Transition Metal Substituted Barium Hexaferrite-Modified Electrode: Application as Electrochemical Sensor of Acetaminophen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Transition-Metal-Substituted Barium Hexaferrites
2.1.1. X-ray Diffraction Analysis
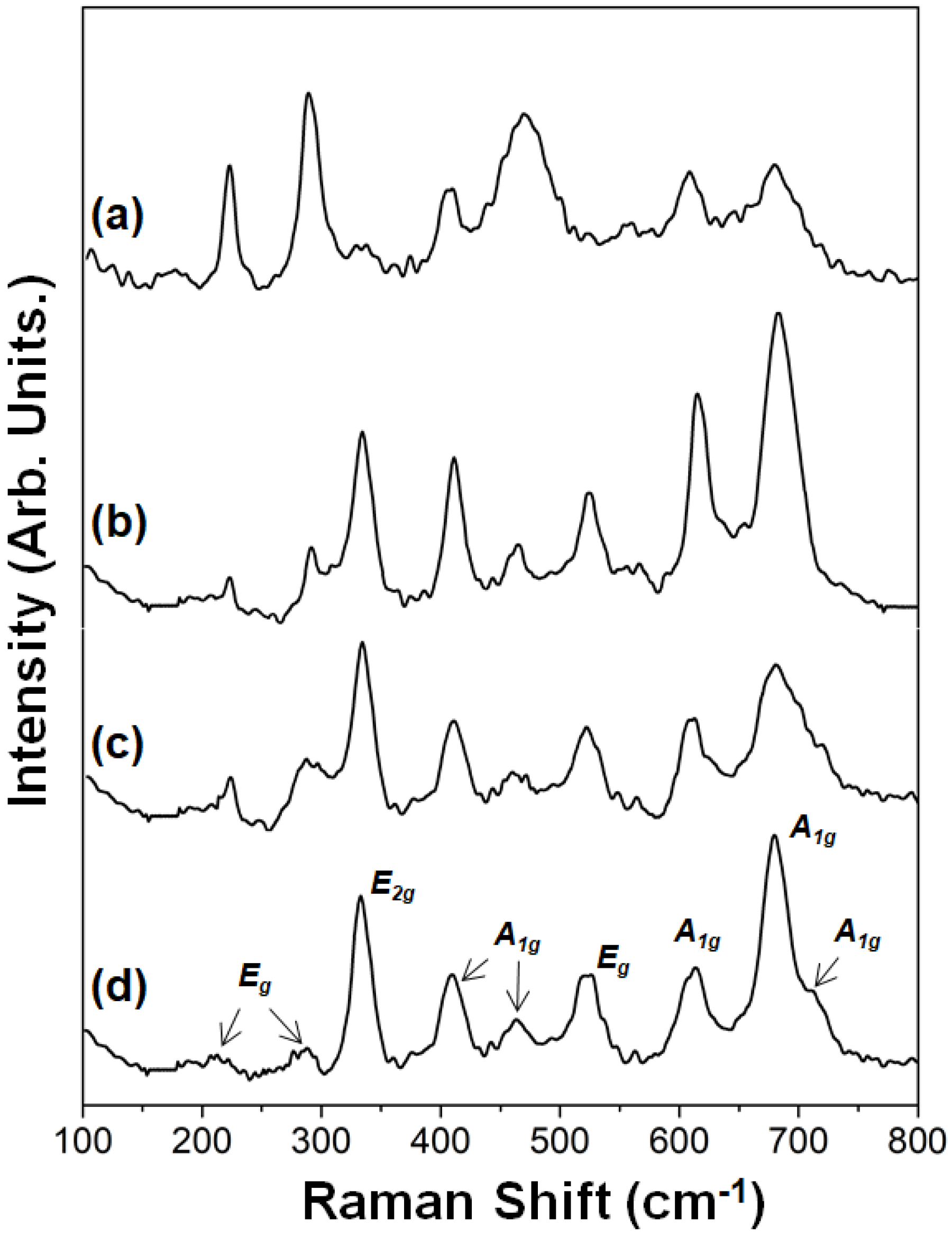
2.1.2. Raman Spectroscopy
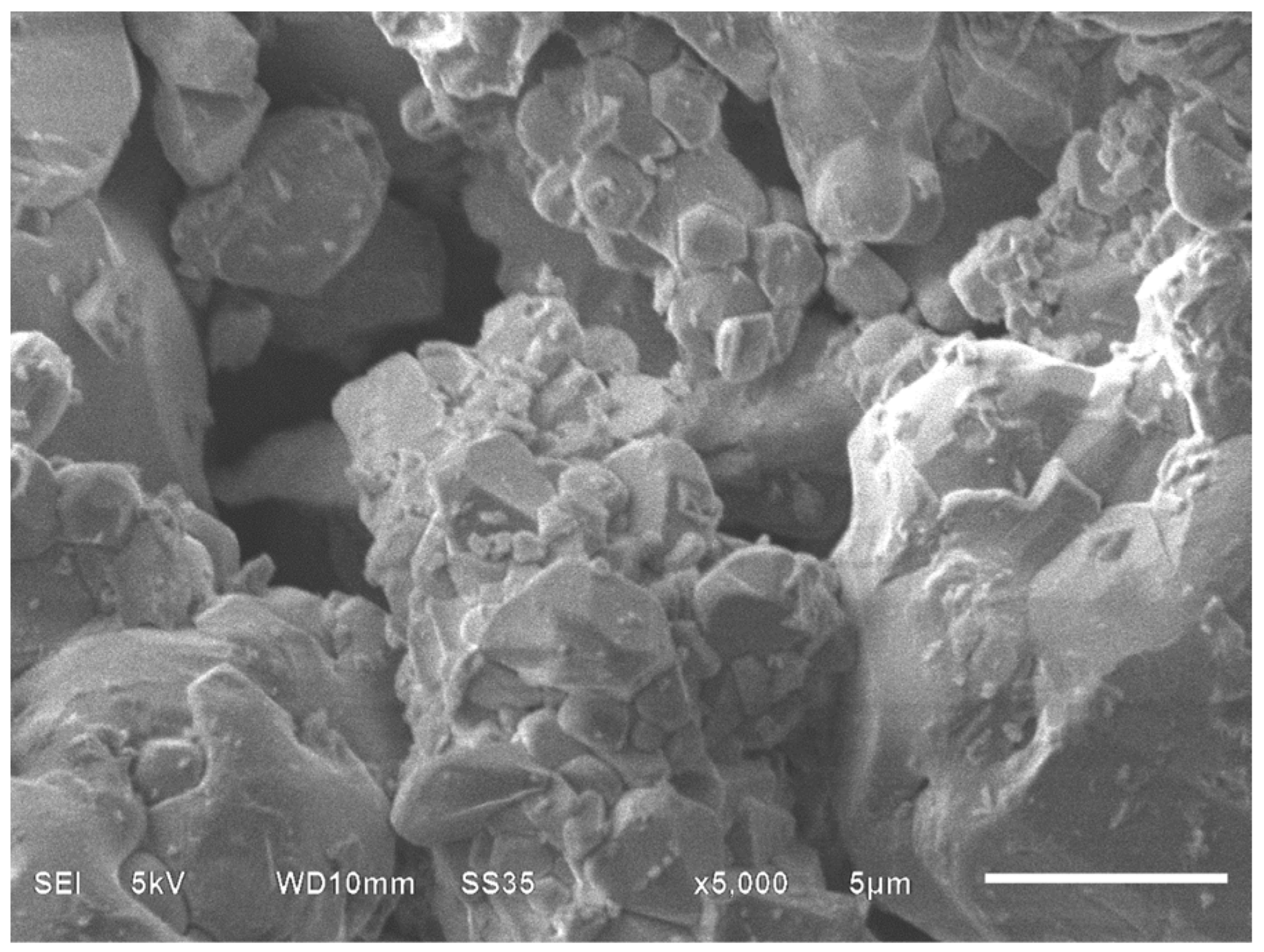
2.1.3. Field-Emission Scanning Electron Microscopy Analysis
2.2. Characterization by Electrocatalysis
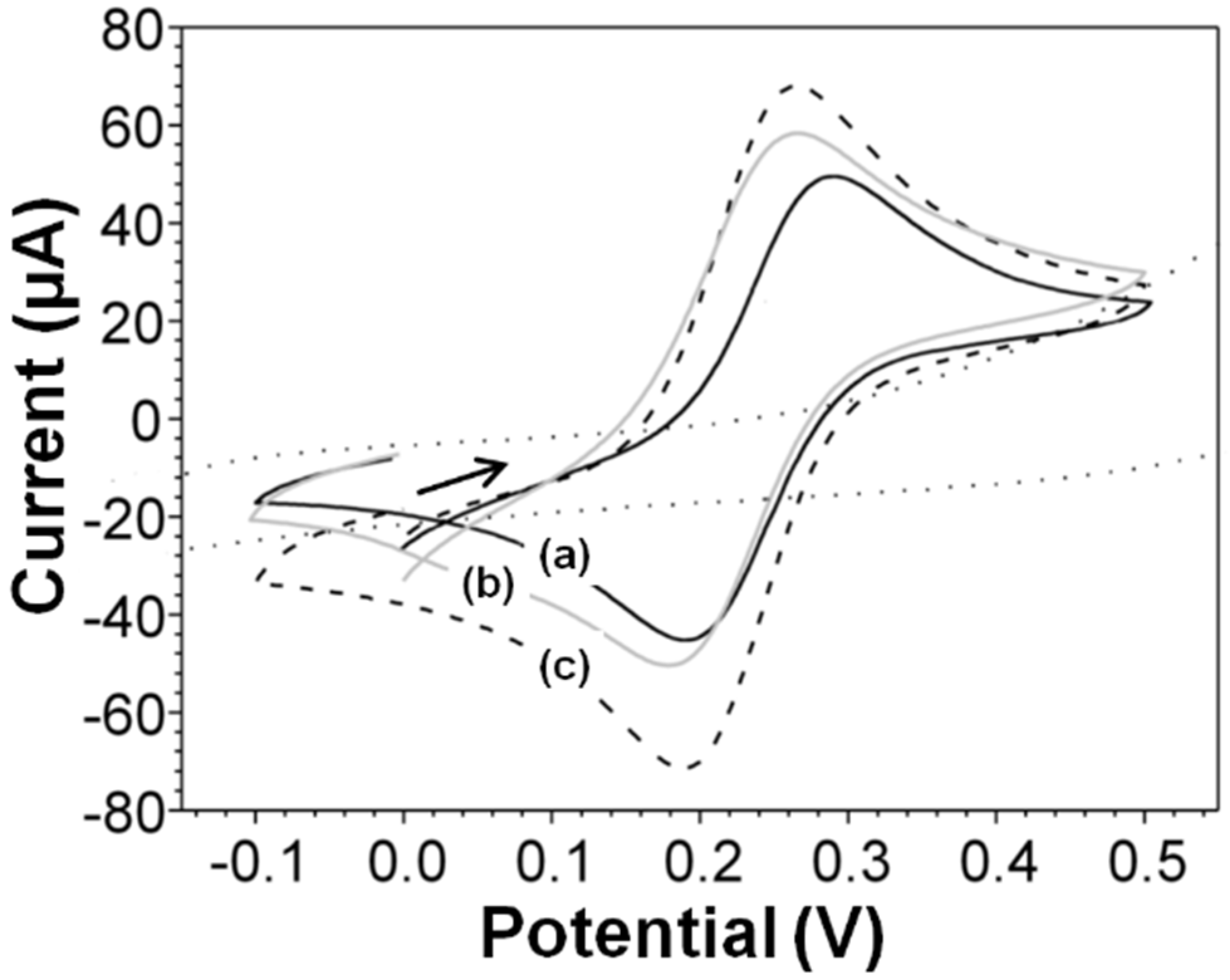
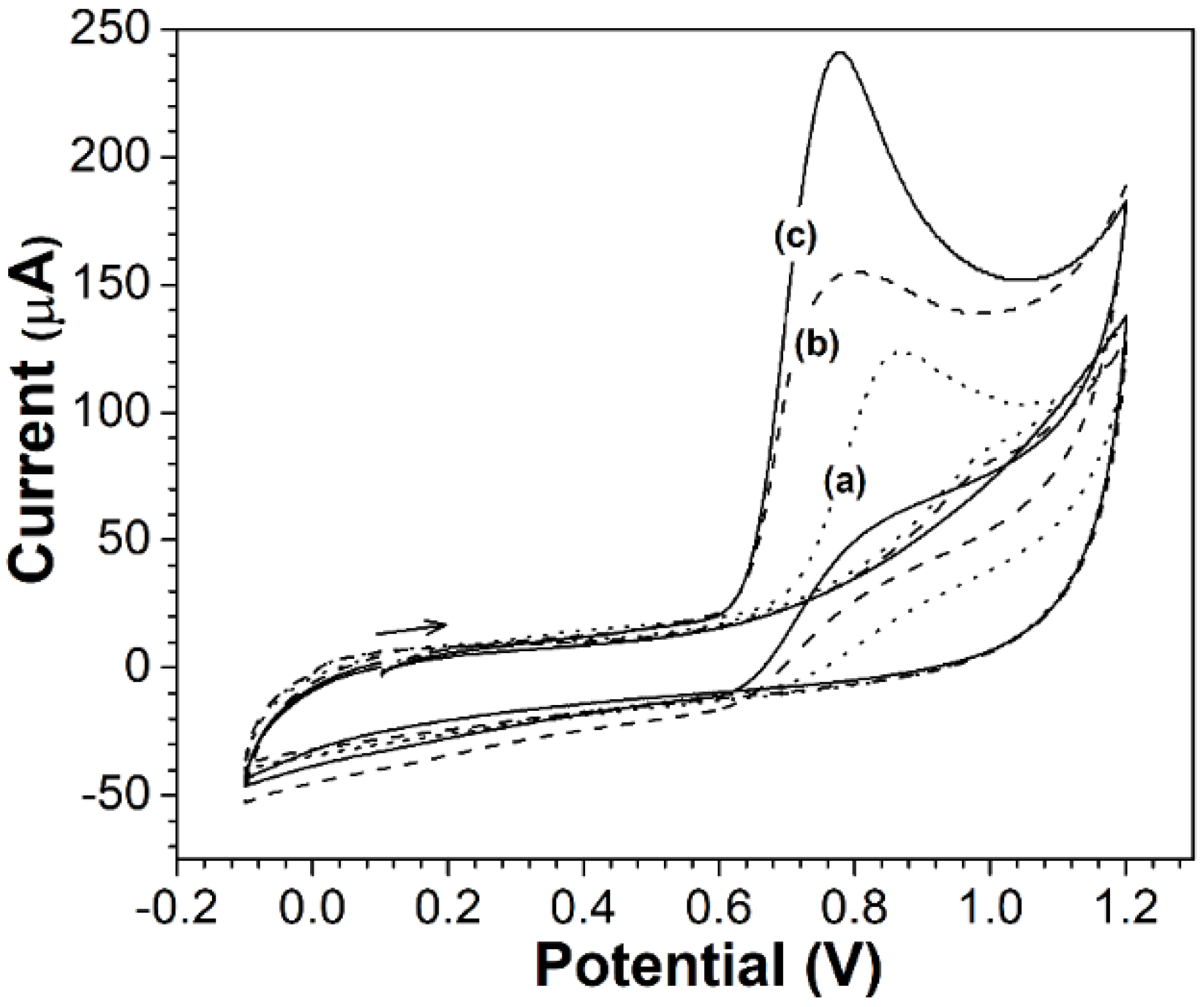
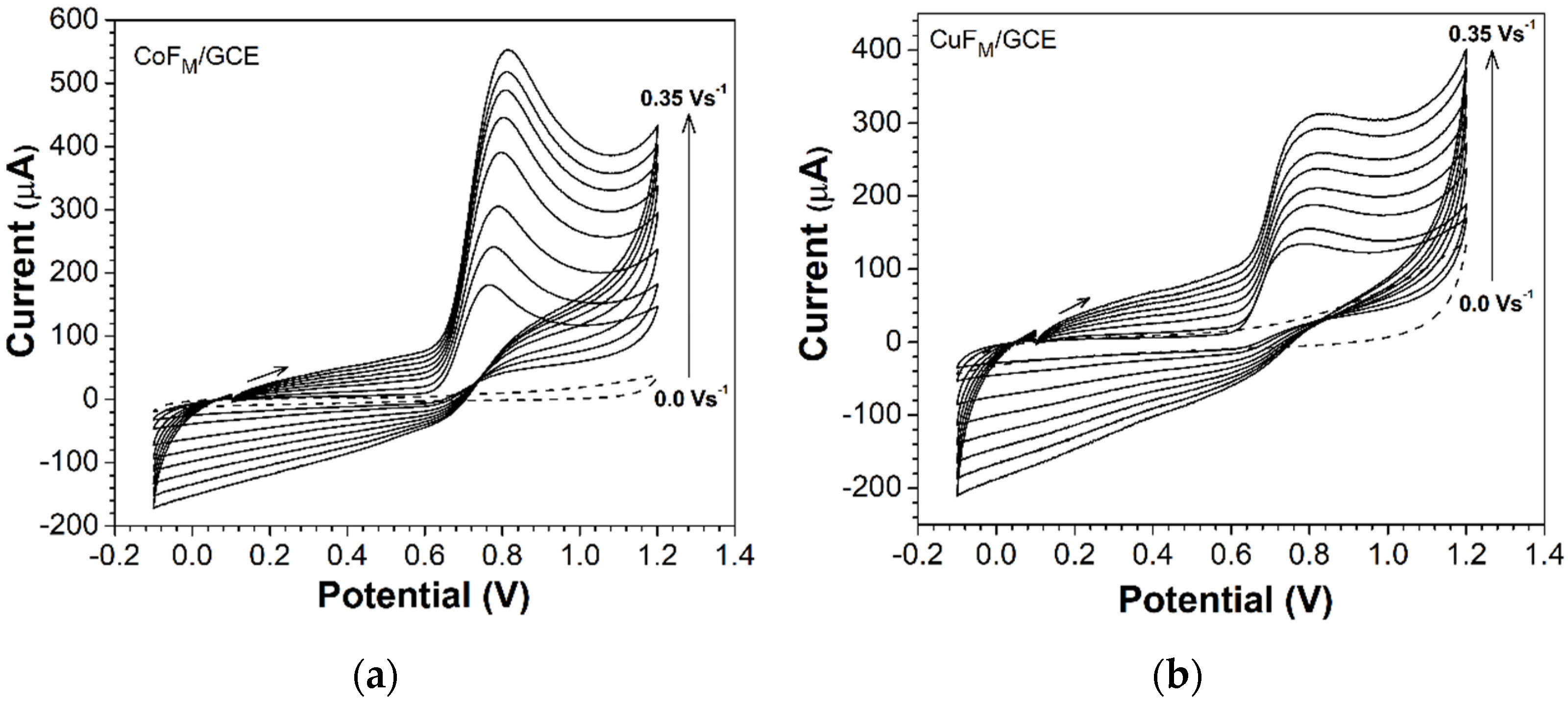
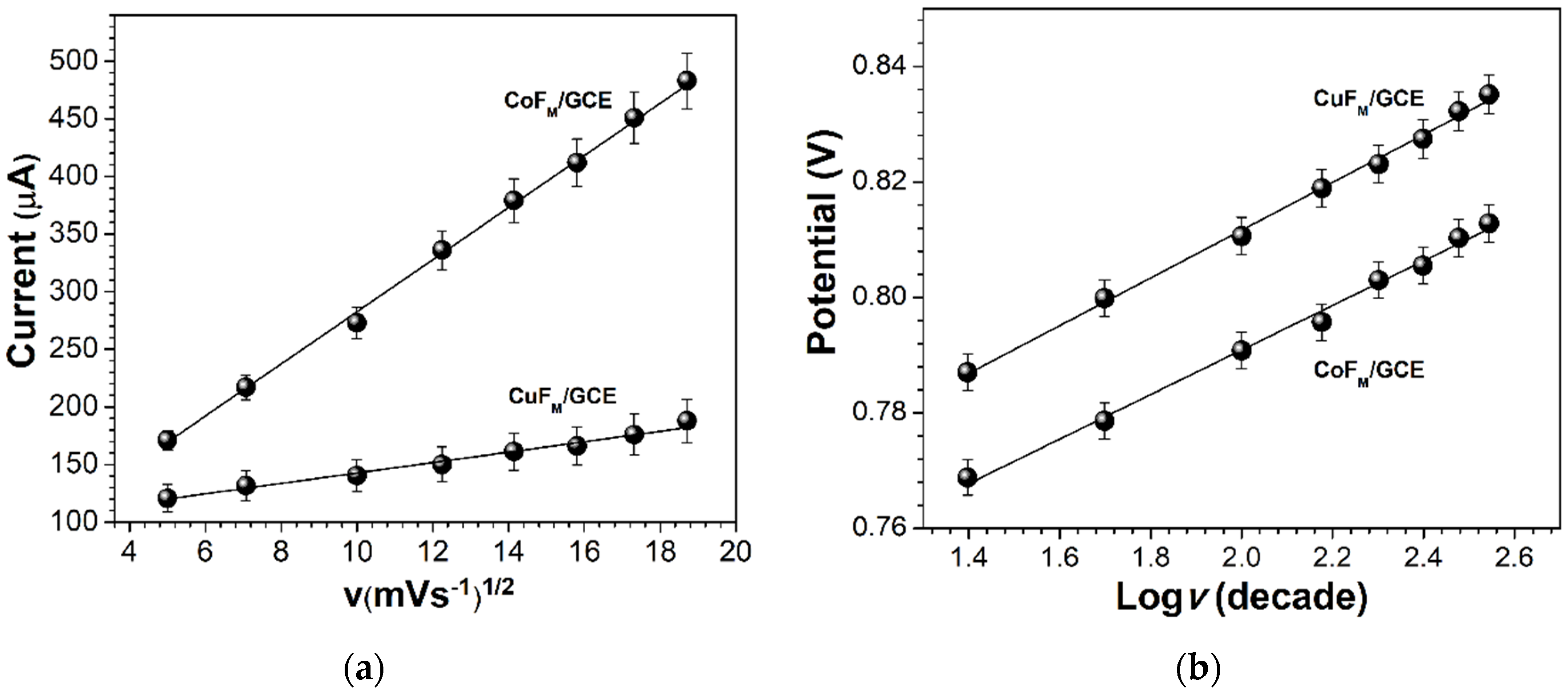
2.2.1. Electrochemical Characterization of Electrodes
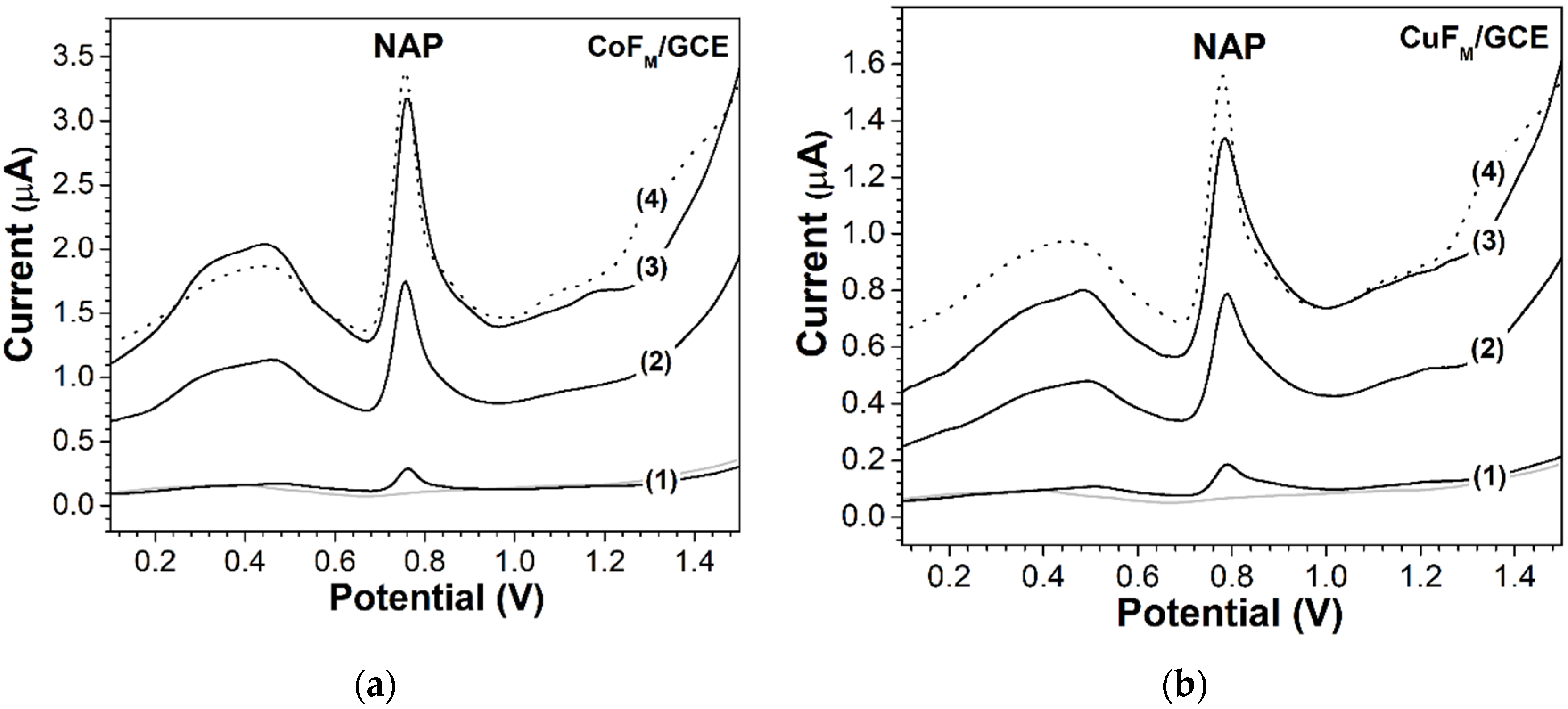
2.2.2. Electrochemical Response of NAP
2.3. Electroanalysis of Acetaminophen NAP in Acidic Medium
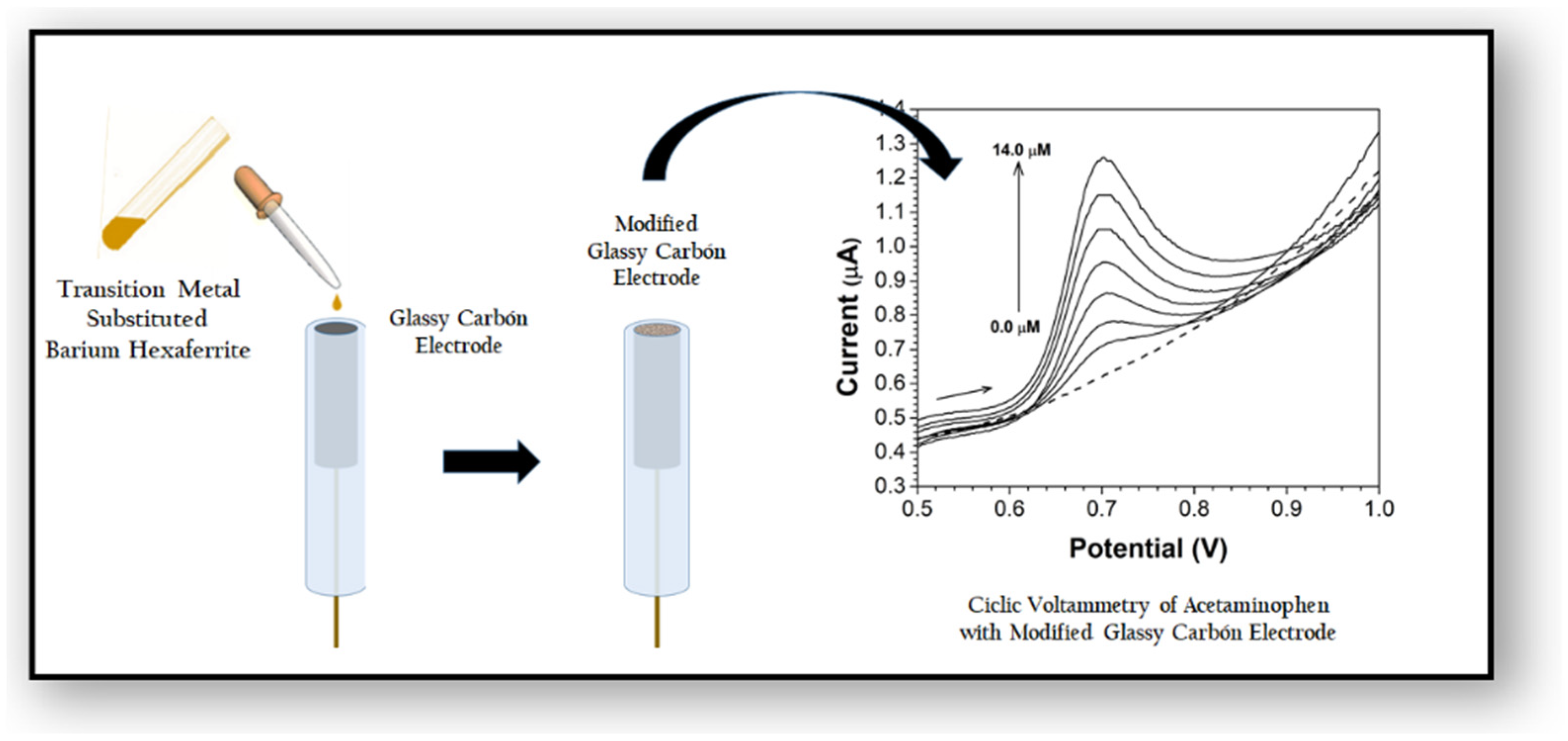
2.3.1. Concentration Analysis of NAP
2.3.2. Reproducibility and Stability in the Analysis of NAP with Modified Electrodes
2.3.3. Analysis of Enriched and Real Urine Samples
3. Materials and Methods
Electrode Modification and Electrochemical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gutes, A.; Calvo, D.; Cespedes, F.; del Valle, M. Automatic sequential injection analysis electronic tongue with integrated reference electrode for the determination of ascorbic acid, uric acid and paracetamol. Microchim. Acta 2007, 157, 1–6. [Google Scholar] [CrossRef]
- Moreira, A.B.; Oliveira, H.P.M.; Atvars, T.D.Z.; Dias, I.L.T.; Neto, G.O.; Zagatto, E.A.G.; Kubota, L.T. Direct determination of paracetamol in powdered pharmaceutical samples by fluorescence spectroscopy. Anal. Chim. Acta 2005, 539, 257–261. [Google Scholar] [CrossRef]
- Erk, N. Application of derivative-differential UV spectrophotometry and ratio derivative spectrophotometric determination of mephenoxalone and acetaminophen in combined tablet preparation. J. Pharm. Biomed. Anal. 1999, 21, 429–437. [Google Scholar] [CrossRef]
