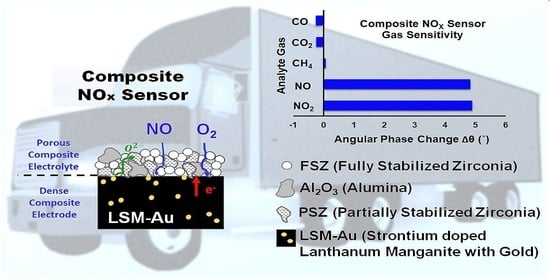
Investigation of an Impedimetric LaSrMnO3-Au/Y2O3-ZrO2-Al2O3 Composite NOx Sensor
Abstract
:1. Introduction
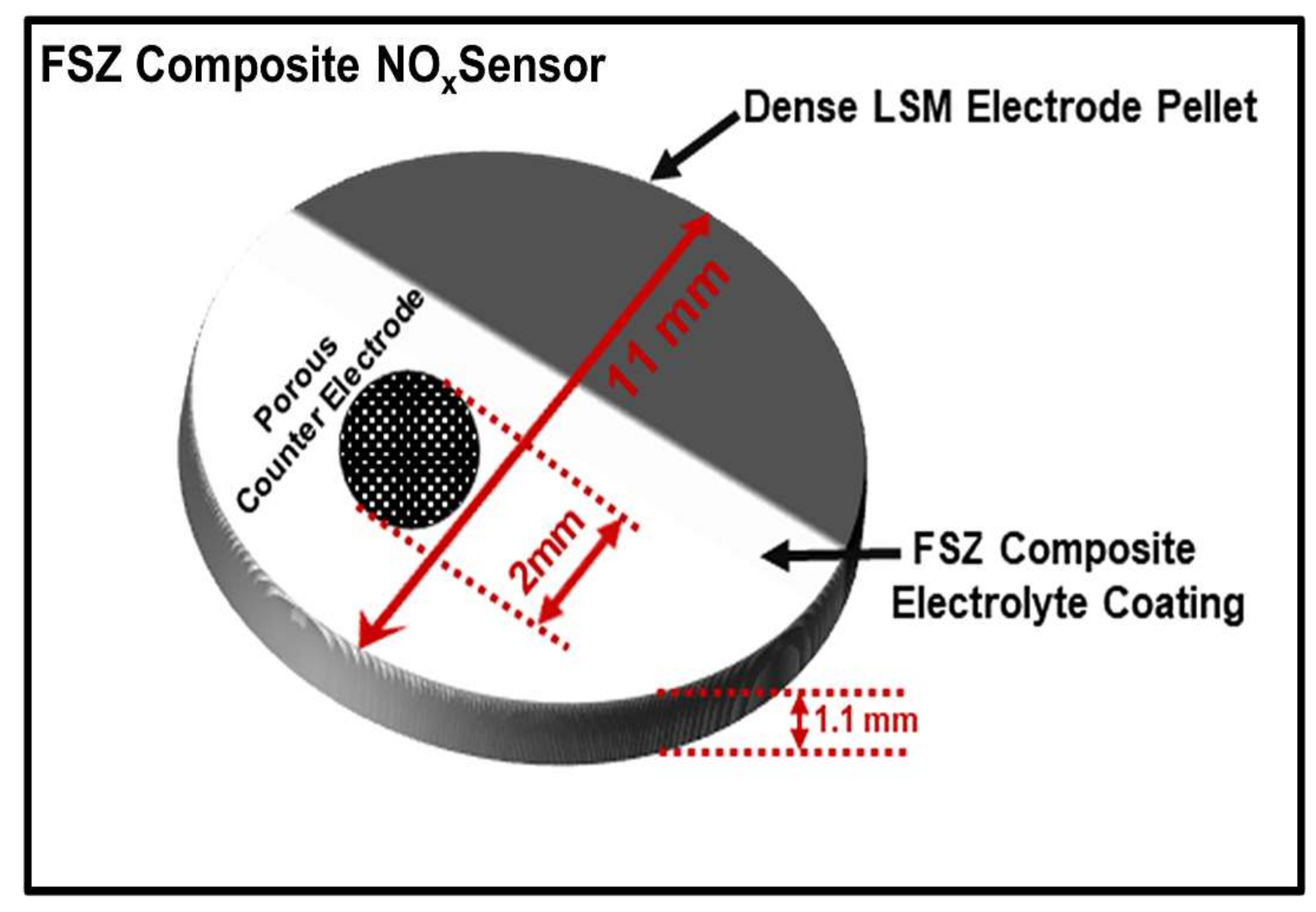
2. Experimental
3. Results and Discussion
3.1. Microstructure and Morphology of NOx Sensors
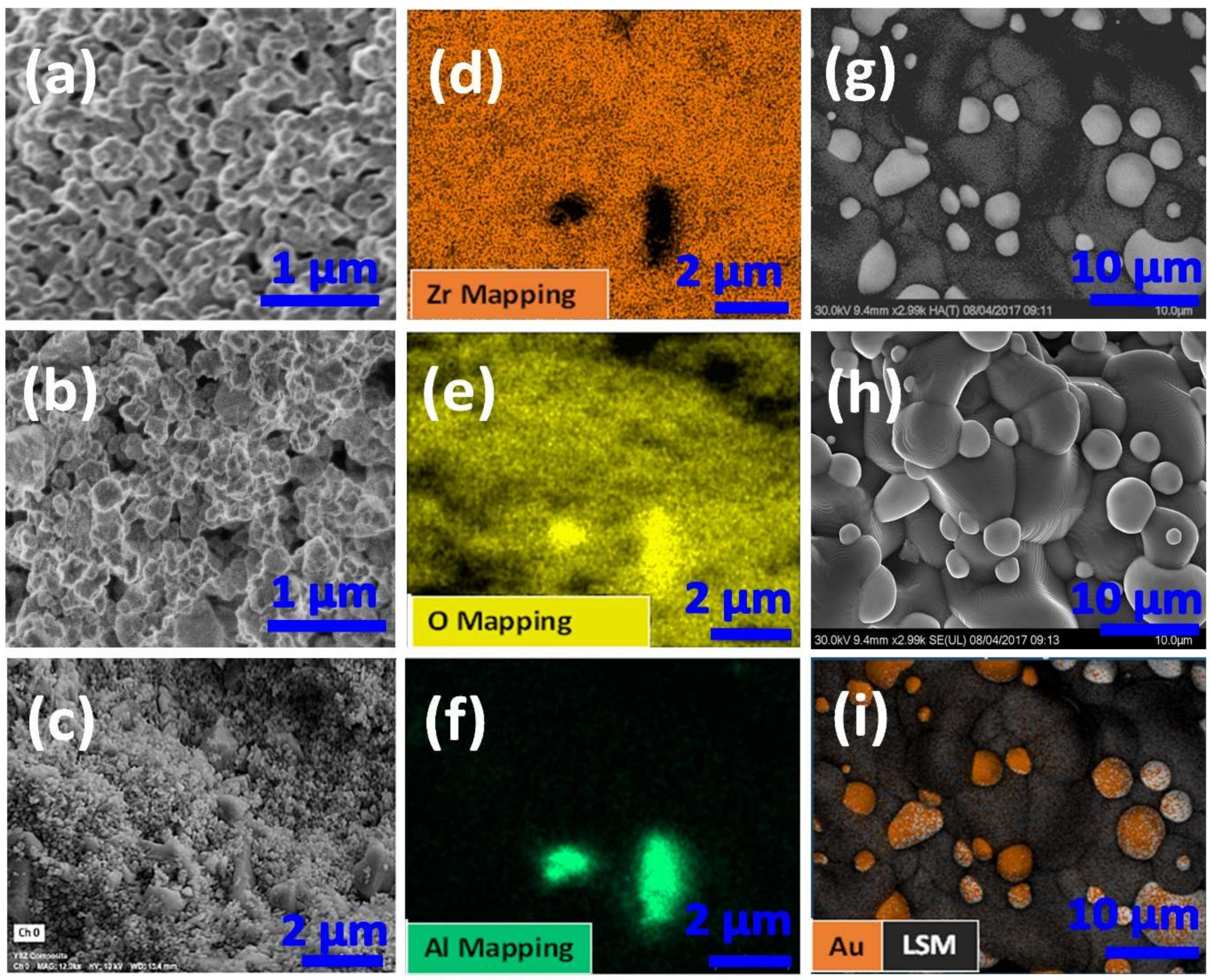
3.1.1. Surface Structure and Elemental Mapping via SEM and EDS
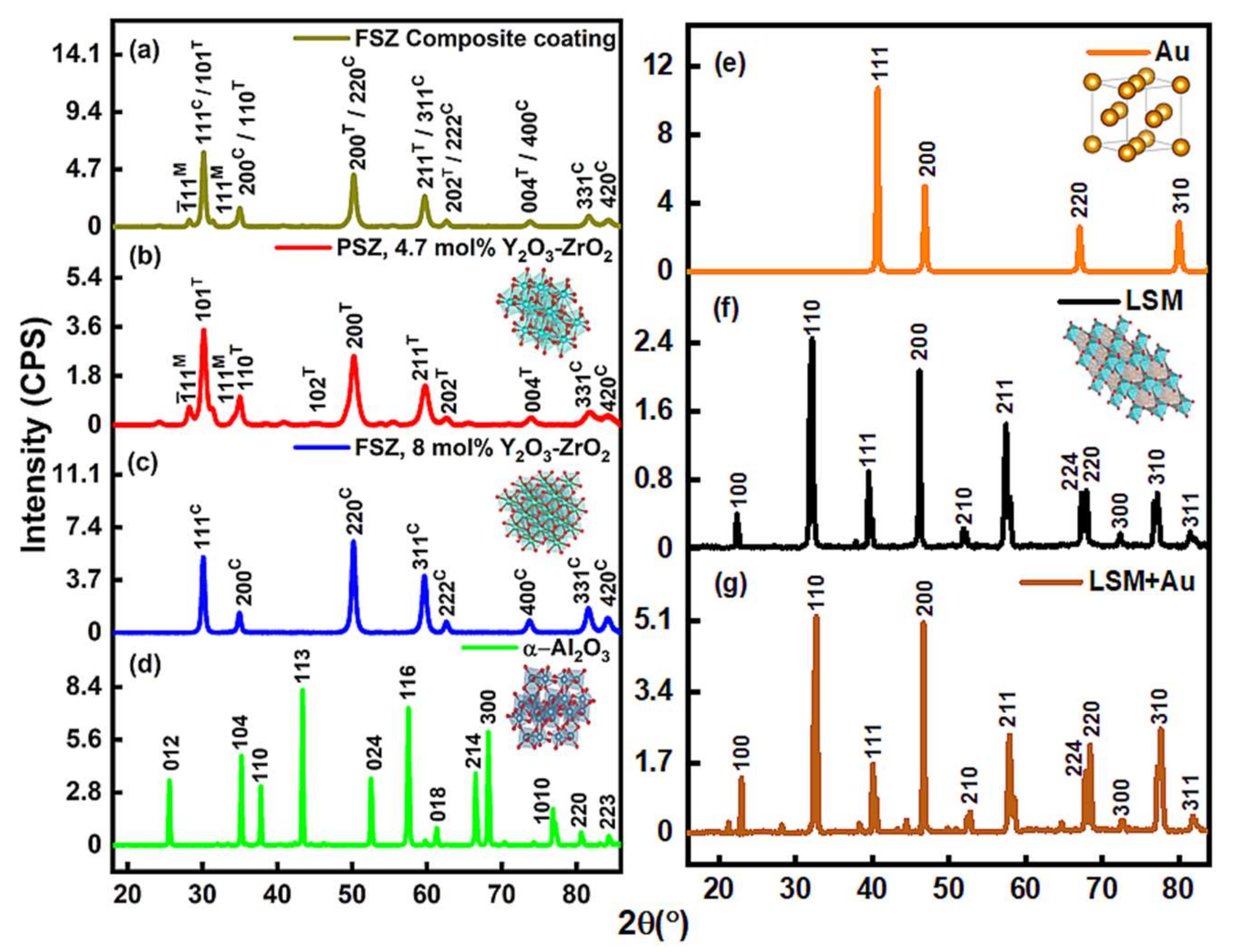
3.1.2. Defect Estimation and Structural Phase Behavior via XRD
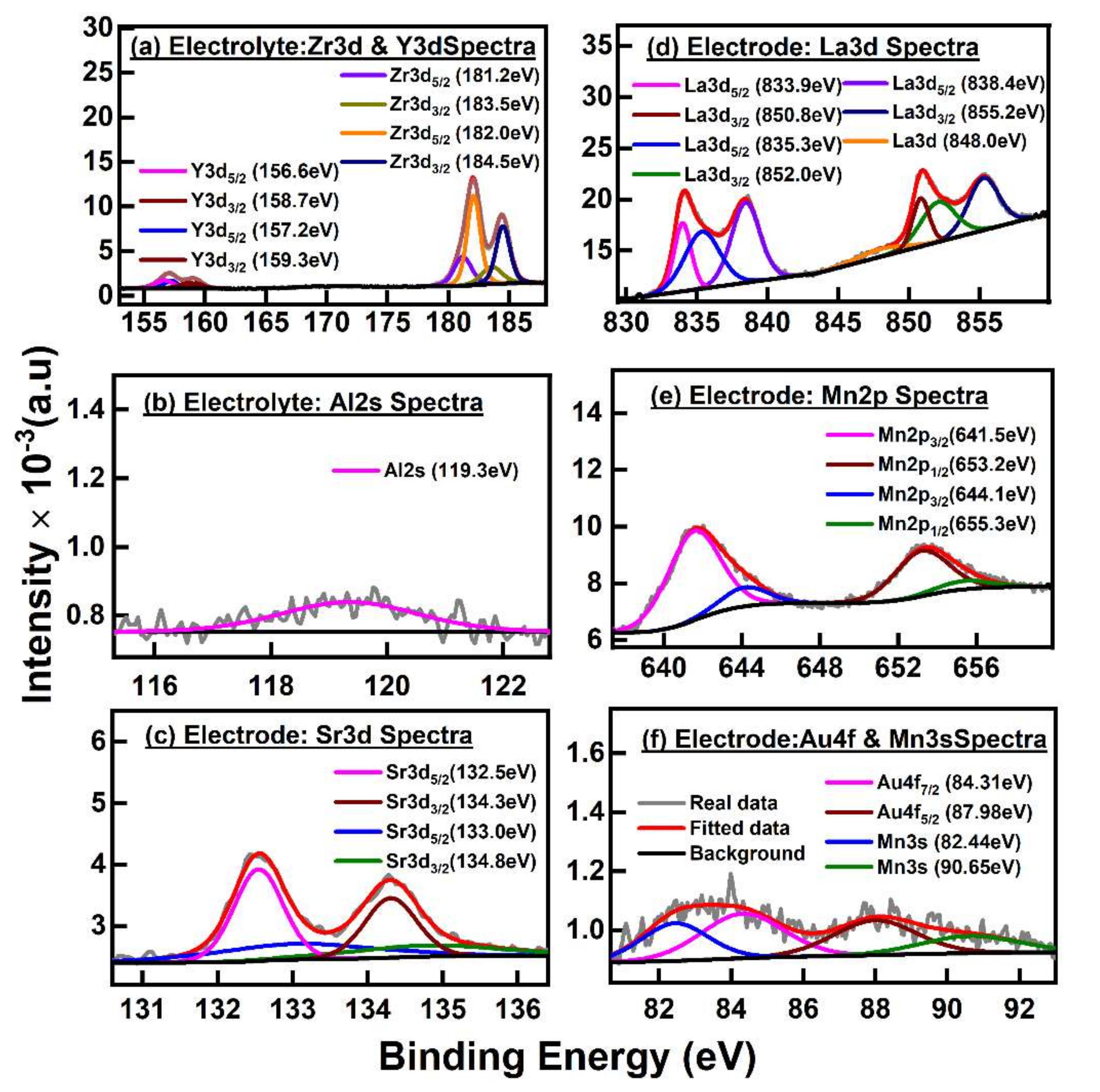
3.1.3. Estimating the Chemical State of Structural Elements via XPS
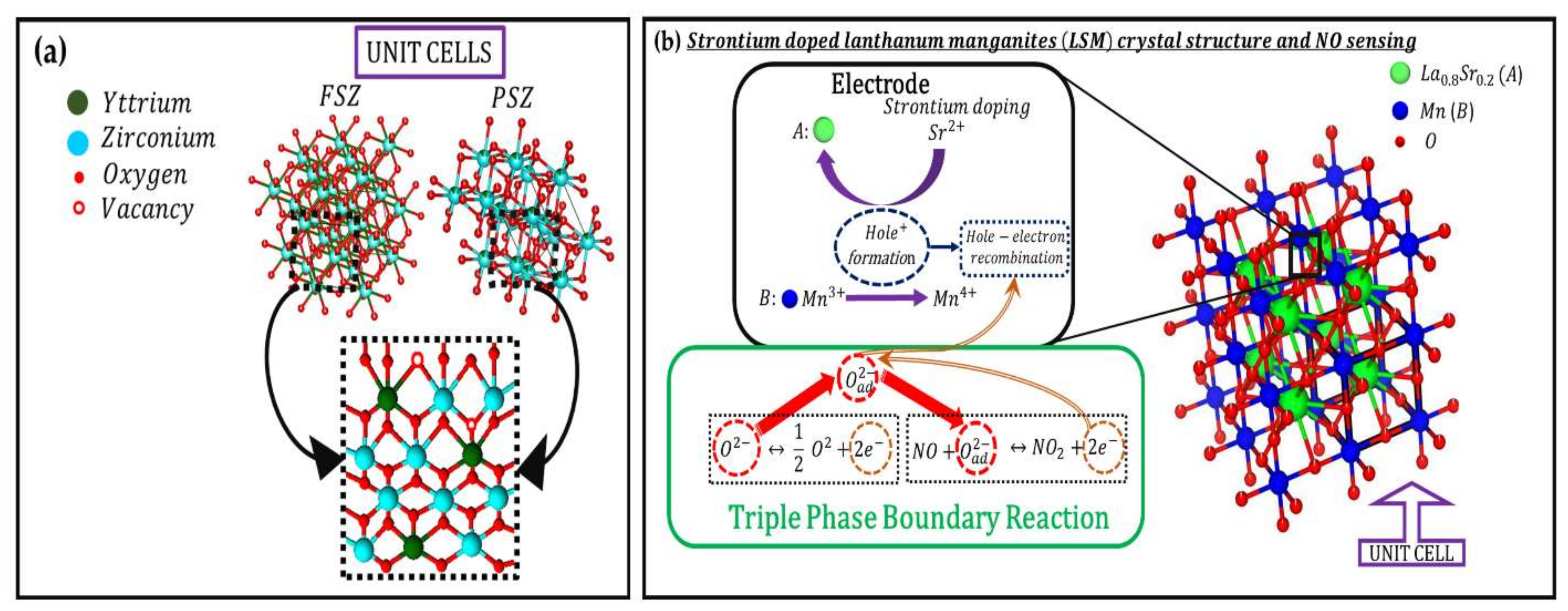
3.2. Working Principle for FSZ Composite NOx Sensor
3.2.1. Oxygen Ion Transport through the Porous FSZ Composite Electrolyte
3.2.2. Charge Transport at LSM-Au Electrode and NOx Sensing Mechanism
3.2.3. Operating Mechanism of Solid-State Electrochemical FSZ Composite NOx Sensor
Electrochemical Behavior LSM-Au/FSZ Composite NOx Sensor
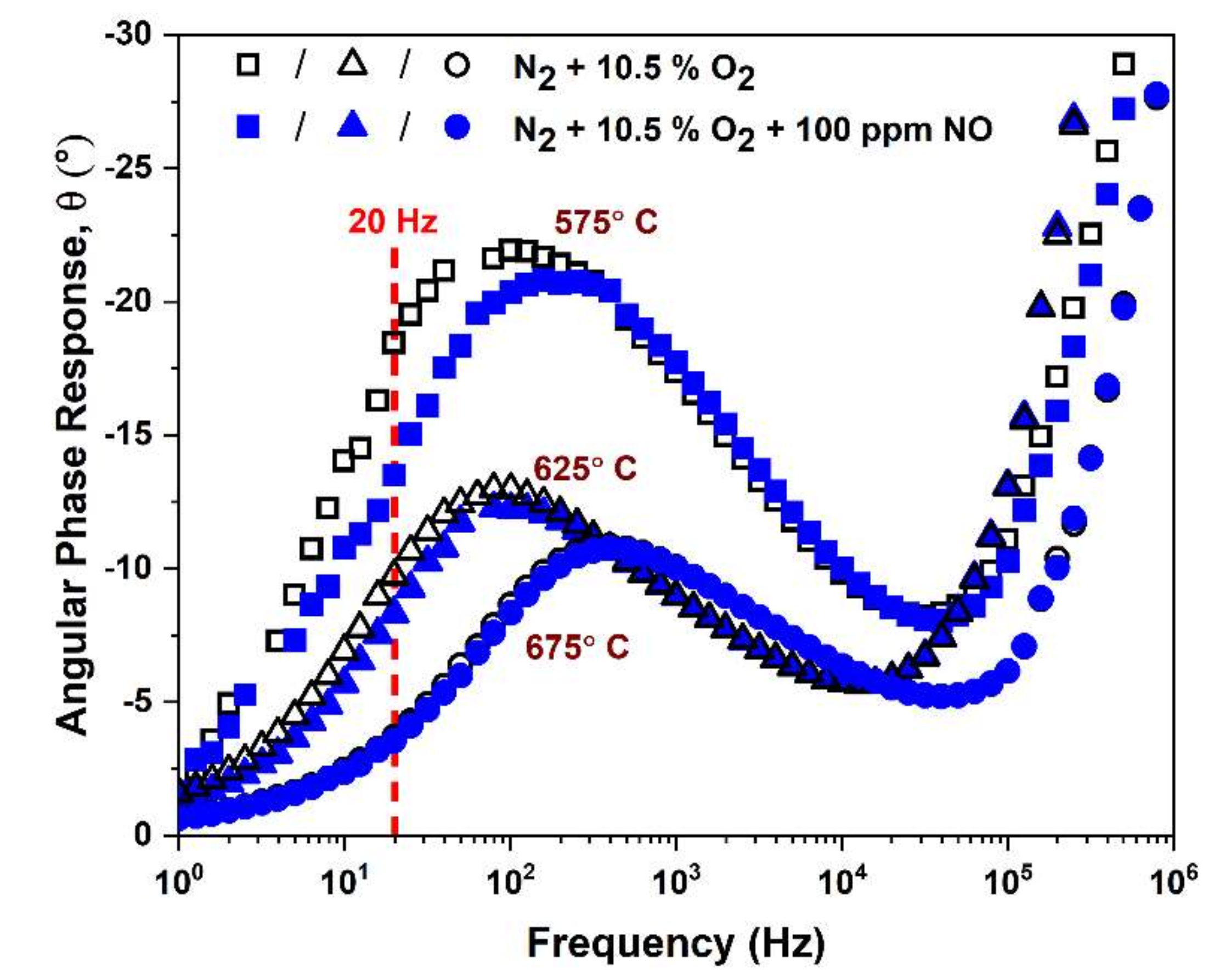
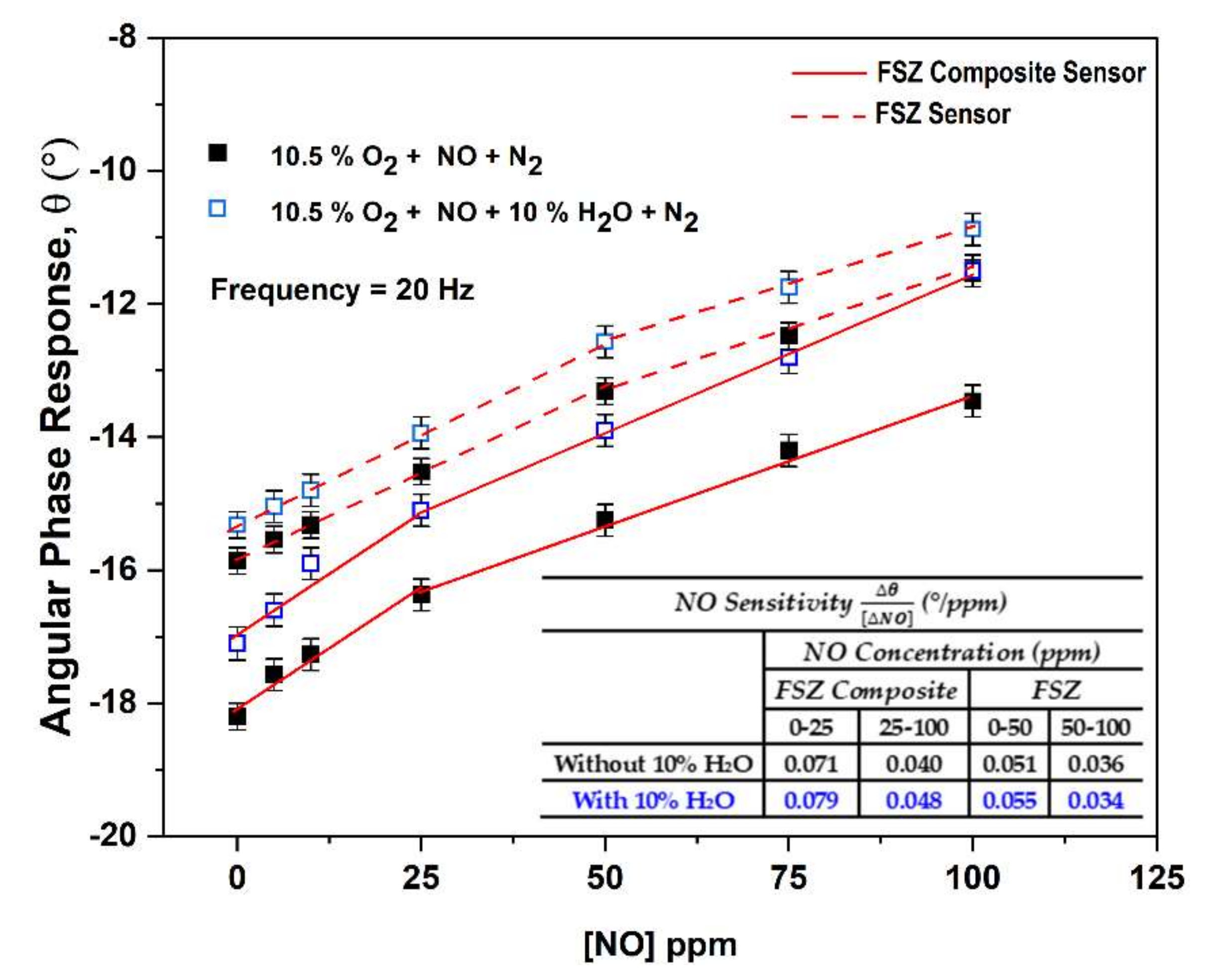
3.2.4. NO Sensitivity of FSZ Composite NOx Sensor
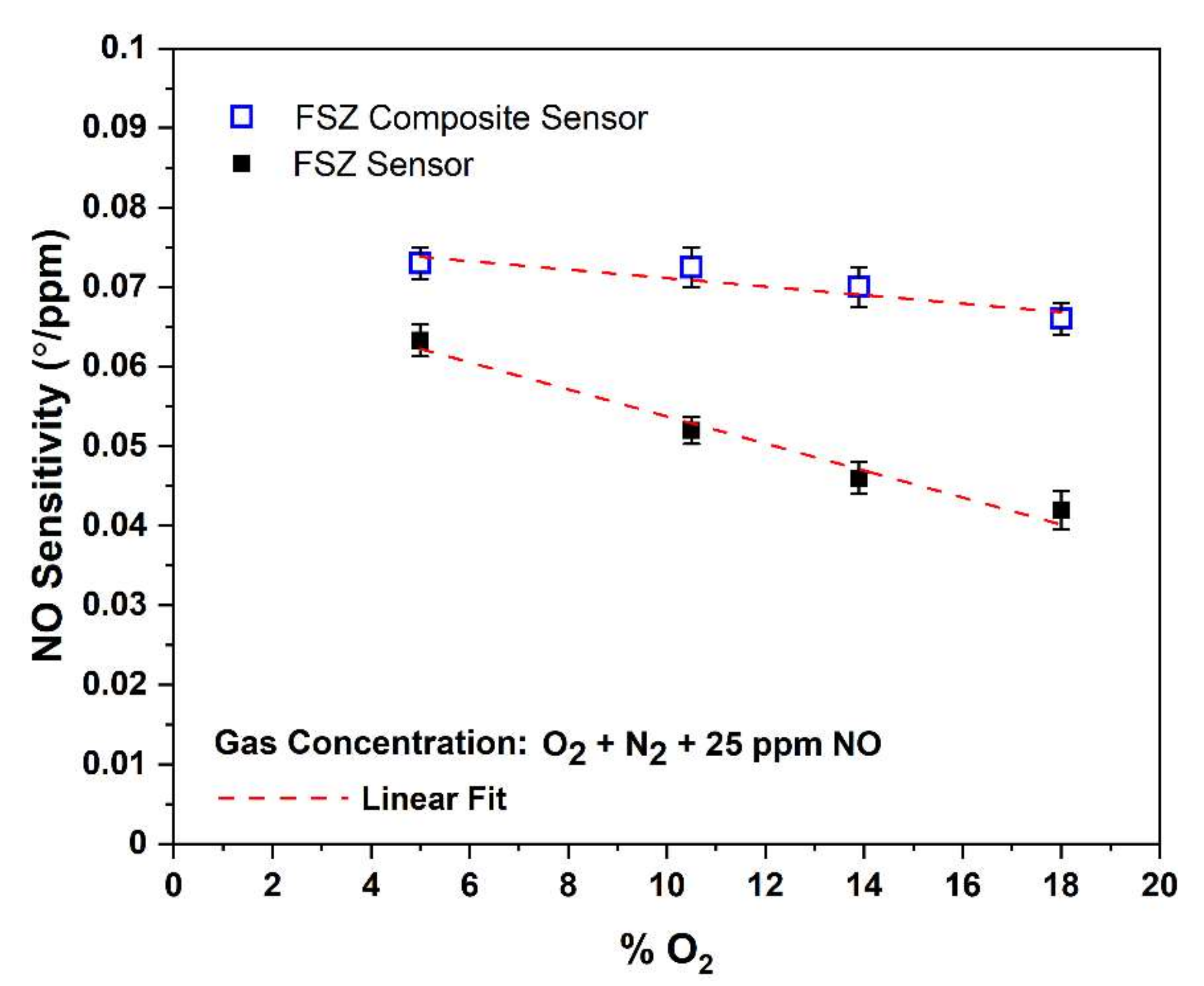
3.2.5. The O2 Influence on NO Sensitivity of The Composite NOx Sensor
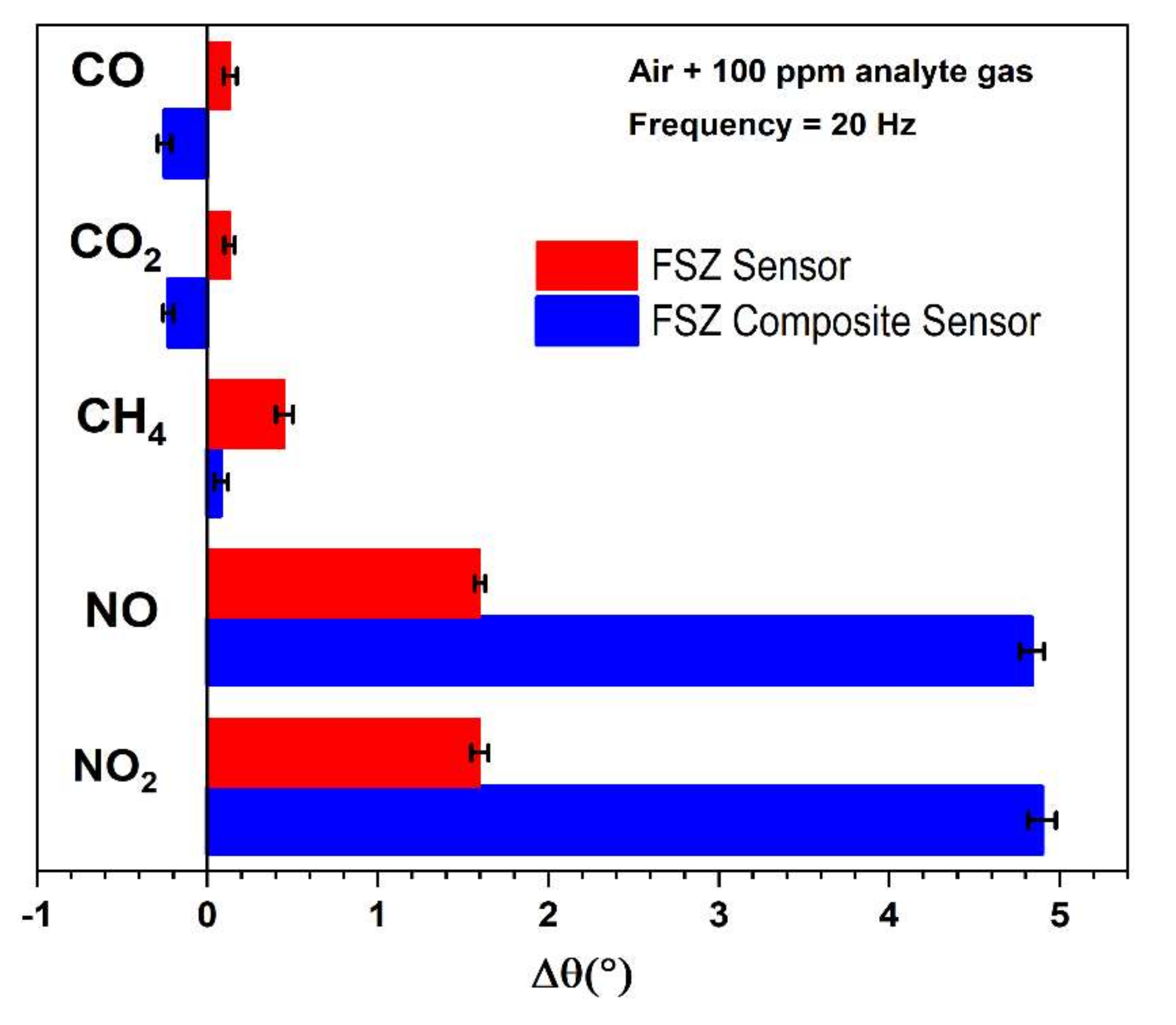
3.2.6. The Influence of CH4, CO2, and CO on NO sensitivity
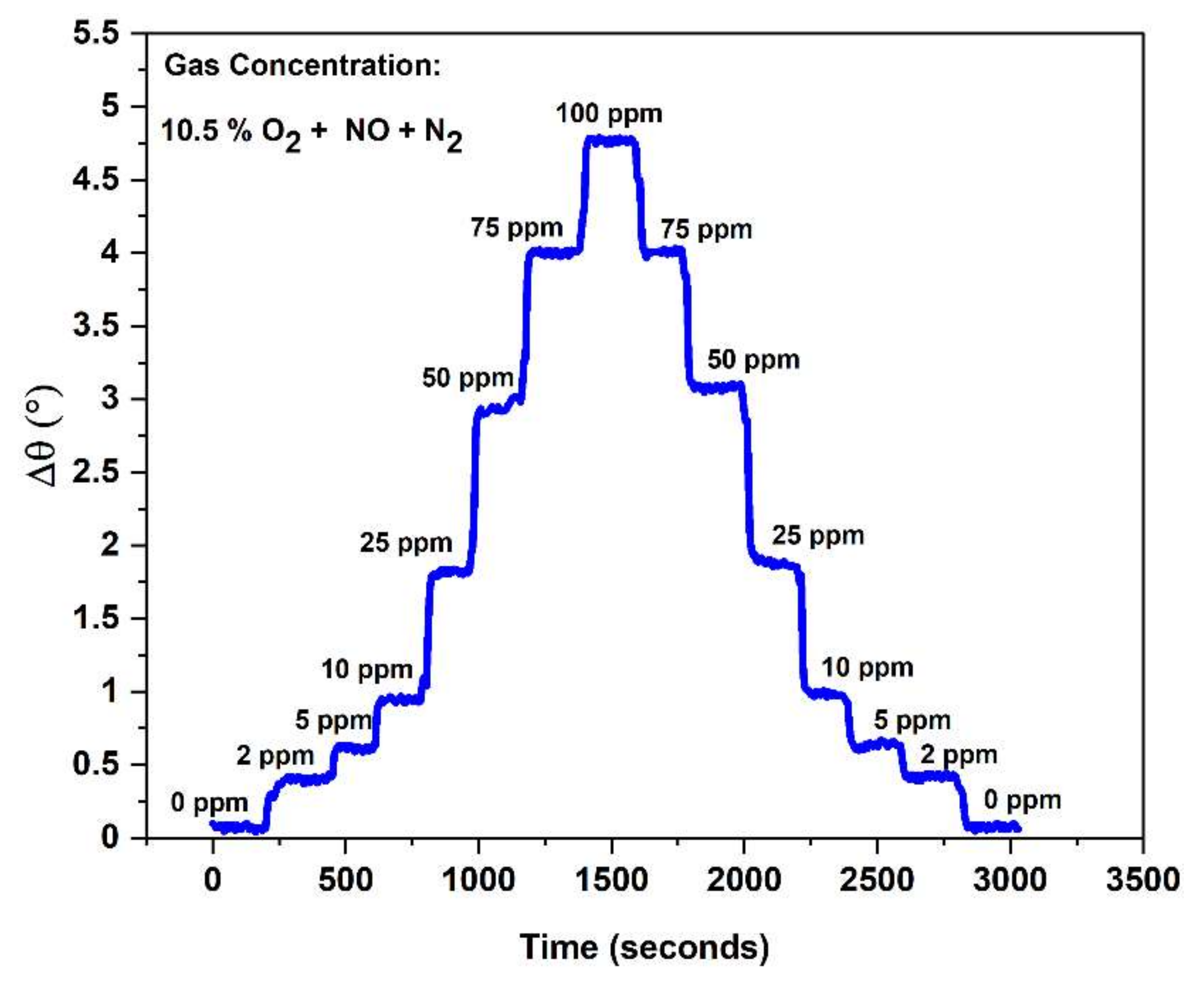
3.2.7. Composite NOx Sensor Response/Recovery Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Westermann, A.; Shi, Y.X.; Cai, N.S.; Rieu, M.; Viricelle, J.-P.; Vernoux, P. Electrochemical Removal of NOx on Ceria-Based Catalyst-Electrodes. Catalysts 2017, 7, 61. