The Integrated Effect of Microbial Inoculants and Biochar Types on Soil Biological Properties, and Plant Growth of Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Plant Dry Biomass
2.2. Plant Beneficial Traits of Microbial Inoculants
2.3. Soil Enzymes
3. Discussion
4. Materials and Methods
4.1. Plant, Soil and Biochars
4.2. Microorganisms
4.3. Plant Growth Experiment
- T0: un-inoculated control plants grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC;
- TB: inoculated plants with Klebsiella sp. BS13 and grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC;
- TF1: inoculated plants with Talaromyces purpureogenus BS16aPP and grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC;
- TF2: inoculated plants with Talaromyces calidicanius RS10bPP and grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC;
- TBF1: inoculated plants with Klebsiella sp. BS13 + Talaromyces calidicanius RS10bPP and grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC;
- TBF2: inoculated plants with Klebsiella sp. BS13 + Talaromyces purpureogenus BS16aPP and grown in soil; (a) without biochar, (b) with CWBC, (c) with WBC, (d) with MBC.
4.4. The Plant Beneficial Traits and Colonization Ability of Microbial Inoculants
4.5. Soil Enzyme Activities
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on the interrelationships among soybean growth, root nodulation, plant P uptake, and soil nutrients in a sandy field. Sustainability 2019, 11, 6542. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Yu, O.Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller-Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil. 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Abd_Allah, E.F.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S.D. Soil amendments with different maize biochars, to varying degrees, improve chickpea growth under drought by improving symbiotic performance with Mesorhizobium ciceri and soil biochemical properties. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef]
- Islami, T.; Curitno, B.; Basuki, N.; Suryanto, A. Maize yield and associated soil quality changes in cassava + maize intercropping system after 3 years of biochar application. J. Agric. Food Technol. 2011, 1, 112–115. [Google Scholar]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; del Campillo, M.D.; Gallardo, A.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475. [Google Scholar] [CrossRef] [Green Version]