- Burgot, G.; Auffret, F.; Burgot, J.L. Determination of acetaminophen by thermometric titrimetry. Anal. Chim. Acta 1997, 343, 125–128. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.C.; Huang, H.J. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Ravisankar, S.; Vasudevan, M.; Gandhimathi, M.; Suresh, B. Reversed-phase HPLC method for the estimation of acetaminophen, ibuprofen and chlorzoxazone in formulations. Talanta 1998, 46, 1577–1581. [Google Scholar] [CrossRef]
- Peng, W.; Li, T.; Li, H.; Wang, E. Direct injection of urine and determination of acetaminophen by micellar liquid chromatography with a wall-jet cell/carbon fibre microelectrode. Anal. Chim. Acta 1994, 298, 415. [Google Scholar] [CrossRef]
- He, F.Y.; Liu, A.L.; Xia, X.H. Poly(dimethylsiloxane) microchip capillary electrophoresis with electrochemical detection for rapid measurement of acetaminophen and its hydrolysate. Anal. Bioanal. Chem. 2004, 379, 1062. [Google Scholar] [CrossRef]
- Heitmer, S.; Blaschke, G. Direct determination of paracetamol and its metabolites in urine and serum by capillary electrophoresis with ultraviolet and mass spectrometric detection. J. Chromotogr. B Biomed. Sci. Appl. 1999, 721, 93. [Google Scholar] [CrossRef]
- Suntornsuk, L.; Pipitharome, O.; Wilairat, P. Simultaneous determination of paracetamol and chlorpheniramine maleate by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2003, 33, 441. [Google Scholar] [CrossRef]
- Valero, E.; Carrion, P.; Varon, R.; Carmona, F.G. Quantification of acetaminophen by oxidation with tyrosinase in the presence of Besthorn’s hydrazine. Anal. Biochem. 2003, 318, 187. [Google Scholar] [CrossRef]
- Malviya, M.; Singh, J.P.; Singh, R.N. Electrochemical characterization of polypyrrole/cobalt ferrite composite films for oxygen reduction. Indian J. Chem. 2005, 44, 2233–2236. [Google Scholar]
