Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis
Abstract
:1. Introduction
2. Materials and Chemical Reagents
2.1. Synthesis of HMS-SO3H Materials
2.2. Synthesis of NRHMS-SO3H Materials
2.3. Characterization of Synthesized Materials
2.4. Catalytic Esterification of LA with Alcohols
3. Results and Discussion
3.1. Physicochemical Properties of HMS-SO3H and NRHMS-SO3H
3.2. The Esterification of LA with Ethanol over Various Solid Acid Catalysts
3.3. Effect of Alcohol Chain Length on LA Esterification
3.4. Effect of Reaction Temperature on the LA Esterification
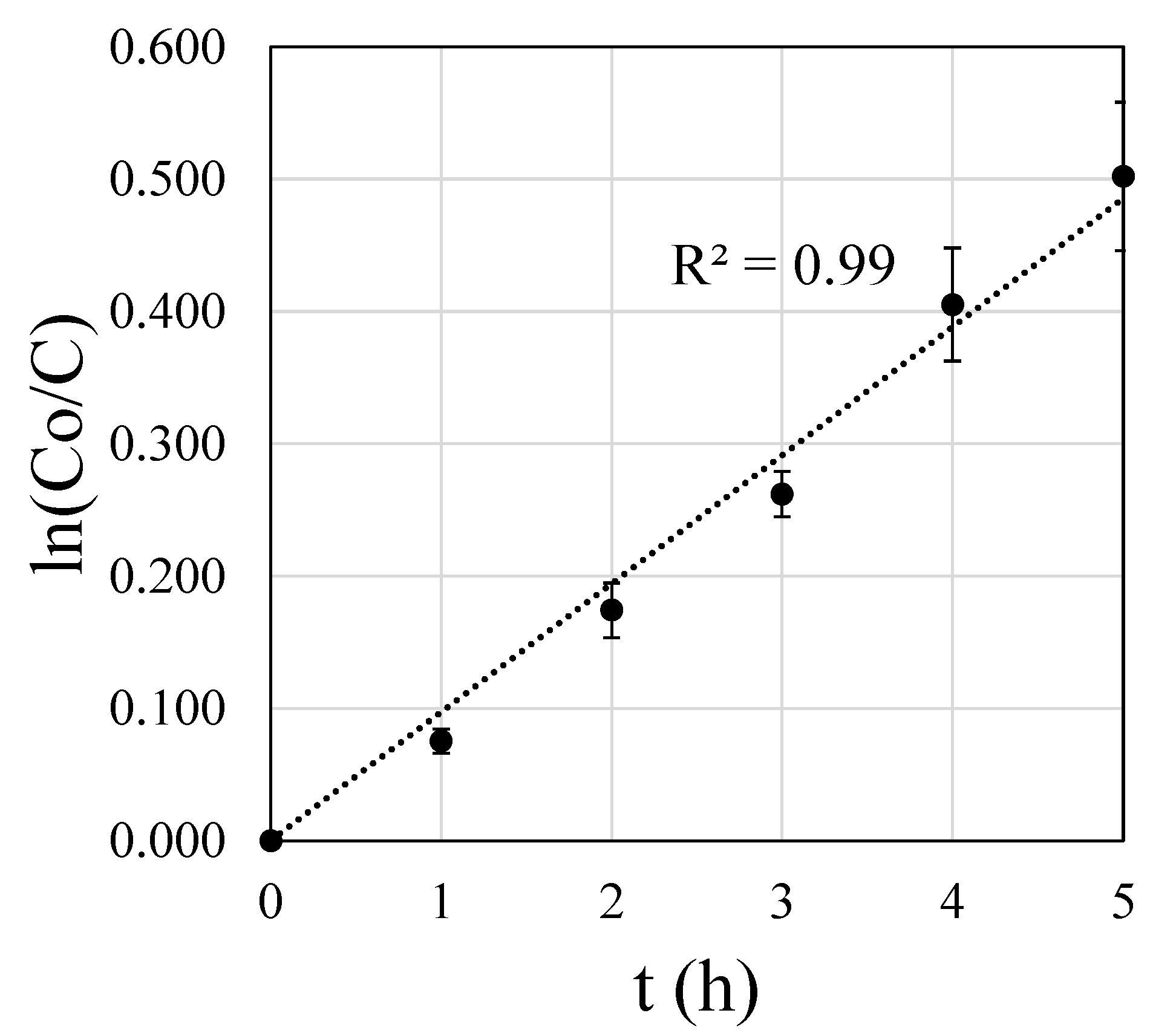
3.4.1. Kinetic Study of LA Esterification
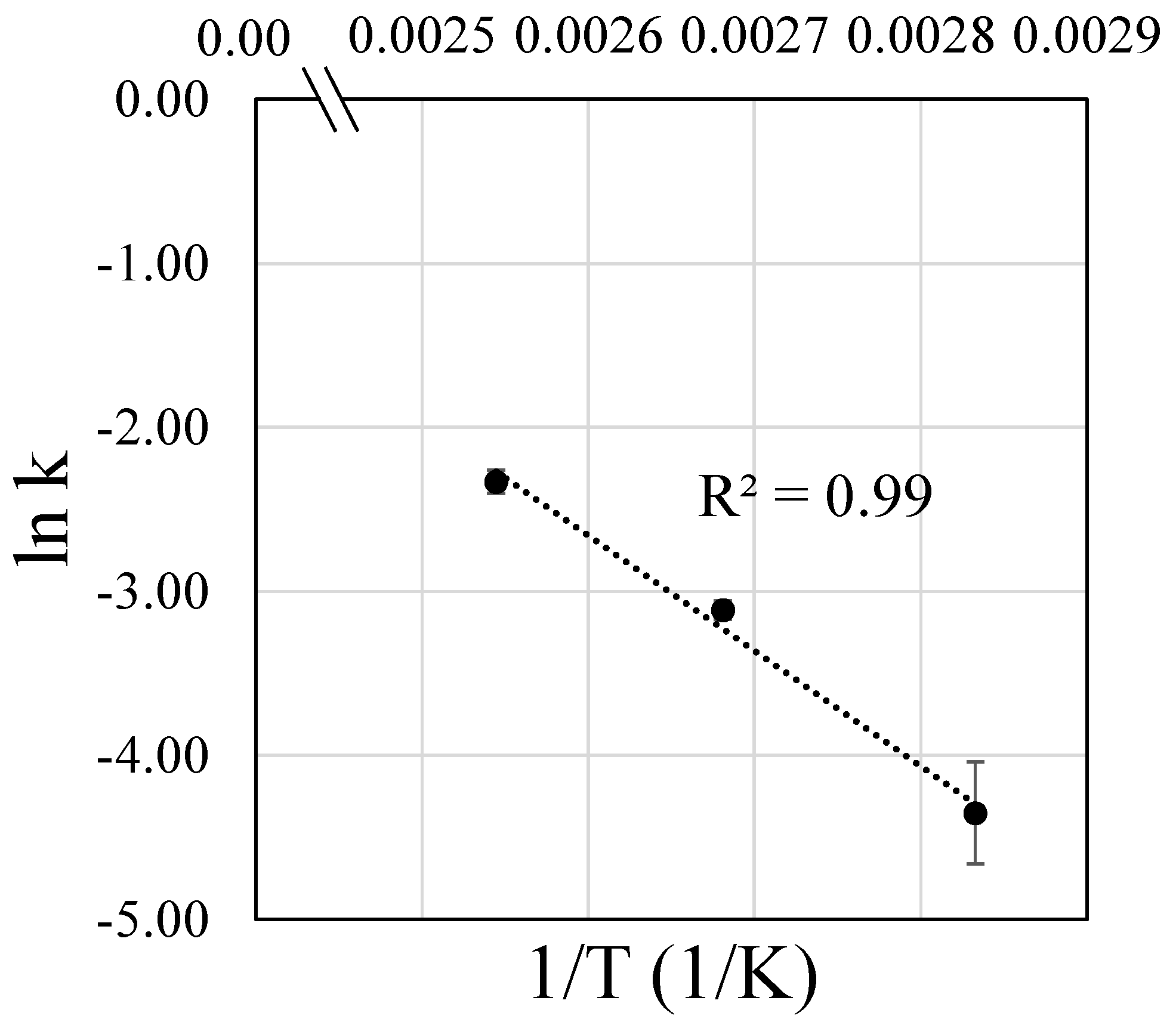
3.4.2. Activation Energy of LA Esterification with n-Butanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of Biomass and Its Derivatives to Levulinic Acid and Levulinate Esters via Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 4749–4766. [Google Scholar] [CrossRef]
- Adeleye, A.; Louis, H.; Akakuru, O.; Joseph, I.; Enudi, O.; Michael, D. A Review on the conversion of levulinic acid and its esters to various useful chemicals. AIMS Energy 2019, 7, 165. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Hayes, D.J. An examination of biorefining processes, catalysts and challenges. Catal. Today 2009, 145, 138–151. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Hu, Y.; Rao, K.T.V.; Xu, C.C.; Yang, S. Advances in production of bio-based ester fuels with heterogeneous bifunctional catalysts. Renew. Sustain. Energy Rev. 2019, 114, 109296. [Google Scholar]
- Ahmad, E.; Alam, I.; Pant, K.K.; Haider, M.A. Catalytic and mechanistic insights into the production of ethyl levulinate from biorenewable feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Bart, H.J.; Reidetschlager, J.; Schatka, K.; Lehmann, A. Kinetics of esterification of levulinic acid with n-butanol by homogeneous catalysis. Ind. Eng. Chem. Res. 1994, 33, 21–25. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin Jr, J.G. A comparison of the esterification of acetic acid with methanol using heterogeneous versus homogeneous acid catalysis. J. Catal. 2006, 242, 278–286. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Mai, E.F.; Mota, C.J.A.; Teixeira da Silva, V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A Gen. 2012, 425–426, 199–204. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Lashgari, N.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A Chem. 2015, 397, 166–191. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Tuning the pore structure and surface properties of carbon-based acid catalysts for liquid-phase reactions. ACS Catal. 2015, 5, 4951–4958. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Radu, D.R.; Lin, V.S.Y.; Shanks, B.H. Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J. Catal. 2003, 219, 329–336. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Trewyn, B.G.; Song, S.; Pruski, M.; Lin, V.S.-Y. Tuning of particle morphology and pore properties in mesoporous silicas with multiple organic functional groups. Chem. Commun. 2003, 18, 2364–2365. [Google Scholar] [CrossRef]
- Anwander, R.; Palm, C.; Stelzer, J.; Groeger, O.; Engelhardt, G. Silazane-Silylation of Mesoporous Silicates: Towards Tailor-Made Support Materials. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 117, pp. 135–142. [Google Scholar]
- Zhao, X.S.; Lu, G.Q. Modification of MCM-41 by Surface Silylation with Trimethylchlorosilane and Adsorption Study. J. Phys. Chem. B 1998, 102, 1556–1561. [Google Scholar] [CrossRef]