- Pietikäinen, J.; Kiikkila, O.; Fritze, H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Kolton, M.; Meller Harel, Y.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Reckling, M.; Wirth, S. Biochar-based inoculum of Bradyrhizobium sp. improves plant growth and yield of lupin (Lupinus albus L.) under drought stress. Eur. J. Soil Biol. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Shurigin, V.; Hashem, A.; Abd Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 28, 1887. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhu, F.; Carrión, V.J.; Cordovez, V. Beyond Plant Microbiome Composition: Exploiting Microbial Functions and Plant Traits via Integrated Approaches. Front. Bioeng. Biotechnol. 2020, 8, 896. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.M.G.; Reis, M.M.; Frazão, L.A.; da Mata Terra, L.E.; Lopes, E.F.; dos Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; Kumar, S.K.; Malo, D.D.; Rice, J.A.; Bleakley, B.; Chilom, G.; Clay, D.E.; Julson, J.L.; Papiernik, S.K.; et al. Molecular characterization of biochars and their influence on microbiological properties of soil. J. Hazard. Mater. 2014, 279, 244–256. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Abid, M.; Fahad, S.; Brtnicky, M.; Dokulilova, T.; Datta, R.; Danish, S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020, 10, 12159. [Google Scholar] [CrossRef] [PubMed]
- Shanta, N.; Schwinghamer, T.; Backer, R.; Allaire, S.E.; Teshler, I.; Vanasse, A.; Whalen, J.; Baril, B.; Lange, S.; MacKay, J.; et al. Biochar and plant growth promoting rhizobacteria effects on switchgrass (Panicum virgatum cv. Cave-in-Rock) for biomass production in southern Québec depend on soil type and location. Biomass Bioenergy 2016, 95, 167–173. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef]
- Dela Cruz, T.E.E.; Din, H.J.F.; Aril-dela Cruz, J.V. Microbes for Sustainable Agriculture: Isolation and Identification of Beneficial Soil- and Plant-Associated Microorganisms; SEARCA Professorial Chair Lecture Monograph No. 6; SEARCA: Los Baños, Philippines, 2021. [Google Scholar]
- Foteinis, S.; Chatzisymeon, E. Life cycle assessment of organic versus conventional agriculture. A case study of lettuce cultivation in Greece. J. Clean. Prod. 2016, 112, 2462–2471. [Google Scholar] [CrossRef]
- Anilakumar, K.R.; Harsha, S.N.; Mallesha, S.; Sharma, R.K. Lettuce: A promising leafy vegetable with functional properties. Def. Life Sci. J. 2017, 2, 178–185. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Chen, M.; Xu, P.; Zhou, Y.; et al. Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl. Microbiol. Biotechnol. 2016, 100, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.F.; Steffens, D.; Reisenauer, H.P.; Schubert, S. Kinetics of carbon mineralisation of biochars compared with wheat straw in three soils. J. Environ. Qual. 2012, 41, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Localisation of nitrate in the rhizosphere of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 2243–2246. [Google Scholar] [CrossRef]
