|---|---|---|---|---|---|
| M1 | 16.9 ± 0.36 * | 8.4 ± 0.01 | 9.9 ± 0.12 * | 421.8 ± 2.28 * | 89.2 ± 4.33 * |
| M2 | 19.4 ± 0.72 | 8.4 ± 0.01 | 7.8 ± 0.41 | 658.4 ± 8.65 * | 223.2 ± 8.98 |
| M3 | 22.6 ± 0.18 | 8.7 ± 0.02 | 8.3 ± 0.04 | 431.6 ± 1.95 | 419.9 ± 3.77 * |
| M4 | 23.1 ± 0.15 * | 8.6 ± 0.02 | 7.9 ± 0.16 | 438.6 ± 1.14 | 416.0 ± 14.4 |
| M5 | 21.9 ± 0.42 | 8.5 ± 0.01 | 7.7 ± 0.07 | 462.4 ± 0.55 * | 575.0 ± 10.22 * |
| M6 | 24.9 ± 0.29 * | 8.3 ± 0.07 | 7.5 ± 0.47 * | 148.7 ± 0.35 * | 52.7 ± 4.90 * |
| Site | Fe | Mn | Cu | Zn | Cr | Cd | Pb |
|---|---|---|---|---|---|---|---|
| Water | |||||||
| M1 | 4.4 ± 0.10 | 1.8 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.03 | 0.03 ± 0.01 | ND | 0.04 ± 0.01 |
| M2 | 7.6 ± 0.05 | 4.3 ± 0.02 | 0.11 ± 0.02 | 0.15 ± 0.03 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| M3 | 4.1 ± 0.02 | 3.4 ± 0.01 | 0.13 ± 0.03 | 0.13 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| M4 | 2.5 ± 0.03 | 3.7 ± 0.01 | 0.17 ± 0.01 | 0.13 ± 0.02 | ND | ND | 0.03 ± 0.01 |
| M5 | 4.4 ± 0.03 | 2.3 ± 0.01 | 0.12 ± 0.02 | 0.11 ± 0.0 | 0.05 ± 0.02 | 0.03 ± 0.01 | ND |
| M6 | 4.5 ± 0.06 | 0.86 ± 0.02 | 0.13 ± 0.03 | 0.11 ± 0.02 | ND | ND | 0.06 ± 0.02 |
| Sediment | |||||||
| M1 | 390.8 ± 5.03 | 160.0 ± 2.58 | 10.24 ± 1.27 | 4.58 ± 0.55 | 1.78 ± 0.25 | 0.78 ± 0.24 | 1.22 ± 0.26 |
| M2 | 520.6 ± 3.92 | 241.8 ± 2.86 | 12.68 ± 1.09 | 6.06 ± 0.52 | 2.56 ± 0.41 | 1.24 ± 0.11 | 1.82 ± 0.23 * |
| M3 | 330.1 ± 4.44 | 98.6 ± 2.95 | 8.02 ± 1.29 | 3.74 ± 0.49 | 1.34 ± 0.23 | 0.68 ± 0.15 | 1.02 ± 0.19 * |
| M4 | 466.6 ± 6.07 | 198.6 ± 3.52 | 11.50 ± 1.25 | 5.38 ± 0.56 | 2.22 ± 0.26 | 1.12 ± 0.15 | 1.66 ± 0.22 |
| M5 | 458.2 ± 4.78 | 192.8 ± 3.22 | 11.38 ± 1.10 | 5.42 ± 0.56 | 2.06 ± 0.30 | 1.06 ± 0.15 | 1.48 ± 0.29 |
| M6 | 390.1 ± 5.94 | 162.0 ± 3.07 | 9.76 ± 1.49 | 4.36 ± 0.77 | 1.82 ± 0.26 | 0.90 ± 0.16 | 1.38 ± 0.24 |
| Drinking Water | Irrigation | Livestock | |||||
|---|---|---|---|---|---|---|---|
| Metal | Current Study | WHO | USEPA | ECE | SA. | SA. | SA. |
| Cu | 0.11–1.17 (0.14) | 2 | 1.3 | 2 | 1.0 | 0.2 | 5 |
| Zn | 0.11–0.16 (0.13) | 3 | 5 | 5 | 3 | 1.0 | 20 |
| Cr | 0.03–0.05 (0.03) | 0.05 | 0.1 | 0.05 | 0.05 | NA | 1.0 |
| Cd | 0.03–0.04 (0.02) | 0.003 | 0.005 | 0.005 | 0.005 | 0.01 | 0.01 |
| Pb | 0.03–0.06 (0.04) | 0.01 | 0.015 | 0.01 | 0.01 | 0.2 | 0.1 |
| Fe | 2.5–7.6 (4.58) | 0.3 | 0.3 | 0.2 | NA | NA | NA |
| Mn | 0.86–4.3 (2.73) | 0.4 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Reference | Cu | Zn | Cr | Cd | Pb | |
|---|---|---|---|---|---|---|
| SQG | TEC | 32 | 120 | 43 | 0.99 | 36 |
| PEC | 150 | 460 | 110 | 5 | 130 | |