- Lim, M.H.; Stein, A. Comparative Studies of Grafting and Direct Syntheses of Inorganic−Organic Hybrid Mesoporous Materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Yang, S.; Guo, Z.; Zhang, T.; Song, H.; Shao, Q. Esterification synthesis of ethyl oleate catalyzed by Brønsted acid–surfactant-combined ionic liquid. Green Chem. Lett. Rev. 2017, 10, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Björk, E.M.; Militello, M.P.; Tamborini, L.H.; Rodriguez, R.C.; Planes, G.A.; Acevedo, D.F.; Moreno, M.S.; Odén, M.; Barbero, C.A. Mesoporous silica and carbon based catalysts for esterification and biodiesel fabrication—The effect of matrix surface composition and porosity. Appl. Catal. A Gen. 2017, 533, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Nuntang, S.; Poompradub, S.; Butnark, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Organosulfonic acid-functionalized mesoporous composites based on natural rubber and hexagonal mesoporous silica. Mater. Chem. Phys. 2014, 147, 583–593. [Google Scholar] [CrossRef]
- Nuntang, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Enhanced esterification of carboxylic acids with ethanol using propylsulfonic acid-functionalized natural rubber/hexagonal mesoporous silica nanocomposites. Catal. Commun. 2016, 80, 5–9. [Google Scholar] [CrossRef]
- Shi, W.; He, B.; Cao, Y.; Li, J.; Yan, F.; Cui, Z.; Zou, Z.; Guo, S.; Qian, X. Continuous esterification to produce biodiesel by SPES/PES/NWF composite catalytic membrane in flow-through membrane reactor: Experimental and kinetic studies. Bioresour. Technol. 2013, 129, 100–107. [Google Scholar] [CrossRef]
- Yadav, G.D.; Yadav, A.R. Synthesis of ethyl levulinate as fuel additives using heterogeneous solid superacidic catalysts: Ef-ficacy and kinetic modeling. Chem. Eng. J. 2014, 243, 556–563. [Google Scholar] [CrossRef]
- Chaowamalee, S.; Ngamcharussrivichai, C. Facile fabrication of mesostructured natural rubber/silica nanocomposites with enhanced thermal stability and hydrophobicity. Nanoscale Res. Lett. 2019, 14, 382. [Google Scholar] [CrossRef]