- Wang, C.; Alidousta, D.; Yng, X.; Isoda, A. Effects of bamboo biochar on soybean root nodulation in multi-elements contaminated soils. Ecotoxicol. Environ. Saf. 2018, 150, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Zoghi, Z.; Nazarov, K.; Wirth, S.; Bellingrath-Kimura, S.D. Plant growth response of broad bean (Vicia faba, L.) to biochar amendment of loamy sand soil under irrigated and drought conditions. Environ. Sustain. 2020, 3, 319–324. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Ma, H.; Müller, M.E.H.; Wirth, S.; Reckling, M.; Bellingrath-Kimura, S.D. The effect of biochars and endophytic bacteria on growth and root rot disease incidence of Fusarium infested narrow-leafed lupin (Lupinus angustifolius L.). Microorganisms 2020, 8, 496. [Google Scholar] [CrossRef] [Green Version]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’-does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Li, Y.; Liu, X.; Meng, L.; Sang, P.; Mu, Y.; Wu, H.; Ma, Z.; Hou, J.; Li, S. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Zheng, B.X.; Ding, K.; Yang, X.R.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.G. Straw biochar increases the abundance of inorganic phosphate solubilizing bacterial community for better rape (Brassica napus) growth and phosphate uptake. Sci. Total Environ. 2019, 647, 1113–1120. [Google Scholar] [CrossRef]
- Hale, L.; Luth, M.; Kenney, R.; Crowley, D. Evaluation of Pinewood Biochar as a Carrier of Bacterial Strain Enterobacter cloacae UW5 for Soil Inoculation. Appl. Soil Ecol. 2014, 84, 192–199. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. Plant growth promoting rhizobacteria isolated from calcisol soil in a semiarid region of Uzbekistan: Biochemical characterisation and effectiveness. Plant Nutr. Soil Sci. 2005, 168, 94–99. [Google Scholar] [CrossRef]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome analysis of Pseudomonas fluorescens PCL1751: A rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 1371, e0140231. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Sarma, B.; Borkotoki, B.; Narzari, R.; Kataki, R.; Gogoi, N. Organic amendments: Effect on carbon mineralization and crop productivity in acidic soil. J. Clean. Prod. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Alaylar, B.; Kistaubayeva, A.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar for improving soil biological properties and mitigating salt stress in plants on salt-affected soils. Comm. Plant Soil Sci. 2021, 140–152. [Google Scholar] [CrossRef]