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakano, H.; Takase, S.; Song, J.-H. Solid electrolyte impedancemetric NOx sensor attached with zeolite receptor. Sens. Actuators B Chem. 2018, 264, 177–183. [Google Scholar] [CrossRef]
- Dietrich, M.; Steiner, C.; Hagen, G.; Moos, R. Radio-Frequency-Based Urea Dosing Control for Diesel Engines with Ammonia SCR Catalysts. SAE Int. J. Engines 2017, 10, 1638–1645. Available online: https://www.jstor.org/stable/26422554 (accessed on 14 January 2021). [CrossRef]
- Bhardwaj, A.; Hong, J.-W.; Kim, I.-H.; Namgung, Y.; Song, S.-J. Effects of electronic probe’s architecture on the sensing performance of mixed-potential based NOX sensor. Sens. Actuators B Chem. 2019, 282, 426–436. [Google Scholar] [CrossRef]
- Ishizuka, S.; Kajiwara, I.; Sato, J.; Hanamura, Y. A New Approach for NOX Soft Sensors for the Aftertreatment of Diesel Engines. J. Physics Conf. Ser. 2016, 744, 12207. [Google Scholar] [CrossRef]
- Zhang, X.; Kohler, H.; Schwotzer, M.; Wu, Y.; Guth, U. Layered Au,Pt-YSZ mixed potential gas sensing electrode: Correlation among sensing response, dynamic electrochemical behavior and structural properties. Sens. Actuators B Chem. 2019, 278, 117–125. [Google Scholar] [CrossRef]
- Dai, L.; Ma, L.; Meng, W.; Li, Y.; He, Z.; Wang, L. Impedancemetric NO2 sensor based on Pd doped perovskite oxide sensing electrode conjunction with phase angle response. Electrochim. Acta 2018, 265, 411–418. [Google Scholar] [CrossRef]