- Peña, L.; Xu, F.; Hohn, K.L.; Li, J.; Wang, D. Propyl-Sulfonic Acid Functionalized Nanoparticles as Catalyst for Pretreatment of Corn Stover. J. Biomater. Nanobiotechnol. 2014, 5, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Amiri, A.A.; Javanshir, S.; Dolatkhah, Z.; Dekamin, M.G. SO3H-functionalized mesoporous silica materials as solid acid catalyst for facile and solvent-free synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11-trione derivatives. New J. Chem. 2015, 39, 9665–9671. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. Sulfonated polyether ether ketone—Sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617–623. [Google Scholar] [CrossRef]
- Estevez, R.; Iglesias, I.; Luna, D.; Bautista, F.M. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol. Molecules 2017, 22, 2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Serrano, E.; Campos-Martin, J.M.; Fierro, J.L.G. Sulfonic acid-functionalized silica through quantitative oxidation of thiol groups. Chem. Commun. 2003, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Rezvani-Boroujeni, A.; Javanbakht, M.; Karimi, M.; Akbari-Adergani, B. Adsorption properties of thiol-functionalized silica nanoparticles prepared for application in poly (ether sulfone) nanocomposite membranes. J. Text. Polym. 2017, 5, 37–47. [Google Scholar]
- Cano-Serrano, E.; Blanco-Brieva, G.; Campos-Martin, J.M.; Fierro, J.L.G. Acid-Functionalized Amorphous Silica by Chemical Grafting−Quantitative Oxidation of Thiol Groups. Langmuir 2003, 19, 7621–7627. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.; Macquarrie, D.J.; Clark, J.H. Structure and reactivity of sol–gel sulphonic acid silicas. Appl. Catal. A Gen. 2002, 228, 127–133. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Chan†, J.C.C. Propylsulfonic Acid-Functionalized Mesoporous Silica Synthesized by in Situ Oxidation of Thiol Groups under Template-Free Condition. J. Phys. Chem. C 2007, 111, 2156–2164. [Google Scholar] [CrossRef]
