Tunneling Nanotubes: A New Target for Nanomedicine?
Abstract
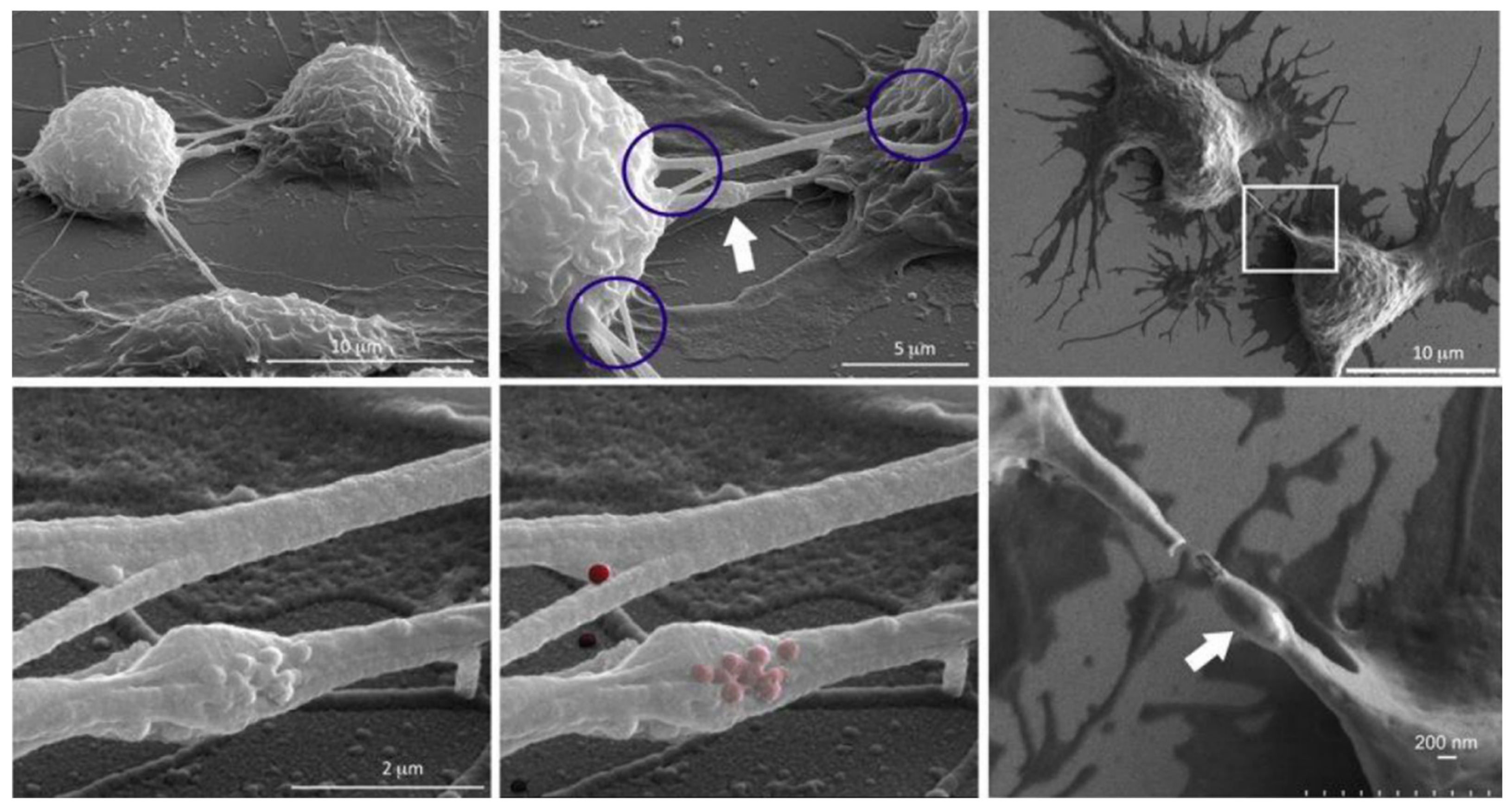
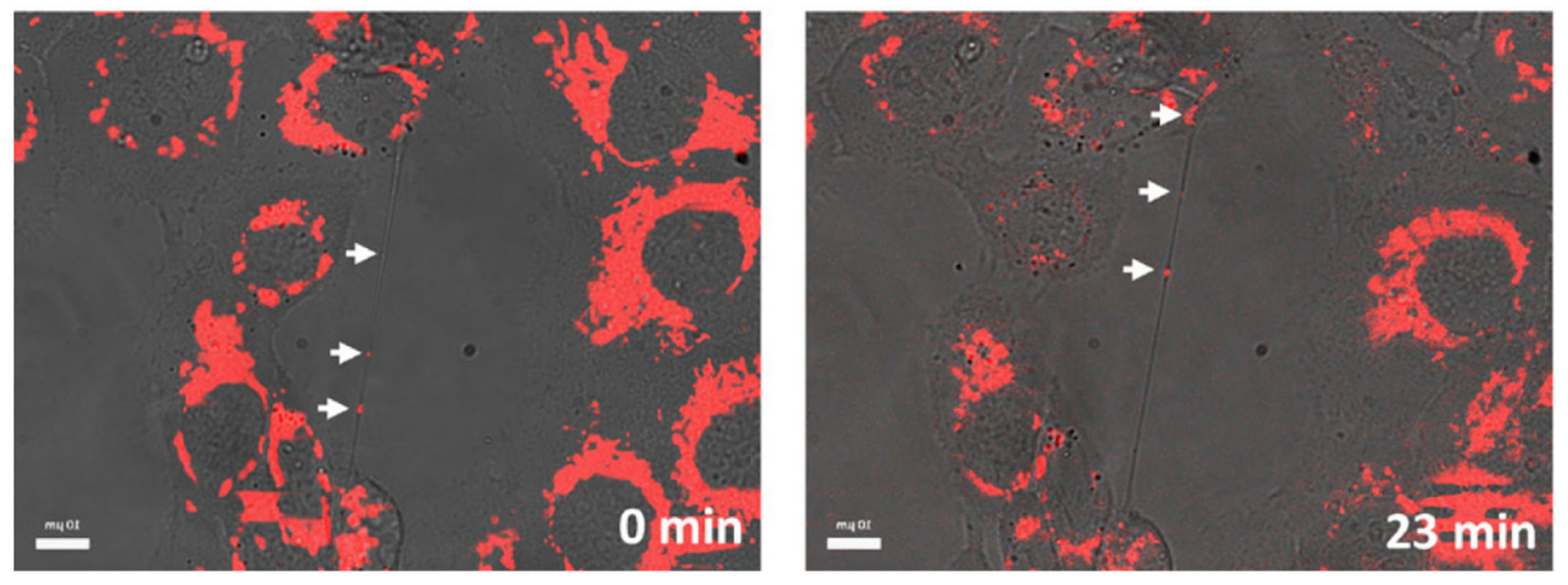
:1. Tunneling Nanotubes
1.1. What Are Tunneling Nanotubes?
1.2. Exogenous Modulation of Tunneling Nanotubes
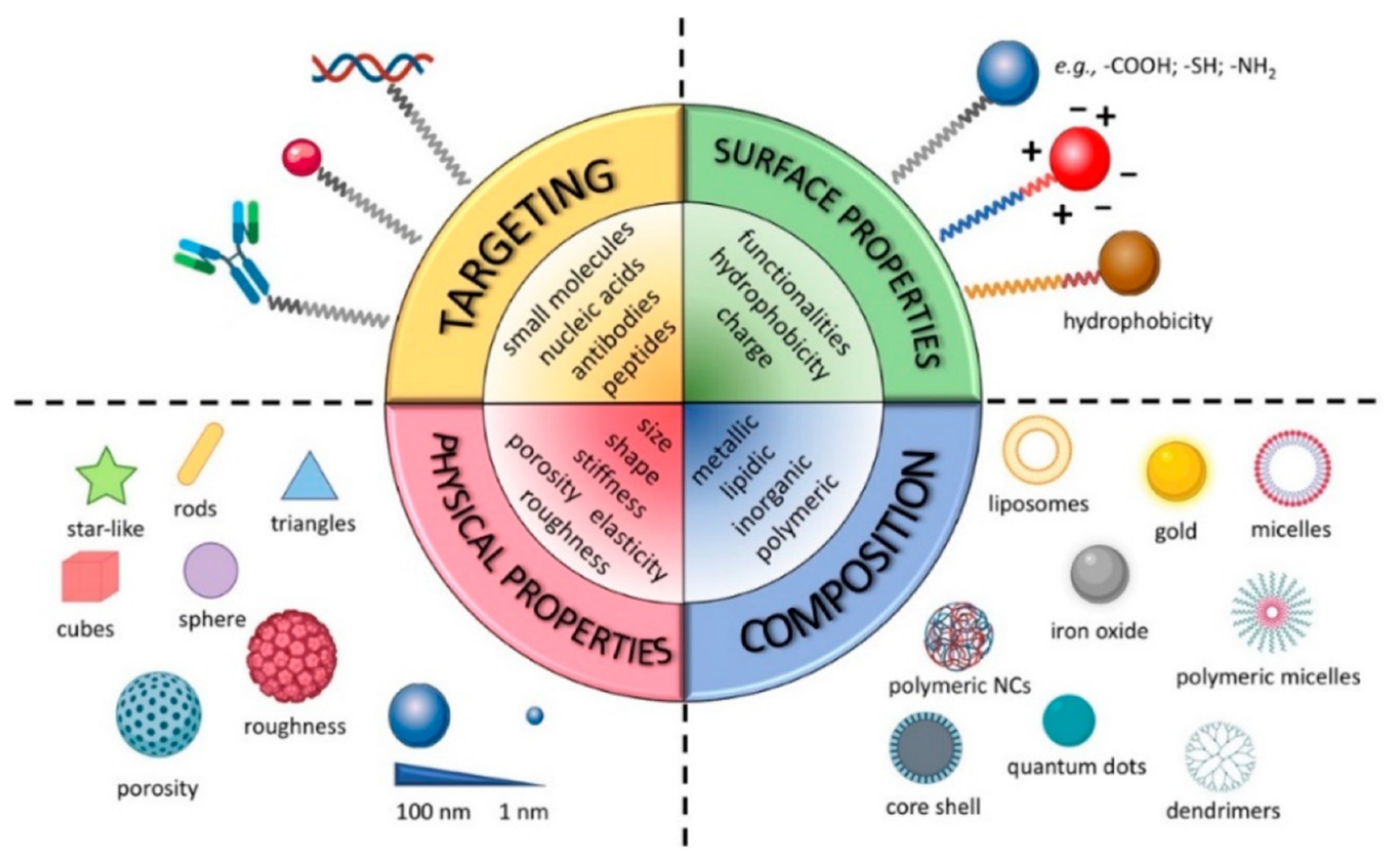
2. Nanomedicine
3. Nanomedicine and TNTs
3.1. Inorganic NMeds
3.2. Organic NMeds
3.2.1. Polymeric NMeds
3.2.2. Lipidic NMeds
4. Limitations in Tunneling Nanotubes Detection
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine Intercellular Connections in Development: TNTs, Cytonemes, or Intercellular Bridges? Cell Stress 2020, 4, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Dubois, F.; Bénard, M.; Jean-Jacques, B.; Schapman, D.; Roberge, H.; Lebon, A.; Goux, D.; Monterroso, B.; Elie, N.; Komuro, H.; et al. Investigating Tunneling Nanotubes in Cancer Cells: Guidelines for Structural and Functional Studies through Cell Imaging. BioMed Res. Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Taiarol, L.; Formicola, B.; Fagioli, S.; Sierri, G.; D’Aloia, A.; Kravicz, M.; Renda, A.; Viale, F.; Dal Magro, R.; Ceriani, M.; et al. The 3.0 Cell Communication: New Insights in the Usefulness of Tunneling Nanotubes for Glioblastoma Treatment. Cancers 2021, 13, 4001. [Google Scholar] [CrossRef]
- Pinto, G.; Brou, C.; Zurzolo, C. Tunneling Nanotubes: The Fuel of Tumor Progression? Trends Cancer 2020, 6, 874–888. [Google Scholar] [CrossRef]
- Pyrgaki, C.; Trainor, P.; Hadjantonakis, A.-K.; Niswander, L. Dynamic Imaging of Mammalian Neural Tube Closure. Dev. Biol. 2010, 344, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Caneparo, L.; Pantazis, P.; Dempsey, W.; Fraser, S.E. Intercellular Bridges in Vertebrate Gastrulation. PLoS ONE 2011, 6, e20230. [Google Scholar] [CrossRef] [Green Version]
- McKinney, M.C.; Stark, D.A.; Teddy, J.; Kulesa, P.M. Neural Crest Cell Communication Involves an Exchange of Cytoplasmic Material through Cellular Bridges Revealed by Photoconversion of KikGR. Dev. Dyn. 2011, 240, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, H.-H.; Rustom, A.; Wang, X. Tunneling Nanotubes, an Emerging Intercellular Communication Route in Development. Mech. Dev. 2013, 130, 381–387. [Google Scholar] [CrossRef]
- Jhala, D.; Rather, H.A.; Vasita, R. Extracellular Matrix Mimicking Polycaprolactone-Chitosan Nanofibers Promote Stemness Maintenance of Mesenchymal Stem Cells via Spheroid Formation. Biomed. Mater. 2020, 15, 035011. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal Stem Cells Rescue Injured Endothelial Cells in an in Vitro Ischemia–Reperfusion Model via Tunneling Nanotube like Structure-Mediated Mitochondrial Transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-J.; Karkossa, I.; Schäfer, I.; Christ, M.; Kühne, H.; Schubert, K.; Rolle-Kampczyk, U.E.; Kalkhof, S.; Nickel, S.; Seibel, P.; et al. Mitochondrial Transfer by Human Mesenchymal Stromal Cells Ameliorates Hepatocyte Lipid Load in a Mouse Model of NASH. Biomedicines 2020, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Boukelmoune, N.; Chiu, G.S.; Kavelaars, A.; Heijnen, C.J. Mitochondrial Transfer from Mesenchymal Stem Cells to Neural Stem Cells Protects against the Neurotoxic Effects of Cisplatin. Acta Neuropathol. Commun. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Gap Junction Protein Connexin43 and Tunneling Nanotubes in Human Trabecular Meshwork Cells. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 212–219. [Google Scholar] [PubMed]
- Chinnery, H.R.; Keller, K.E. Tunneling Nanotubes and the Eye: Intercellular Communication and Implications for Ocular Health and Disease. BioMed Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Keller, K. Tunneling Nanotubes and Actin Cytoskeleton Dynamics in Glaucoma. Neural Regen. Res. 2020, 15, 2031. [Google Scholar] [CrossRef]
- Wittig, D.; Wang, X.; Walter, C.; Gerdes, H.-H.; Funk, R.H.W.; Roehlecke, C. Multi-Level Communication of Human Retinal Pigment Epithelial Cells via Tunneling Nanotubes. PLoS ONE 2012, 7, e33195. [Google Scholar] [CrossRef] [Green Version]
- Alarcon-Martinez, L.; Villafranca-Baughman, D.; Quintero, H.; Kacerovsky, J.B.; Dotigny, F.; Murai, K.K.; Prat, A.; Drapeau, P.; Di Polo, A. Interpericyte Tunnelling Nanotubes Regulate Neurovascular Coupling. Nature 2020, 585, 91–95. [Google Scholar] [CrossRef]