- Todo, Y.; Ichikawa, H.; Yotou, H.; Aoki, K.; Kawai, M. Development of High Accuracy and Quick Light-off NOx Sensor; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Balamurugan, C.; Song, S.-J.; Kim, H.-S. Enhancing Gas Response Characteristics of Mixed Metal Oxide Gas Sensors. J. Korean Ceram. Soc. 2018, 55, 1–20. [Google Scholar] [CrossRef]
- Pal, N.; Murray, E. Dense LaSrMnO3 composite electrodes for NOx sensing. Sens. Actuators B Chem. 2018, 256, 351–358. [Google Scholar] [CrossRef]
- Ikeda, H.; Iio, A.; Anggraini, S.A.; Miura, N. Impedancemetric YSZ-based oxygen sensor using BaFeO3 sensing-electrode. Sens. Actuators B Chem. 2017, 243, 279–282. [Google Scholar] [CrossRef]
- Romanytsia, I.; Viricelle, J.-P.; Vernoux, P.; Pijolat, C. Application of advanced morphology Au–X (X=YSZ, ZrO2) composites as sensing electrode for solid state mixed-potential exhaust NOx sensor. Sens. Actuators B Chem. 2015, 207, 391–397. [Google Scholar] [CrossRef]
- Adhyapak, P.V.; Bang, A.D.; More, P.; Munirathnam, N.R. Nanostructured WO3/graphene composites for sensing NOx at room temperature. RSC Adv. 2018, 8, 34035–34040. [Google Scholar] [CrossRef]
- Woo, L.Y.; Glass, R.S. FY2011 Progress Report: Agreement 8697-NOx Sensor Development; No. LLNL-TR-510234; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2011. [Google Scholar] [CrossRef]
- Striker, T.; Ramaswamy, V.; Armstrong, E.N.; Willson, P.D.; Wachsman, E.D.; Ruud, J.A. Effect of nanocomposite Au–YSZ electrodes on potentiometric sensor response to NOx and CO. Sens. Actuators B Chem. 2013, 181, 312–318. [Google Scholar] [CrossRef]