- Iijima, M.; Yamane, K.; Izumi, Y.; Daimon, H.; Motonaga, T. Continuous application of biochar inoculated with root nodule bacteria to subsoil enhances yield of soybean by the nodulation control using crack fertilization technique. Plant Prod. Sci. 2015, 18, 197–208. [Google Scholar] [CrossRef]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar based inoculants improve soybean growth and nodulation. J. Agric. Sci. 2017, 8, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Fall, D.; Bakhoum, N.; Nourou Sall Zoubeirou, A.M.; Sylla, S.N.; Diouf, D. Rhizobial inoculation increases soil microbial functioning and gum arabic production of 13-year-old Senegalia senegal (L.) Britton, trees in the north part of Senegal. Front. Plant Sci. 2016, 7, 1355. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.M.; Rahman, M.M.; Morshed, M.M.; Islam, M.S.; Afrad, M.S.I. Biochar on soil fertility and crop productivity. Agriculturists 2019, 17, 76–88. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Shoaf, N.L. Biochar and vermicompost amendments in vegetable cropping systems: Impacts on soil quality, soil-borne pathogens and crop productivity. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2014. [Google Scholar]
- Li, Q.; Lei, Z.; Song, X.; Zhang, Z.; Ying, Y.; Peng, C. Biochar amendment decreases soil microbial biomass and increases bacterial diversity in Moso bamboo (Phyllostachys edulis) plantations under simulated nitrogen deposition. Environ. Res. Lett. 2018, 13, 044029. [Google Scholar] [CrossRef]
- Dempster, D.N.; Glesson, D.B.; Solaiman, Z.M.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Blackwell, P.; Krull, E.; Butler, G.; Herbert, A.; Solaiman, Z. Effect of banded biochar on dryland wheat production and fertiliser use in south-western Australia: An agronomic and economic perspective. Aust. J. Soil Res. 2010, 48, 531–545. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichhornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Rijavec, T.; Lapanje, A. Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Front. Microbiol. 2016, 7, 1785. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.; Yang, F.; Wang, Z.; Tian, C. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.L.; Liang, G.Q.; Zhang, Q.; Ai, C.; Zhou, W. Maise biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Reibe, K.; Götz, K.P.; Ross, C.L.; Doering, T.F.; Ellmer, F.; Ruess, L. Impact of quality and quantity of biochar and hydrochar on soil collembola and growth of spring wheat. Soil Biol. Biochem. 2015, 8, 84–87. [Google Scholar] [CrossRef]
