A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
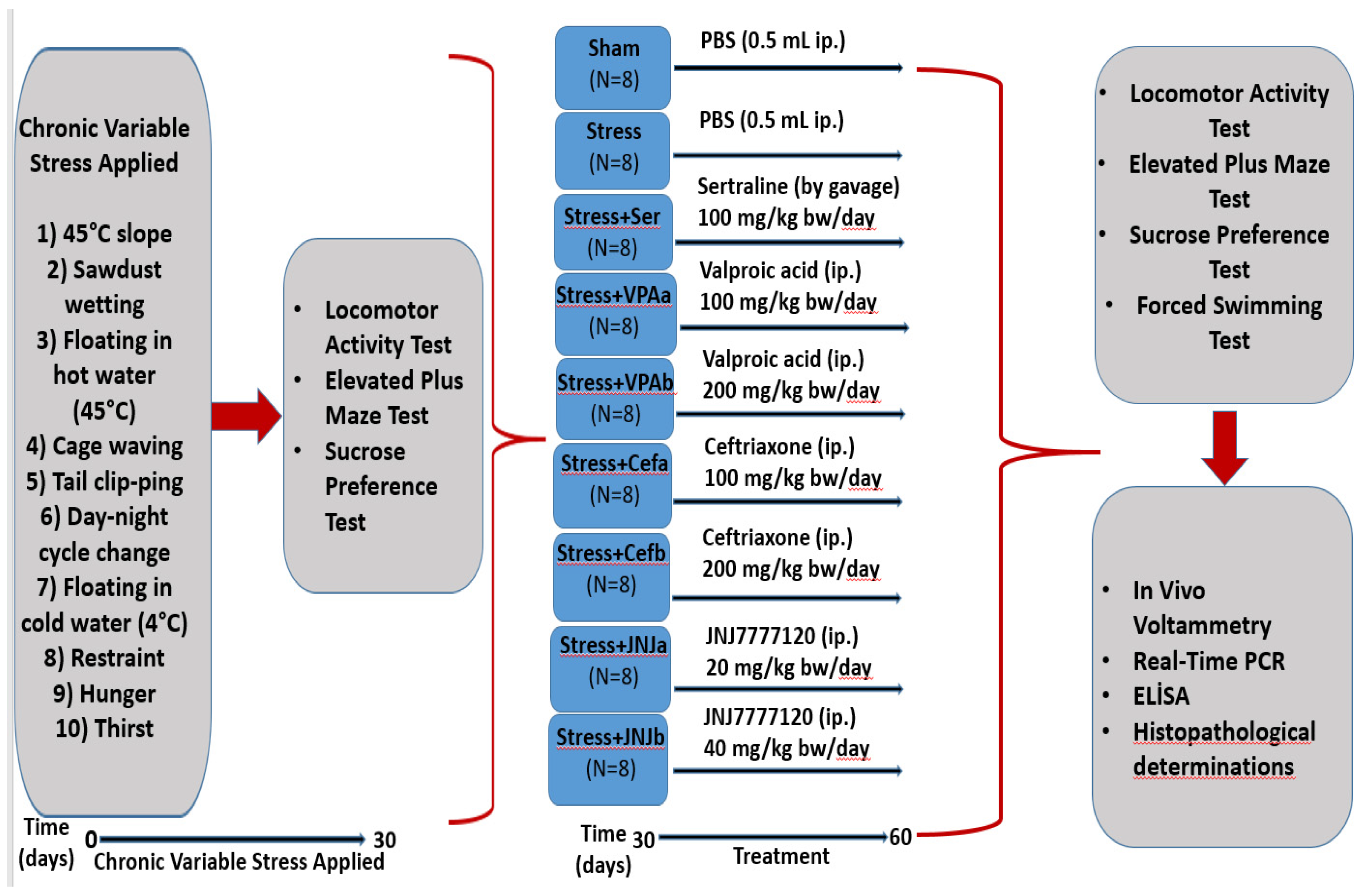
2.2. Experiment Animals
2.3. Chronic Unpredictable Mild Stress Model
2.4. Treatment
2.5. Locomotor Activity Test
2.6. Elevated plus Maze Test
2.7. Forced Swimming Test
2.8. Sucrose Preference Test
2.9. RNA Isolation and cDNA Synthesis
2.10. Real-Time PCR
2.11. In Vivo Voltammetry
2.12. Glutathione (GSH), Lactate Dehydrogenase (LDH) and Acetylcholinesterase (AChE) Analysis
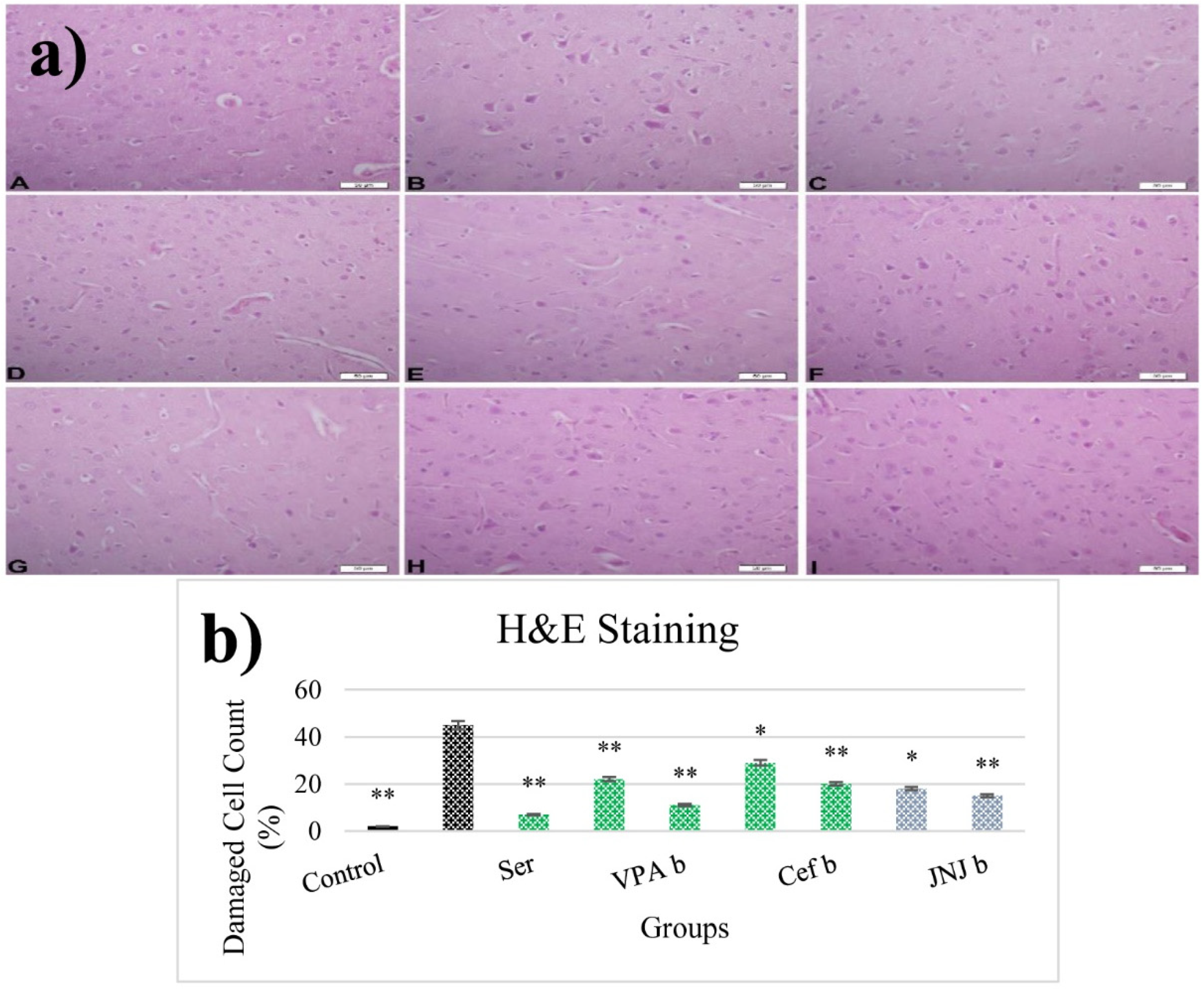
2.13. Histopathological Examinations
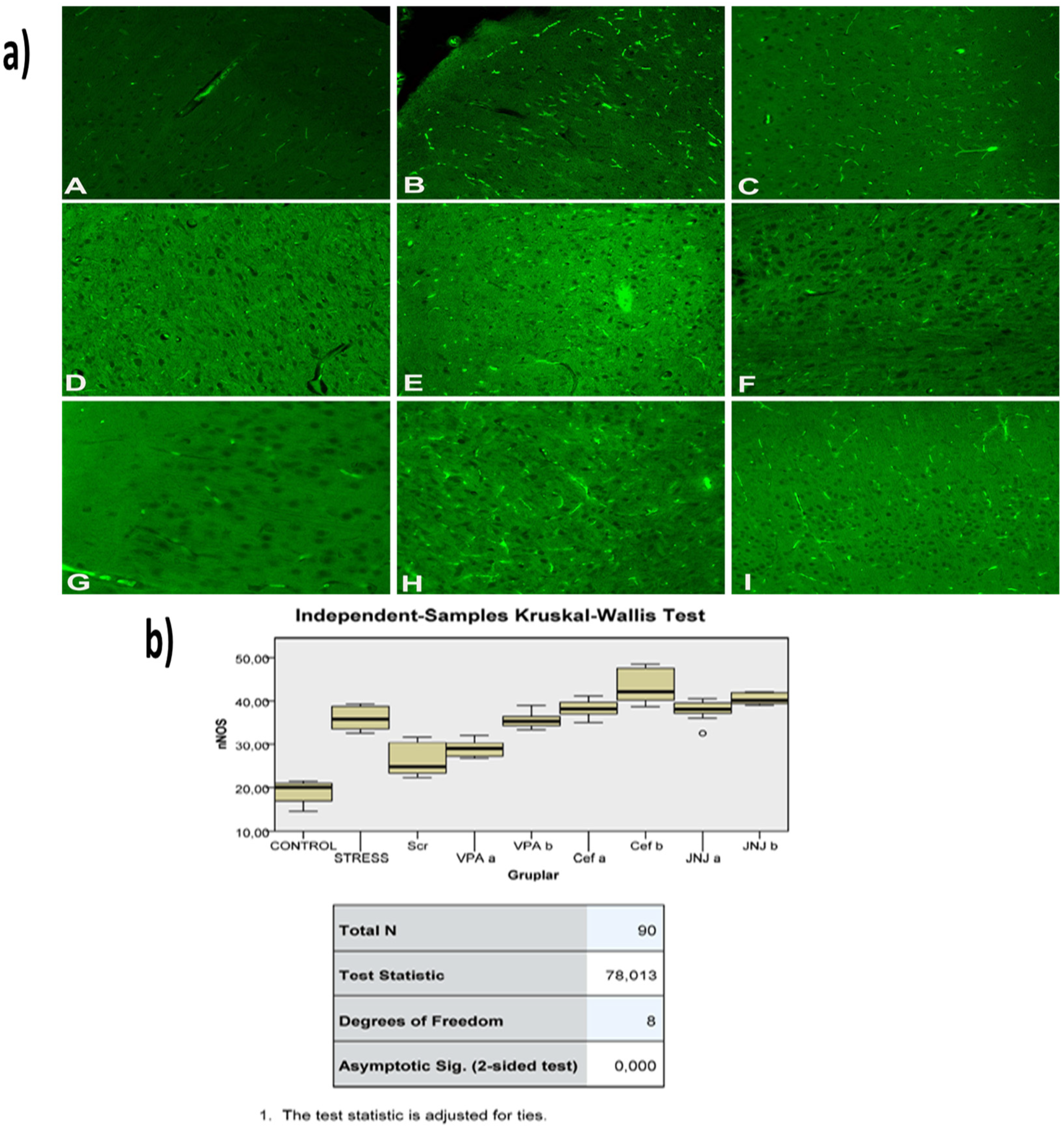
2.14. Immunohistochemical Studies
2.15. Statistical Analysis
3. Results
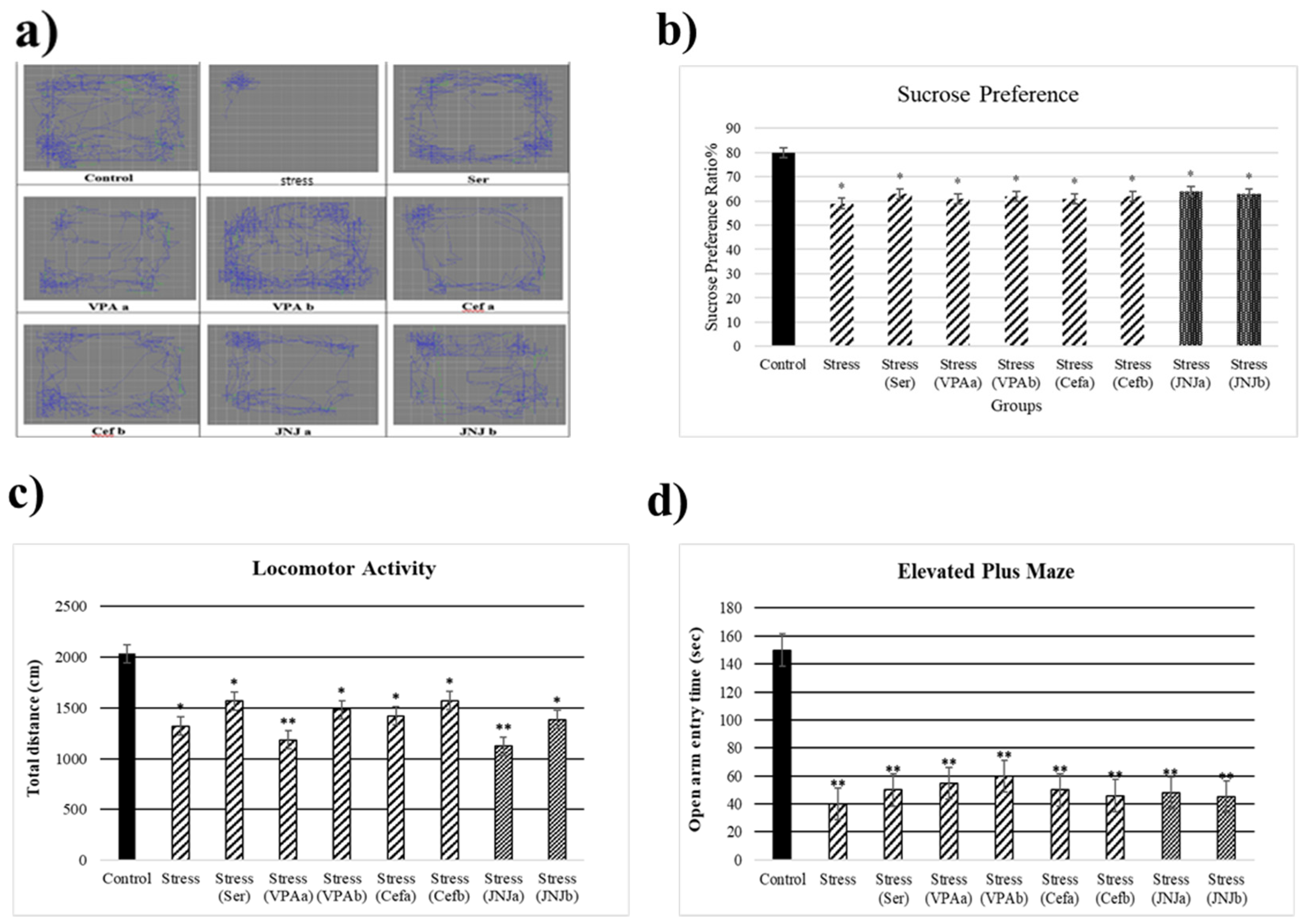
3.1. Validation of Mild Stress Model of Rats
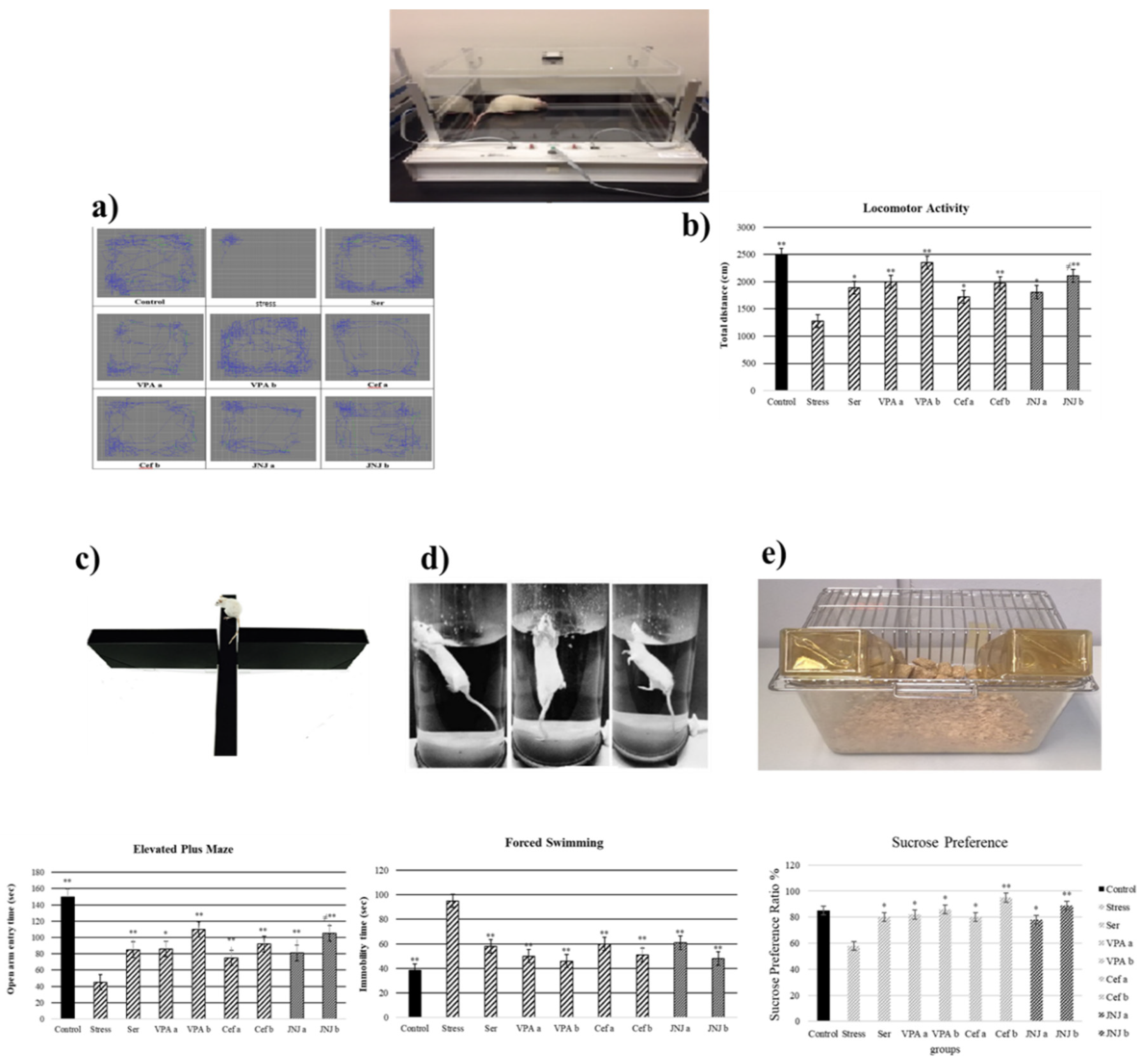
3.2. JNJ Treatment Increases Locomotor Activity in a Mild Stress Model of Rats
3.3. JNJ Treatment Increases Locomotor Activity in a Mild Stress Model of Rats
3.4. JNJ Treatment Increases Time Spent in Open Arm in Elevated plus Maze Test in a Mild Stress Model of Rats
3.5. JNJ Treatment Decreases Immobility Time in Forced Swimming Test in a Mild Stress Model of Rats
3.6. JNJ Treatment Increase Sucrose Preference in a Mild Stress Model of Rats
3.7. JNJ Treatment Decreased T80 Time of Glut Reuptake in CA3 and DS Regions in a Mild Stress Model of Rats
3.8. JNJ Treatment Increased SLC1A2, SLC1A3, and GLUL Gene Expression and Decreased JWA Gene Expression in a Mild Stress Model of Rats
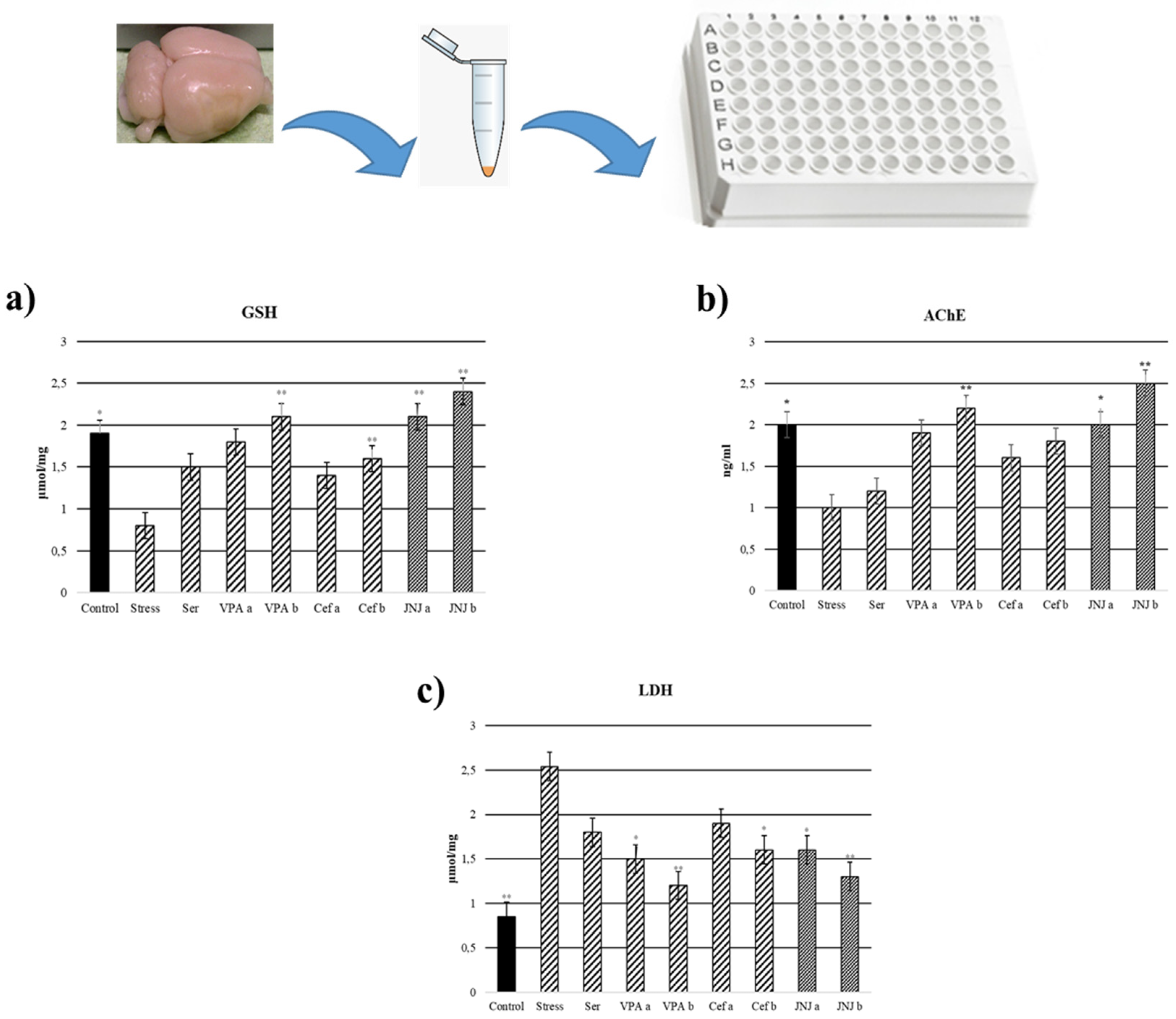
3.9. JNJ Treatment Increase GSH Levels in Brain Tissue in a Mild Stress Model of Rats
3.10. JNJ Treatment Decreased LDH Levels in Brain Tissue in a Mild Stress Model of Rats
3.11. JNJ Treatment Increased AChE Activity in Brain Tissue in a Mild Stress Model of Rats