- Ueda, T.; Umeda, M.; Okawa, H.; Takahashi, S. Effects of Sr Addition to La-Based Perovskite Sensing-Electrode on YSZ-Based Amperometric-Type NOx Sensor. IOP Conf. Series Mater. Sci. Eng. 2011, 18, 212012. [Google Scholar] [CrossRef]
- Raza, R.; Ahmed, A.; Akram, N.; Saleem, M.; Akhtar, M.N.; Sherazi, T.A.; Khan, M.A.; Abbas, G.; Shakir, I.; Mohsin, M.; et al. Composite electrolyte with proton conductivity for low-temperature solid oxide fuel cell. Appl. Phys. Lett. 2015, 107, 183903. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Javed, F.; Ahsan, M.; Shakir, I.; Rafique, M.S. High performance of SDC and GDC core shell type composite electrolytes using methane as a fuel for low temperature SOFC. AIP Adv. 2016, 6, 025202. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Q.; Wu, F.; Wang, H. A novel MgO doped Zr0.92Y0.08O2-α(8YSZ) with NaCl/KCl composite electrolyte for intermediate temperature fuel cells. Ceram. Int. 2018, 45, 1605–1608. [Google Scholar] [CrossRef]
- Feighery, A.; Irvine, J. Effect of alumina additions upon electrical properties of 8 mol.% yttria-stabilised zirconia. Solid State Ion. 1999, 121, 209–216. [Google Scholar] [CrossRef]
- Drożdż, E. The influence of the method of addition of Al2O3 to 3YSZ material on its thermal and electrical properties. J. Therm. Anal. 2014, 118, 1345–1353. [Google Scholar] [CrossRef]
- Kharashi, K.; Murray, E.P. Effect of Al2O3in Porous Zirconia Electrolytes for NO Sensing. J. Electrochem. Soc. 2016, 163, B633–B637. [Google Scholar] [CrossRef]
- Guo, X. Roles of Alumina in Zirconia for Functional Applications. J. Am. Ceram. Soc. 2003, 86, 1867–1873. [Google Scholar] [CrossRef]