- Simonsen, V.L.E.; Find, D.; Lilliedal, M.; Petersen, R.; Kammer, K. Spinels as cathodes for the electrochemical reduction of O2 and NO. Top. Catal. 2007, 45, 143. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Song, F.; Shen, X.; Xiang, J.; Zhu, Y. Characterization and magnetic properties of BaxSr1 − xFe12O19 (x = 0 − 1)ferrte hollow fibers via gel-precursor transformation process. J. Alloy. Compd. 2010, 507, 297–301. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Veisi, S.S.; Yousefi, M.; Amini, M.M.; Shakeri, A.R.; Bagherzadeh, M. Magnetic and microwave absorption properties of Cu/Zr doped M+type Ba/Sr hexaferrites prepared via sol-gel auto-combustion method. J. Alloy. Compd. 2019, 773, 1187–1194. [Google Scholar] [CrossRef]
- Valenzuela, R. Magnetic Ceramics. In Chemistry of Solid State Materials Part No. 4; Cambridge University Press: Cambridge, UK, 1994; p. 50. [Google Scholar]
- Basante-Delgado, S.F.; González-Vidal, D.; Morales-Morales, J.A.; Aperador-Chaparro, W.A.; Gómez-Cuaspud, J.A. A preliminary study of oxides of Fe doped with Ba, Co, Cu and synthetized by the citrate sol–gel combustion route. J. Phys. Conf. Ser. 2020, 1541, 012013. [Google Scholar] [CrossRef]
- Zhon, W.; Ding, W.P.; Zhang, N.; Hong, J.M.; Yan, Q.J.; Du, Y.W. Key step in synthesis of ultrafine BaFe12O19 by sol-gel technique. J. Magn. Magn. Mater. 1997, 168, 196–202. [Google Scholar] [CrossRef]
- Vadivelan, S.; Victor Jaya, N. Investigation of magnetic and structural properties of copper substituted barium ferrite powder particles via co-precipitation method. Results Phys. 2016, 6, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.-T.; Wu, M.-S.; Tsai, J.-D. 13C NMR study of the solution chemistry of barium titanium citrate gels prepared using pechini process. J. Am. Ceram. Soc. 2002, 85, 2984–2988. [Google Scholar] [CrossRef]
- Gonzalez-Carreño, T.; Morales, M.P.; Serna, C.J. Barium ferrite nanoparticles prepared directly by aerosol pirolisis. Mater. Lett. 2000, 43, 97–101. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gan, L.-M.; Ng, S.-C. Improving the magnetic properties of hydrothermally synthesized barium ferrite. J. Magn. Magn. Mater. 1999, 195, 452–459. [Google Scholar] [CrossRef]
- Müller, R.; Hiergeist, R.; Steinmetz, H.; Ayoub, N.; Fujisaki, M.; Schüppel, W. Barium hexaferrite ferrofluids—Preparation and physical properties. J. Magn. Magn. Mater. 1999, 201, 34–37. [Google Scholar] [CrossRef]