3.12. JNJ Treatment Prevent Severe Histopathological Degeneration of Brain Tissue Produced by Mild Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Bauer, M.; Carvalho, A.F.; Eyre, H.; Fava, M.; Kasper, S.; Kennedy, S.H.; Khoo, J.P.; Lopez Jaramillo, C.; Malhi, G.S.; et al. A clinical approach to treatment resistance in depressed patients: What to do when the usual treatments don’t work well enough? World J. Biol. Psychiatry 2021, 22, 483–494. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, M.; Hillemacher, T.; Bleich, S.; Frieling, H. The Epigenetic Code in Depression: Implications for Treatment. Clin. Pharmacol. Ther. 2012, 91, 310–314. [Google Scholar] [CrossRef]
- Almeida, R.F.; Thomazi, A.P.; Godinho, G.F.; Saute, J.A.M.; Wofchuk, S.T.; Souza, D.O.; Ganzella, M. Effects of Depressive-Like Behavior of Rats on Brain Glutamate Uptake. Neurochem. Res. 2010, 35, 1164–1171. [Google Scholar] [CrossRef]
- De Vasconcellos-Bittencourt, A.P.; Vendite, D.A.; Nassif, M.; Crema, L.M.; Frozza, R.; Thomazi, A.P.; Nieto, F.B.; Wofchuk, S.; Salbego, C.; da Rocha, E.R.; et al. Chronic stress and lithium treatments alter hippocampal glutamate uptake and release in the rat and potentiate necrotic cellular death after oxygen and glucose deprivation. Neurochem. Res 2011, 36, 793–800. [Google Scholar] [CrossRef]
- Zink, M.; Vollmayr, B.; Gebicke-Haerter, P.J.; Henn, F.A. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology 2010, 58, 465–473. [Google Scholar] [CrossRef]
- Altshuler, L.L.; Abulseoud, O.A.; Foland-Ross, L.; Bartzokis, G.; Chang, S.; Mintz, J.; Hellemann, G.; Vinters, H.V. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010, 12, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Kerman, I.A.; Thompson, R.C.; Jones, E.G.; Bunney, W.E.; Barchas, J.D.; Schatzberg, A.F.; Myers, R.M.; Akil, H.; Watson, S.J. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol. Psychiatr. 2011, 16, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel-Hidalgo, J.J.; Waltzer, R.; Whittom, A.A.; Austin, M.C.; Rajkowska, G.; Stockmeier, C.A. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J. Affect. Disord. 