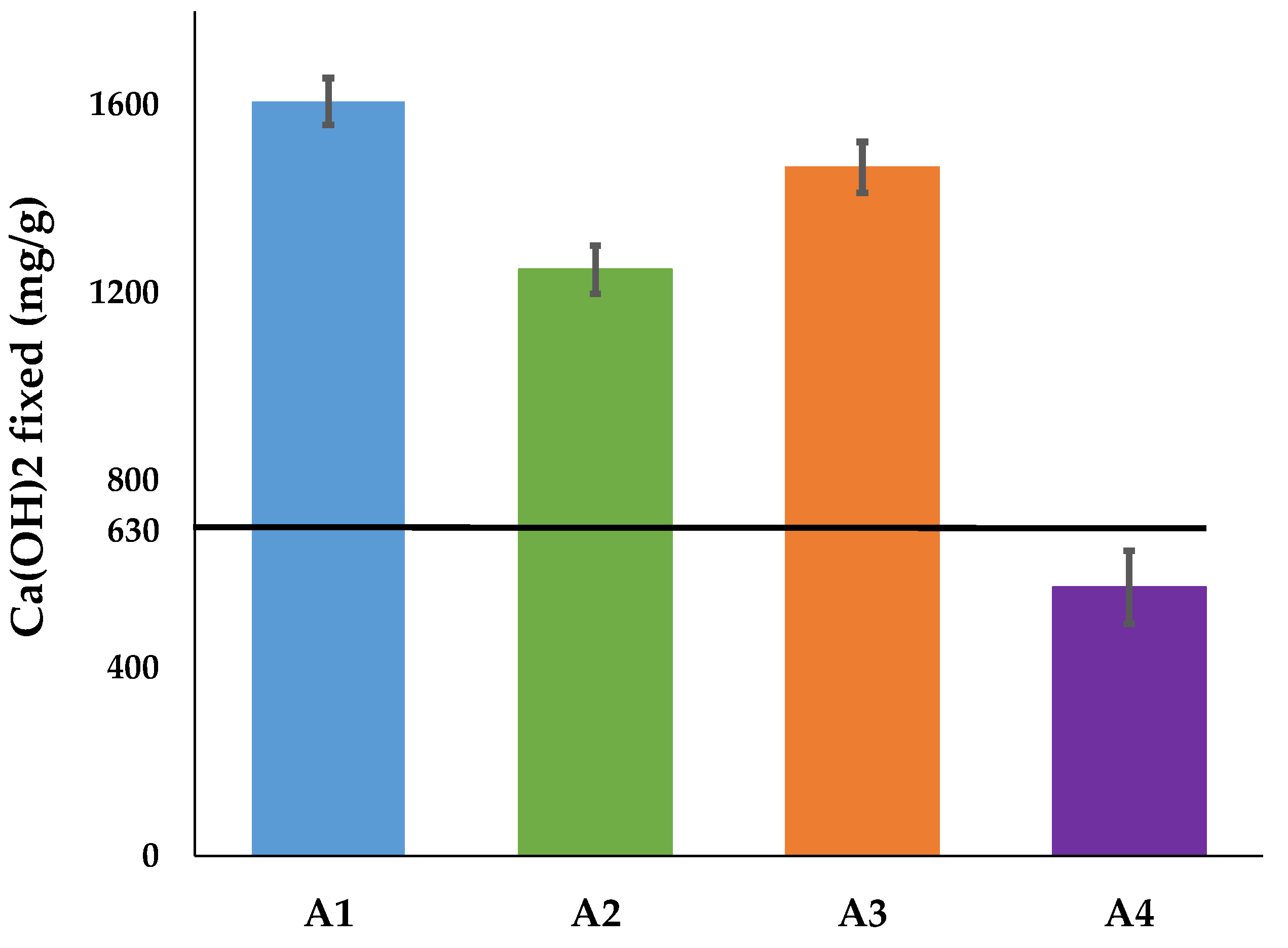2.2.2. Microstructural Analyses: Set-up of Calcination Conditions and Monitoring of Hydration
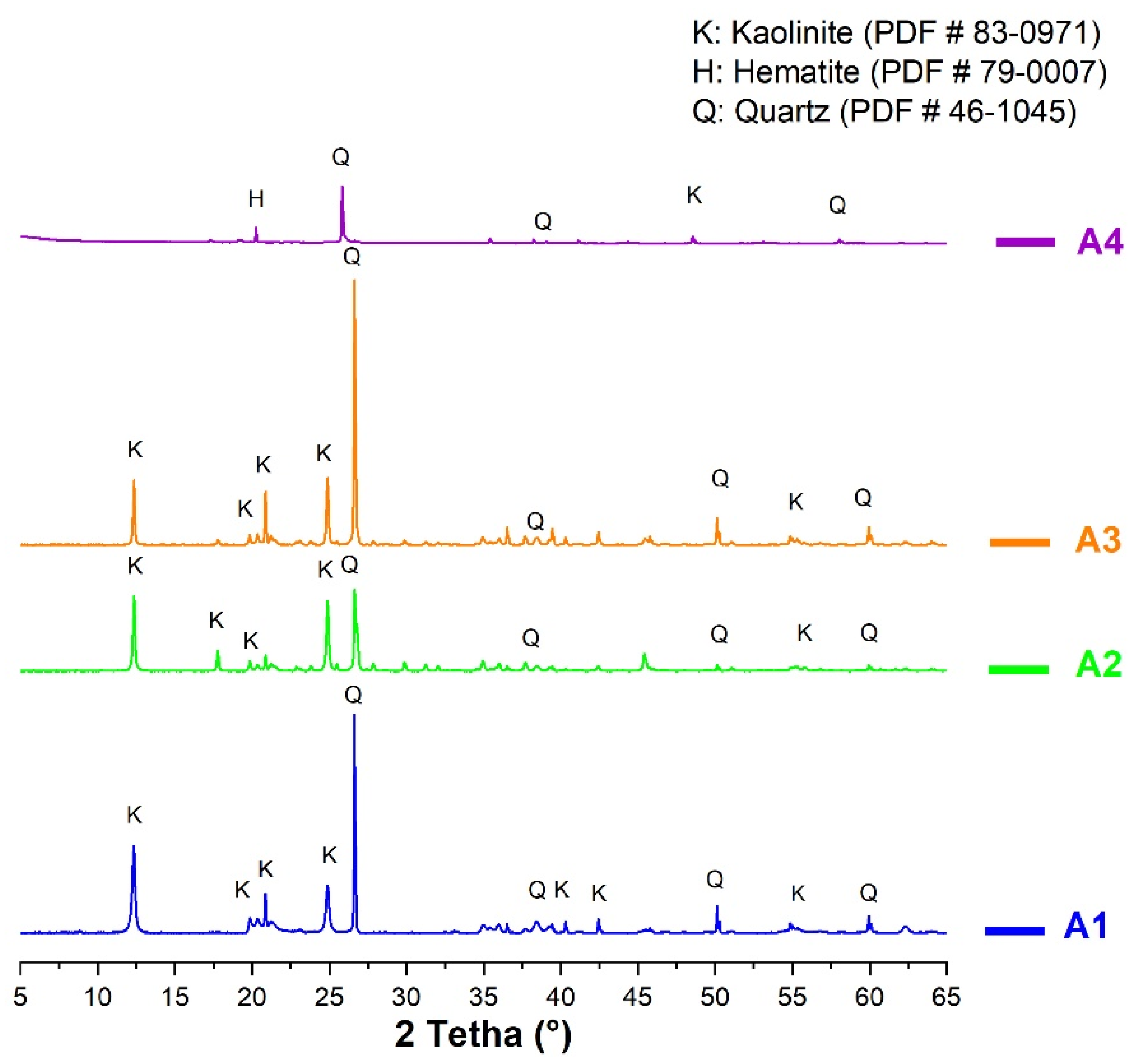
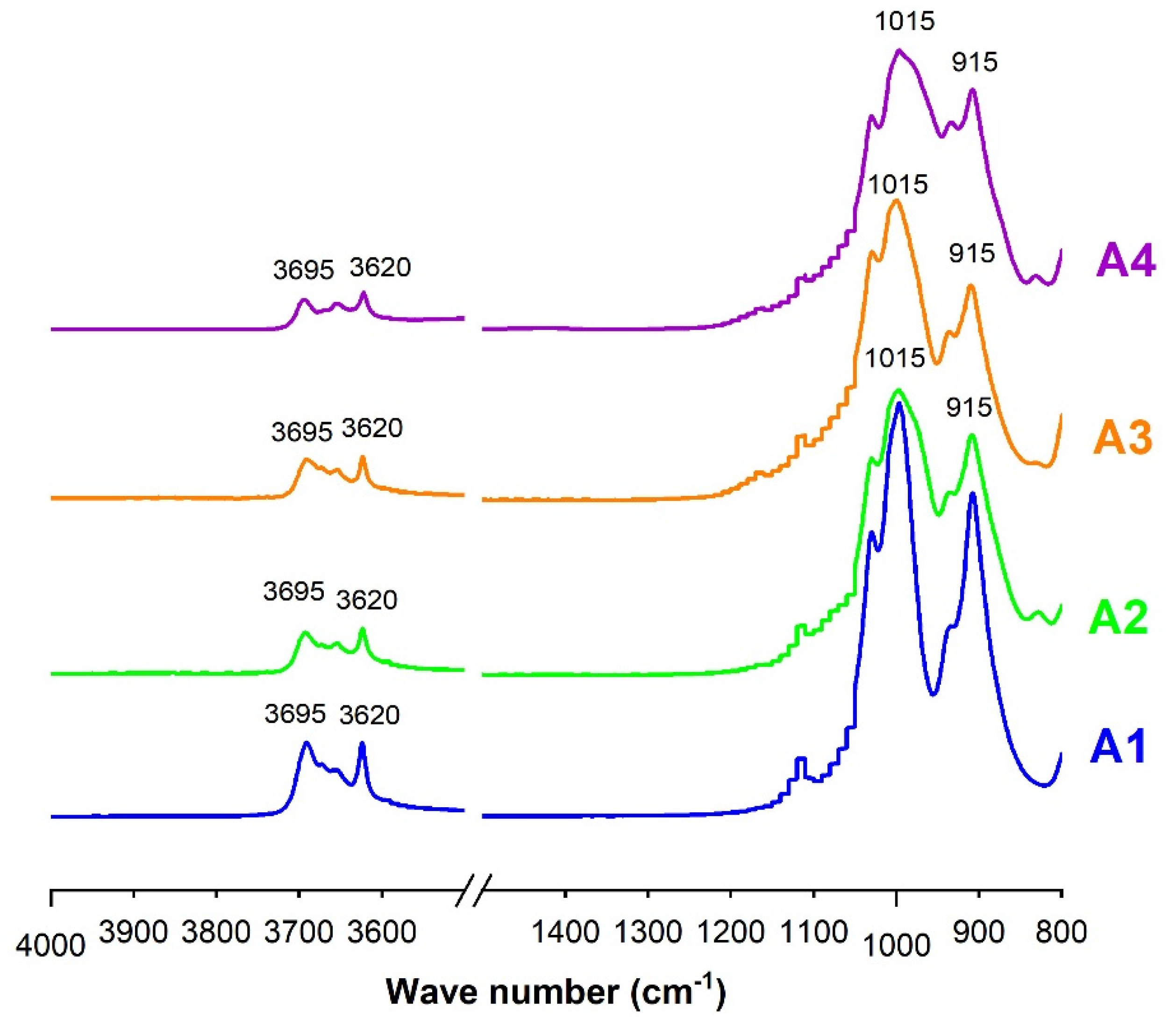
Before characterization, all the samples were ground to 80 µm using a type RS200 vibratory crusher (Haan, Germany). After grinding, the samples were subjected to various microstructural analyses: X Ray diffraction (XRD), infrared spectroscopy and thermogravimetric analysis (TGA). XRD was performed to identify the mineral composition of the samples. The acquisition of X-ray diffraction (XRD) patterns was performed using a D8 Advance diffractometer (Bruker, Mannheim, Germany) equipped with CuKα radiation (λKα = 0.154186 nm), at a step size of 0.02° (2θ) between 5° and 70° (2θ), and a step time of 1.3 s. The XRD data were analyzed using EVA software 13.0.0.3. The infrared (IR) analysis was performed using a ThermoFisher Scientific IS50 spectrometer in ATR (Attenuated Transmittance Resonance) mode. This test allowed determination of the amorphous, semi-amorphous or crystallized structure of the samples from different approaches. The TGA was performed using a Setaram Setsys device, in the temperature range between 30 and 1100 °C, with a heating rate of 10 °C/min under air environment at a flow rate of 20 mL/min. For each sample, the initial mass was 50 mg. This analysis was used to determine the kaolinite content in each sample and the appropriate range of calcination temperature.