- Castric, P.A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 1975, 21, 613–618. [Google Scholar] [CrossRef]
- Bano, N.; Musarrat, J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 2003, 46, 324–328. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorous in soil in the connection with vital activity of some microbial species. Mikorobiologiya 1948, 17, 362–370. [Google Scholar]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimalization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for the assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Phosphorus cycle enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; SSSA: Madison, WI, USA, 2011; pp. 161–183. [Google Scholar]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]


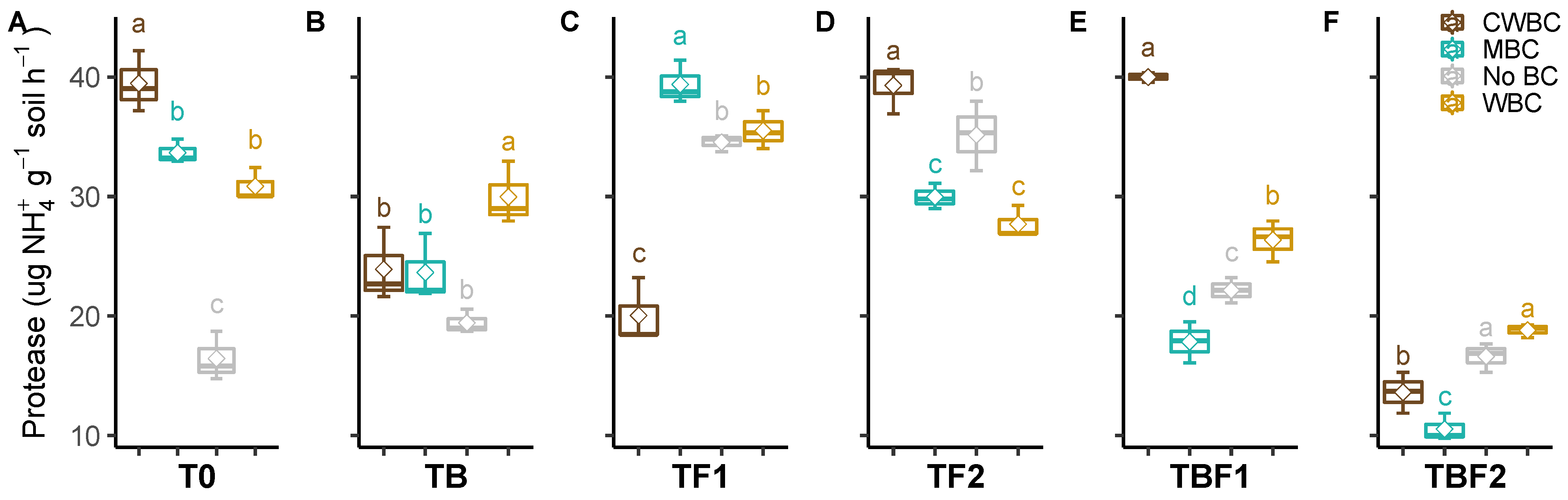
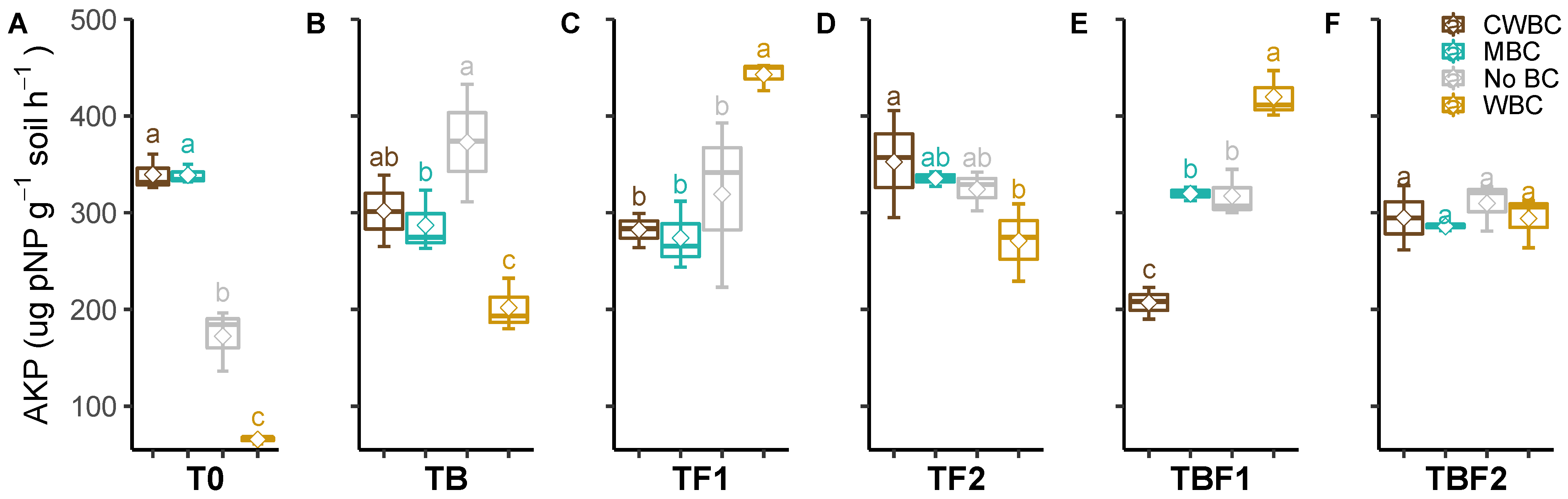
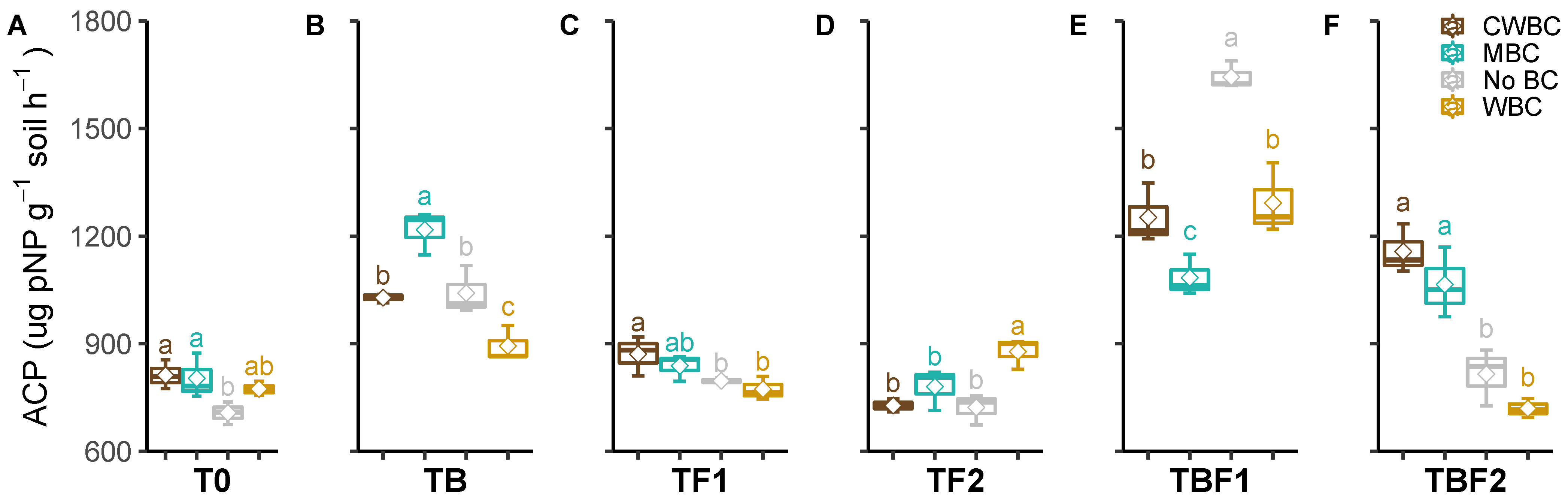
| Interaction Effects | Plant Dry Weight | Soil FDA | Soil Protease | Soil AKP | Soil ACP |
|---|---|---|---|---|---|
| Biochar | *** | *** | *** | ns | *** |
| Microbes | *** | *** | *** | *** | *** |
| Biochar × Microbes | ** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Shurigin, V.; Jabborova, D.; dela Cruz, J.A.; dela Cruz, T.E.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. The Integrated Effect of Microbial Inoculants and Biochar Types on Soil Biological Properties, and Plant Growth of Lettuce (Lactuca sativa L.). Plants 2022, 11, 423. https://doi.org/10.3390/plants11030423
Ma H, Shurigin V, Jabborova D, dela Cruz JA, dela Cruz TE, Wirth S, Bellingrath-Kimura SD, Egamberdieva D. The Integrated Effect of Microbial Inoculants and Biochar Types on Soil Biological Properties, and Plant Growth of Lettuce (Lactuca sativa L.). Plants. 2022; 11(3):423. https://doi.org/10.3390/plants11030423
Chicago/Turabian StyleMa, Hua, Vyacheslav Shurigin, Dilfuza Jabborova, Jeane Aril dela Cruz, Thomas Edison dela Cruz, Stephan Wirth, Sonoko Dorothea Bellingrath-Kimura, and Dilfuza Egamberdieva. 2022. "The Integrated Effect of Microbial Inoculants and Biochar Types on Soil Biological Properties, and Plant Growth of Lettuce (Lactuca sativa L.)" Plants 11, no. 3: 423. https://doi.org/10.3390/plants11030423