- Murray, E.P.; Kharashi, K.; Adedeji, K. Managing H2O Cross-Sensitivity Using Composite Electrolyte NOx Sensors. In Electrochemical Sensors Technology; Rahman, M.M., Asiri, A.M., Eds.; IntechOpen Ltd.: London, UK, 2017. [Google Scholar] [CrossRef]
- Pavone, M.; García, A.B.M.; Ritzmann, A.; Carter, E.A. First-Principles Study of Lanthanum Strontium Manganite: Insights into Electronic Structure and Oxygen Vacancy Formation. J. Phys. Chem. C 2014, 118, 13346–13356. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Zheng, F.; Pederson, L.R. Phase Behavior of Lanthanum Strontium Manganites. J. Electrochem. Soc. 1999, 146, 2810–2816. [Google Scholar] [CrossRef]
- Zhang, F.; Van Meerbeek, B.; Vleugels, J. Importance of tetragonal phase in high-translucent partially stabilized zirconia for dental restorations. Dent. Mater. 2020, 36, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. One-step preparation of CaO-doped partially stabilized zirconia from fused zirconia. Ceram. Int. 2020, 46, 6484–6490. [Google Scholar] [CrossRef]
- Liang, B.; Ding, C.; Liao, H.; Coddet, C. Phase composition and stability of nanostructured 4.7 wt.% yttria-stabilized zirconia coatings deposited by atmospheric plasma spraying. Surf. Coat. Technol. 2006, 200, 4549–4556. [Google Scholar] [CrossRef]
- Costantini, J.-M.; Beuneu, F. Threshold displacement energy in yttria-stabilized zirconia. Phys. Status Solidi c 2007, 4, 1258–1263. [Google Scholar] [CrossRef]
- Elm, M.T.; Hofmann, J.D.; Suchomski, C.; Janek, J.; Brezesinski, T. Ionic Conductivity of Mesostructured Yttria-Stabilized Zirconia Thin Films with Cubic Pore Symmetry—On the Influence of Water on the Surface Oxygen Ion Transport. ACS Appl. Mater. Interfaces 2015, 7, 11792–11801. [Google Scholar] [CrossRef]