- Hamoudi, S.; Kaliaguine, S. Sulfonic acid-functionalized periodic mesoporous organosilica. Microporous Mesoporous Mater. 2003, 59, 195–204. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Nuntang, S.; Poompradub, S.; Butnark, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Novel mesoporous composites based on natural rubber and hexagonal mesoporous silica: Synthesis and characterization. Mater. Chem. Phys. 2014, 143, 1199–1208. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Influence of Surface Hydrophobicity on the Esterification of Fatty Acids over Solid Catalysts. Energy Fuels 2010, 24, 2154–2161. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid Acid Catalysts for Biodiesel Production—Towards Sustainable Energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Chaowamalee, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Mesostructured natural rubber/in situ formed silica nanocomposites: A simple way to prepare mesoporous silica with hydrophobic properties. Microporous Mesoporous Mater. 2018, 259, 79–88. [Google Scholar] [CrossRef]
- Wu, J.; Ling, L.; Xie, J.; Ma, G.; Wang, B. Surface modification of nanosilica with 3-mercaptopropyl trimethoxysilane: Ex-perimental and theoretical study on the surface interaction. Chem. Phys. Lett. 2014, 591, 227–232. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Zhang, L.; Zhang, S. Efficient production of n-butyl levulinate fuel additive from levulinic acid using amor-phous carbon enriched with oxygenated groups. Catalysts 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Mbaraka, I.; Shanks, B. Design of multifunctionalized mesoporous silicas for esterification of fatty acid. J. Catal. 2005, 229, 365–373. [Google Scholar] [CrossRef]
