Carbon, Nitrogen and Water Footprints of Organic Rice and Conventional Rice Production over 4 Years of Cultivation: A Case Study in the Lower North of Thailand
Abstract
:1. Introduction
2. Materials and Methods
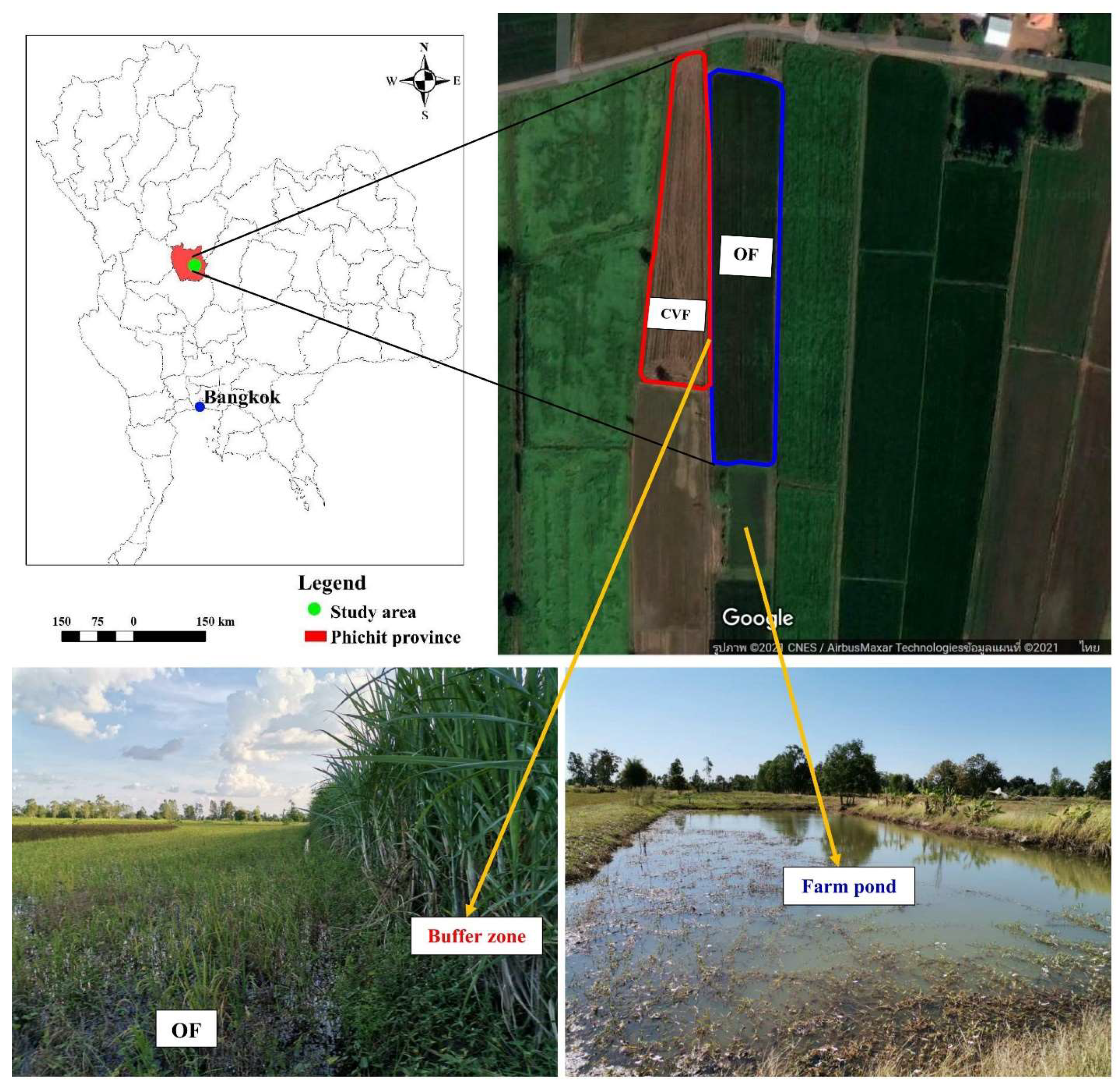
2.1. Study Sites
2.2. Data Collection
2.2.1. Farm Management Practice Data
2.2.2. Soil Sampling and Analysis
2.2.3. Soil Organic Carbon Calculation
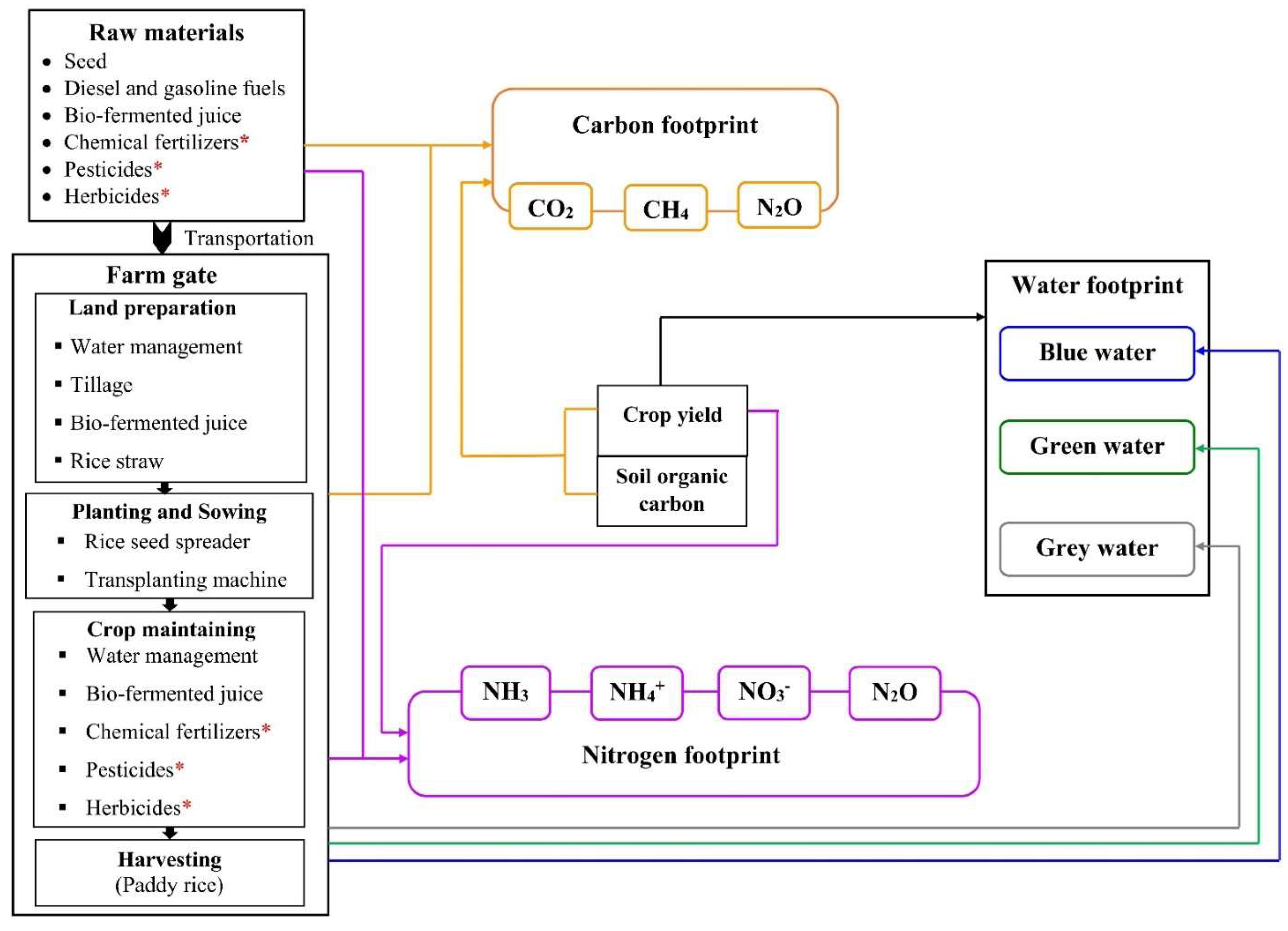
2.3. System Boundary and Functional Unit
2.4. Carbon Footprint Calculation
2.5. Nitrogen Footprint Calculation
2.6. Water Footprint Calculation
2.7. Calculation of Economic Return and CF, NF, and WF Per Net Return
2.8. Statistical Analysis
3. Results and Discussion
3.1. Input Inventory Analysis and Soil Physical and Chemical Properties
3.2. Soil Organic Carbon Stock of OF and CVF
3.3. Greenhouse Gas Emissions and Carbon Footprint of OF and CVF
3.4. Reactive Nitrogen Emissions and Nitrogen Footprints of OF and CVF
3.5. Water Footprint of OF and CVF
3.6. Economic Return and CF, NF, and WF per Net Return
3.7. Limitations and Recommendations for Further Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Field, C.B.; Barros, V.R. (Eds.) Climate Change 2014: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2014; p. 1820. [Google Scholar]
- Office of Agricultural Economics (OAE). Agricultural Statistics of Thailand; Office of Agricultural Economics: Bangkok, Thailand, 2020; Available online: http://www.oae.go.th/assets/portals/1/files/jounal/2564/yearbook2563.pdf (accessed on 28 May 2021). (In Thai)
- Ding, Y.; Liu, Y.X.; Wu, W.X.; Shi, D.Z.; Yang, M.; Zhong, Z.K. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Pandey, D.; Agrawal, M. Carbon Footprint Estimation in the Agriculture Sector. In Assessment of Carbon Footprint in Different Industrial Sectors; Springer: Singapore, 2014; Volume 1, pp. 25–47. [Google Scholar]
- Arunrat, N.; Wang, C.; Pumijumnong, N. Alternative cropping systems for greenhouse gases mitigation in rice field: A case study in Phichit province of Thailand. J. Clean. Prod. 2016, 133, 657–671. [Google Scholar] [CrossRef]
- Maraseni, T.N.; Deo, R.C.; Qu, J.; Gentle, P.; Neupane, P.R. An international comparison of rice consumption behaviours and greenhouse gas emissions from rice production. J. Clean. Prod. 2018, 172, 2288–2300. [Google Scholar] [CrossRef]