- Hüseyin, S.; Hüsnü, O.; Nader, G. Properties of YBCO superconductors prepared by ammonium nitrate melt and solid-state reaction methods. J. Alloys Compd. 2007, 428, 1–7. [Google Scholar]
- Hüseyin, S. Simple recipe to synthesize single-domain BaFe12O19 with high saturation magnetization. J. Magn. Magn. Mater. 2009, 321, 2717–2722. [Google Scholar]
- Hüseyin, S. Effect of pelletization on magnetic properties of BaFe12O19. J. Alloys Compd. 2009, 486, 809. [Google Scholar]
- Ebrahim, P.; Ali, G.; Abdolhamid, J.; Hassan, S. Influence of acid catalysts on the structural and magnetic properties of nanocrystalline barium ferrite prepared by sol–gel method. J. Magn. Magn. Mater. 2008, 320, L137. [Google Scholar]
- Chimarro, E.; Moreno, B.; Martin, D.; Gozález, L.; Villanueva, E.; Guinea, D.; Jurado, J.R. Posibilidades del análisis de imagen para el estudio de la síntesis de materiales por combustión. Bol. Soc. Esp. Ceram. V 2005, 44, 105–112. [Google Scholar] [CrossRef]
- Rostami, M.; Reza, M.; Vahdani, K.; Moradi, M. Structural, magnetic, and microwave absorption properties of Mg-TiZr-Co-substituted barium hexaferrites nanoparticles synthesized via sol–gel auto-combustion method. J. Sol.-Gel. Sci. Technol. 2017, 82, 783–794. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineering 2016, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangchang, L.; Xiaojing, Q.; Mingyu, L.; Ting, L.; Peng, H.X. La and Co substituted M-type barium ferrites processed by sol–gel combustion synthesis. Mater. Res. Bull. 2013, 48, 4449–4453. [Google Scholar]
- Sonal, S.; Kirandish, K.; Sheenu, J.; Santosh, B.; Bansal, S. Structural and Magnetic Properties of BaCoxFe12xO19 (x = 0.2, 0.4, 0.6, & 1.0) Nanoferrites Synthesized via Citrate Sol-Gel Method. World J. Condens. Matter Phys. 2011, 1, 101–104. [Google Scholar]
- Briceño, S.; Sagredo, V.; del Castillo, H. Synergy in the selective catalytic reduction of nox over cu1-xcoxfe2o4 ferrites. Rev. Latinam. Metal. Mat. 2012, 32, 129–135. [Google Scholar]