- Victoria, G.S.; Zurzolo, C. The Spread of Prion-like Proteins by Lysosomes and Tunneling Nanotubes: Implications for Neurodegenerative Diseases. J. Cell Biol. 2017, 216, 2633–2644. [Google Scholar] [CrossRef]
- Costanzo, M.; Abounit, S.; Marzo, L.; Danckaert, A.; Chamoun, Z.; Roux, P.; Zurzolo, C. Transfer of Polyglutamine Aggregates in Neuronal Cells Occurs in Tunneling Nanotubes. J. Cell Sci. 2013, 126, 3678–3685. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Subramaniam, S. Rhes Travels from Cell to Cell and Transports Huntington Disease Protein via TNT-like Protrusion. J. Cell Biol. 2019, 218, 1972–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieriks, B.V.; Park, T.I.-H.; Fourie, C.; Faull, R.L.M.; Dragunow, M.; Curtis, M.A. α-Synuclein Transfer through Tunneling Nanotubes Occurs in SH-SY5Y Cells and Primary Brain Pericytes from Parkinson’s Disease Patients. Sci. Rep. 2017, 7, 42984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, S.; Witt, S.N. Trojan Horses and Tunneling Nanotubes Enable α-Synuclein Pathology to Spread in Parkinson Disease. PLoS Biol. 2021, 19, e3001331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, Z.; Chen, X.; Zhang, Y.; Guo, A.; Zhang, Y. Intercellular Transport of Tau Protein and β-Amyloid Mediated by Tunneling Nanotubes. Am. J. Transl. Res. 2021, 13, 12509–12522. [Google Scholar]
- Sisakhtnezhad, S.; Khosravi, L. Emerging Physiological and Pathological Implications of Tunneling Nanotubes Formation between Cells. Eur. J. Cell Biol. 2015, 94, 429–443. [Google Scholar] [CrossRef]
- Wang, X.-T.; Sun, H.; Chen, N.-H.; Yuan, Y.-H. Tunneling Nanotubes: A Novel Pharmacological Target for Neurodegenerative Diseases? Pharmacol. Res. 2021, 170, 105541. [Google Scholar] [CrossRef]
- Hanna, S.J.; McCoy-Simandle, K.; Leung, E.; Genna, A.; Condeelis, J.; Cox, D. Tunneling Nanotubes, a Novel Mode of Tumor Cell–Macrophage Communication in Tumor Cell Invasion. J. Cell Sci. 2019, 132, jcs223321. [Google Scholar] [CrossRef] [Green Version]
- Matejka, N.; Reindl, J. Perspectives of Cellular Communication through Tunneling Nanotubes in Cancer Cells and the Connection to Radiation Effects. Radiat. Oncol. 2019, 14, 218. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, A.; Zhang, F.; Somasekharan, S.P.; Tse, C.; Leachman, L.; Gleave, A.; Li, B.; Asmaro, I.; Huang, T.; Kotula, L.; et al. Stress-Induced Tunneling Nanotubes Support Treatment Adaptation in Prostate Cancer. Sci. Rep. 2019, 9, 7826. [Google Scholar] [CrossRef]
- Lu, J.J.; Yang, W.M.; Li, F.; Zhu, W.; Chen, Z. Tunneling Nanotubes Mediated MicroRNA-155 Intercellular Transportation Promotes Bladder Cancer Cells’ Invasive and Proliferative Capacity. Int. J. Nanomed. 2019, 14, 9731–9743. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zheng, X.; Li, F.; Yu, Y.; Chen, Z.; Liu, Z.; Wang, Z.; Xu, H.; Yang, W. Tunneling Nanotubes Promote Intercellular Mitochondria Transfer Followed by Increased Invasiveness in Bladder Cancer Cells. Oncotarget 2017, 8, 15539–15552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aloia, A.; Arrigoni, E.; Costa, B.; Berruti, G.; Martegani, E.; Sacco, E.; Ceriani, M. RalGPS2 Interacts with Akt and PDK1 Promoting Tunneling Nanotubes Formation in Bladder Cancer and Kidney Cells Microenvironment. Cancers 2021, 13, 6330. [Google Scholar] [CrossRef] [PubMed]
- Desir, S.; O’Hare, P.; Vogel, R.I.; Sperduto, W.; Sarkari, A.; Dickson, E.L.; Wong, P.; Nelson, A.C.; Fong, Y.; Steer, C.J.; et al. Chemotherapy-Induced Tunneling Nanotubes Mediate Intercellular Drug Efflux in Pancreatic Cancer. Sci. Rep. 2018, 8, 9484. [Google Scholar] [CrossRef]
- Franchi, M.; Piperigkou, Z.; Riti, E.; Masola, V.; Onisto, M.; Karamanos, N.K. Long Filopodia and Tunneling Nanotubes Define New Phenotypes of Breast Cancer Cells in 3D Cultures. Matrix Biol. Plus 2020, 6–7, 100026. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; de Rooij, B.; Pieters, R.; den Boer, M.L. B-Cell Precursor Acute Lymphoblastic Leukemia Cells Use Tunneling Nanotubes to Orchestrate Their Microenvironment. Blood 2015, 126, 2404–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omsland, M.; Andresen, V.; Gullaksen, S.; Ayuda-Durán, P.; Popa, M.; Hovland, R.; Brendehaug, A.; Enserink, J.; McCormack, E.; Gjertsen, B.T. Tyrosine Kinase Inhibitors and Interferon-α Increase Tunneling Nanotube (TNT) Formation and Cell Adhesion in Chronic Myeloid Leukemia (CML) Cell Lines. FASEB J. 2020, 34, 3773–3791. [Google Scholar] [CrossRef] [Green Version]
