Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Tetrahydrocurcumin-Loaded Solid Lipid Nanoparticles (THC-SLNs)
2.3. Preparation of THC-SLNs-Based Gel
2.4. Characterization of THC-SLNs
2.4.1. Total Drug Content (TDC) and Entrapment Efficiency (EE)
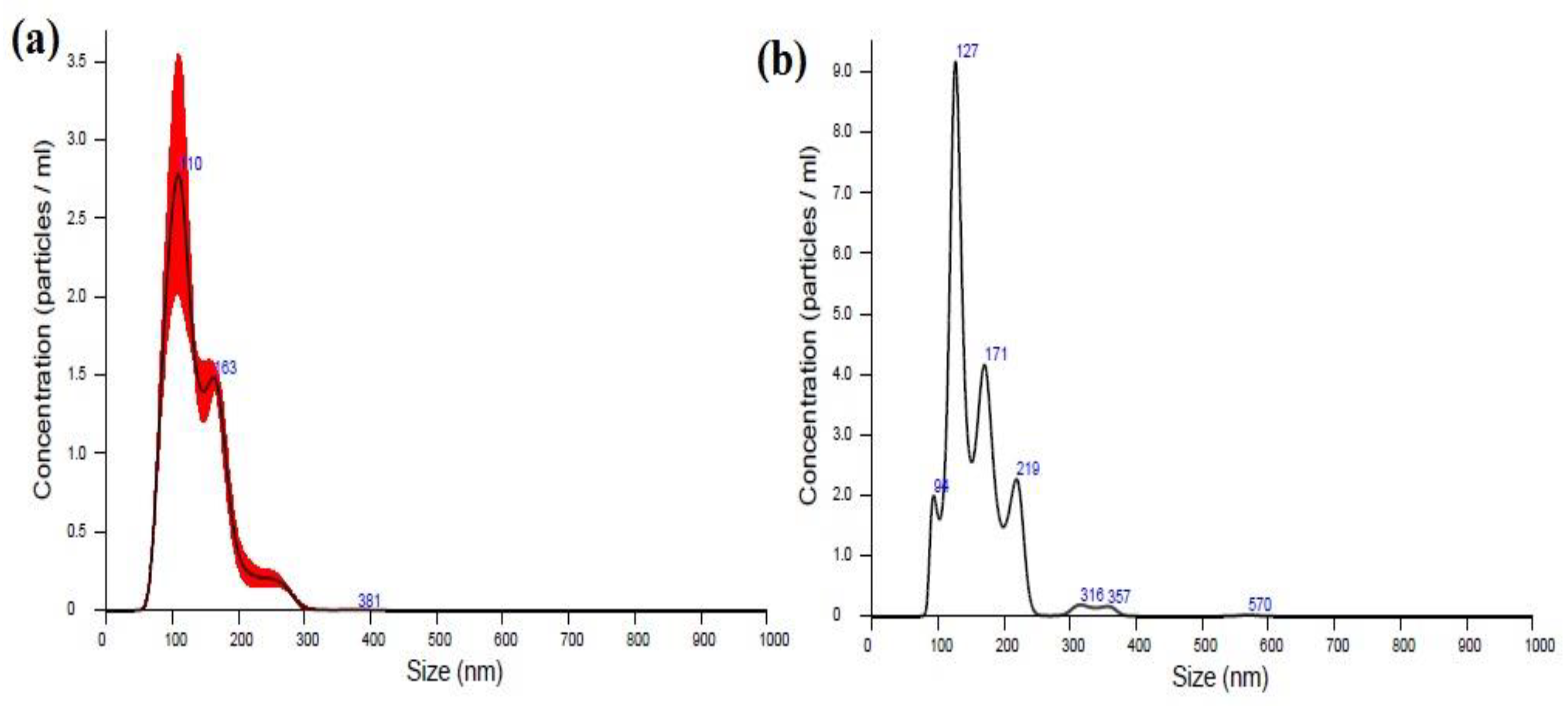
2.4.2. Nanoparticle Tracking Analysis
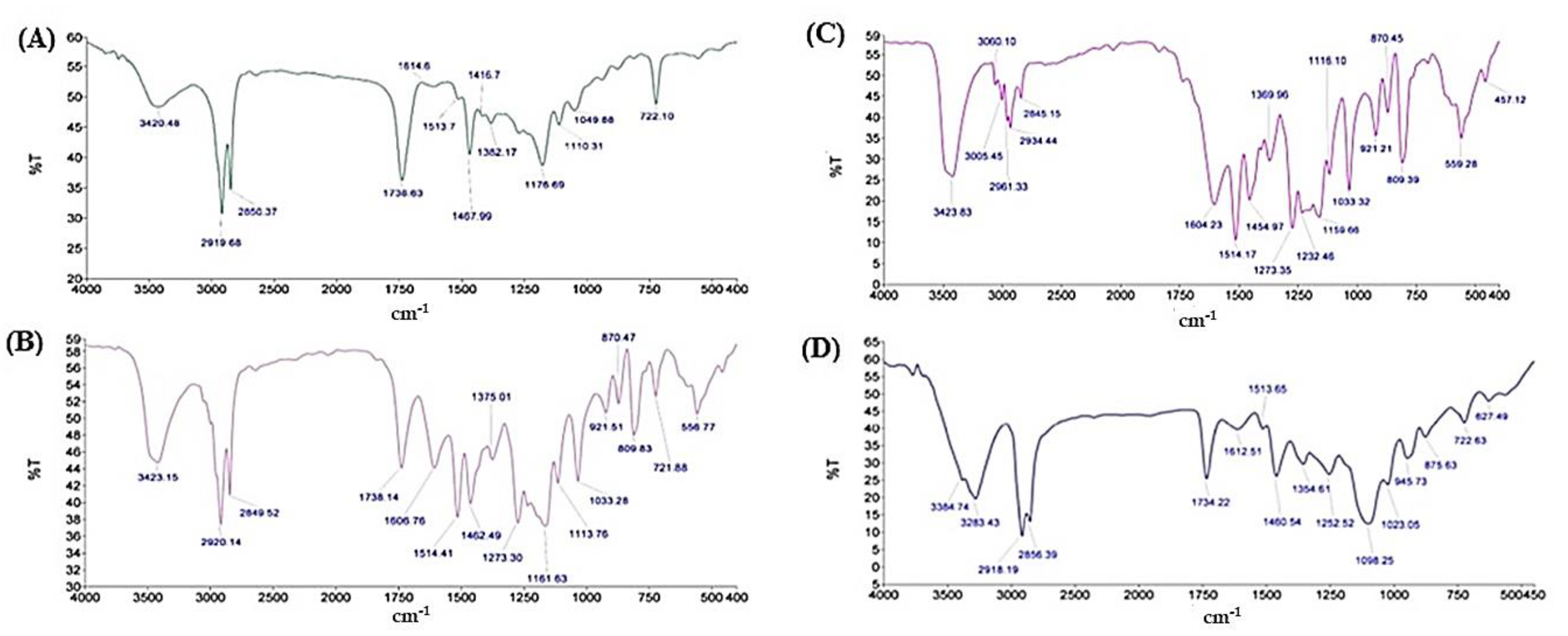
2.4.3. Fourier Transform Infra-Red Spectroscopy (FTIR)
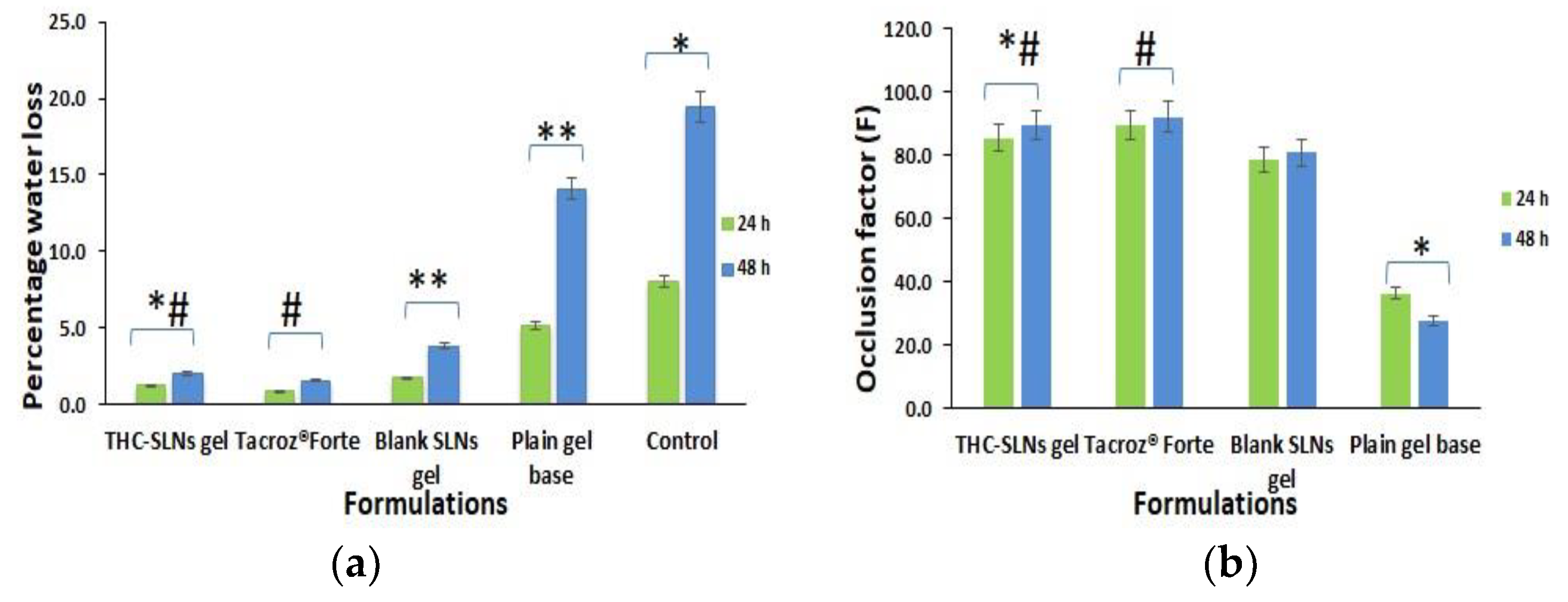
2.5. Skin Hydration Studies
2.5.1. Ex Vivo Skin Hydration Studies
2.5.2. In Vivo Skin Hydration Studies
2.6. Skin Penetration of THC-SLNs
2.6.1. Ex Vivo Penetration and Dermatokinetics
2.6.2. In Vivo Penetration Using Confocal Laser Scanning Microscopy (CLSM)
2.7. Pharmacodynamic Activity in a Murine Atopic Dermatitis (AD) Model
2.7.1. Effectiveness against AD
2.7.2. Biochemical Estimation
2.7.3. Evaluation of Molecular Markers (IL-6 and TNF-α) in Skin
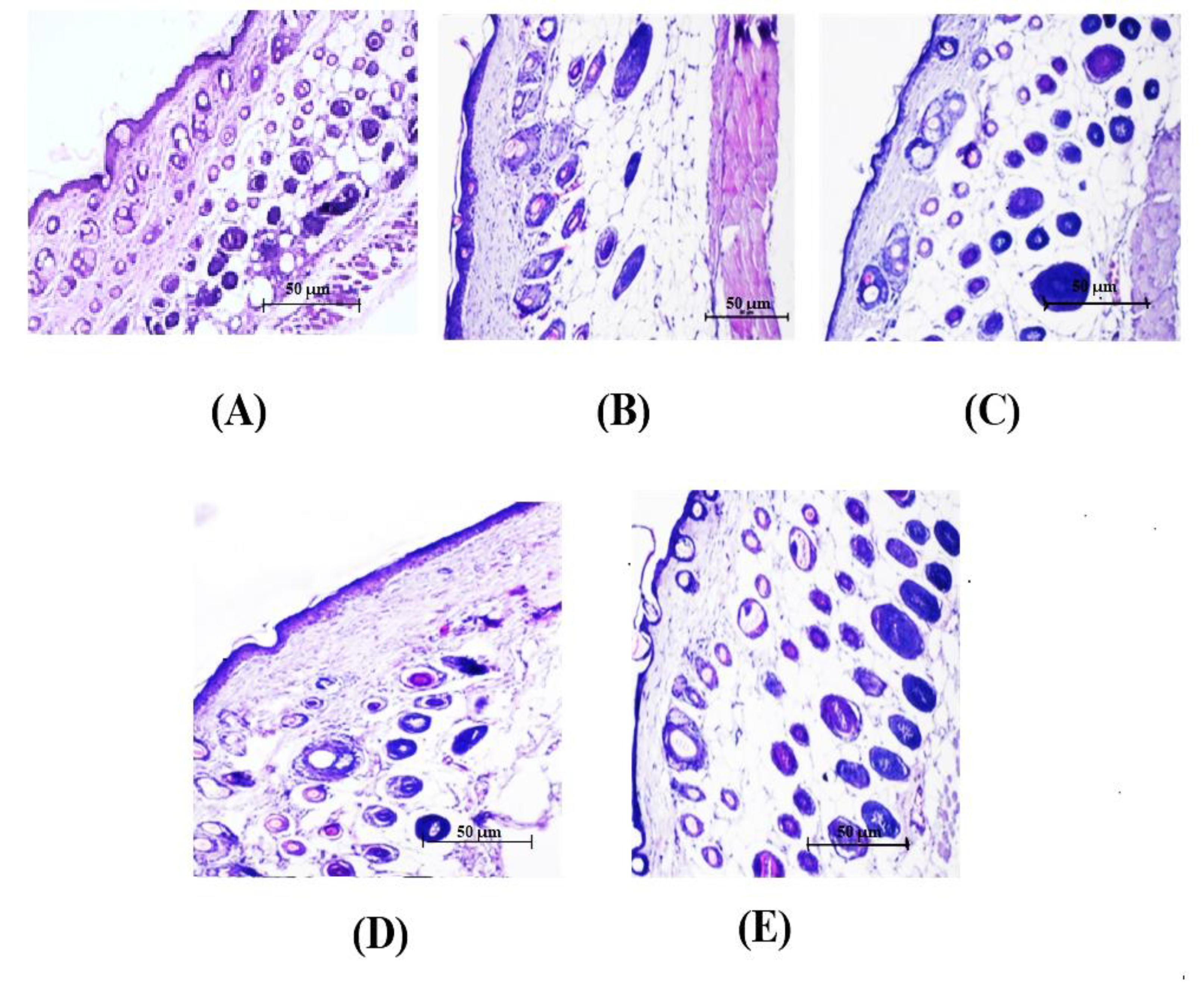
2.7.4. Histopathological Studies
2.8. Statistical Analysis
3. Results
3.1. Formulation of Lipidic Nanoparticles Loaded with Tetrahydrocurcumin (THC-SLNs)
3.2. Characterization of THC-SLNs
3.2.1. Total Drug Content (TDC) and Entrapment Efficiency (EE)
3.2.2. Nanoparticle Tracking Analysis (NTA)
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Skin Hydration
3.3.1. Ex Vivo Studies
3.3.2. In Vivo Studies
3.4. Skin Penetration of THC-SLNs
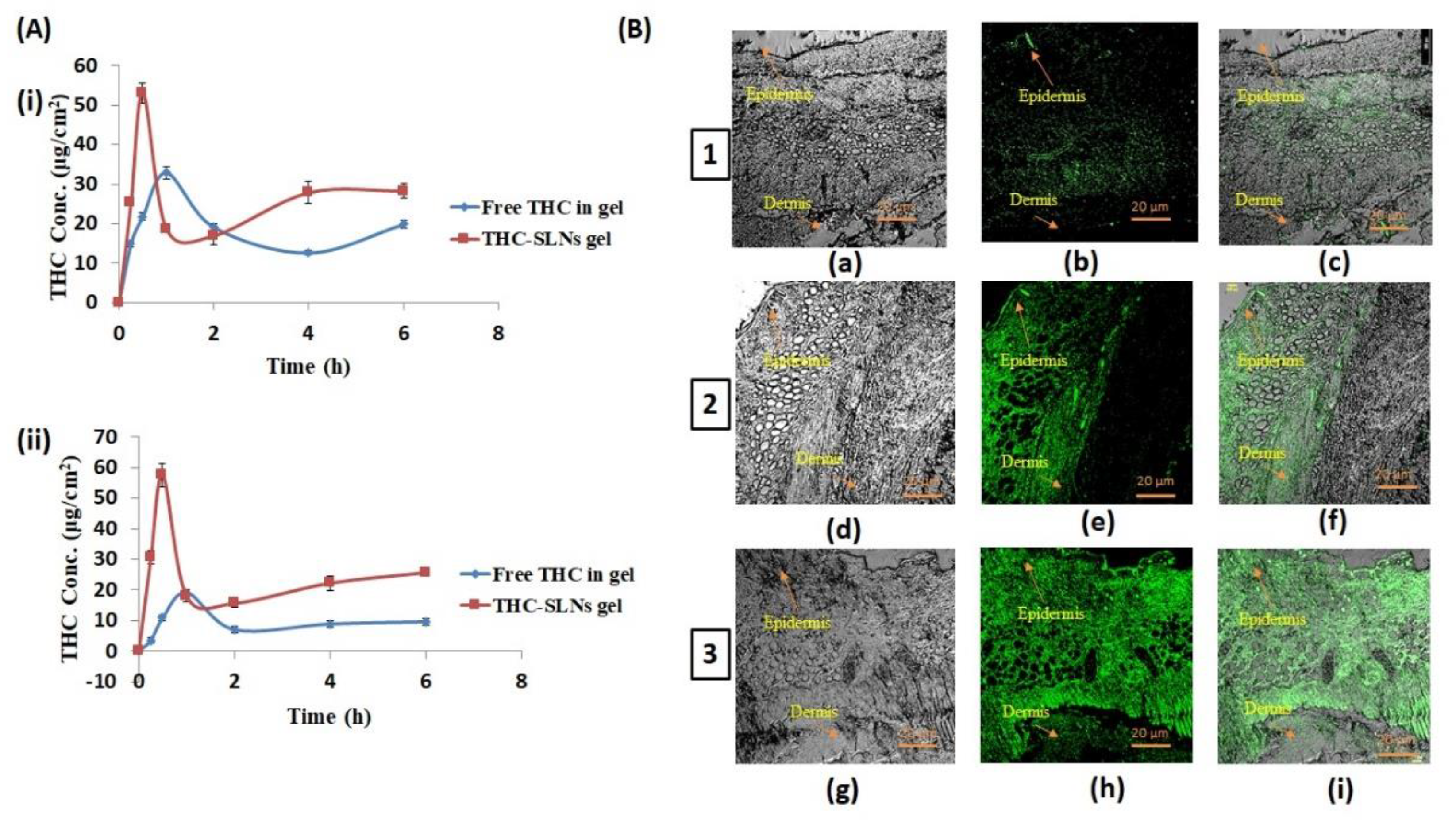
3.4.1. Ex Vivo Penetration and Dermatokinetics
3.4.2. In Vivo Penetration Using Confocal Laser Scanning Microscopy (CLSM)
3.5. Pharmacodynamic Activity
3.5.1. Evaluation of Atopic Dermatitis (AD)
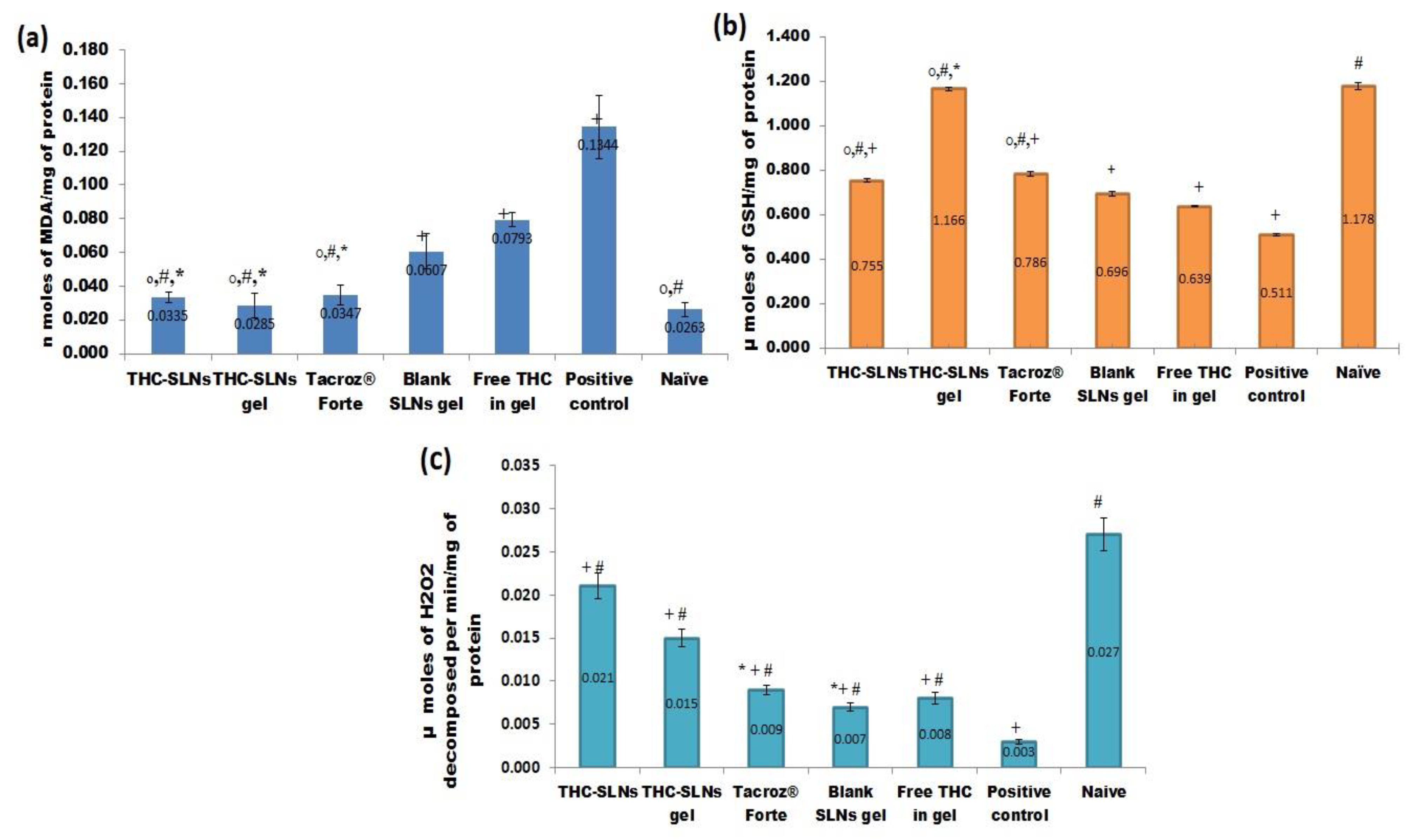
3.5.2. Biochemical Estimation
Lipid Peroxidation (LPO)
Reduced Glutathione (GSH)
Catalase H2O2