- Zabiszak, M.; Nowak, M.; Taras-Goslinska, K.; Kaczmarek, M.T.; Hnatejko, Z.; Jastrzab, R. Carboxyl groups of citric acid in the process of complex formation with bivalent and trivalent metal ions in biological systems. J. Inorg. Biochem. 2018, 182, 37–47. [Google Scholar] [CrossRef]
- Bagherinasab, Z.; Beitollahi, H.; Yousefi, M.; Bagherzadeh, M.; Hekmati, M. Rapid sol gel synthesis of BaFe12O19 nanoparticles: An excellent catalytic application in the electrochemical detection of tramadol in the presence of acetaminophen. Microchem. J. 2020, 156, 104803. [Google Scholar] [CrossRef]
- Kreisel, J.; Lucazeau, G.; Vincent, H. Raman Spectra and Vibrational Analysis of BaFe12O19 Hexagonal Ferrite. J. Solid State Chem. 1998, 137, 127. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Wei, P.; Wu, X.Y.; Wang, W.; Zhang, Q.J. Lattice vibration characterization and magnetic properties of M-type barium hexaferrite with excessive iron. J. Appl. Phys. 2008, 103, 063902. [Google Scholar] [CrossRef]
- Ali, M.M. Synthesis and Study the Structural and Magnetic Properties of Cobalt Substituted Strontium Hexaferrite. Int. J. Nanoelectron. Mater. 2020, 13, 283–294. [Google Scholar]
- Morales-Morales, J.A. Synthesis of Hematite α-Fe2O3 Nano Powders by the controlled precipitation method. Cienc. Desarro. 2017, 8, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Shah, J.; Negi, N.S.; Sharma, C.; Kotnala, R.K. Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting. Int. J. Energy Res. 2019, 43, 4743–4755. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmosphere. CrstEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Wu, M.S.; Chung, C.J.; Ceng, Z.Z. Cyclic voltammetric deposition of discrete nickel phosphide clusters with mesoporous nanoparticles on fluorine-doped tin oxide glass as a counter electrode for dye-sensitized solar cells. RSC Adv. 2015, 5, 4561–4567. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.A.; Guzmán-Vargas, A.; Manzo-Robledo, A.; Martínez-Ortiz, M.J.; Flores-Moreno, J.L. Modified electrode with hydrotalcite-like materials and their response during electrochemical oxidation of blue 69. Catal. Today 2011, 166, 194–200. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef] [Green Version]