- De Rooij, B.; Polak, R.; Stalpers, F.; Pieters, R.; den Boer, M.L. Tunneling Nanotubes Facilitate Autophagosome Transfer in the Leukemic Niche. Leukemia 2017, 31, 1651–1654. [Google Scholar] [CrossRef] [Green Version]
- Civita, P.; M Leite, D.; Pilkington, G.J. Pre-Clinical Drug Testing in 2D and 3D Human In Vitro Models of Glioblastoma Incorporating Non-Neoplastic Astrocytes: Tunneling Nano Tubules and Mitochondrial Transfer Modulates Cell Behavior and Therapeutic Respons. Int. J. Mol. Sci. 2019, 20, 6017. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, S.; Malik, S.; Luu, R.; Loudig, O.; Mitchell, M.; Okafo, G.; Bhat, K.; Prideaux, B.; Eugenin, E.A. Tunneling Nanotubes, TNT, Communicate Glioblastoma with Surrounding Non-Tumor Astrocytes to Adapt Them to Hypoxic and Metabolic Tumor Conditions. Sci. Rep. 2021, 11, 14556. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.S.; Lou, E. Tunneling Nanotubes: A Bridge for Heterogeneity in Glioblastoma and a New Therapeutic Target? Cancer Rep. 2019, 2, e1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurzolo, C. Tunneling Nanotubes: Reshaping Connectivity. Curr. Opin. Cell Biol. 2021, 71, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nasoni, M.G.; Carloni, S.; Canonico, B.; Burattini, S.; Cesarini, E.; Papa, S.; Pagliarini, M.; Ambrogini, P.; Balduini, W.; Luchetti, F. Melatonin Reshapes the Mitochondrial Network and Promotes Intercellular Mitochondrial Transfer via Tunneling Nanotubes after Ischemic-like Injury in Hippocampal HT22 Cells. J. Pineal Res. 2021, 71, e12747. [Google Scholar] [CrossRef]
- Damodaran, N.; Dilna, A.; Kielkopf, C.S.; Kagedal, K.; Ollinger, K.; Nath, S. Amyloid-β Induced Membrane Damage Instigates Tunneling Nanotubes by Exploiting PAK1 Dependent Actin Remodulation. bioRxiv 2020, 655340. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Hou, Y.; Xu, J.; Zhong, L.; Zhou, J.; Zhang, G.; Sun, J. Mitochondria Transfer via Tunneling Nanotubes Is an Important Mechanism by Which CD133+ Scattered Tubular Cells Eliminate Hypoxic Tubular Cell Injury. Biochem. Biophys. Res. Commun. 2020, 522, 205–212. [Google Scholar] [CrossRef]
- Dupont, M.; Souriant, S.; Balboa, L.; Vu Manh, T.-P.; Pingris, K.; Rousset, S.; Cougoule, C.; Rombouts, Y.; Poincloux, R.; Ben Neji, M.; et al. Tuberculosis-Associated IFN-I Induces Siglec-1 on Tunneling Nanotubes and Favors HIV-1 Spread in Macrophages. eLife 2020, 9, e52535. [Google Scholar] [CrossRef] [Green Version]
- Okura, T.; Taneno, A.; Oishi, E. Cell-to-Cell Transmission of Turkey Herpesvirus in Chicken Embryo Cells via Tunneling Nanotubes. Avian Dis. 2021, 65, 335–339. [Google Scholar] [CrossRef]
- Panasiuk, M.; Rychłowski, M.; Derewońko, N.; Bieńkowska-Szewczyk, K. Tunneling Nanotubes as a Novel Route of Cell-to-Cell Spread of Herpesviruses. J. Virol. 2018, 92, e00090-18. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Koganti, R.; Russell, G.; Sharma, A.; Shukla, D. Role of Tunneling Nanotubes in Viral Infection, Neurodegenerative Disease, and Cancer. Front. Immunol. 2021, 12, 2256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kim, J.H.; Ranjan, P.; Metcalfe, M.G.; Cao, W.; Mishina, M.; Gangappa, S.; Guo, Z.; Boyden, E.S.; Zaki, S.; et al. Influenza Virus Exploits Tunneling Nanotubes for Cell-to-Cell Spread. Sci. Rep. 2017, 7, 40360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, Challenges, and Future of Nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Pepe, A.; Pietropaoli, S.; Vos, M.; Barba-Spaeth, G.; Zurzolo, C. Tunneling Nanotubes Provide a Novel Route for SARS-CoV-2 Spreading between Permissive Cells and to Non-Permissive Neuronal Cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jansens, R.J.J.; Tishchenko, A.; Favoreel, H.W. Bridging the Gap: Virus Long-Distance Spread via Tunneling Nanotubes. J. Virol. 2020, 94, e02120-19. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Yang, Z.; Peng, H.; Wei, R.; Wang, C.; Feng, M. Tunneling Nanotubular Expressways for Ultrafast and Accurate M1 Macrophage Delivery of Anticancer Drugs to Metastatic Ovarian Carcinoma. ACS Nano 2019, 13, 1078–1096. [Google Scholar] [CrossRef]