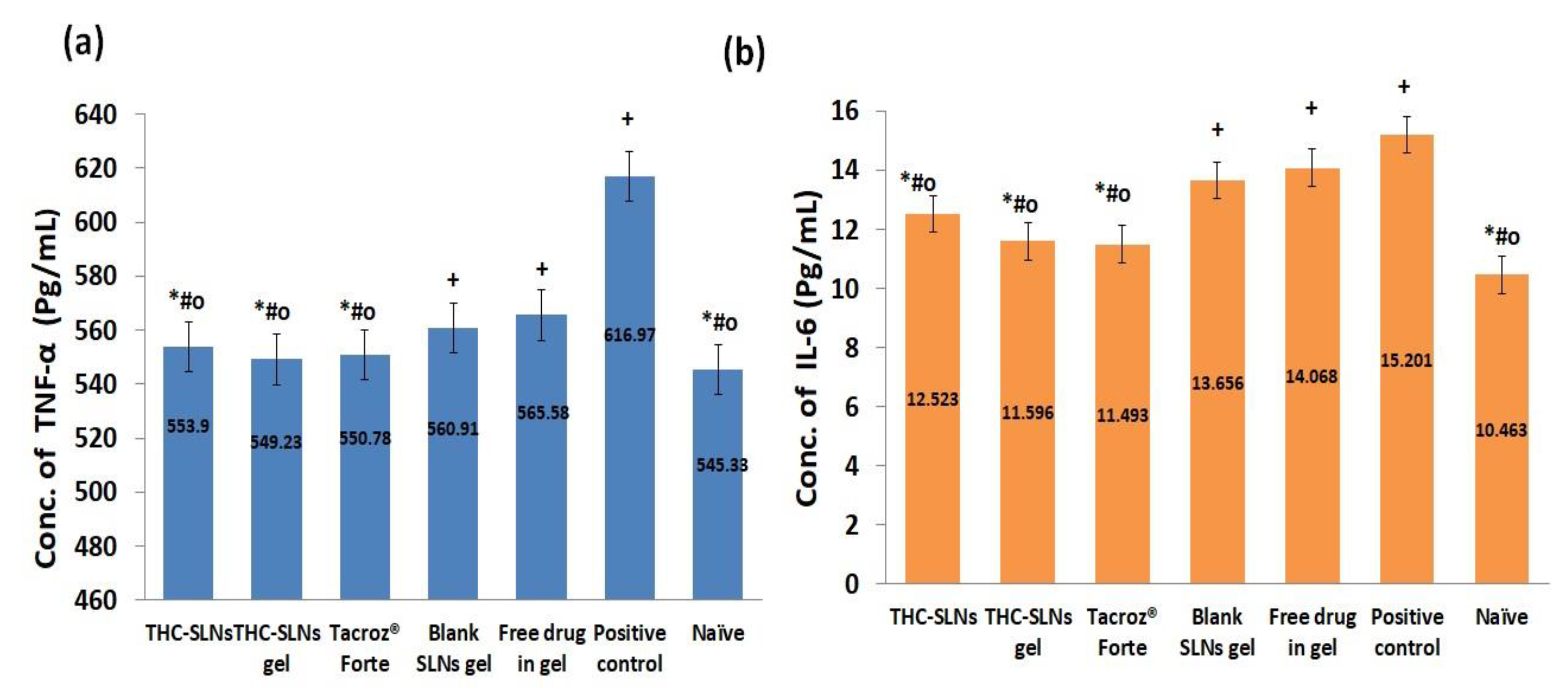
3.5.3. Molecular Markers (Interleukins (IL-6) and TNF-α) in Skin Homogenates
Effect on the Level of TNF-α
Effect on the Level of IL-6
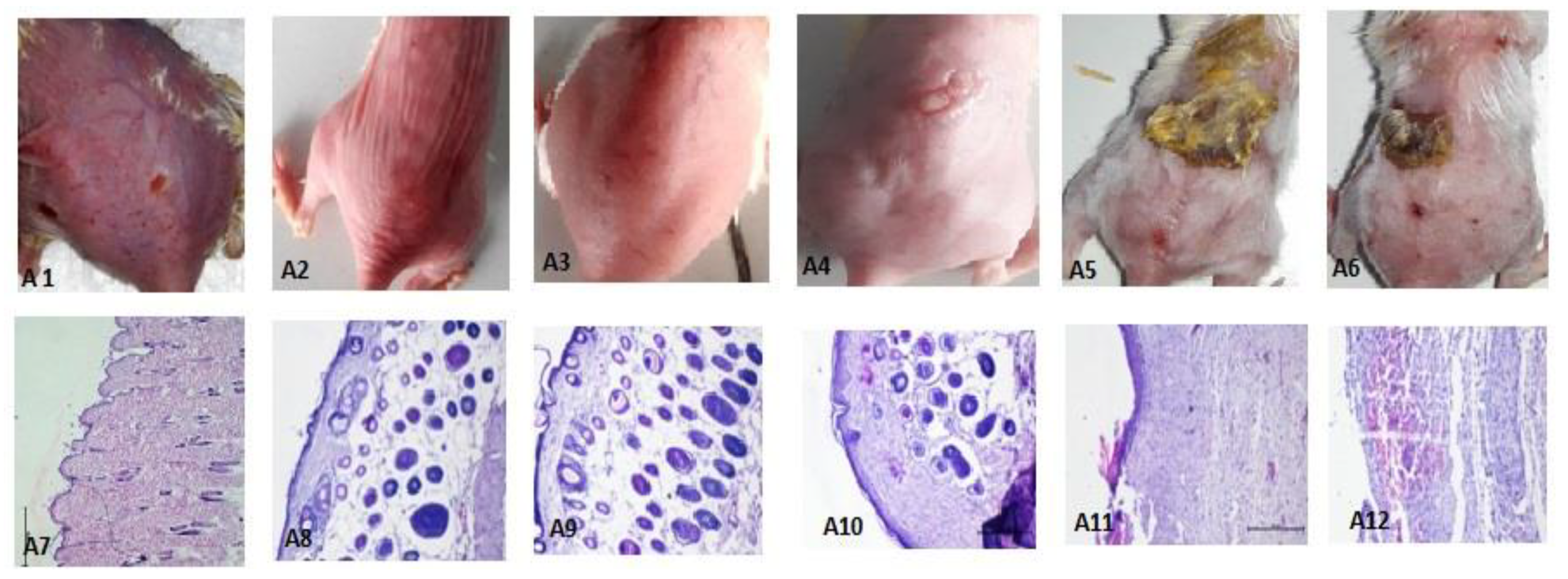
3.5.4. Histopathological Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, H.; Li, X.K. Oxidative Stress in Atopic Dermatitis. Oxidative Med. Cell Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Kaya, G.; Rollason, V. Topical corticosteroid-induced skin atrophy: A comprehensive review. Drug Saf. 2015, 38, 493–509. [Google Scholar] [CrossRef]
- Carr, W.W. Topical calcineurin inhibitors for atopic dermatitis: Review and treatment recommendations. Paediatr. Drugs. 2013, 15, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.L.; Lio, P.A. Alternative, Complementary, and Forgotten Remedies for Atopic Dermatitis. Evid. Based Complement. Altern. Med. 2015, 2015, 676897. [Google Scholar] [CrossRef][Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Luo, D.D.; Xie, J.H.; Xian, Y.F.; Lai, Z.Q.; Liu, Y.H.; Liu, W.H.; Chen, J.N.; Lai, X.P.; Lin, Z.X.; et al. Curcumin’s Metabolites, Tetrahydrocurcumin and Octahydrocurcumin, Possess Superior Anti-inflammatory Effects in vivo Through Suppression of TAK1-NF-κB Pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Pople, P.; Singh, K.K. Development and evaluation of colloidal modified nanolipid carrier: Application to topical delivery of tacrolimus. Eur. J. Pharm. Biopharm. 2011, 79, 82–94. [Google Scholar] [CrossRef]
- Kakadia, P.G.; Conway, B.R. Lipid nanoparticles for dermal drug delivery. Curr. Pharm. Des. 2015, 21, 2823–2829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakkar, V.; Kaur, I.P.; Pal, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Kaur, I.P. Preparation, characterization and scale-up of sesamol loaded solid lipid nanoparticles. Nanotechnol. Dev. 2012, 2, 8. [Google Scholar] [CrossRef]