Following the TGA, the temperature of calcination of the clays was set at 700 °C. The calcination was conducted on clays ground and sieved on 80 µm, in an electric furnace in two phases: three hours of temperature increase from the ambient temperature (30 °C) to 700 °C, and three hours at the soaking temperature (700 °C), followed by cooling in the furnace. After the calcination, the clays were analyzed for chemical and mineral compositions using X Ray fluorescence, XRD, TGA and IR. The physical properties were also characterized: the particle size analysis was carried out by laser diffraction using Malvern Mastersizer 200 device using the dry method. The specific gravity was determined using a helium pycnometer. The specific surface area was determined by Blaine method.
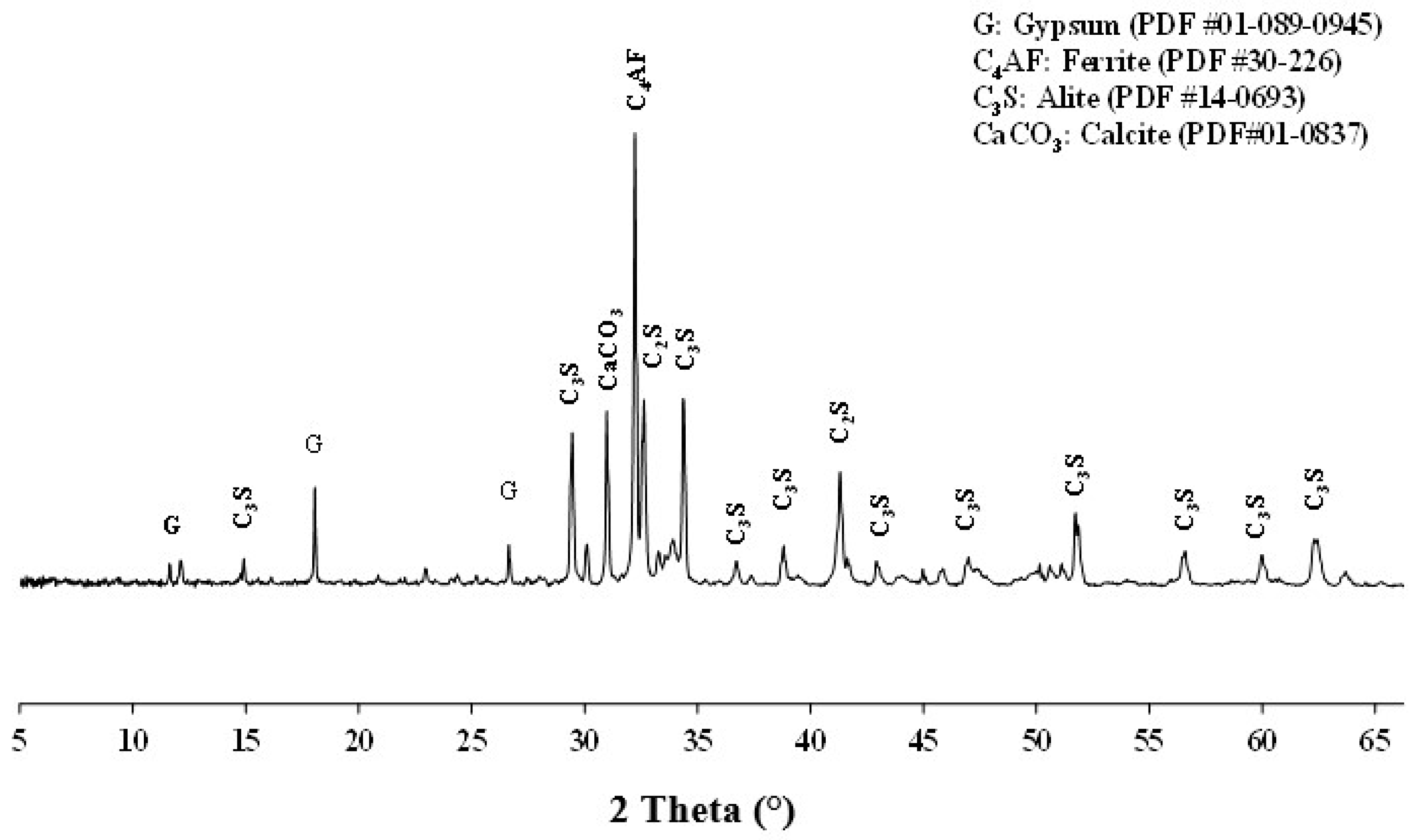
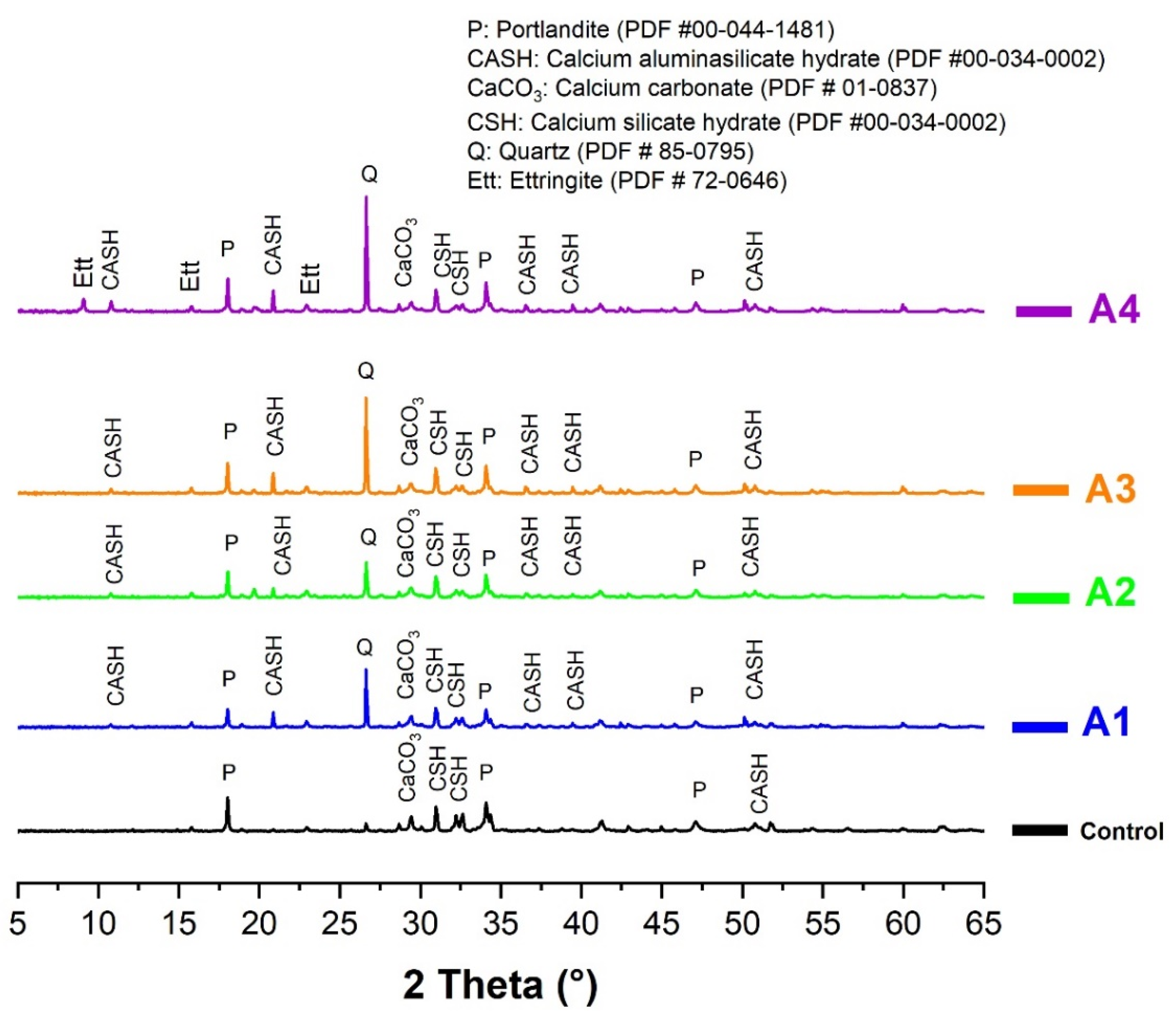
A hydration monitoring was conducted to analyze the influence of additions (calcined clays) on the microstructure of cement paste. For this purpose, the pastes were prepared at the same consistency by mixing 75% CEM I and 25% calcined clay by mass percentage. The water demand for reaching normal consistency evolved in the range of 0.35 to 0.33 for clay A to A4. A 100% CEM I paste was used as a reference. After mixing, the pastes were kept in sealed plastic boxes to avoid water evaporation. After 28 days of hydration, all samples were ground under the same conditions to the same fineness (80 µm) and subjected to XRD and TGA using the same method and equipment.