| Present study | Min | 8.02 | 3.74 | 1.34 | 0.68 | 1.02 |
| Max | 12.68 | 6.06 | 2.56 | 1.24 | 1.82 | |
| Mean | 10.60 | 4.92 | 1.96 | 0.96 | 1.43 | |
| Other studies (2015–2018) | L.Akaki [1] | 59.06 | 228.53 | 70.96 | ND | 238.17 |
| G. Akaki [5] | 4.2 | 21.8 | 24.5 | 2.6 | 137.7 | |
| Aba Yohannes [5] | 45 | 10 | 25 | 2.6 | 136.8 | |
| Elalla [11] | 40.03 | 387.5 | 47.2 | 16.25 | 1.58 | |
| Awash [4] | 79.43 | 382.73 | 120.58 | 2.60 | 13.53 | |
| Tendaho [30] | 10.35 | 21.89 | 2.52 | 1.03 | 5.78 | |
| L. Awassa [53] | 8.69 | 93.80 | 8.27 | 0.21 | 15.7 |
| Fe | Mn | Cu | Zn | Cr | Cd | Pb | |
|---|---|---|---|---|---|---|---|
| Sediment | |||||||
| Fe | 1 | ||||||
| Mn | 0.94 ** | 1 | |||||
| Cu | 0.91 ** | 0.96 ** | 1 | ||||
| Zn | 0.95 ** | 0.94 ** | 0.93 ** | 1 | |||
| Cr | 0.89 ** | 0.95 ** | 0.87 ** | 0.92 ** | 1 | ||
| Cd | 0.92 ** | 0.89 ** | 0.866 ** | 0.92 ** | 0.87 ** | 1 | |
| Pb | 0.89 ** | 0.91 ** | 0.866 ** | 0.869 ** | 0.87 ** | 0.92 * | 1 |
| Water | |||||||
| Fe | 1 | ||||||
| Mn | −0.60 ** | 1 | |||||
| Cu | −0.62 ** | 0.28 | 1 | ||||
| Zn | 0.44 * | −0.35 | −0.03 | 1 | |||
| Cr | 0.55 ** | −0.13 | −0.35 | 0.12 | 1 | ||
| Cd | 0.51 ** | 0.12 | −0.37 | 0.14 | 0.66 ** | 1 | |
| Pb | 0.08 | −0.44 * | 0.11 | 0.05 | −0.48 ** | −0.32 | 1 |
| Variable | PC1 | PC2 |
|---|---|---|
| Eigenvalue | 7.38 | 3.73 |
| Variance | 38.85 | 23.87 |
| Cumulative variance | 38.85 | 62.72 |
| pH | −0.21 | 0.82 |
| DO | 0.11 | −0.79 |
| EC | 0.61 | 0.31 |
| Tur | 0.08 | 0.97 |
| Fe-s | 0.95 | 0.06 |
| Mn-s | 0.96 | −0.07 |
| Pb-s | 0.89 | 0.02 |
| Pb-w | −0.27 | −0.67 |
| Mn-w | −0.23 | 0.91 |
| Cr-s | 0.92 | −0.04 |
| Fe-w | 0.56 | −0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engdaw, F.; Hein, T.; Beneberu, G. Heavy Metal Distribution in Surface Water and Sediment of Megech River, a Tributary of Lake Tana, Ethiopia. Sustainability 2022, 14, 2791. https://doi.org/10.3390/su14052791
Engdaw F, Hein T, Beneberu G. Heavy Metal Distribution in Surface Water and Sediment of Megech River, a Tributary of Lake Tana, Ethiopia. Sustainability. 2022; 14(5):2791. https://doi.org/10.3390/su14052791
Chicago/Turabian StyleEngdaw, Flipos, Thomas Hein, and Getachew Beneberu. 2022. "Heavy Metal Distribution in Surface Water and Sediment of Megech River, a Tributary of Lake Tana, Ethiopia" Sustainability 14, no. 5: 2791. https://doi.org/10.3390/su14052791
APA StyleEngdaw, F., Hein, T., & Beneberu, G. (2022). Heavy Metal Distribution in Surface Water and Sediment of Megech River, a Tributary of Lake Tana, Ethiopia. Sustainability, 14(5), 2791. https://doi.org/10.3390/su14052791