- Kong, P.S.; Pérès, Y.; Wan Daud, W.M.A.; Cognet, P.; Aroua, M.K. Esterification of Glycerol with Oleic Acid Over Hydrophobic Zirconia-Silica Acid Catalyst and Commercial Acid Catalyst: Optimization and Influence of Catalyst Acidity. Front. Chem. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Bassan, I.A.; Nascimento, D.R.; San Gil, R.A.; da Silva, M.I.P.; Moreira, C.R.; Gonzalez, W.A.; Faro Jr, A.C.; Onfroy, T.; Lachter, E.R. Esterification of fatty acids with alcohols over niobium phosphate. Fuel Processing Technol. 2013, 106, 619–624. [Google Scholar] [CrossRef]
- Pappu, V.K.; Kanyi, V.; Santhanakrishnan, A.; Lira, C.T.; Miller, D.J. Butyric acid esterification kinetics over Amberlyst solid acid catalysts: The effect of alcohol carbon chain length. Bioresour. Technol. 2013, 130, 793–797. [Google Scholar] [CrossRef]
- Erdem, B.; Cebe, M. Determination of steric effect on the esterification of different alcohols with propanoic acid over cati-on-exchange resin catalyst Dowex 50Wx4. Z. Für Phys. Chem. 2011, 225, 125–136. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Thermo-chemical energy assessment for production of energy-rich fuel additive compounds by using levulinic acid and immobilized lipase. Fuel Process. Technol. 2015, 138, 139–146. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Sivasubramaniam, D.; Amin, N.A.S. Esterification of Levulinic Acid Using ZrO2-Supported Phosphotungstic Acid Catalyst for Ethyl Levulinate Production. BioEnergy Res. 2017, 10, 1105–1116. [Google Scholar] [CrossRef]
- Zainol, M.M.; Amin, N.A.S.; Asmadi, M. Kinetics and thermodynamic analysis of levulinic acid esterification using lig-nin-furfural carbon cryogel catalyst. Renew. Energy 2019, 130, 547–557. [Google Scholar] [CrossRef]
- Escobar, A.M.; Blanco, M.N.; Martínez, J.J.; Cubillos, J.A.; Romanelli, G.P.; Pizzio, L.R. Biomass Derivative Valorization Using Nano Core-Shell Magnetic Materials Based on Keggin-Heteropolyacids: Levulinic Acid Esterification Kinetic Study with N-Butanol. J. Nanomater. 2019, 2019, 5710708. [Google Scholar] [CrossRef] [Green Version]