- Kaur, A.; Goindi, S.; Katare, O.P. Formulation, characterisation and in vivo evaluation of lipid-based nanocarrier for topical delivery of diflunisal. J. Microencapsul. 2016, 33, 475–486. [Google Scholar] [CrossRef]
- Bhandari, R.; Kaur, I.P. A Method to Prepare Solid Lipid Nanoparticles with Improved Entrapment Efficiency of Hydrophilic Drugs. Curr. Nanosci. 2013, 9, 211. [Google Scholar]
- Dantas, M.G.; Reis, S.A.; Damasceno, C.M.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G.S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Ganna, S.; Gutturu, R.; Borelli, D.P.; Rao, K.M.; Mallikarjuna, K.; Nannepaga, J.S. Formulation, optimization, and in vitro characterization of omega-3-rich binary lipid carriers for curcumin delivery: In vitro evaluation of sustained release and its potential antioxidant behavior. Polym. Bull. 2021, 79, 307–330. [Google Scholar] [CrossRef]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—In vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Pople, P.V.; Singh, K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech 2006, 7, 91. [Google Scholar] [CrossRef]
- Wagner, J.G. Application of the Wagner-nelson absorption method to the Two-compartment open model. J. Pharm. Biopharm. 1974, 2, 469–486. [Google Scholar] [CrossRef]
- Zou, Y.; Celli, A.; Zhu, H.; Elmahdy, A.; Cao, Y.; Hui, X.; Maibach, H. Confocal laser scanning microscopy to estimate nanoparticles’ human skin penetration in vitro. Int. J. Nanomed. 2017, 12, 8035–8041. [Google Scholar] [CrossRef]