- Pasquier, J.; Guerrouahen, B.S.; Al Thawadi, H.; Ghiabi, P.; Maleki, M.; Abu-Kaoud, N.; Jacob, A.; Mirshahi, M.; Galas, L.; Rafii, S.; et al. Preferential Transfer of Mitochondria from Endothelial to Cancer Cells through Tunneling Nanotubes Modulates Chemoresistance. J. Transl. Med. 2013, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, J.; Galas, L.; Boulangé-Lecomte, C.; Rioult, D.; Bultelle, F.; Magal, P.; Webb, G.; Le Foll, F. Different Modalities of Intercellular Membrane Exchanges Mediate Cell-to-Cell P-Glycoprotein Transfers in MCF-7 Breast Cancer Cells. J. Biol. Chem. 2012, 287, 7374–7387. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Hazari, S.; Mehra, S.; Kaushal, D.; Moroz, K.; Dash, S. Increased Expression of P-Glycoprotein and Doxorubicin Chemoresistance of Metastatic Breast Cancer is Regulated by MiR-298. Am. J. Pathol. 2012, 180, 2490–2503. [Google Scholar] [CrossRef] [Green Version]
- Abad, E.; Lyakhovich, A. Movement of Mitochondria with Mutant DNA through Extracellular Vesicles Helps Cancer Cells Acquire Chemoresistance. ChemMedChem 2021, 16, 642. [Google Scholar] [CrossRef]
- Dash, C.; Saha, T.; Sengupta, S.; Jang, H.L. Inhibition of Tunneling Nanotubes between Cancer Cell and the Endothelium Alters the Metastatic Phenotype. Int. J. Mol. Sci. 2021, 22, 6161. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Yang, Y.-F.; Keller, K.E. Myosin-X Silencing in the Trabecular Meshwork Suggests a Role for Tunneling Nanotubes in Outflow Regulation. Investig. Ophthalmol. Vis. Sci. 2019, 60, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A.S. Tunneling Nanotubes Provide a Unique Conduit for Intercellular Transfer of Cellular Contents in Human Malignant Pleural Mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, P.; Jena, S.R.; Samanta, L. Tunneling Nanotubes: A Versatile Target for Cancer Therapy. Curr. Cancer Drug Targets 2018, 18, 514–521. [Google Scholar] [CrossRef]
- Dilsizoglu Senol, A.; Pepe, A.; Grudina, C.; Sassoon, N.; Reiko, U.; Bousset, L.; Melki, R.; Piel, J.; Gugger, M.; Zurzolo, C. Effect of Tolytoxin on Tunneling Nanotube Formation and Function. Sci. Rep. 2019, 9, 5741. [Google Scholar] [CrossRef]
- Pergu, R.; Dagar, S.; Kumar, H.; Kumar, R.; Bhattacharya, J.; Mylavarapu, S.V.S. The Chaperone ERp29 Is Required for Tunneling Nanotube Formation by Stabilizing MSec. J. Biol. Chem. 2019, 294, 7177–7193. [Google Scholar] [CrossRef]
- Barutta, F.; Kimura, S.; Hase, K.; Bellini, S.; Corbetta, B.; Corbelli, A.; Fiordaliso, F.; Barreca, A.; Papotti, M.G.; Ghiggeri, G.M.; et al. Protective Role of the M-Sec–Tunneling Nanotube System in Podocytes. J. Am. Soc. Nephrol. 2021, 32, 1114–1130. [Google Scholar] [CrossRef]
- Yasuda, K.; Park, H.-C.; Ratliff, B.; Addabbo, F.; Hatzopoulos, A.K.; Chander, P.; Goligorsky, M.S. Adriamycin Nephropathy: A Failure of Endothelial Progenitor Cell-Induced Repair. Am. J. Pathol. 2010, 176, 1685–1695. [Google Scholar] [CrossRef]
- Kato, K.; Nguyen, K.T.; Decker, C.W.; Silkwood, K.H.; Eck, S.M.; Hernandez, J.B.; Garcia, J.; Han, D. Tunneling Nanotube Formation Promotes Survival against 5-Fluorouracil in MCF-7 Breast Cancer Cells. FEBS Open Bio 2022, 12, 203–210. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Nakhle, J.; Galloni, M.; Vignais, M.-L. The Role of Metabolism and Tunneling Nanotube-Mediated Intercellular Mitochondria Exchange in Cancer Drug Resistance. Biochem. J. 2018, 475, 2305–2328. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic Nano-Carriers Based Smart Drug Delivery Systems for Tumor Therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug Delivery across the Blood–Brain Barrier: Recent Advances in the Use of Nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as Carriers for Drug Delivery of Macromolecules across the Blood-Brain Barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef]
- Righeschi, C.; Coronnello, M.; Mastrantoni, A.; Isacchi, B.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Strategy to Provide a Useful Solution to Effective Delivery of Dihydroartemisinin: Development, Characterization and in Vitro Studies of Liposomal Formulations. Colloids Surf. B Biointerfaces 2014, 116, 121–127. [Google Scholar] [CrossRef]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous Silica Nanoparticles as Potential Carriers for Enhanced Drug Solubility of Paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Matranga, M.A.; Cortinas, A.B.; Delcassian, D.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-Responsive Nanoparticles for Rapid and Extended Self-Regulated Insulin Delivery. ACS Nano 2020, 14, 488–497. [Google Scholar] [CrossRef]