2.2.3. Pozzolanicity
The ASTM C618 standard [
27] defines a “pozzolan as a material which in itself has no binding properties, but which, under certain conditions of temperature and humidity, can react with calcium hydroxide to form compounds with binding properties”. Firstly, the pozzolanicity of an addition is assessed based on its chemical composition. The standard stipulates that the sum in mass percentages of the oxides (SiO
2 + Al
2O
3 + Fe
2O
3) contained in the addition must be greater or equal to 70%, the mass content of SO
3 must be less than or equal to 4% and the glass content (%SiO
2 + %CaO) must be ≥34%. Secondly, for a 25% substitution rate in the cement, the addition must have a strength activity index at 7 and 28 days of at least 75%. If these two conditions are verified, the material can be considered as Class N pozzolan. Therefore, this method was used to assess the pozzolanicity of calcined clays. Additional methods, both qualitative and quantitative, such as the amorphicity test, Frattini test, modified Chapelle test and electrical conductivity were used to confirm the pozzolanicity of the calcined clays.
Calcination is the process of amorphization of materials, so after this process it is important to determine their amorphous phases. The determination of the amorphous phase consists of monitoring the dissolution kinetics of aluminosilicates in a solution of hydrofluoric acid at 1% volumetric concentration. This test was developed by Keyser in the ceramics domain, and previously used by Sore [
23] for the assessment of the amorphicity of calcined clays. It consisted of introducing 1 g (m
1) of the sample of calcined clays which passed through an 80 µm sieve into 200 mL of the acidic solution and keeping it therein for 40 min. The solution was filtered, the residue was dried in an oven at 105 °C for 24 h and a second mass m
2 was weighed. The amorphous content (AC) was determined using Equation (1).
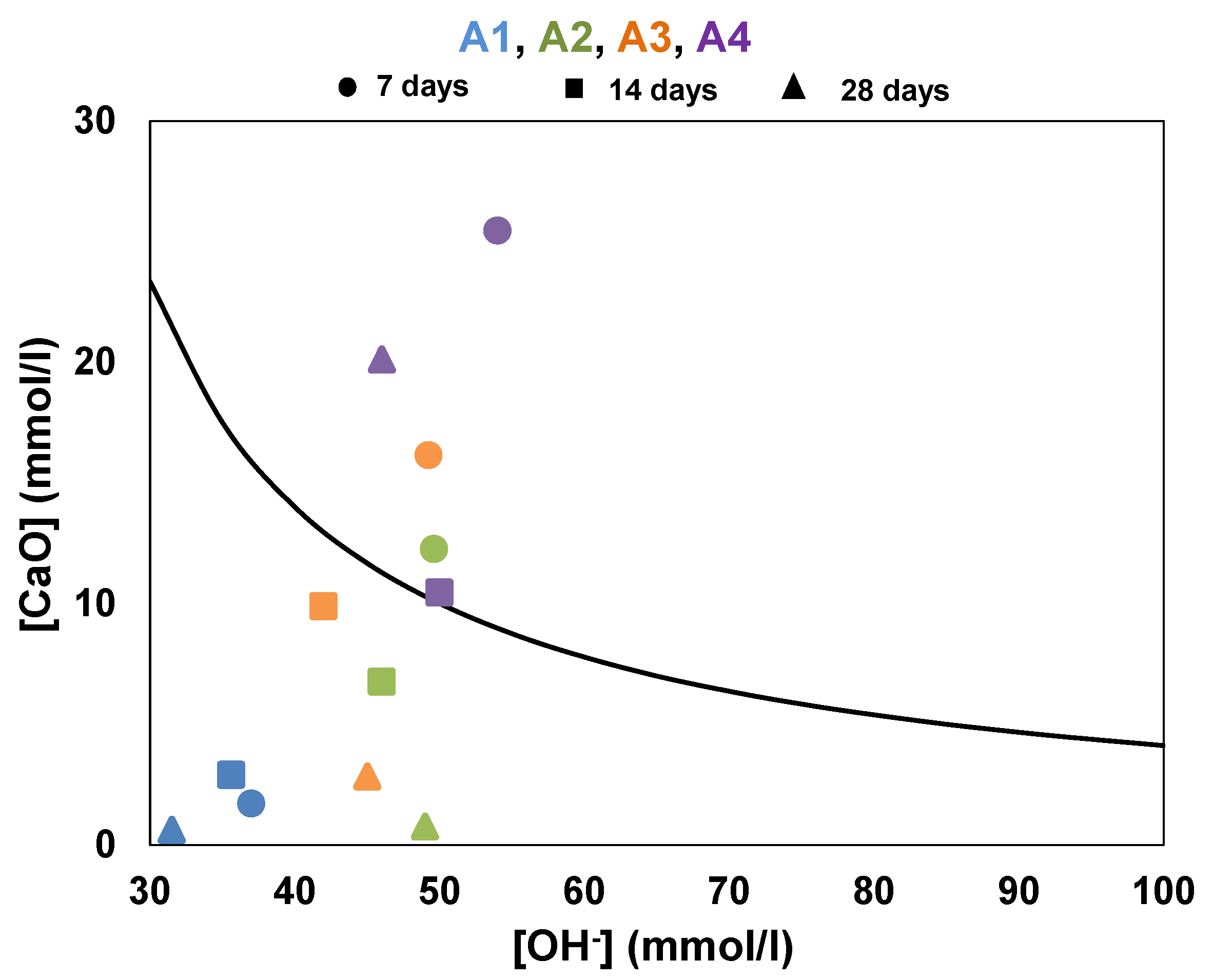
In the Frattini test, the aqueous solution containing samples of calcined clays was made from 20 g of blended binder (15 g of CEM I and 5 g of calcined clay) and mixed with 100 mL of distilled water. The samples were left to cure for 7, 14 and 28 days in sealed containers and kept in an oven at 40 °C. At the test time, the samples were filtered through an 8 µm pore-size filter paper and cooled to ambient temperature. The filtrate was analyzed by titration to determine the concentration of OH