2010, 127, 230–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, F.J.; Lluch, M.; Dot, J.; Blanco, I.; RodriguezAlvarez, J. Histamine modulation of glutamate release from hippocampal synaptosomes. Eur. J. Pharmacol. 1997, 323, 283–286. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Chang, C.Y.; Huang, S.K.; Wang, S.J. Ciproxifan, a histamine H-3 receptor antagonist and inverse agonist, presynaptically inhibits glutamate release in rat hippocampus. Toxicol. Appl. Pharm. 2017, 319, 12–21. [Google Scholar] [CrossRef]
- Karpati, A.; Yoshikawa, T.; Nakamura, T.; Iida, T.; Matsuzawa, T.; Kitano, H.; Harada, R.; Yanai, K. Histamine elicits glutamate release from cultured astrocytes. J. Pharmacol. Sci. 2018, 137, 122–128. [Google Scholar] [CrossRef]
- Leurs, R.; Chazot, P.L.; Shenton, F.C.; Lim, H.D.; de Esch, I.J. Molecular and biochemical pharmacology of the histamine H4 receptor. Br. J. Pharmacol. 2009, 157, 14–23. [Google Scholar] [CrossRef]
- Terzioglu Bebitoglu, B.; Oguz, E.; Gokce, A. Effect of valproic acid on oxidative stress parameters of glutamate-induced excitotoxicity in SH-SY5Y cells. Exp. Ther. Med. 2020, 20, 1321–1328. [Google Scholar] [CrossRef]
- Kobayashi, H.; Iwata, M.; Mitani, H.; Yamada, T.; Nakagome, K.; Kaneko, K. Valproic acid improves the tolerance for the stress in learned helplessness rats. Neurosci. Res. 2012, 72, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Tikhonova, M.A.; Amstislavskaya, T.G.; Ho, Y.J.; Akopyan, A.A.; Tenditnik, M.V.; Ovsyukova, M.V.; Bashirzade, A.A.; Dubrovina, N.I.; Aftanas, L.I. Neuroprotective Effects of Ceftriaxone Involve the Reduction of Abeta Burden and Neuroinflammatory Response in a Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 736786. [Google Scholar] [CrossRef]