- Chen, T.Y.; Tsai, M.J.; Lin, I.L.; Chang, L.C.; Wu, P.C. Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study. Pharmaceuticals 2020, 13, 329. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, Y.S.; Jun, S.S.; Koo, J.S.; Cheon, H.G.; Shin, B.C. Topical application of liposomal cobalamin hydrogel for atopic dermatitis therapy. Pharmazie 2011, 66, 430–435. [Google Scholar]
- Lim, S.K.; Kwon, M.S.; Lee, J.; Oh, Y.J.; Jang, J.J.; Jong-Hee Lee, J.H.; Park, H.W.; Nam, Y.D.; Myung-Ji Seo, M.J.; Roh, S.W.; et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci. Rep. 2017, 7, 40040. [Google Scholar] [CrossRef]
- Bhor, U.; Pande, S. Scoring systems in dermatology. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Stalder, J.F.; Taieb, A.; Atherton, D.J.; Bieber, P.; Bonifazi, E.; Broberg, A.; Calza, A.; Coleman, R.; De Prost, Y.; Stalder, J.F.; et al. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar]
- Jemec, G.B.; Wulf, H.C. The applicability of clinical scoring systems: SCORAD and PASI in psoriasis and atopic dermatitis. Acta Derm. Venereol. 1997, 77, 392–393. [Google Scholar] [PubMed]
- Puri, R.; Jain, S.K. Use of Biochemical and Microscopic Techniques to Determine Enhanced Skin Permeation of Nanovesicles: A Mechanistic Study. Pharmaceu. Nanotechnol. 2013, 1, 213. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Robak, E.L.; Sysa-Jedrzejowska, A.; Robak, T.; Stepień, H.; Woźniacka, A.; Waszczykowsk, E. Tumour necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6) and their soluble receptors (sTNF-alpha-Rp55 and slL-6R) serum levels in systemic lupus erythematodes. Mediat. Inflamm. 1996, 5, 435–441. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Upadhyay, S.U.; Patel, J.K.; Patel, V.A.; Saluja, A.K. Effect of different lipids and surfactants on formulation of solid lipid nanoparticles incorporating tamoxifen citrate. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S1), S112–S113. [Google Scholar] [CrossRef]