- Duskey, J.T.; Ottonelli, I.; Rinaldi, A.; Parmeggiani, I.; Zambelli, B.; Wang, L.Z.; Prud’homme, R.K.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Tween® Preserves Enzyme Activity and Stability in PLGA Nanoparticles. Nanomaterials 2021, 11, 2946. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; da Ros, F.; Ottonelli, I.; Zambelli, B.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Enzyme Stability in Nanoparticle Preparations Part 1: Bovine Serum Albumin Improves Enzyme Function. Molecules 2020, 25, 4593. [Google Scholar] [CrossRef]
- Rigon, L.; Salvalaio, M.; Pederzoli, F.; Legnini, E.; Duskey, J.T.; D’Avanzo, F.; De Filippis, C.; Ruozi, B.; Marin, O.; Vandelli, M.A.; et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Xu, S.; Wang, H.; Ling, Y.; Dong, J.; Xia, R.; Sun, X. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Chen, Y.-S.; Green, C.R.; Rupenthal, I.D. Hyaluronic Acid Coated Albumin Nanoparticles for Targeted Peptide Delivery in the Treatment of Retinal Ischaemia. Biomaterials 2018, 168, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Ruozi, B.; Duskey, J.; Hagmeyer, S.; Sauer, A.K.; Grabrucker, S.; Coelho, R.; Oddone, N.; Ottonelli, I.; Daini, E.; et al. Nanomedicine Against Aβ Aggregation by β-Sheet Breaker Peptide Delivery: In Vitro Evidence. Pharmaceutics 2019, 11, E572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowaidar, M.; Nasser Abdelhamid, H.; Hällbrink, M.; Langel, Ü.; Zou, X. Chitosan Enhances Gene Delivery of Oligonucleotide Complexes with Magnetic Nanoparticles–Cell-Penetrating Peptide. J. Biomater. Appl. 2018, 33, 392–401. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Ling, D. Surface Engineering of Nanoparticles for Targeted Delivery to Hepatocellular Carcinoma. Small 2018, 14, 1702037. [Google Scholar] [CrossRef]
- Xiao, Y.; Shi, K.; Qu, Y.; Chu, B.; Qian, Z. Engineering Nanoparticles for Targeted Delivery of Nucleic Acid Therapeutics in Tumor. Mol. Ther.-Methods Clin. Dev. 2019, 12, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-Loaded Nanoparticles Targeted to the Site of Infection Enhance Antibacterial Efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Su, R.; Huang, X.; Wang, Y.; Kuang, X.; Zhou, S.; Liu, H. Triphenylphosphonium-Modified Mitochondria-Targeted Paclitaxel Nanocrystals for Overcoming Multidrug Resistance. Asian J. Pharm. Sci. 2019, 14, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor Targeted Delivery of Umbelliferone via a Smart Mesoporous Silica Nanoparticles Controlled-Release Drug Delivery System for Increased Anticancer Efficiency. Mater. Sci. Eng. C 2020, 116, 111239. [Google Scholar] [CrossRef]
- Salehiabar, M.; Nosrati, H.; Javani, E.; Aliakbarzadeh, F.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. Production of Biological Nanoparticles from Bovine Serum Albumin as Controlled Release Carrier for Curcumin Delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Jiang, P.; Choi, A.; Swindle-Reilly, K.E. Controlled Release of Anti-VEGF by Redox-Responsive Polydopamine Nanoparticles. Nanoscale 2020, 12, 17298–17311. [Google Scholar] [CrossRef]
- Cano, A.; Turowski, P.; Ettcheto, M.; Duskey, J.T.; Tosi, G.; Sánchez-López, E.; García, M.L.; Camins, A.; Souto, E.B.; Ruiz, A.; et al. Nanomedicine-Based Technologies and Novel Biomarkers for the Diagnosis and Treatment of Alzheimer’s Disease: From Current to Future Challenges. J. Nanobiotechnol. 2021, 19, 122. [Google Scholar] [CrossRef]
- Baskin, J.; Jeon, J.E.; Lewis, S.J.G. Nanoparticles for Drug Delivery in Parkinson’s Disease. J. Neurol. 2021, 268, 1981–1994. [Google Scholar] [CrossRef]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Passoni, A.; Favagrossa, M.; Duskey, J.T.; Bombaci, M.; Vandelli, M.A.; Colombo, L.; Bagnati, R.; et al. Insights into Kinetics, Release, and Behavioral Effects of Brain-Targeted Hybrid Nanoparticles for Cholesterol Delivery in Huntington’s Disease. J. Control. Release 2021, 330, 587–598. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.-L. Nanomedicine Applications in the Treatment of Breast Cancer: Current State of the Art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatar, A.-S.; Nagy-Simon, T.; Tomuleasa, C.; Boca, S.; Astilean, S. Nanomedicine Approaches in Acute Lymphoblastic Leukemia. J. Control. Release 2016, 238, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; Belletti, D.; Pederzoli, F.; Vandelli, M.A.; Forni, F.; Ruozi, B.; Tosi, G. Current Strategies for the Delivery of Therapeutic Proteins and Enzymes to Treat Brain Disorders. Int. Rev. Neurobiol. 