- Lee, S.G.; Su, Z.Z.; Emdad, L.; Gupta, P.; Sarkar, D.; Borjabad, A.; Volsky, D.J.; Fisher, P.B. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J. Biol. Chem. 2008, 283, 13116–13123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Liu, Z.; Yu, J.; Li, S.M.; Tang, M.; Zeng, L.; Wang, H.Y.; Xie, H.; Peng, L.; Huang, H.J.; et al. Quantitative Proteomic Analysis Reveals Synaptic Dysfunction in the Amygdala of Rats Susceptible to Chronic Mild Stress. Neuroscience 2018, 376, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Tyshko, N.V.; Docea, A.O.; Shestakova, S.I.; Sidorova, Y.S.; Petrov, N.A.; Zlatian, O.; Mach, M.; Hartung, T.; Tutelyan, V.A. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes-A real-life risk simulation approach. Toxicol. Lett. 2019, 315, 96–106. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Zlatian, O.; Mitroi, M.; Renieri, E.; Tsatsakis, A.; Izotov, B.N.; Burada, F.; Sosoi, S.; Burada, E.; Buga, A.M.; et al. A Novel Nutraceutical Formulation Can Improve Motor Activity and Decrease the Stress Level in a Murine Model of Middle-Age Animals. J. Clin. Med. 2021, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shao, W.; Liu, Z.; Fan, S.; Yu, J.; Chen, J.; Qiao, R.; Zhou, J.; Xie, P. iTRAQ-based quantitative analysis of hippocampal postsynaptic density-associated proteins in a rat chronic mild stress model of depression. Neuroscience 2015, 298, 220–292. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic Status of Gdnf in the Ventral Striatum Determines Susceptibility and Adaptation to Daily Stressful Events. Neuron 2011, 69, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Hu, C.L.; Luo, Y.; Wang, H.; Kuang, S.N.; Liang, G.J.; Yang, Y.; Mai, S.S.; Yang, J.Q. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE 2017, 12, e0185129. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Martorell, M.; Sharopov, F.; Tumer, T.B.; Kurt, B.; Lankatillake, C.; Docea, A.O.; Moreira, A.C.; et al. A Pharmacological Perspective on Plant-derived Bioactive Molecules for Epilepsy. Neurochem. Res. 2021, 46, 2205–2225. [Google Scholar] [CrossRef]