− using 0.1 mol/L HCl solution with methyl orange indicator, and the concentration of CaO was analyzed by pH adjustment to 12.5 followed by titration with 0.03 mol/L EDTA (Ethylenediaminetetraacetic acid) solution. This test compares the concentrations of CaO and OH
− ([CaO], [OH
−]) contained in the aqueous solution at 7, 14 and 28 days of hydration with the solubility curve in an alkaline solution at the same temperature. According to the EN 196-5 [
28], the sample is considered as active pozzolan when the couple ([CaO], [OH
−]) is under the solubility curve. The calcium ion saturation curve is given by Equation (2).
This test consists of putting the calcined clay in a saturated lime solution and evaluating its pozzolanic reactivity by the quantity of lime it can fix. According to the conformity criteria of metakaolin of the NF P 18-513 standard [
29], the minimum quantity to be fixed is 630 mg per gram of addition. The test consists of preparing a solution in which 1 g of calcined clay and 2 g of CaO are added to 250 mL of water at 90 °C in a plastic bottle. After 16 h storage in an oven (90 °C), the solution is cooled to room temperature and filtered. A measurement of 25 mL of the filtrate is titrated with a 0.1 mol/l HCl solution using phenolphthalein as indicator, while 25 mL of the white solution (calcined clay-free), prepared under the same conditions by dissolving 60 g of sucrose in 250 mL of water, is also titrated under the same conditions. The quantity of lime fixed by the calcined clay is given by Equation (3); where V
1 and V
2 represent the volumes of HCl used to titrate, respectively, the white solution of sucrose and the solution with calcined clay.
This test was proposed by Luxan et al. [
30] and modified by Yu et al. [
31]. This test consists of dissolving 2 g of the addition in 200 mL of a saturated lime solution and measuring the evolution of the electrical conductivity of the solution over time. The principle of this test is based on the hypothesis that the electrical conductivity of the aqueous solution is controlled by the presence of Ca
2+ ions released by the lime. In the presence of the mineral addition, the Ca
2+ ions are fixed by the silica and/or alumina contained in the addition. This decreases the concentration of Ca
2+ ions and consequently results in a decrease in the electrical conductivity of the solution. The reactivity of the addition is evaluated by the decrease in the electrical conductivity of the solution.