- Inactive Ingredient Search for Approved Drug Products. 2021. Available online: www.fda.gov (accessed on 18 August 2021).
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Souto, E.B.; Mehnert, W.; Muller, R.H. Polymorphic behaviour of Compritol888 ATO as bulk lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef] [PubMed]
- El-Gizawy, S.A.; El-Maghraby, G.M.; Hedaya, A.A. Formulation of acyclovir-loaded solid lipid nanoparticles: Design, optimization, and in-vitro characterization. Pharm. Dev. Technol. 2019, 24, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Nanoparticle Tracking Analysis. 2011. Available online: www.nanosight.com (accessed on 19 August 2021).
- Bobbi, E.; Sabagh, B.; Cryan, S.A.; Wilson, J.A.; Heise, A. Anisotropic polymer nanoparticles with solvent and temperature dependent shape and size from triblock copolymers. Polym. Chem. 2019, 10, 3436–3443. [Google Scholar] [CrossRef]
- Zwain, T.; Alder, J.; Sabagh, B.; Shaw, A.; Burrow, A.J.; Singh, K.K. Tailoring functional nanostructured lipid carriers for glioblastoma treatment with enhanced permeability through in-vitro 3D BBB/BBTB models. Mater. Sci. Eng. C 2021, 121, 111774. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4802–4808. [Google Scholar] [CrossRef]
- Khurana, R.K.; Bansal, A.K.; Beg, S.; Burrow, A.J.; Katare, O.P.; Singh, K.K.; Singh, B. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: Systematic development, characterization and evaluation. Int. J. Pharm. 2017, 518, 289–306. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Wissing, S.A.; Lippacher, A.; Muller, R.H. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–323. [Google Scholar]