- Santosh, B.K.; Sharanappa, T.N. Electrochemical Behavior of 2-Aminothiazole at Poly Glycine Modified Pencil Graphite Electrode. Anal. Bioanal. Electrochem. 2020, 12, 208–222. [Google Scholar]
- Ali, M.F.B.; Abdel-aal, F.A.M. In situ polymerization and FT-IR characterization of polyglycine on pencil graphite electrode for sensitive determination of antiemetic drug, granisetron in injections and human plasma. RSC Adv. 2019, 9, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley Interscience: New York, NY, USA, 2004. [Google Scholar]
- Asadpour-Zeynali, K.; Amini, R. Nanostructured hexacyanoferrate intercalated Ni/Al layered double hydroxide modified electrode as a sensitive electrochemical sensor for paracetamol determination. Electroanalysis 2017, 29, 635–642. [Google Scholar] [CrossRef]
- Andawiyah, R.; Mulyasuryani, A.; Sulistyarti, H. Voltammetric Determination of Paracetamol using Polyvinyl Alcohol (PVA)-Fe3O4 Modified Glassy Carbon Electrode. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012059. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Electroanalysis of estriol hormone using electrochemical sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Pasban, A.A.; Nia, E.H.; Piryaei, M. Determination of Acetaminophen Via TiO2/MWCNT Modified Electrode. J. Nanoanalysis 2017, 4, 142–149. [Google Scholar]
- OmymenaJelen, W.M.; Rogana, J.R.; Jugović, B.Z.; Gvozdenović, M.M.; Grgur, B.N. Photo-assisted electrochemical oxidation of the urea onto TiO2-nanotubes modified by hematite. J. Saudi Chem. Soc. 2017, 21, 990–997. [Google Scholar] [CrossRef]
- Osman, N.S.E.; Thapliyal, N.; Alwan, W.S.; Karpoormath, R.; Moyo, T. Synthesis and characterization of Ba0.5Co0.5Fe2O4 nanoparticle ferrites: Application as electrochemical sensor for ciprofloxacin. J. Mater. Sci. Mater. Electron. 2015, 26, 5097–5105. [Google Scholar] [CrossRef]