- Nussbaum, L.; Hogea, L.M.; Calina, D.; Andreescu, N.; Gradinaru, R.; Stefanescu, R.; Puiu, M. Modern treatment approaches in psychoses. pharmacogenetic, neuroimagistic and clinical implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douen, A.G.; Akiyama, K.; Hogan, M.J.; Wang, F.; Dong, L.; Chow, A.K.; Hakim, A. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebal cortex plasma membranes. J. Neurochem. 2000, 75, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Orlov, I.; Ruggiero, A.M.; Dykes-Hoberg, M.; Lee, A.; Jackson, M.; Rothstein, J.D. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3-18. Nature 2001, 410, 84–88. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Calina, D.; Buga, A.M.; Mitroi, M.; Buha, A.; Caruntu, C.; Scheau, C.; Bouyahya, A.; El Omari, N.; El Menyiy, N.; Docea, A.O. The Treatment of Cognitive, Behavioural and Motor Impairments from Brain Injury and Neurodegenerative Diseases through Cannabinoid System Modulation-Evidence from In Vivo Studies. J. Clin. Med. 2020, 9, 28. [Google Scholar] [CrossRef]
- Pan, L.L.; Qi, R.R.; Wang, J.Q.; Zhou, W.; Liu, J.L.; Cai, Y.L. Evidence for a Role of Orexin/Hypocretin System in Vestibular Lesion-Induced Locomotor Abnormalities in Rats. Front. Neurosci.-Switz 2016, 10, 2395. [Google Scholar] [CrossRef] [Green Version]
- Henningsen, K.; Palmfeldt, J.; Christiansen, S.; Baiges, I.; Bak, S.; Jensen, O.N.; Gregersen, N.; Wiborg, O. Candidate hippocampal biomarkers of susceptibility and resilience to stress in a rat model of depression. Mol. Cell Proteom. 2012, 11, M111-016428. [Google Scholar] [CrossRef] [Green Version]
- Chandley, M.J.; Szebeni, K.; Szebeni, A.; Crawford, J.; Stockmeier, C.A.; Turecki, G.; Miguel-Hidalgo, J.J.; Ordway, G.A. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J. Psychiatr. Neurosci. 