2017, 137, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Naserifar, M.; Hosseinzadeh, H.; Abnous, K.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Oral Delivery of Folate-Targeted Resveratrol-Loaded Nanoparticles for Inflammatory Bowel Disease Therapy in Rats. Life Sci. 2020, 262, 118555. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhong, Z.; Wang, Y.; Feng, Y.; Mei, Z.; Li, H.; Chen, X.; Cai, L.; Li, C. Exosome-Based Biomimetic Nanoparticles Targeted to Inflamed Joints for Enhanced Treatment of Rheumatoid Arthritis. J. Nanobiotechnol. 2020, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Sun, S.; Varner, J.A.; Howell, S.B.; Ruoslahti, E.; Sailor, M.J. Securing the Payload, Finding the Cell, and Avoiding the Endosome: Peptide-Targeted, Fusogenic Porous Silicon Nanoparticles for Delivery of SiRNA. Adv. Mater. 2019, 31, 1902952. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood–Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Duskey, J.T.; Ottonelli, I.; Da Ros, F.; Vilella, A.; Zoli, M.; Kovachka, S.; Spyrakis, F.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Novel Peptide-Conjugated Nanomedicines for Brain Targeting: In Vivo Evidence. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102226. [Google Scholar] [CrossRef]
- Bu, J.; Nair, A.; Iida, M.; Jeong, W.; Poellmann, M.J.; Mudd, K.; Kubiatowicz, L.J.; Liu, E.W.; Wheeler, D.L.; Hong, S. An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy. Nano Lett. 2020, 20, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Johnston, M.C.; Scott, C.J. Antibody Conjugated Nanoparticles as a Novel Form of Antibody Drug Conjugate Chemotherapy. Drug Discov. Today Technol. 2018, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ucak, S.; Sudagidan, M.; Borsa, B.A.; Mansuroglu, B.; Ozalp, V.C. Inhibitory Effects of Aptamer Targeted Teicoplanin Encapsulated PLGA Nanoparticles for Staphylococcus Aureus Strains. World J. Microbiol. Biotechnol. 2020, 36, 69. [Google Scholar] [CrossRef] [PubMed]
- Zununi Vahed, S.; Fathi, N.; Samiei, M.; Maleki Dizaj, S.; Sharifi, S. Targeted Cancer Drug Delivery with Aptamer-Functionalized Polymeric Nanoparticles. J. Drug Target. 2019, 27, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A Potential Carrier for Anti-Tumor Targeted Delivery-Hyaluronic Acid Nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Hyaluronic Acid Coated Albumin Nanoparticles for Targeted Peptide Delivery to the Retina. Mol. Pharm. 2017, 14, 533–545. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Liu, X.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Leng, X.; Song, C.; Kong, D.; et al. Hyaluronic Acid-Modified Cationic Lipid–PLGA Hybrid Nanoparticles as a Nanovaccine Induce Robust Humoral and Cellular Immune Responses. ACS Appl. Mater. Interfaces 2016, 8, 11969–11979. [Google Scholar] [CrossRef]
- Sabri, T.; Pawelek, P.D.; Capobianco, J.A. Dual Activity of Rose Bengal Functionalized to Albumin-Coated Lanthanide-Doped Upconverting Nanoparticles: Targeting and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 26947–26953. [Google Scholar] [CrossRef]
- Rao, L.; Yu, G.-T.; Meng, Q.-F.; Bu, L.-L.; Tian, R.; Lin, L.-S.; Deng, H.; Yang, W.; Zan, M.; Ding, J.; et al. Cancer Cell Membrane-Coated Nanoparticles for Personalized Therapy in Patient-Derived Xenograft Models. Adv. Funct. Mater. 2019, 29, 1905671. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, X.; Yu, X.; He, X.; An, Y.; Lu, H. Hyaluronidase Enzyme-Responsive Targeted Nanoparticles for Effective Delivery of 5-Fluorouracil in Colon Cancer. Pharm. Res. 2018, 35, 73. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management. Adv. Mater. 2018, 30, 1803618. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Oddone, N.; Boury, F.; Garcion, E.; Grabrucker, A.M.; Martinez, M.C.; Da Ros, F.; Janaszewska, A.; Forni, F.; Vandelli, M.A.; Tosi, G.; et al. Synthesis, Characterization, and In Vitro Studies of an Reactive Oxygen Species (ROS)-Responsive Methoxy Polyethylene Glycol-Thioketal-Melphalan Prodrug for Glioblastoma Treatment. Front. Pharmacol. 2020, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Chen, G.-B.; Chen, H.-H.; Zhang, J.-B.; Li, H.-Z.; Sheng, M.-X.; Weng, W.-B.; Guo, S.-M. Cancer Cell Membrane-Cloaked Mesoporous Silica Nanoparticles with a PH-Sensitive Gatekeeper for Cancer Treatment. Colloids Surf. B Biointerfaces 2019, 175, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Sahandi Zangabad, P.; Barar, J.; Aghanejad, A.; Erfan-Niya, H.; Omidi, Y. Thermo-Sensitive Chitosan Copolymer-Gold Hybrid Nanoparticles as a Nanocarrier for Delivery of Erlotinib. Int. J. Biol. Macromol. 2018, 106, 266–276. [Google Scholar] [CrossRef]