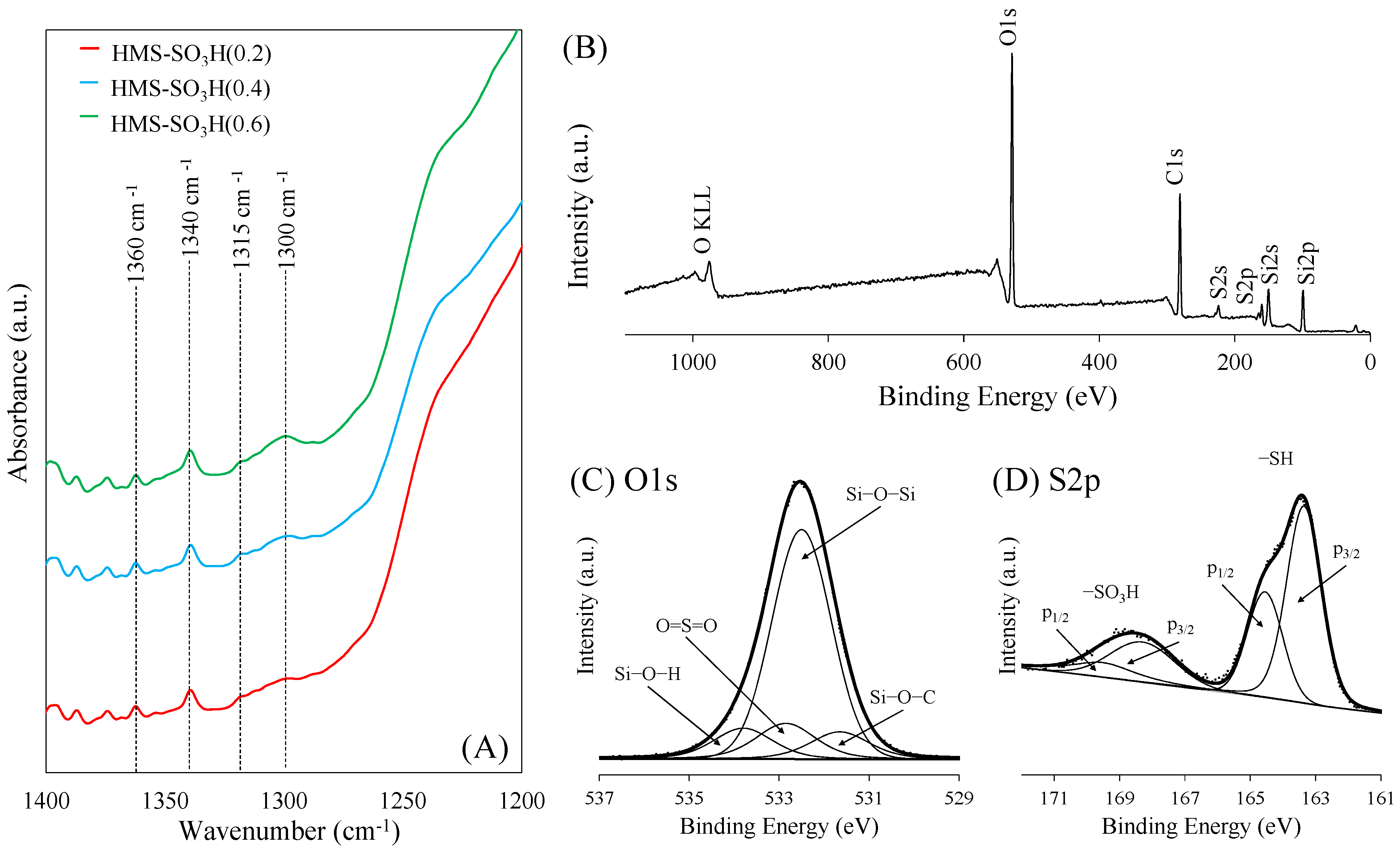

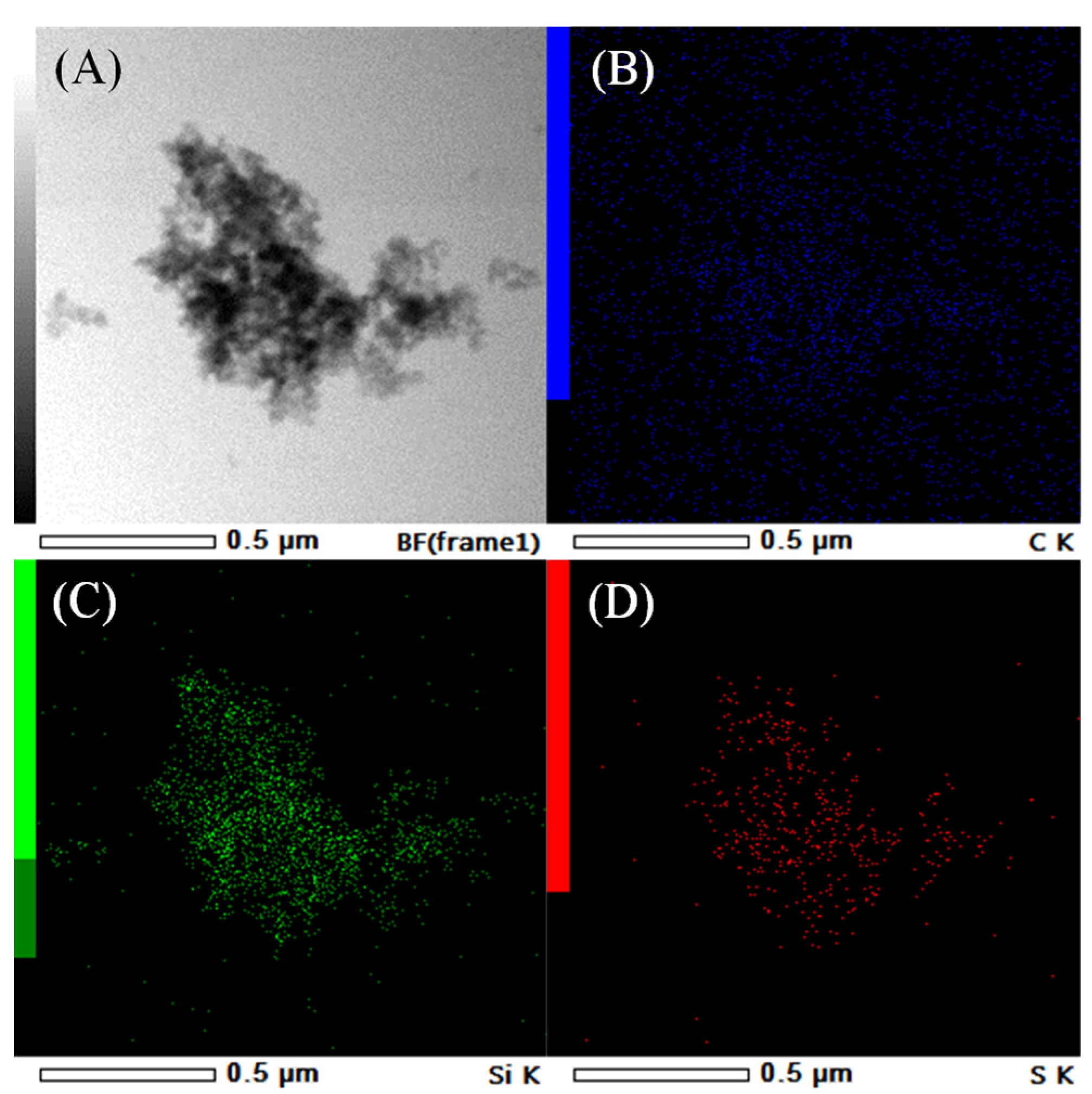
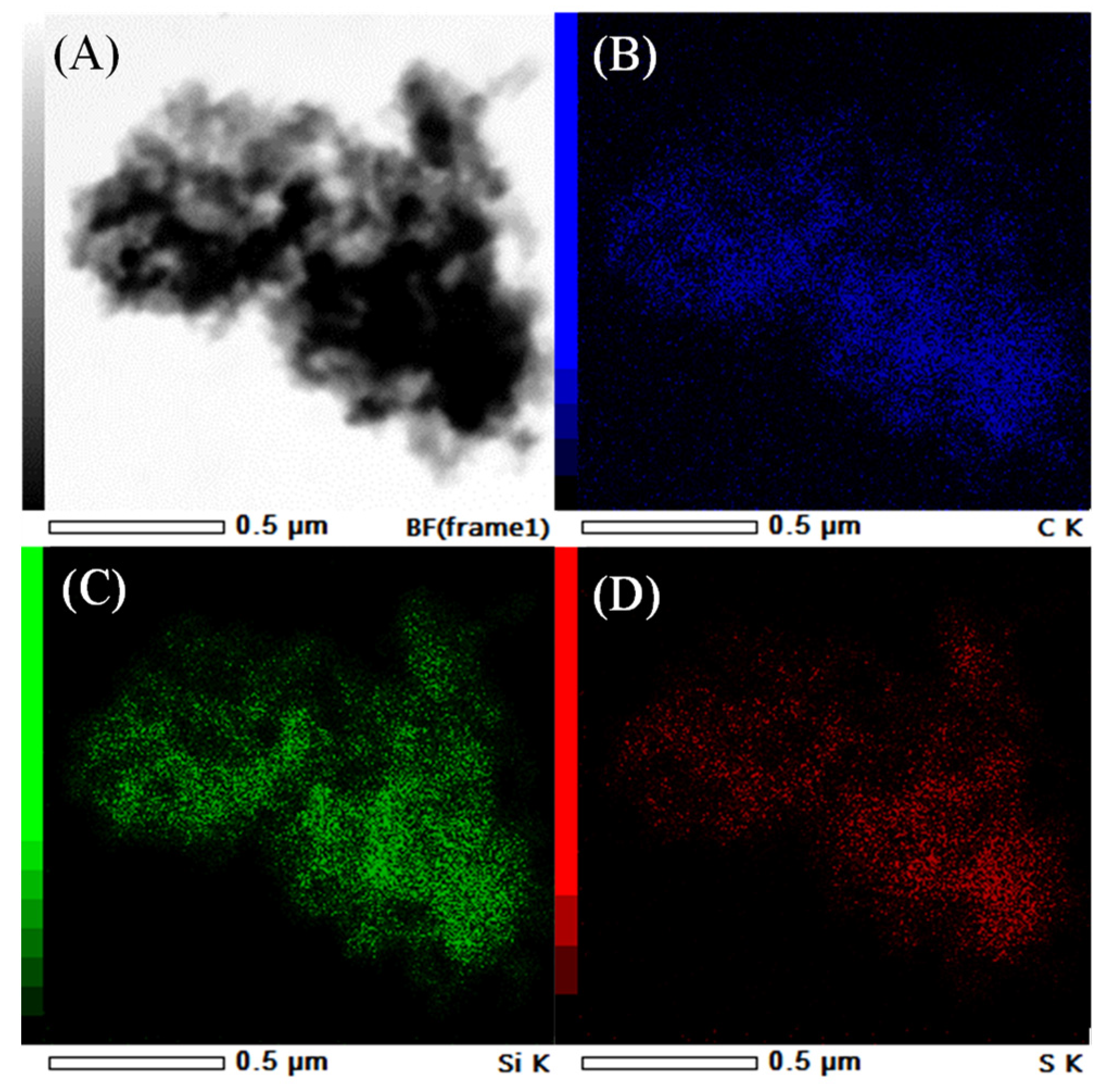


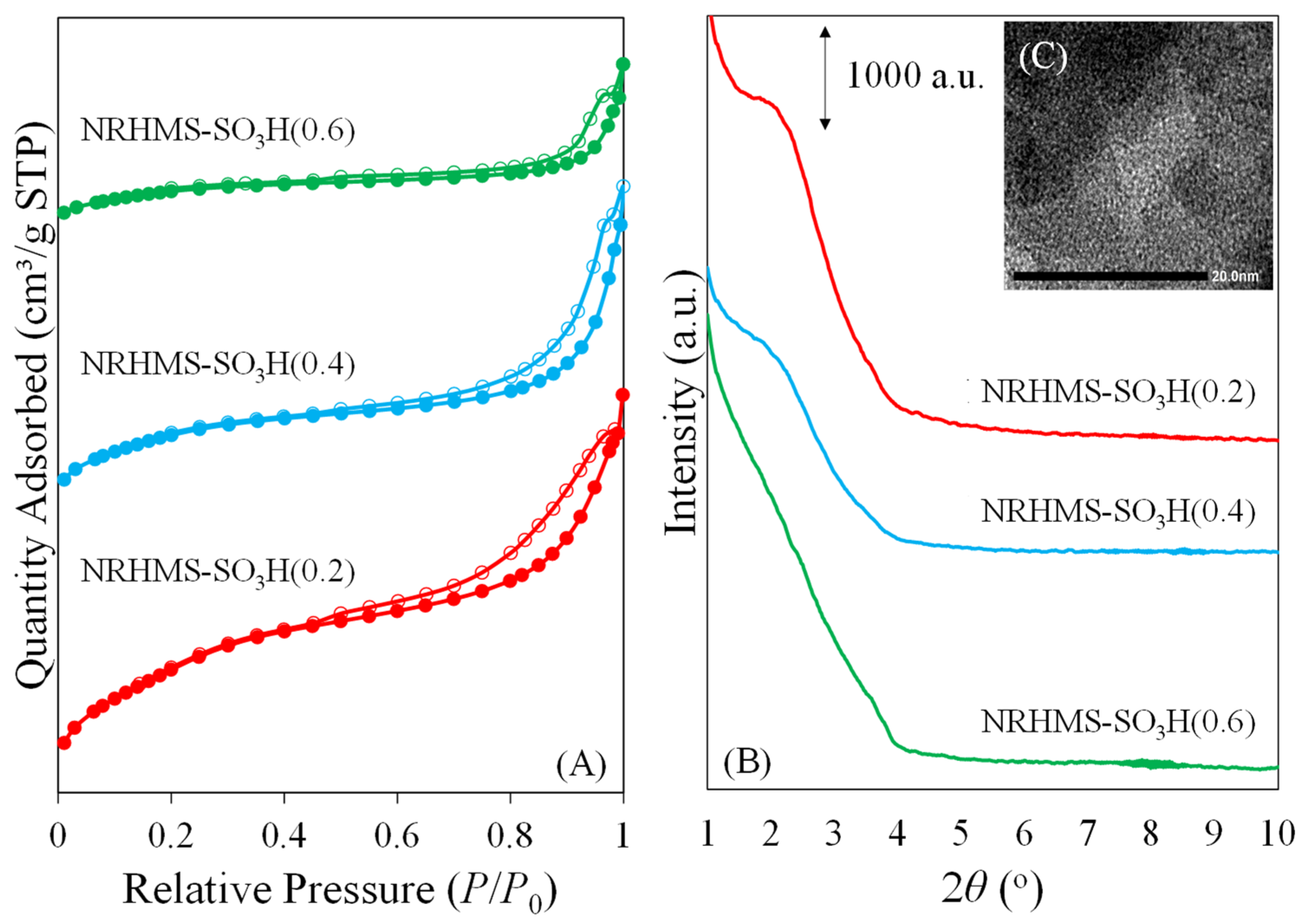
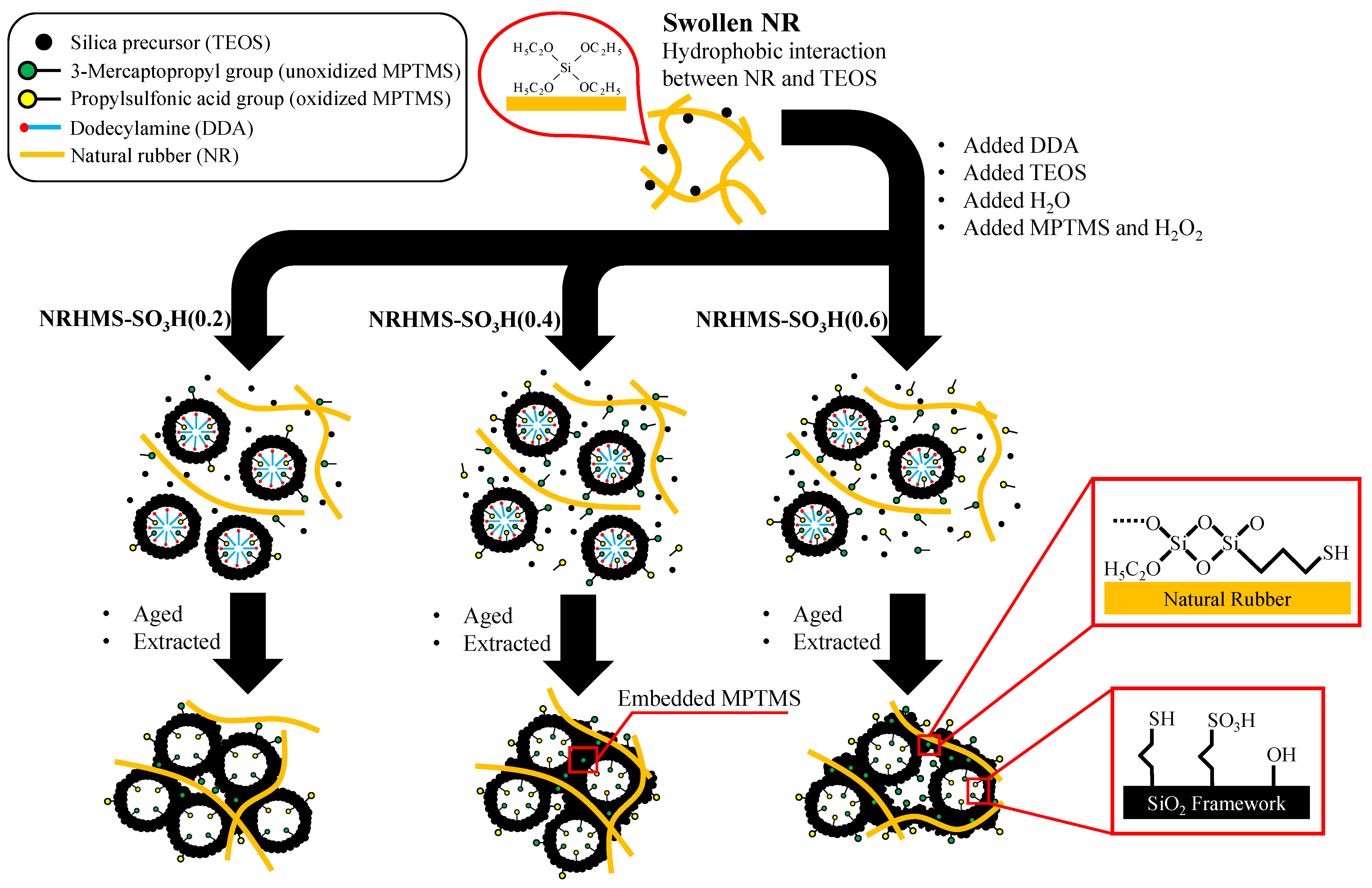
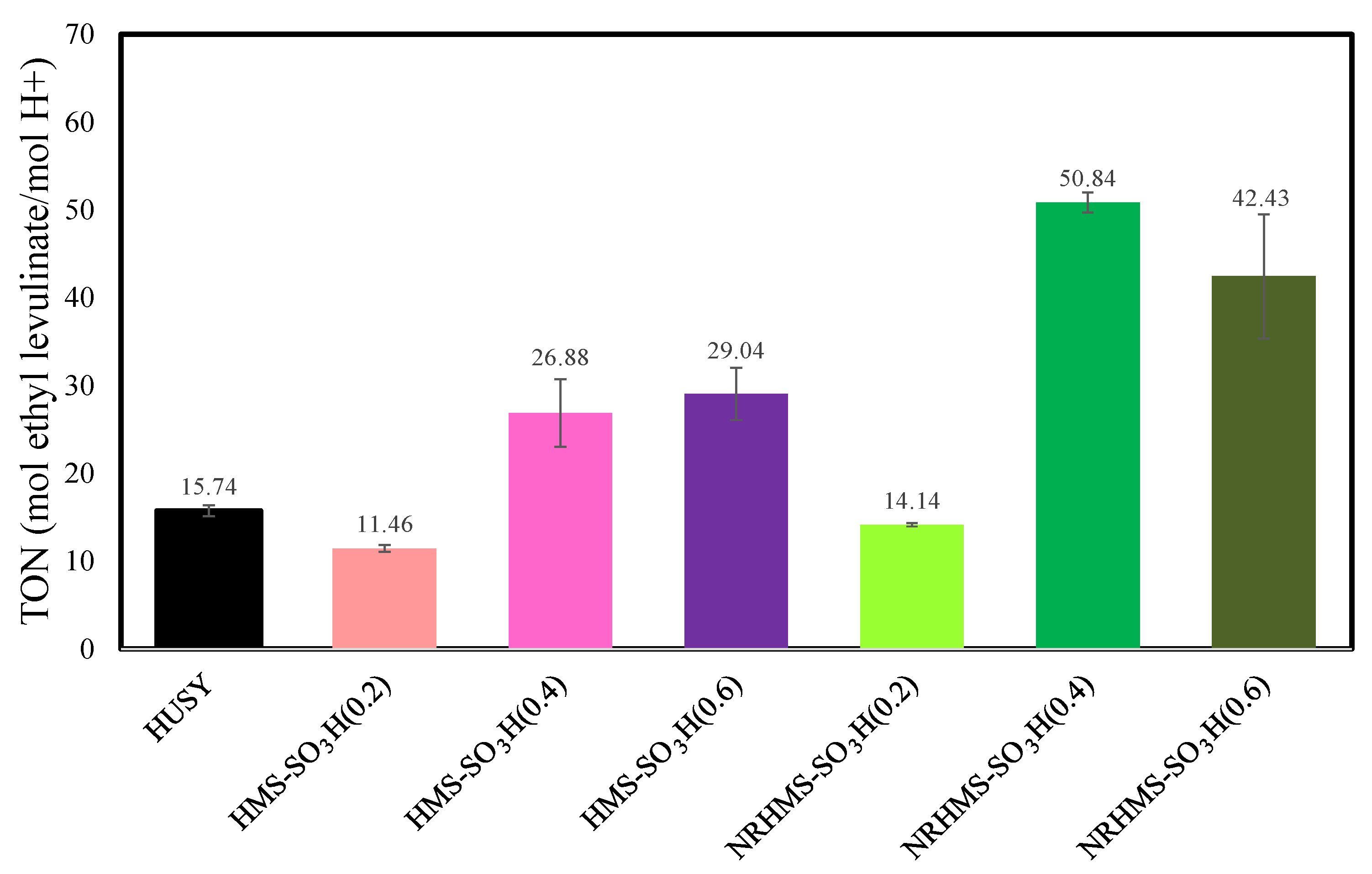
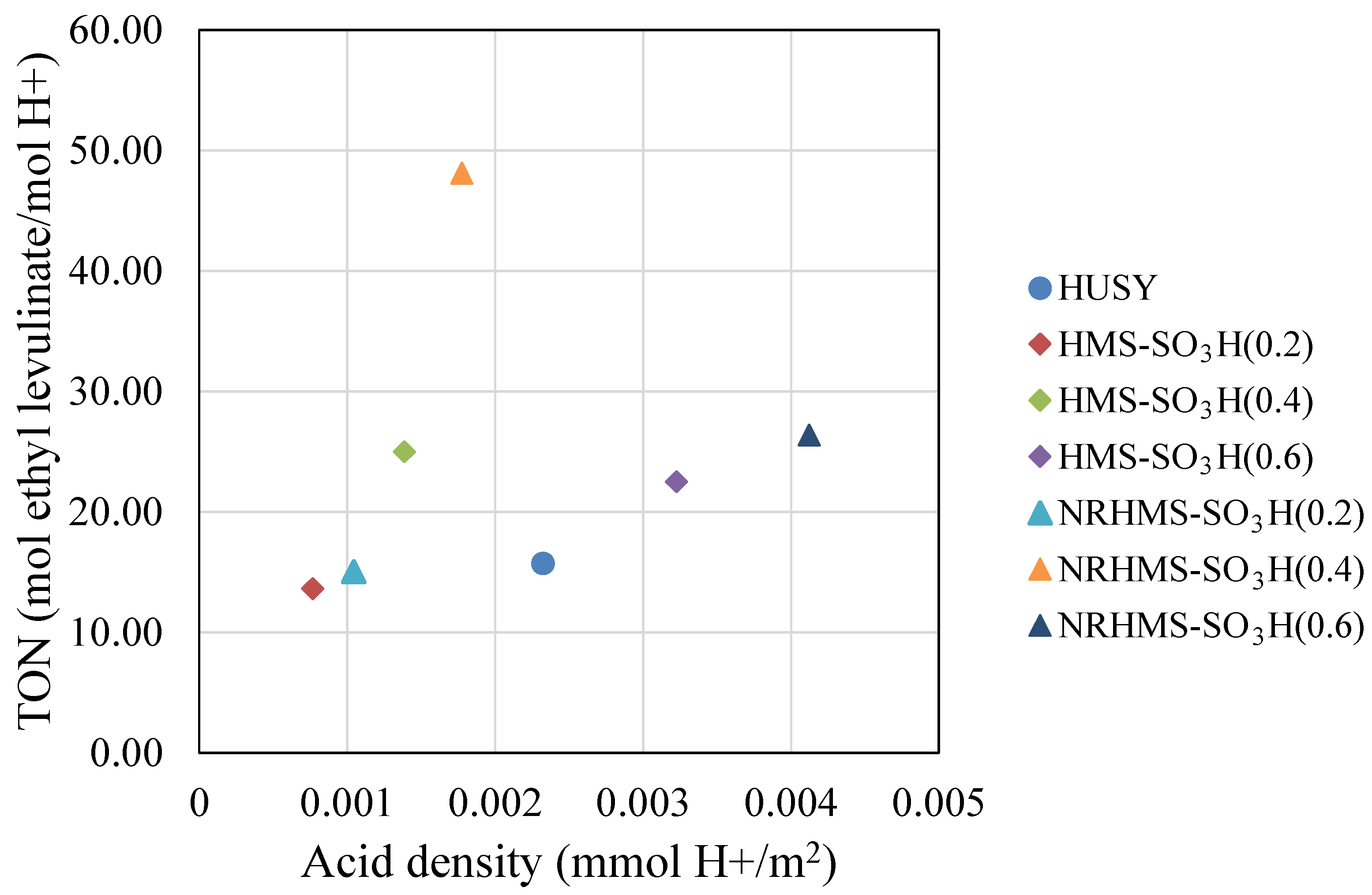
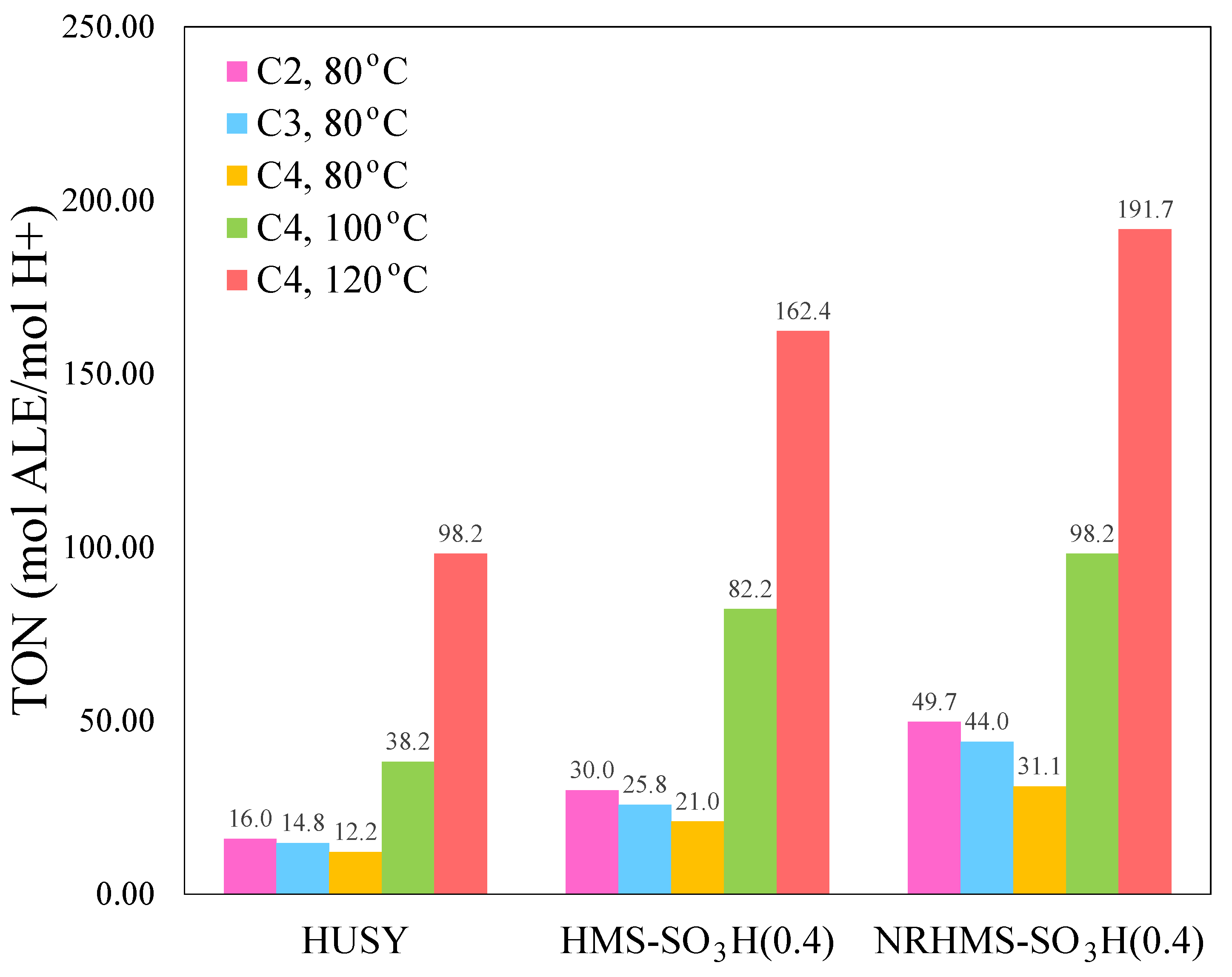
| Sample | SiO2 Content a (wt.%) | S Content b (wt.%) | Theoretical Acidity c (mmol/g) | Total Acidity d (mmol/g) | Sulfur Species e (mmol/g) | |
|---|---|---|---|---|---|---|
| −SH | −SO3H | |||||
| HMS-SO3H (0.2) | 67.8 | 7.8 | 2.45 | 0.69 | 1.87 | 0.58 |
| HMS-SO3H (0.4) | 64.4 | 10.3 | 3.22 | 0.79 | 2.36 | 0.85 |
| HMS-SO3H (0.6) | 55.4 | 12.2 | 3.80 | 0.90 | 2.64 | 1.16 |
| NRHMS-SO3H (0.2) | 62.6 | 7.1 | 2.21 | 0.62 | 1.63 | 0.58 |
| NRHMS-SO3H (0.4) | 56.0 | 8.6 | 2.68 | 0.71 | 1.95 | 0.74 |
| NRHMS-SO3H (0.6) | 54.6 | 11.4 | 3.56 | 0.80 | 2.27 | 1.29 |
| Sample | SBET a (m2 g−1) | Sext b (m2 g−1) | VT c (cm3 g−1) | VP d (cm3 g−1) | Dp e (nm) | d100 f (nm) | ao g (nm) | WT h (nm) | Vm, H2O i (cm3 STP g−1) |
|---|---|---|---|---|---|---|---|---|---|
| HMS-SO3H (0.2) | 901 | 211 | 0.97 | 0.35 | 2.20 | 3.84 | 4.43 | 2.23 | 63.8 |
| HMS-SO3H (0.4) | 580 | 117 | 0.80 | 0.16 | 2.05 | 4.01 | 4.63 | 2.58 | 68.2 |
| HMS-SO3H (0.6) | 279 | 37 | 0.41 | 0.12 | 2.05 | n.d. | n.d. | n.d. | 72.8 |
| NRHMS-SO3H (0.2) | 594 | 177 | 0.81 | 0.17 | 2.15 | 4.01 | 4.63 | 2.48 | 56.7 |
| NRHMS-SO3H (0.4) | 400 | 125 | 0.71 | 0.13 | 2.05 | 4.30 | 4.97 | 2.92 | 58.1 |
| NRHMS-SO3H (0.6) | 194 | 47 | 0.36 | 0.07 | 2.05 | n.d. | n.d. | n.d. | 65.8 |
| Catalyst | Alcohol | Temperature (°C) | Rate of Reaction (mmol ALE/h) | k (h−1) |
|---|---|---|---|---|
| Blank | ethanol | 80 | 0.165 ± 0.046 | 0.0065 ± 0.0017 |
| n-propanol | 80 | 0.163 ± 0.023 | 0.0063 ± 0.0006 | |
| n-butanol | 80 | 0.150 ± 0.049 | 0.0052 ± 0.0008 | |