2013, 38, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ding, X.F.; Wang, X.X.; Zou, X.J.; Li, X.J.; Liu, Y.E.Y.; Li, J.; Qian, X.Y.; Chen, J.X. Xiaoyaosan exerts antidepressant-like effects by regulating the functions of astrocytes and EAATs in the prefrontal cortex of mice. BMC Complem. Altern. Med. 2019, 19, 215. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Su, R.; Zhang, X.; Wen, J.; Yao, D.; Gao, X.; Zhu, Z.; Li, H. Hippocampal GR- and CB1-mediated mGluR5 differentially produces susceptibility and resilience to acute and chronic mild stress in rats. Neuroscience 2017, 357, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Reiterer, V.; Ruggiero, A.M.; Rothstein, J.D.; Thomas, S.; Dahm, R.; Sitte, H.H.; Farhan, H. GTRAP3-18 serves as a negative regulator of Rab1 in protein transport and neuronal differentiation. J. Cell Mol. Med. 2009, 13, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic JWA deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Vawter, M.P.; Ginsberg, S.D. Target Identification for CNS Diseases by Transcriptional Profiling. Neuropsychopharmacology 2009, 34, 18–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloizou, A.M.; Siokas, V.; Pateraki, G.; Liampas, I.; Bakirtzis, C.; Tsouris, Z.; Lazopoulos, G.; Calina, D.; Docea, A.O.; Tsatsakis, A.; et al. Thinking Outside the Ischemia Box: Advancements in the Use of Multiple Sclerosis Drugs in Ischemic Stroke. J. Clin. Med. 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.; Oliveira, C.R. Glutamate toxicity on a PC12 cell line involves glutathione (GSH) depletion and oxidative stress. Free Radic. Biol. Med. 1997, 23, 637–647. [Google Scholar] [CrossRef]
- Shaw, C.A.; Bains, J.S. Synergistic versus antagonistic actions of glutamate and glutathione: The role of excitotoxicity and oxidative stress in neuronal disease. Cell Mol. Biol. 2002, 48, 127–136. [Google Scholar]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.-M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008, 9, 148–158. [Google Scholar] [CrossRef]