- Scialabba, N.E.-H.; Müller-Lindenlauf, M. Organic agriculture and climate change. Renew. Agric. Food Syst. 2010, 25, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Meena, A.L.; Pandey, R.N.; Kumar, D.; Dotaniya, M.L.; Sharma, V.K.; Singh, G.; Meena, B.P.; Kumar, A.; Bhanu, C. Impact of 12-year-long rice based organic farming on soil quality in terms of soil physical properties, available micronutrients and rice yield in a typic Ustochrept soil of India. Commun. Soil Sci. Plant Anal. 2020, 51(18), 2331–2348. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Meemken, E.M.; Qaim, M. Organic agriculture, food security, and the environment. Ann. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Arunrat, N.; Sereenonchai, S.; Wang, C. Carbon footprint and predicting the impact of climate change on carbon sequestration ecosystem services of organic rice farming and conventional rice farming: A case study in Phichit province, Thailand. J. Environ. Manag. 2021, 289, 112458. [Google Scholar] [CrossRef]
- Shahane, A.A.; Shivay, Y.S.; Prasanna, R.; Kumar, D. Improving water and nutrient use efficiency in rice by changing crop establishment methods, application of microbial inoculations, and Zn fertilization. Glob. Chall. 2019, 3, 1800005. [Google Scholar] [CrossRef]
- Wiedmann, T.; Minx, J. A Definition of Carbon Footprint. Ecol. Econ. Res. Trends 2008, 1, 1–11. [Google Scholar]
- Hoekstra, A.Y. 2003. Virtual Water Trade. In Proceedings of the International Expert Meeting on Virtual Water Trade, Delft, The Netherlands, 12–13 December 2002. [Google Scholar]
- Hoekstra, A.Y.; Chapagain, A.K. Water footprints of nations: Water use by people as a function of their consumption pattern. Water Resour. Manag. 2010, 21, 35–48. [Google Scholar] [CrossRef]
- Siebert, S.; Döll, P. Quantifying blue and green virtual water contents in global crop production as well as potential production losses without irrigation. J. Hydrol. 2010, 384, 198–207. [Google Scholar] [CrossRef]
- Hoekstra, A.Y.; Chapagain, A.K.; Aldaya, M.M.; Mekonnen, M.M. The Water Footprint Assessment Manual: Setting the Global Standard; Earthscan: London, UK, 2011. [Google Scholar]
- Hoekstra, A.Y.; Mekonnen, M.M. The water footprint of humanity. Proc. Natl. Acad. Sci. USA 2012, 109, 3232–3237. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Liang, C.; Hamel, C.; Cutforth, H.; Wang, H. Strategies for reducing the carbon footprint of field crops for semiarid areas. A review. Agron. Sustain. Dev. 2011, 31, 643–656. [Google Scholar] [CrossRef] [Green Version]