- Dariva, C.G.; Coelho, J.F.J.; Serra, A.C. Near Infrared Light-Triggered Nanoparticles Using Singlet Oxygen Photocleavage for Drug Delivery Systems. J. Control. Release 2019, 294, 337–354. [Google Scholar] [CrossRef]
- Oddone, N.; Pederzoli, F.; Duskey, J.T.; De Benedictis, C.A.; Grabrucker, A.M.; Forni, F.; Angela Vandelli, M.; Ruozi, B.; Tosi, G. ROS-Responsive “Smart” Polymeric Conjugate: Synthesis, Characterization and Proof-of-Concept Study. Int. J. Pharm. 2019, 570, 118655. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle Crossing of Blood–Brain Barrier: A Road to New Therapeutic Approaches to Central Nervous System Diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Ranganath, S.H.; Thanuja, M.Y.; Anupama, C.; Manjunatha, T.D. Systemic drug delivery to the posterior segment of the eye: Overcoming blood–retinal barrier through smart drug design and nanotechnology. In Immobilization Strategies: Biomedical, Bioengineering and Environmental Applications; Immobilization Strategies: Biomedical, Bioengineering and Environmental Applications; Tripathi, A., Melo, J.S., Eds.; Springer: Singapore, 2021; pp. 219–269. ISBN 9789811579981. [Google Scholar]
- He, K.; Luo, W.; Zhang, Y.; Liu, F.; Liu, D.; Xu, L.; Qin, L.; Xiong, C.; Lu, Z.; Fang, X.; et al. Intercellular Transportation of Quantum Dots Mediated by Membrane Nanotubes. ACS Nano 2010, 4, 3015–3022. [Google Scholar] [CrossRef]
- Mittal, R.; Karhu, E.; Wang, J.-S.; Delgado, S.; Zukerman, R.; Mittal, J.; Jhaveri, V.M. Cell Communication by Tunneling Nanotubes: Implications in Disease and Therapeutic Applications. J. Cell. Physiol. 2019, 234, 1130–1146. [Google Scholar] [CrossRef]
- Dagar, S.; Pathak, D.; Oza, H.V.; Mylavarapu, S.V.S. Tunneling Nanotubes and Related Structures: Molecular Mechanisms of Formation and Function. Biochem. J. 2021, 478, 3977–3998. [Google Scholar] [CrossRef]
- Mi, L.; Xiong, R.; Zhang, Y.; Yang, W.; Chen, J.-Y.; Wang, P.-N. Microscopic Observation of the Intercellular Transport of CdTe Quantum Dot Aggregates through Tunneling-Nanotubes. J. Biomater. Nanobiotechnol. 2011, 2, 172. [Google Scholar] [CrossRef] [Green Version]
- Domhan, S.; Ma, L.; Tai, A.; Anaya, Z.; Beheshti, A.; Zeier, M.; Hlatky, L.; Abdollahi, A. Intercellular Communication by Exchange of Cytoplasmic Material via Tunneling Nano-Tube Like Structures in Primary Human Renal Epithelial Cells. PLoS ONE 2011, 6, e21283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehberg, M.; Nekolla, K.; Sellner, S.; Praetner, M.; Mildner, K.; Zeuschner, D.; Krombach, F. Intercellular Transport of Nanomaterials Is Mediated by Membrane Nanotubes In Vivo. Small 2016, 12, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Epperla, C.P.; Mohan, N.; Chang, C.-W.; Chen, C.-C.; Chang, H.-C. Nanodiamond-Mediated Intercellular Transport of Proteins through Membrane Tunneling Nanotubes. Small 2015, 11, 6097–6105. [Google Scholar] [CrossRef] [PubMed]
- Franco, S.; Noureddine, A.; Guo, J.; Keth, J.; Paffett, M.L.; Brinker, C.J.; Serda, R.E. Direct Transfer of Mesoporous Silica Nanoparticles between Macrophages and Cancer Cells. Cancers 2020, 12, 2892. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.; Zappavigna, S.; Sannolo, N.; Caraglia, M. Advantages and Risks of Nanotechnologies in Cancer Patients and Occupationally Exposed Workers. Expert Opin. Drug Deliv. 2014, 11, 1087–1101. [Google Scholar] [CrossRef]
- Ingle, N.P.; Hexum, J.K.; Reineke, T.M. Polyplexes Are Endocytosed by and Trafficked within Filopodia. Biomacromolecules 2020, 21, 1379–1392. [Google Scholar] [CrossRef]