- Cai, W.; Ma, W.; Wang, G.T.; Li, Y.J.; Shen, W.D. Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog-signaling pathway in a rat model of poststroke depression. Neuropsychiatr. Dis. Treat. 2019, 15, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.G.; Takeuchi, K.; Rodenas-Ruano, A.; Takayasu, Y.; Murphy, J.; Bennett, M.V.L.; Zukin, R.S. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem. Soc. Trans. 2009, 37, 1369–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 1403–1411. [Google Scholar] [CrossRef]
- Dong, H.; Xiang, Y.Y.; Farchi, N.; Ju, W.; Wu, Y.; Chen, L.; Wang, Y.; Hochner, B.; Yang, B.; Soreq, H.; et al. Excessive expression of acetylcholinesterase impairs glutamatergic synaptogenesis in hippocampal neurons. J. Neurosci. 2004, 24, 8950–8960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilsaver, S.C.; Peck, J.A.; Overstreet, D.H. The Flinders Sensitive Line exhibits enhanced thermic responsiveness to nicotine relative to the Sprague-Dawley rat. Pharmacol. Biochem. Behav. 1992, 41, 23–27. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. NeuroBiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Sestito, S.; Rapposelli, S.; Peron, G.; Calina, D.; Sharifi-Rad, M.; Sharopov, F.; Martins, N.; Sharifi-Rad, J. Epibatidine: A Promising Natural Alkaloid in Health. Biomolecules 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Buga, A.M.; Docea, A.O.; Albu, C.; Malin, R.D.; Branisteanu, D.E.; Ianosi, G.; Ianosi, S.L.; Iordache, A.; Calina, D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol. Lett. 2019, 17, 4170–4175. [Google Scholar] [CrossRef] [Green Version]
- Li, L.L.; Ginet, V.; Liu, X.N.; Vergun, O.; Tuittila, M.; Mathieu, M.; Bonny, C.; Puyal, J.; Truttmann, A.C.; Courtney, M.J. The nNOS-p38MAPK Pathway Is Mediated by NOS1AP during Neuronal Death. J. Neurosci. 2013, 33, 8185–8201. [Google Scholar] [CrossRef] [Green Version]