- Leach, A.M.; Galloway, J.N.; Bleeker, A.; Erisman, J.W.; Kohn, R.; Kitzes, J. A nitrogen Footprint model to help consumers understand their role in nitrogen losses to the environment. Environ. Dev. 2012, 1, 40–66. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xu, C.; Ji, L.; Feng, J.; Li, F.; Zhou, X.; Fang, F. Effects of multi-cropping system on temporal and spatial distribution of carbon and nitrogen footprint of major crops in China. Glob. Ecol. Conserv. 2020, 22, e00895. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Pan, G.; Li, L.; Wu, L.; Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Global Chang. Biol. 2004, 10, 79–92. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.; Han, B.; Ouyang, Z.; Duan, X.; Zheng, H.; Miao, H. Soil carbon sequestrations by nitrogen fertilizer application, straw return and no-tillage in China’s cropland. Glob. Chang. Biol. 2009, 15, 281–305. [Google Scholar] [CrossRef]
- Buysse, P.; Roisin, C.; Aubinet, M. Fifty years of contrasted residue management of an agricultural crop: Impacts on the soil carbon budget and on soil heterotrophic respiration. Agri. Ecosyst. Environ. 2013, 167, 52–59. [Google Scholar] [CrossRef]
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S.; Chareonwong, U.; Wang, C. Assessment of climate change impact on rice yield and water footprint of large-scale and individual farming in Thailand. Sci. Total Environ. 2020, 726, 137864. [Google Scholar] [CrossRef] [PubMed]
- Wachter, J.M.; Reganold, J.P. Organic agricultural production: Plants. Encycl. Agr. Food Syst. 2014, 265–286. [Google Scholar]
- Willer, H.; Schlatter, B.; Trávníček, J.; Kemper, L.; Lernoud, J. The World of Organic Agriculture—Statistics and Emerging Trends 2020; Research Institute of Organic Agriculture (FiBL): Frick, Switzerland; IFOAM—Organics International: Bonn, Germany, 2020. [Google Scholar]
- Pongsrihadulchai, A. 2018. Thailand’s Rice Industry and Current Policies Towards High Value Rice Products. In Proceedings of the International Seminar on Promoting Rice Farmers’ Market Through Value-Adding Activities, Bangkok, Thailand, 6–7 June 2018; Food and Fertilizer Technology Center: Taipei, Taiwan. Available online: https://ap.fftc.org.tw/article/1297 (accessed on 20 August 2021).