- Khaskheli, A.R.; Fischerb, J.; Barekb, J.; Vyskocil, V.; Uddin, S.; Iqbal Bhanger, M. Differential pulse voltammetric determination of paracetamol in tablet and urine samples at a micro-crystalline natural graphite–polystyrene composite film modified electrode. Electrochim. Acta 2013, 101, 238–242. [Google Scholar] [CrossRef]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.S.; Díaz-Cruz, J.M. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. [Google Scholar]
- Siegers, C.P.; Schütt, A. Dose-Dependent Biliary and Renal Excretion of Paracetamol in the Rat. Pharmacology 1979, 18, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Bales, J.R.; Sadler, P.J.; Nicholson, J.K.; Timbrell, J.A. Urinary Excretion of Acetaminophen and Its Metabolites as Studied by Proton NMR Spectroscopy. Clin. Chem. 1984, 30, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Roberts, R.J.; Fischer, L.J. Acetaminophen elimination kinetics in neonates, children, and adults. Clin. Pharmacol Ther. 1975, 19, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Foroozan, H.; Masoumeh, T.; Somayeh, T. Ultra-sensitive electrochemical sensing of acetaminophen and codeine in biological fluids using CuO/CuFe2O4 nanoparticles as a novel electrocatalyst. J. Food Drug Anal. 2018, 26, 879–886. [Google Scholar]
- Bose, D.; Durgbanshi, A.; Martinavarro-Domínguez, A.; Capella-Peiró, M.E.; Carda-Broch, S.; Esteve-Romero, J.S.; Gil-Agustí, M.T. Rapid Determination of Acetaminophen in Physiological Fluids by Liquid Chromatography Using SDS Mobile Phase and ED Detection. J. Chromatogr. Sci. 2005, 43, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Surface | Peak Current Ipa (µA) | Peak Potential Epa (V) |
|---|---|---|
| GCE | 96.12 ± 4.73 | 0.864 ± 0.028 |
| CuFM/GCE | 131.24 ± 4.51 | 0.793 ± 0.031 |
| CoFM/GCE | 201.37 ± 4.22 | 0.777 ± 0.027 |
| Modified Electrode | Methods | Linear Range(µM) | Analyte | LoD (µM) | Ref |
|---|---|---|---|---|---|
| GCE-arene-ruthenium (II) complex | Amperometry | 1.99–31 | NAP | 0.58 | [52] |