| n-butanol | 100 | 0.521 ± 0.084 | 0.0220 ± 0.0037 | |
| n-butanol | 120 | 1.281 ± 0.035 | 0.0671 ± 0.0040 | |
| HUSY | ethanol | 80 | 0.242 ± 0.058 | 0.0097 ± 0.0023 |
| n-propanol | 80 | 0.223 ± 0.052 | 0.0085 ± 0.0018 | |
| n-butanol | 80 | 0.184 ± 0.052 | 0.0072 ± 0.0021 | |
| n-butanol | 100 | 0.576 ± 0.011 | 0.0246 ± 0.0007 | |
| n-butanol | 120 | 1.481 ± 0.100 | 0.0715 ± 0.0065 | |
| HMS-SO3H (0.4) | ethanol | 80 | 0.344 ± 0.151 | 0.0138 ± 0.0059 |
| n-propanol | 80 | 0.295 ± 0.053 | 0.0117 ± 0.0019 | |
| n-butanol | 80 | 0.241 ± 0.093 | 0.0094 ± 0.0034 | |
| n-butanol | 100 | 0.942 ± 0.164 | 0.0406 ± 0.0071 | |
| n-butanol | 120 | 1.860 ± 0.269 | 0.0892 ± 0.0137 | |
| NRHMS-SO3H (0.4) | ethanol | 80 | 0.512 ± 0.214 | 0.0209 ± 0.0089 |
| n-propanol | 80 | 0.452 ± 0.157 | 0.0185 ± 0.0063 | |
| n-butanol | 80 | 0.320 ± 0.127 | 0.0129 ± 0.0033 | |
| n-butanol | 100 | 1.011 ± 0.042 | 0.0444 ± 0.0026 | |
| n-butanol | 120 | 1.973 ± 0.136 | 0.0971 ± 0.0066 |
| Catalyst | Ea (kJ/mol) |
|---|---|
| Blank | 71.3 ± 5.0 |
| HUSY | 66.2 ± 8.4 |
| HMS-SO3H (0.4) | 65.2 ± 9.7 |
| NRHMS-SO3H (0.4) | 58.2 ± 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaowamalee, S.; Yan, N.; Ngamcharussrivichai, C. Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis. Nanomaterials 2022, 12, 604. https://doi.org/10.3390/nano12040604
Chaowamalee S, Yan N, Ngamcharussrivichai C. Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis. Nanomaterials. 2022; 12(4):604. https://doi.org/10.3390/nano12040604
Chicago/Turabian StyleChaowamalee, Supphathee, Ning Yan, and Chawalit Ngamcharussrivichai. 2022. "Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis" Nanomaterials 12, no. 4: 604. https://doi.org/10.3390/nano12040604
APA StyleChaowamalee, S., Yan, N., & Ngamcharussrivichai, C. (2022). Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis. Nanomaterials, 12(4), 604. https://doi.org/10.3390/nano12040604