- Ostle, N.J.; Levy, P.E.; Evans, C.D.; Smith, P. UK land use and soil carbon sequestration. Land Use Pol. 2009, 26, S274–S283. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Chai, Q.; Lemke, R.L.; Campbell, C.A.; Zentner, R.P. Improving farming practices reduces the carbon footprint of spring wheat production. Nat. Commun. 2014, 5, 5012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adewale, C.; Reganold, J.P.; Higgins, S.; Evans, R.D.; Carpenter-Boggs, L. Improving carbon footprinting of agricultural systems: Boundaries, tiers, and organic farming. Environ. Impact Assess. Rev. 2018, 71, 41–48. [Google Scholar] [CrossRef]
- Alam, M.K.; Bell, R.W.; Biswas, W.K. Decreasing the carbon footprint of an intensive rice-based cropping system using conservation agriculture on the Eastern Gangetic Plains. J. Clean. Prod. 2019, 218, 259–272. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. In Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- National Soil Survey Center. Soil Survey Laboratory Methods Manual. In Soil Survey Investigations Report No. 42, Version 3.0; Natural Conservation Service: Washington, DC, USA, 1996. [Google Scholar]
- Bray, R.A.; Kurtz, L.T. Determination of total organic and available form of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Thomas, G.W. Method of Soil Analysis, Part 3: Chemical Methods. In Soil pH and Soil Acidity; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Walkley, A.; Black, J.A. An examination of the dichromate method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- IPCC (Intergovernmental Panel on Climate Change). 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basic Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- The National Technical Committee on Product Carbon Footprinting. The National Guideline on Product Carbon Footprint, 3rd ed.; Amarin Publishing: Bangkok, Thailand, 2011. [Google Scholar]
- TGO (Thailand Greenhouse Gas Management Organization). Product Category Rules (PCRS) of Rice in Thailand. 2014. Available online: http://thaicarbonlabel.tgo.or.th/PCR/A4.pdf (accessed on 20 September 2018).
- EPA. Emission Factors for Greenhouse Gas Inventories; United States Environmental Protection Agency: Washington, DC, USA, 2014. Available online: https://www.epa.gov/sites/production/files/2015-07/documents/emission-factors_2014.pdf (accessed on 18 May 2016).
- The National Technical Committee on Product Carbon Footprinting. National Guideline on Product National Guideline on Product Carbon Footprint; Amarin Publishing: Bangkok, Thailand, 2015. [Google Scholar]
- Ecoinvent Centre. Ecoinvent Database v.3.2; Swiss Centre for Life Cycle Inventories: Duebendorf, Switzerland, 2015. Available online: http://www.ecoinvent.org/ (accessed on 25 June 2016).
- Maciel, V.G.; Zortea, R.B.; da Silva, W.M.; Cybis, L.F.A.; Einloft, S.; Seferin, M. Life Cycle Inventory for the agricultural stages of soybean production in the state of Rio Grande do Sul. Braz. J. Clean. Prod. 2015, 93, 65–74. [Google Scholar] [CrossRef]
- Gu, B.; Leach, A.M.; Ma, L.; Galloway, J.N.; Chang, S.X.; Ge, Y. Nitrogen footprint in China: Food, energy, and nonfood goods. Environ. Sci. Technol. 2013, 47, 9217–9224. [Google Scholar] [CrossRef] [PubMed]
- ISO 14044; Environmental Management e Life Cycle Assessment e Requirements and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006.
- Guinée, J.B.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; de Koning, A.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Udo de Haes, H.; et al. Life Cycle Assessment: An Operational Guide to the ISO Standards; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Allan, J.A. Virtual water: A strategic resource global solutions to regional deficits. Ground Water 1998, 36, 545–546. [Google Scholar] [CrossRef]
- Hoekstra, A.Y.; Hung, P.Q. Virtual water trade. A quantification of virtual water flows between nations in relation to international crop trade. Value Water Res. Rep. Ser. 2002, 11, 166. [Google Scholar]
- Hoekstra, A.Y.; Chapagain, A.K. Globalization of Water: Sharing the Planet’s Freshwater Resources; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. Global gray water footprint and water pollution levels related to anthropogenic nitrogen loads to fresh water. Environ. Sci. Technol. 2015, 49, 12860–12868. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. Agriculture, Forestry and Other Land Use. In IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Pollution Control Department. The Quality of Surface Water Standard; Ministry of Natural Resources and Environment: Bangkok, Thailand, 1994. (In Thai). Available online: http://infofile.pcd.go.th/law/3_14_water.pdf (accessed on 7 September 2019).