- Pople, P.; Singh, K.K. Development and evaluation of colloidal modified nanolipid carrier: Application to topical delivery of tacrolimus, Part II—In vivo assessment, drug targeting, efficacy and safety in treatment of atopic dermatitis. Eur. J. Pharm. Biopharm. 2013, 72, 72–83. [Google Scholar] [CrossRef]
- Wissing, S.; Müller, R. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Pople, P.V.; Singh, K.K. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis. Int. J. Pharm. 2010, 398, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kamboj, S.; Thakur, K.; Negi, P.; Raza, K.; Katare, O.P. Delivery of Thermoresponsive-Tailored Mixed Micellar Nanogel of Lidocaine and Prilocaine with Improved Dermatokinetic Profile and Therapeutic Efficacy in Topical Anaesthesia. AAPS PharmSciTech 2017, 18, 790–802. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Katare, O.P. Nano-engineered lipid-polymer hybrid nanoparticles of fusidic acid: An investigative study on dermatokinetics profile and MRSA-infected burn wound model. Drug Deliv. Transl. Res. 2019, 9, 748–763. [Google Scholar] [CrossRef]
- Rangsimawong, W.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Terpene-containing PEGylated liposomes as transdermal carriers of a hydrophilic compound. Biol. Pharm. Bull. 2014, 37, 1936–1943. [Google Scholar] [CrossRef]
- Sharma, G.; Devi, N.; Thakur, K.; Jain, A.; Katare, O.P. Lanolin-based organogel of salicylic acid: Evidences of better dermatokinetic profile in imiquimod-induced keratolytic therapy in BALB/c mice model. Drug Deliv. Transl. Res. 2018, 8, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Pople, P.V.; Singh, K.K. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis—Part II: In vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Int. J. Pharm. 2012, 434, 70–79. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Wan, T.; Pan, W.; Long, Y.; Yu, K.; Liu, S.; Ruan, W.; Pan, J.; Qin, M.; Wu, C.; Xu, Y. Effects of nanoparticles with hydrotropic nicotinamide on tacrolimus: Permeability through psoriatic skin and antipsoriatic and antiproliferative activities. Int. J. Nanomed. 2017, 12, 1485–1497. [Google Scholar] [CrossRef]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. S5), 1229–1234. [Google Scholar]
- Lone, A.M.; Taskén, K. Proinflammatory and immunoregulatory roles of eicosanoids in T cells. Front. Immunol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Ishihara, K. Antioxidant activity and active sites of phospholipids as antioxidants. J. Am. Oil Chem. Soc. 1997, 74, 1531–1536. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, M.J.; Kyo, M.; Morozumi, K.; Yamaguchi, Y.; Nickeleit, V.; Ryffel, B. The side-effects of ciclosporine-A and tacrolimus. Clin. Nephrol. 1998, 49, 356–363. [Google Scholar]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef]
- Żelechowska, P.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes Rev. 2018, 19, 686–697. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.Q.; Ma, B.H. Tetrahydrocurcumin, a major metabolite of curcumin, ameliorates allergic airway inflammation by attenuating Th2 response and suppressing the IL-4Rα-Jak1-STAT6 and Jagged1/Jagged2 -Notch1/Notch2 pathways in asthmatic mice. Clin. Exp. Allergy 2018, 48, 1494–1508. [Google Scholar] [CrossRef]
| Groups | Formulation |
|---|---|
| 1 | THC-SLN dispersion |
| 2 | THC-SLN gel |
| 3 | Tacroz® Forte (0.1% w/w tacrolimus ointment) |
| 4 | Blank SLN gel |
| 5 | Free drug gel |
| 6 | Positive control (DNCB, 0.5% w/v in a 3:1 of acetone: olive oil) |
| 7 | Negative control (Naive) |