- Ding, H.; Virkar, A.V.; Liu, F. Defect configuration and phase stability of cubic versus tetragonal yttria-stabilized zirconia. Solid State Ion. 2012, 215, 16–23. [Google Scholar] [CrossRef]
- Zhu, J.; Van Ommen, J.; Bouwmeester, H.J.; Lefferts, L. Activation of O2 and CH4 on yttrium-stabilized zirconia for the partial oxidation of methane to synthesis gas. J. Catal. 2005, 233, 434–441. [Google Scholar] [CrossRef]
- Syzgantseva, O.A.; Calatayud, M.; Minot, C. Revealing the Surface Reactivity of Zirconia by Periodic DFT Calculations. J. Phys. Chem. C 2012, 116, 6636–6644. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Kumar, V. A novel yellowish white Dy3+ activated α-Al2O3 phosphor: Photoluminescence and optical studies. Funct. Mater. Lett. 2015, 8, 1550061. [Google Scholar] [CrossRef]
- Kumar, S.; Mote, V.D.; Prakash, R.; Kumar, V. X-ray Analysis of α-Al2O3 Particles by Williamson–Hall Methods. Mater. Focus 2016, 5, 545–549. [Google Scholar] [CrossRef]
- Kumar, B.R.; Hymavathi, B. X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson–Hall and size-strain plot methods. J. Asian Ceram. Soc. 2017, 5, 94–103. [Google Scholar] [CrossRef]
- Ponkumar, S.; Janaki, K.; Babu, D.P.; Munirathnam, K.; Kumar, M.M. ZrO2-Al2O3 nanocomposite: Synthesis, characterization and influence of electron beam irradiation on the structural and PL properties. AIP Conf. Proc. 2018, 1966, 020009. [Google Scholar] [CrossRef]