- Sáenz-de-Santa-María, I.; Bernardo-Castiñeira, C.; Enciso, E.; García-Moreno, I.; Chiara, J.L.; Suarez, C.; Chiara, M.-D. Control of Long-Distance Cell-to-Cell Communication and Autophagosome Transfer in Squamous Cell Carcinoma via Tunneling Nanotubes. Oncotarget 2017, 8, 20939–20960. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK Signaling in Human Cancer as a Target for Therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Tosi, G.; Vilella, A.; Chhabra, R.; Schmeisser, M.J.; Boeckers, T.M.; Ruozi, B.; Vandelli, M.A.; Forni, F.; Zoli, M.; Grabrucker, A.M. Insight on the Fate of CNS-Targeted Nanoparticles. Part II: Intercellular Neuronal Cell-to-Cell Transport. J. Control. Release 2014, 177, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Costantino, L.; Rivasi, F.; Ruozi, B.; Leo, E.; Vergoni, A.V.; Tacchi, R.; Bertolini, A.; Vandelli, M.A.; Forni, F. Targeting the Central Nervous System: In Vivo Experiments with Peptide-Derivatized Nanoparticles Loaded with Loperamide and Rhodamine-123. J. Control. Release 2007, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salvalaio, M.; Rigon, L.; Belletti, D.; D’Avanzo, F.; Pederzoli, F.; Ruozi, B.; Marin, O.; Vandelli, M.A.; Forni, F.; Scarpa, M.; et al. Targeted Polymeric Nanoparticles for Brain Delivery of High Molecular Weight Molecules in Lysosomal Storage Disorders. PLoS ONE 2016, 11, e0156452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Kristl, J.; Plajnšek, K.T.; Kreft, M.E.; Janković, B.; Kocbek, P. Intracellular Trafficking of Solid Lipid Nanoparticles and Their Distribution between Cells through Tunneling Nanotubes. Eur. J. Pharm. Sci. 2013, 50, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Astanina, K.; Koch, M.; Jüngst, C.; Zumbusch, A.; Kiemer, A.K. Lipid Droplets as a Novel Cargo of Tunnelling Nanotubes in Endothelial Cells. Sci. Rep. 2015, 5, 11453. [Google Scholar] [CrossRef] [Green Version]
- Rossen, N.S.; Hansen, A.J.; Selhuber-Unkel, C.; Oddershede, L.B. Arachidonic Acid Randomizes Endothelial Cell Motion and Regulates Adhesion and Migration. PLoS ONE 2011, 6, e25196. [Google Scholar] [CrossRef]
- Formicola, B.; D’Aloia, A.; Dal Magro, R.; Stucchi, S.; Rigolio, R.; Ceriani, M.; Re, F. Differential Exchange of Multifunctional Liposomes Between Glioblastoma Cells and Healthy Astrocytes via Tunneling Nanotubes. Front. Bioeng. Biotechnol. 2019, 7, 403. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Jiang, Y.; Zhang, J.; Deng, C.; Zhong, Z. Oncoprotein Inhibitor Rigosertib Loaded in ApoE-Targeted Smart Polymersomes Reveals High Safety and Potency against Human Glioblastoma in Mice. Mol. Pharm. 2019, 16, 3711–3719. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, Y.; Deng, C.; Zhong, Z.; Lan, Q. Doxorubicin Delivered via ApoE-Directed Reduction-Sensitive Polymersomes Potently Inhibit Orthotopic Human Glioblastoma Xenografts in Nude Mice. Int. J. Nanomed. 2021, 16, 4105–4115. [Google Scholar] [CrossRef]
- Costa, P.M.; Cardoso, A.L.; Mendonça, L.S.; Serani, A.; Custódia, C.; Conceição, M.; Simões, S.; Moreira, J.N.; Pereira de Almeida, L.; Pedroso de Lima, M.C. Tumor-Targeted Chlorotoxin-Coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Mol. Ther.-Nucleic Acids 2013, 2, e100. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma Multiforme Targeted Therapy: The Chlorotoxin Story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Austefjord, M.W.; Gerdes, H.-H.; Wang, X. Tunneling Nanotubes. Commun. Integr. Biol. 2014, 7, e27934. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Saenz-de-Santa-Maria, I.; Chastagner, P.; Perthame, E.; Delmas, C.; Toulas, C.; Moyal-Jonathan-Cohen, E.; Brou, C.; Zurzolo, C. Patient-Derived Glioblastoma Stem Cells Transfer Mitochondria through Tunneling Nanotubes in Tumor Organoids. Biochem. J. 2021, 478, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Pearlman, E.; McMenamin, P.G. Cutting Edge: Membrane Nanotubes In Vivo: A Feature of MHC Class II + Cells in the Mouse Cornea. J. Immunol. 2008, 180, 5779–5783. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ye, S.; Guo, J.; Wang, H.; Yan, W.; Song, J.; Qu, J. Biocompatible Carbon Dots with Low-Saturation-Intensity and High-Photobleaching-Resistance for STED Nanoscopy Imaging of the Nucleolus and Tunneling Nanotubes in Living Cells. Nano Res. 2019, 12, 3075–3084. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottonelli, I.; Caraffi, R.; Tosi, G.; Vandelli, M.A.; Duskey, J.T.; Ruozi, B. Tunneling Nanotubes: A New Target for Nanomedicine? Int. J. Mol. Sci. 2022, 23, 2237. https://doi.org/10.3390/ijms23042237
Ottonelli I, Caraffi R, Tosi G, Vandelli MA, Duskey JT, Ruozi B. Tunneling Nanotubes: A New Target for Nanomedicine? International Journal of Molecular Sciences. 2022; 23(4):2237. https://doi.org/10.3390/ijms23042237
Chicago/Turabian StyleOttonelli, Ilaria, Riccardo Caraffi, Giovanni Tosi, Maria Angela Vandelli, Jason Thomas Duskey, and Barbara Ruozi. 2022. "Tunneling Nanotubes: A New Target for Nanomedicine?" International Journal of Molecular Sciences 23, no. 4: 2237. https://doi.org/10.3390/ijms23042237