| S.No. | Formulation | Thickness of Skin (cm) (n = 3) | Skin Hydration Potential (SHP) |
|---|---|---|---|
| 1 | THC- SLN gel | 0.13 ± 0.01 | 216.7 ± 16.70 |
| 2 | THC-SLN dispersion | 0.12 ± 0.01 | 200 ± 16.70 |
| 3 | Free THC gel | 0.09 ± 0.02 | 150 ± 33.30 |
| 4 | Blank SLN gel | 0.10 ± 0.02 | 166.7 ± 33.30 |
| 5 | Tacroz® Forte, | 0.12 ± 0.01 | 200 ± 16.70 |
| 6 | Control skin (naïve) | 0.06 ± 0.01 | 100 ± 16.70 |
| S.No. | Dermatokinetic Parameters | THC-SLNs Gel | Free THC Gel | ||
|---|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | ||
| 1. | AUC0–6h (μg cm−2 h) | 149.56 ± 0.028 | 136.21 ± 0.071 | 68.75 ± 0.097 | 23.35 ± 0.072 |
| 2. | AUC0–∞ (μg cm−2 h) | 157.09 ± 0.059 | 141.94 ± 0.069 | 147.63 ± 0.073 | 41.40 ± 0.071 |
| 3. | Cmax (μg cm−2) | 52.98 ± 0.035 | 57.51 ± 0.036 | 33 ± 0.045 | 20.01 ± 0.040 |
| 4. | Tmax (h) | 0.56 ± 0.026 | 0.48 ± 0.020 | 1.09 ± 0.025 | 1.02 ± 0.027 |
| 5. | Kp | 6.72 ± 0.049 | 4.14 ± 0.043 | 0.66 ± 0.050 | 0.44 ± 0.055 |
| 6. | Ke | 0.16 ± 0.087 | 0.33 ± 0.089 | 3.75 ± 0.094 | 4.48 ± 0.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, K.; Modgill, N.; Singh, K.K.; Kakkar, V. Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials 2022, 12, 636. https://doi.org/10.3390/nano12040636
Saini K, Modgill N, Singh KK, Kakkar V. Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials. 2022; 12(4):636. https://doi.org/10.3390/nano12040636
Chicago/Turabian StyleSaini, Komal, Nancy Modgill, Kamalinder K. Singh, and Vandita Kakkar. 2022. "Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model" Nanomaterials 12, no. 4: 636. https://doi.org/10.3390/nano12040636
APA StyleSaini, K., Modgill, N., Singh, K. K., & Kakkar, V. (2022). Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials, 12(4), 636. https://doi.org/10.3390/nano12040636