- Nakai, I.; Abe, Y. Portable X-ray powder diffractometer for the analysis of art and archaeological materials. Appl. Phys. A 2011, 106, 279–293. [Google Scholar] [CrossRef]
- Thangadurai, P.; Bose, A.C.; Ramasamy, S. Phase stabilization and structural studies of nanocrystalline La2O3-ZrO2. J. Mater. Sci. 2005, 40, 3963–3968. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Wang, Y.; Sun, H.; Xie, S.; Bai, B.; Liu, Y.; Ji, K.; Li, J. Three-Dimensionally Ordered Macroporous La0.6Sr0.4MnO3 Supported Ag Nanoparticles for the Combustion of Methane. J. Phys. Chem. C 2014, 118, 14913–14928. [Google Scholar] [CrossRef]
- Yan, K.-L.; Fan, R.-H.; Chen, M.; Sun, K.; Yin, L.-W.; Li, H.; Pan, S.-B.; Yu, M.-X. Perovskite (La,Sr)MnO3 with tunable electrical properties by the Sr-doping effect. J. Alloys Compd. 2015, 628, 429–432. [Google Scholar] [CrossRef]
- Hasegawa, S.; Sugimoto, T.; Hashimoto, T. Investigation of structural phase transition behavior of SrZrO3 by thermal analyses and high-temperature X-ray diffraction. Solid State Ion. 2010, 181, 1091–1097. [Google Scholar] [CrossRef]
- Majumdar, D.; Chatterjee, D.K. X-ray photoelectron spectroscopic studies on yttria, zirconia, and yttria-stabilized zirconia. J. Appl. Phys. 1991, 70, 988–992. [Google Scholar] [CrossRef]
- Zhu, W.; Nakashima, S.; Marin, E.; Gu, H.; Pezzotti, G. Microscopic mapping of dopant content and its link to the structural and thermal stability of yttria-stabilized zirconia polycrystals. J. Mater. Sci. 2020, 55, 524–534. [Google Scholar] [CrossRef]
- Pomfret, M.B.; Stoltz, C.; Varughese, B.; Walker, R.A. Structural and Compositional Characterization of Yttria-Stabilized Zirconia: Evidence of Surface-Stabilized, Low-Valence Metal Species. Anal. Chem. 2005, 77, 1791–1795. [Google Scholar] [CrossRef]