- Ehsanifar, M.; Tameh, A.A.; Farzadkia, M.; Kalantari, R.R.; Zavareh, M.S.; Nikzaad, H.; Jafari, A.J. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019, 168, 338–347. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, A.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Orlovsky, M.A.; Dosenko, V.E.; Spiga, F.; Skibo, G.G.; Lightman, S.L. Hippocampus remodeling by chronic stress accompanied by GR, proteasome and caspase-3 overexpression. Brain Res. 2014, 1593, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Santha, P.; Veszelka, S.; Hoyk, Z.; Meszaros, M.; Walter, F.R.; Toth, A.E.; Kiss, L.; Kincses, A.; Olah, Z.; Seprenyi, G.; et al. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front. Mol. Neurosci. 2016, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeni, Y.; Cakir, Z.; Hacimuftuoglu, A.; Taghizadehghalehjoughi, A.; Okkay, U.; Genc, S.; Yildirim, S.; Saglam, Y.S.; Calina, D.; Tsatsakis, A.; et al. A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression. J. Pers. Med. 2022, 12, 246. https://doi.org/10.3390/jpm12020246
Yeni Y, Cakir Z, Hacimuftuoglu A, Taghizadehghalehjoughi A, Okkay U, Genc S, Yildirim S, Saglam YS, Calina D, Tsatsakis A, et al. A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression. Journal of Personalized Medicine. 2022; 12(2):246. https://doi.org/10.3390/jpm12020246
Chicago/Turabian StyleYeni, Yesim, Zeynep Cakir, Ahmet Hacimuftuoglu, Ali Taghizadehghalehjoughi, Ufuk Okkay, Sidika Genc, Serkan Yildirim, Yavuz Selim Saglam, Daniela Calina, Aristidis Tsatsakis, and et al. 2022. "A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression" Journal of Personalized Medicine 12, no. 2: 246. https://doi.org/10.3390/jpm12020246
APA StyleYeni, Y., Cakir, Z., Hacimuftuoglu, A., Taghizadehghalehjoughi, A., Okkay, U., Genc, S., Yildirim, S., Saglam, Y. S., Calina, D., Tsatsakis, A., & Docea, A. O. (2022). A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression. Journal of Personalized Medicine, 12(2), 246. https://doi.org/10.3390/jpm12020246