- Yang, X.; Gao, W.; Zhang, M.; Chen, Y.; Sui, P. Reducing agricultural carbon footprint through diversified crop rotation systems in the North China Plain. J. Clean. Prod. 2014, 76, 131–139. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Xie, J.; Luo, Z.; Zhang, R.; Cai, L.; Coulter, J.A.; Palta, J.A. Managing the trade-offs among yield, economic benefits and carbon and nitrogen footprints of wheat cropping in a semi-arid region of China. Sci. Total Environ. 2021, 768, 145280. [Google Scholar] [CrossRef]
- Bennett, M.; Franzell, S. Can organic and resource-conserving agriculture improve livelihood. Int. J. Agric. Sustain. 2013, 11, 193–215. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, T.; Ning, Z.; Xiao, E.; Sun, W. Microbial community analysis in rice paddy soils irrigated by acid mine drainage contaminated water. Appl. Microbiol. Biotechnol. 2015, 99, 2911–2922. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. Microbiota Biofertil. 2021, 2, 1–20. [Google Scholar]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Rukshana, F.; Butterly, C.R.; Xu, J.-M.; Baldock, J.A.; Tang, C. Organic anion-to- acid ratio influences pH change of soils differing in initial pH. Int. J. Soil Sediment Water 2013, 14, 407–414. [Google Scholar] [CrossRef]
- Gathala, M.K.; Ladha, J.K.; Saharawat, Y.S.; Kumar, V.; Kumar, V.; Sharma, P.K. Effect of tillage and crop establishment methods on physical properties of a medium-textured soil under a seven-year Rice-Wheat rotation. Soil Sci. Soc. Am. J. 2011, 75, 1851–1862. [Google Scholar] [CrossRef]
- Gelybó, G.; Tóth, E.; Farkas, C.; Horel, A.; Kása, I.; Bakacsi, Z. Potential impacts of climate change on soil properties. Agrokem. Talajt. 2018, 67, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Jat, H.S.; Datta, A.; Sharma, P.C.; Kumar, V.; Yadav, A.K.; Choudhary, M.; Choudhary, V.; Gathala, M.K.; Sharma, D.K.; Jat, M.L.; et al. Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arch. Agron. Soil Sci. 2018, 64, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, Y.; Yang, X.; Cui, J.; Sui, P. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ. Sci. Pollut. Res. 2017, 24, 24019–24028. [Google Scholar] [CrossRef]
- Hiederer, R.; Köchy, M. Global Soil Organic Carbon Estimates and the Harmonized World Soil Database; Publication Office of the European Union: Brussels, Belgium, 2011; Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/Other/EUR25225.pdf (accessed on 2 July 2021).
- Zdruli, P.; Lal, R.; Cherlet, M.; Kapur, S. New World Atlas of Desertification and Issues of Carbon Sequestration, Organic Carbon Stocks, Nutrient Depletion and Implications for Food Security. In Carbon Management, Technologies, and Trends in Mediterranean Ecosystems; Erşahin, S., Kapur, S., Akça, E., Namlı, A., Erdoğan, H.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 13–25. [Google Scholar]
- Adamu, H.; Umar, Y.A.; Akanang, H.; Sabo, A. Evaluation of Carbon Sequestration Potential of Soils―What Is Missing? J. Geosci. Environ. Prot. 2021, 9, 39–47. [Google Scholar] [CrossRef]
- Pan, G.; Xu, X.; Smith, P.; Pan, W.; Lal, R. An increase in topsoil SOC stock of China’s croplands between 1985 and 2006 revealed by soil monitoring. Agr. Ecosyst. Environ. 2010, 136, 133–138. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon sequestration in agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Knudsen, M.T.; Meyer-Aurich, A.; Olesen, J.E.; Chirinda, N.; Hermansen, J.E. Carbon footprints of crops from organic and conventional arable crop rotationse using a life cycle assessment approach. J. Clean. Prod. 2014, 64, 609–618. [Google Scholar] [CrossRef]
- Ghimire, R.; Lamichhane, S.; Acharya, B.S.; Bista, P.; Sainju, U.M. Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: A review. J. Integr. Agric. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Smith, P. Soil Organic Carbon Dynamics and Land-Use Change. In Land Use and Soil Resources; Braimoh, A.K., Vlek, P.L.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 9–22. [Google Scholar]