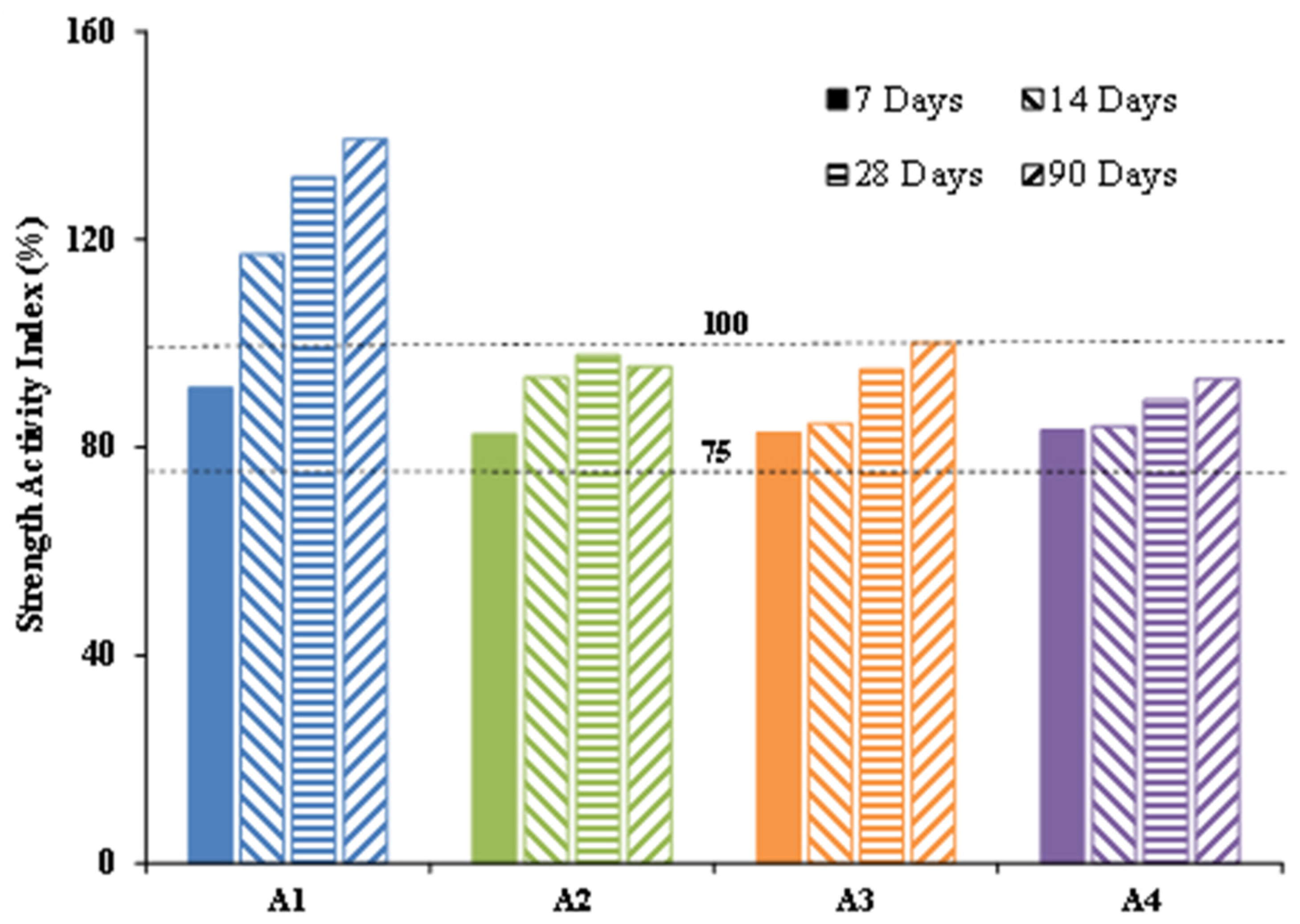
The strength activity index is calculated from the compressive strength of a standard mortar. The standard mortar is produced as described by the EN 196-1 standard [
32], mixing 1350 g of standard sand, 450 g of cement CEM I and 225 g of water. Test mortars are prepared by substitution (mass substitution) of 25% CEM I for each type of calcined clay, e.g., 337.5 g of CEM I for 112.5 g of calcined clay, keeping the other constituents the same. All samples were demolded after 24 h and stored to cure in lime-saturated water at 20 °C with relative humidity at 100%, and crushed at 7, 14, 28 and 90 days to determine their compressive strengths. The strength activity index (SAI), expressed as a percentage, is defined by Equation (4); where CS
i (MPa) and CS
0 (MPa) respectively represent the strength of the test mortar and the control mortar.