| GCE-PVA-Fe3O4 | CV | 0–100 | NAP | 8 | [53] |
| GCE-hexacyanoferrate(III) intercalated Ni Al LDH | CV | 3.0–1500 | NAP | 0.80 | [54] |
| GCE-MWCNT/TiO2 | CV | 10–120 | NAP | 11.77 | [55] |
| GCE-Ba1.0Co1.22Fe11.41O18.11 | DPV | 1–12 | NAP | 0.530 | This work |
| GCE-Ba1.14Cu0.82Fe11.65O18.02 | DPV | 1–12 | NAP | 0.895 | This work |
| Urine | Added (μM) | (a) Detected (µM) | Recovery (%) | SD ± RSD (%) | |||
|---|---|---|---|---|---|---|---|
| CoFM/GCE | CuFM/GCE | CoFM/GCE | CuFM/GCE | CoFM/GCE | CuFM/GCE | ||
| Sample 1 | 1 | 0.98 | 0.95 | 98.0 | 95.0 | 0.019 ± 1.939 | 0.032 ± 3.371 |
| Sample 2 | 5 | 4.99 | 4.89 | 99.8 | 97.8 | 0.023 ± 0.460 | 0.021 ± 0.430 |
| Sample 3 | 10 | 10.01 | 9.84 | 101.0 | 98.4 | 0.021 ± 0.210 | 0.041 ± 0.417 |
| (b) Sample 4 | - | 10.56 | 9.18 | - | - | - | - |
| 10 | 19.86 | 18.01 | 96.6 | 93.9 | 0.015 ± 0.076 | 0.031 ± 0.172 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granja-Banguera, C.P.; Silgado-Cortázar, D.G.; Morales-Morales, J.A. Transition Metal Substituted Barium Hexaferrite-Modified Electrode: Application as Electrochemical Sensor of Acetaminophen. Molecules 2022, 27, 1550. https://doi.org/10.3390/molecules27051550
Granja-Banguera CP, Silgado-Cortázar DG, Morales-Morales JA. Transition Metal Substituted Barium Hexaferrite-Modified Electrode: Application as Electrochemical Sensor of Acetaminophen. Molecules. 2022; 27(5):1550. https://doi.org/10.3390/molecules27051550
Chicago/Turabian StyleGranja-Banguera, Claudia Patricia, Daniel Gerardo Silgado-Cortázar, and Jimmy Alexander Morales-Morales. 2022. "Transition Metal Substituted Barium Hexaferrite-Modified Electrode: Application as Electrochemical Sensor of Acetaminophen" Molecules 27, no. 5: 1550. https://doi.org/10.3390/molecules27051550
APA StyleGranja-Banguera, C. P., Silgado-Cortázar, D. G., & Morales-Morales, J. A. (2022). Transition Metal Substituted Barium Hexaferrite-Modified Electrode: Application as Electrochemical Sensor of Acetaminophen. Molecules, 27(5), 1550. https://doi.org/10.3390/molecules27051550