- Paustian, K.; Andrén, O.; Janzen, H.H.; Lal, R.; Smith, P.; Tian, G.; Tiessen, H.; van Noordwijk, M.; Woomer, P.L. Agricultural soils as a sink to mitigate CO2 emissions. Soil Use Manag. 1997, 13, 229–244. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Gas Inventories: Workbook (Revised 1996); Intergovernmental Panel on Climate Change: Paris, France, 1997. [Google Scholar]
- Smith, P.; Powlson, D.S. Sustainability of soil management practices: A global perspective. In Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture; Abbott, L.K., Murphy, D.V., Eds.; Kluwer Academic Publishers: Dodrecht, The Netherlands, 2003; pp. 241–254. [Google Scholar]
- Mohammadi, A.; Omid, M. Economical analysis and relation between energy inputs and yield of greenhouse cucumber production in Iran. Appl. Energy 2010, 87, 191–196. [Google Scholar] [CrossRef]
- Banaeian, N.; Namdari, M. Effect of ownership energy use efficiency in watermelon farms–A data envelopment analysis approach. Int. J. Renew. Energy Res. 2011, 1, 75–82. [Google Scholar]
- Khoshnevisan, B.; Rafiee, S.; Omid, M.; Mousazadeh, H. Reduction of CO2 emission by improving energy use efficiency of greenhouse cucumber production using DEA approach. Energy 2013, 55, 676–682. [Google Scholar] [CrossRef]
- Zhang, W.; Dou, Z.; He, P.; Ju, X.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.; Zhang, Y.; Wu, L. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.K. Methane emission and rice agriculture. Curr. Sci. 2001, 81, 345–346. [Google Scholar]
- Yu, K.; Böhme, F.; Rinklebe, J.; Neue, H.U.; Delaune, R.D. Major biogeochemical processes in soils-a microcosm incubation from reducing to oxidizing conditions. Soil Sci. Soc. Am. J. 2007, 71, 1406–1417. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Akiyama, H.; Yagi, K.; Akimoto, H. Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 Intergovernmental Panel on Climate Change Guidelines. Glob. Biogeochem. Cycles 2009, 23, GB2002. [Google Scholar] [CrossRef]
- Alam, M.K.; Biswas, W.K.; Bell, R.W. Greenhouse gas implications of novel and conventional rice production technologies in the Eastern-Gangetic plains. J. Clean. Prod. 2016, 112, 3977–3987. [Google Scholar] [CrossRef]
- Bacenetti, J.; Fusi, A.; Negri, M.; Fiala, M. Impact of cropping system and soil tillage on environmental performance of cereal silage productions. J. Clean. Prod. 2015, 86, 49–59. [Google Scholar] [CrossRef]
- Kanter, D.R.; Searchinger, T.D. A technology-forcing approach to reduce nitrogen pollution. Nat. Sustain. 2018, 1, 544–552. [Google Scholar] [CrossRef]
- Fan, D.J.; Zhang, H.R.; Liu, T.Q.; Cougui, C.; Chengfeng, L. Control effects of Chelonus munakatae against Chilo suppressalis and impact on greenhouse gas emissions from paddy fields. Front. Plant Sci. 2020, 11, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunrat, N.; Pumijumnong, N. Practices for reducing greenhouse gas emissions from rice production in Northeast Thailand. Agriculture 2017, 7, 4. [Google Scholar]
- Champrasert, P.; Sampattagul, S.; Yodkhum, S.; Wangpakapattanawong, P. Assessment of carbon footprint of upland rice production in Northern Thailand. CMU J. Nat. Sci. 2020, 19, 427–446. [Google Scholar] [CrossRef]
- Thanawong, K.; Perret, S.R.; Basset-Mens, C. Eco-efficiency of paddy rice production in Northeastern Thailand: A comparison of rain-fed and irrigated cropping systems. J. Clean. Prod. 2014, 73, 204–217. [Google Scholar] [CrossRef]
- Yodkhum, S.; Gheewala, S.H.; Sampattagul, S. Life cycle GHG evaluation of organic rice production in northern Thailand. J. Environ. Manag. 2017, 196, 217–223. [Google Scholar] [CrossRef]
- Yodkhum, S.; Sampattagul, S.; Gheewala, S.H. Energy and environmental impact analysis of rice cultivation and straw management in northern Thailand. Environ. Sci. Pollut. Res. 2018, 25, 17654–17664. [Google Scholar] [CrossRef]
- Mungkung, R.; Sitthikitpanya, S.; Dangsiri, S.; Gheewala, S.H. Life cycle assessment of Thai Hom Mali rice to support the policy decision on organic farming area expansion. Sustainability 2020, 12, 6003. [Google Scholar] [CrossRef]
- Petersen, B.M.; Knudsen, M.T.; Hermansen, J.E.; Halberg, N. An approach to include soil carbon changes in life cycle assessments. J. Clean. Prod. 2013, 52, 217–224. [Google Scholar] [CrossRef]
- Goglio, P.; Smith, W.N.; Grant, B.B.; Desjardins, R.L.; McConkey, B.G.; Campbell, C.A.; Nemecek, T. Accounting for soil carbon changes in agricultural life cycle assessment (LCA): A review. J. Clean. Prod. 2015, 104, 23–39. [Google Scholar] [CrossRef]
- Alam, M.K.; Bell, R.W.; Haque, M.E.; Kader, M.A. Minimal soil disturbance and increased residue retention increase soil carbon in rice-based cropping systems on the Eastern Gangetic Plain. Soil Till. Res. 2018, 183, 28–41. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhong, Y.M.; Yang, J.P.; Wu, Y.X.Y.; Hui, L.; Zheng, L. Effect of nitrogen fertilizer rates on carbon footprint and ecosystem service of carbon sequestration in rice production. Sci. Total Environ. 2019, 670, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Yang, H.S.; Liu, J.; Wu, J.; Chen, W.; Wu, J.; Zhu, L.; Bian, X. Effects of ditch-buried straw return on soil organic carbon and rice yields in a rice-wheat rotation system. Catena 2015, 127, 56–63. [Google Scholar] [CrossRef]
- Zhang, J.; Bei, S.; Li, B.; Zhang, J.; Christie, P.; Li, X. Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Appl. Soil Ecol. 2019, 136, 67–79. [Google Scholar] [CrossRef]
- Xue, X.B.; Landis, A.E. Eutrophication potential of food consumption patterns. Environ. Sci. Technol. 2010, 44, 6450–6456. [Google Scholar] [CrossRef] [PubMed]
- Pierer, M.; Winiwarter, W.; Leach, A.M.; Galloway, J.N. The nitrogen footprint of food products and general consumption patterns in Austria. Food Policy 2014, 49, 128–136. [Google Scholar] [CrossRef]
- Xue, J.F.; Pu, C.; Liu, S.L. Carbon and nitrogen footprint of double rice production in Southern China. Ecol. Indicat. 2016, 64, 249–257. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Chapagain, A.K.; Hoekstra, A.Y. The Green, Blue and Grey Water Footprint of Rice from Both a Production and Consumption Perspective. In Value of Water Research Report Series No. 40; IHE Institute for Water Education: Delft, The Netherlands, 2010. [Google Scholar]
- Johannes, H.P.; Priadi, C.R.; Herdiansyah, H.; Novalia, I. Water Footprint Saving through Organic Rice Commodity; AIP Publishing: College Park, MD, USA, 2020. [Google Scholar]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Benbi, D.K. Carbon footprint and agricultural sustainability nexus in an intensively cultivated region of Indo-Gangetic Plains. Sci. Total Environ. 2018, 644, 611–623. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef] [Green Version]

| Input | Unit | Quantity | |
|---|---|---|---|
| Organic Rice Farming | Conventional Rice Farming | ||
| Rice seeds | kg ha−1 crop−1 | 62.5 ± 31.3a | 93.8 ± 31.3b |
| Gasoline | L ha−1 crop−1 | 62.5 ± 18.8a | 125.0 ± 31.3b |
| Diesel | L ha−1 crop−1 | 125.0 ± 31.3a | 156.3 ± 18.8a |
| Straw | kg ha−1 crop−1 | 3375.0 ± 1250.0a | 3100.0 ± 1250.0a |
| Bio-fermented juice * | kg ha−1 crop−1 | 312.5 ± 125.0 | - |
| Fertilizer 16-20-0 | kg ha−1 crop−1 | - | 156.3 ± 62.5 |
| Fertilizer 46-0-0 | kg ha−1 crop−1 | - | 125.0 ± 31.3 |
| Glyphosate 48% w/v SL | L ha−1 crop−1 | - | 218.8 ± 31.3 (0.75 L (a.i) ha−1) |
| Alachlor 48% w/v EC | L ha−1 crop−1 | - | 187.5 ± 31.3 (0.56 L (a.i) ha−1) |
| Acephate 75% S | L ha−1 crop−1 | - | 406.3 ± 62.5 (0.41 kg (a.i) ha−1) |
| Chlorpyrifos 40% EC | L ha−1 crop−1 | - | 375.0 ± 62.5 (0.75 L (a.i) ha−1) |
| Organic Rice Farming | Conventional Rice Farming | |||||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2018 | 2019 | 2020 | 2021 | |
| pH (1:2.5) | 5.61 ± 0.31a | 5.38 ± 0.56a | 5.59 ± 0.54a | 5.65 ± 0.52a | 5.53 ± 0.42a | 5.03 ± 0.37a | 5.48 ± 0.61a | 5.54 ± 0.57a |
| BD (g cm−3) | 1.36 ± 0.28a | 1.36 ± 0.25a | 1.35 ± 0.41a | 1.36 ± 0.55a | 1.40 ± 0.32a | 1.37 ± 0.35a | 1.40 ± 0.46a | 1.38 ± 0.43a |
| OM (%) | 3.16 ± 0.14a | 3.18 ± 0.17a | 3.20 ± 0.16a | 3.20 ± 0.17a | 2.75 ± 0.15b | 2.80 ± 0.14b | 2.82 ± 0.15b | 2.81 ± 0.13b |
| ECe (dS m−1) | 0.49 ± 0.01a | 0.57 ± 0.01a | 0.25 ± 0.03a | 0.37 ± 0.02a | 0.36 ± 0.02b | 0.42 ± 0.03b | 0.30 ± 0.02a | 0.41 ± 0.02a |
| Avail. P (mg kg−1) | 13.01 ± 8.32a | 15.06 ± 12.10a | 17.57 ± 15.32a | 18.6 ± 10.51a | 11.69 ± 4.32a | 15.34 ± 17.54a | 18.96 ± 21.07a | 13.65 ± 9.65a |
| Avail. K (mg kg−1) | 142.98 ± 31.20a | 151.54 ± 12.63a | 184.97 ± 27.50a | 176.5 ± 19.54a | 164.96 ± 33.8a | 188.53 ± 15.07a | 107.96 ± 21.18b | 157.3 ± 25.01a |
| Avail. Ca (mg kg−1) | 1897.06 ± 613.5a | 1965.45 ± 498.3a | 2872.59 ± 572.3a | 2373.34 ± 315.3a | 3770.40 ± 743.2b | 3653.13 ± 631.4b | 1564.00 ± 287.56b | 2567.40 ± 267.4a |
| Avail. Mg (mg kg−1) | 138.14 ± 54.3a | 157.89 ± 48.6a | 178.93 ± 74.2a | 168.9 ± 39.6a | 216.38 ± 61.5b | 274.65 ± 71.3b | 100.31 ± 49.5b | 218.5 ± 55.2a |
| Sand (%) | 9.8a ± 3.75a | 10.1 ± 3.60a | 8.5 ± 3.11a | 9.61 ± 4.21a | 12.3 ± 4.07a | 15.3 ± 4.31a | 14.3 ± 4.25a | 16.3 ± 6.72a |
| Silt (%) | 44.9 ± 10.43a | 47.3 ± 9.11a | 46.2 ± 8.75a | 43.77 ± 7.85a | 44.6 ± 8.54a | 43.9 ± 9.23a | 44.2 ± 8.06a | 43.3 ± 7.91a |
| Clay (%) | 45.3 ± 6.21a | 42.6 ± 4.98a | 45.3 ± 7.61a | 46.62 ± 6.99a | 43.1 ± 5.55a | 40.8 ± 5.08a | 41.5 ± 9.43a | 40.4 ± 7.03a |
| Soil Texture | Silty Clay | Silty Clay | Silty Clay | Silty Clay | Silty Clay | Silty Clay | Silty Clay | Silty Clay |
| Practice | SOC 2018 (kg C ha−1) | SOC 2021 (kg C ha−1) | ΔSOCS (kg C ha−1 year−1) | ΔSOCS (kg CO2eq ha−1 year−1) |
|---|---|---|---|---|
| Organic rice farming | 74,784.2a | 75,226.0a | 147.3 | 539.9 |
| Conventional rice farming | 66,995.4a | 67,254.2a | 86.3 | 316.3 |
| Life Cycle Stage | Organic Rice Farming | Conventional Rice Farming | |
|---|---|---|---|
| Raw material production (kg CO2eq ha−1 year−1) | Seeds | 15.6 ± 0.3a | 23.5 ± 0.5b |
| Gasoline | 21.3 ± 0.7a | 42.6 ± 0.7b | |
| Diesel | 48.3 ± 1.2a | 60.4 ± 1.9b | |
| Bio-fermented juice | 79.8 ± 5.1 | 0 | |
| Chemical fertilizers | 0 | 884.2 ± 221.1 | |
| Herbicides | 0 | 72.6 ± 9.5 | |
| Pesticides | 0 | 48.8 ± 9.8 | |
| Field emission (kg CO2eq ha−1 year−1) | Gasoline | 144.9 ± 4.6a | 289.9 ± 4.6b |
| Diesel | 343.1 ± 8.2a | 428.8 ± 13.7b | |
| Herbicides | 0 | 100.6 ± 15.1 | |
| Pesticides | 0 | 31.9 ± 6.4 | |
| CH4 | 2932.2 ± 1570.0a | 2876.8 ± 1684.5a | |
| direct N2O | 122.5 ± 17.9a | 256.8 ± 43.4b | |
| Harvesting | 121.3 ± 15.2a | 121.3 ± 15.2a | |
| Total GHG (kg CO2eq ha−1 year−1) | 3829.0 ± 1623.1a | 5238.0 ± 2026.1b | |
| Net GHG emissions (kg CO2eq ha−1 year−1) | 3289.1 ± 1085.2a | 4921.7 ± 1254.8b | |
| Yield (kg ha−1 year−1) | 2812.5 ± 625.0a | 5312.5 ± 750.0b | |
| CF intensity (kg CO2eq kg−1 rice yield) | 1.17 ± 0.78a | 0.93 ± 0.64b | |
| Practice | Nitrogen Footprint (kg Neq ha−1 Year−1) | Total (kg Neq ha−1 Year−1) | NF Intensity (kg Neq kg−1 Rice Yield) | ||||
|---|---|---|---|---|---|---|---|
| Agricultural Inputs | N2O | NH3 | NO3− | NH4+ | |||
| Organic rice farming | 0.014 ± 0.010a | 2.11 ± 1.1a | 322.4 ± 167.8a | 303.2 ± 104.1a | 323.1 ± 112.0a | 950.9 ± 378.8a | 0.34 ± 0.21a |
| Conventional rice farming | 173.3 ± 25.2b | 140.8 ± 45.2b | 21,448.5 ± 2105.6b | 20,167.6 ± 1780.6b | 21,492.2 ± 1967.5b | 63,422.5 ± 7866.3b | 11.94 ± 5.3b |
| Practice | Total Cost (THB ha−1 Year−1) | Total Revenue (THB ha−1 Year−1) | Net Economic Return (THB ha−1 Year−1) | CF Per Net Return (kg CO2eq THB−1 Year−1) | NF Per Net Return (kg Neq THB−1 Year−1) | WF Per Net Return (m3 THB−1 Year−1) |
|---|---|---|---|---|---|---|
| Organic rice farming | 10,420.0 ± 8125.0a | 45,000.0 ± 9375.0a | 34,580 ± 9375.0a | 0.09 ± 0.03a | 0.03 ± 0.02a | 98.9 ± 41.34a |
| Conventional rice farming | 21,612.8 ± 13,625.0b | 39,843.8 ± 12,500.0b | 18,231 ± 12,500.0b | 0.27 ± 0.08b | 3.48 ± 1.88b | 428.4 ± 253.7b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunrat, N.; Sereenonchai, S.; Chaowiwat, W.; Wang, C.; Hatano, R. Carbon, Nitrogen and Water Footprints of Organic Rice and Conventional Rice Production over 4 Years of Cultivation: A Case Study in the Lower North of Thailand. Agronomy 2022, 12, 380. https://doi.org/10.3390/agronomy12020380
Arunrat N, Sereenonchai S, Chaowiwat W, Wang C, Hatano R. Carbon, Nitrogen and Water Footprints of Organic Rice and Conventional Rice Production over 4 Years of Cultivation: A Case Study in the Lower North of Thailand. Agronomy. 2022; 12(2):380. https://doi.org/10.3390/agronomy12020380
Chicago/Turabian StyleArunrat, Noppol, Sukanya Sereenonchai, Winai Chaowiwat, Can Wang, and Ryusuke Hatano. 2022. "Carbon, Nitrogen and Water Footprints of Organic Rice and Conventional Rice Production over 4 Years of Cultivation: A Case Study in the Lower North of Thailand" Agronomy 12, no. 2: 380. https://doi.org/10.3390/agronomy12020380