- Prakash, R.; Kumar, S.; Kumar, V.; Choudhary, R.J. Phase, Optical and x-ray photoelectron spectroscopy studies of α-Al2O3. AIP Conf. Proc. 2016, 1731, 050097. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Liu, M.; Jaegermann, W. X-ray photoelectron spectroscopy of La0.5Sr0.5MnO3. Mater. Lett. 2005, 59, 1980–1983. [Google Scholar] [CrossRef]
- Villa-Bustamante, R.A.; Jativa, J.A.; Ospina, R.; Londono-Menjura, R.F.; Jurado, J.F.; Quintero, J.; Restrepo-Parra, E.; Mello, A. Thermal treatment influence on structural, morphological and magnetic properties of La1-xSrxMnO3 powders produced by the hydrothermal technique. Contemp. Eng. Sci. 2018, 11, 4021–4039. [Google Scholar] [CrossRef]
- Muhammad, A.; Tahir, M.; Al-Shahrani, S.S.A.; Ali, A.M.; Rather, S.U. Template free synthesis of graphitic carbon nitride nanotubes mediated by lanthanum (La/g-CNT) for selective photocatalytic CO2 reduction via dry reforming of methane (DRM) to fuels. Appl. Surf. Sci. 2020, 504, 144177. [Google Scholar] [CrossRef]
- Chen, K.; Hyodo, J.; Ai, N.; Ishihara, T.; Jiang, S.P. Boron deposition and poisoning of La0.8Sr0.2MnO3 oxygen electrodes of solid oxide electrolysis cells under accelerated operation conditions. Int. J. Hydrog. Energy 2016, 41, 1419–1431. [Google Scholar] [CrossRef]
- Kozakov, A.; Kochur, A.; Nikolskii, A.; Torgashev, V.; Trotsenko, V.; Bush, A. Valence state of manganese and iron ions in La1−A MnO3 (A = Ca, Sr) and Bi1−Sr FeO systems from Mn2p, Mn3s, Fe2p and Fe3s X-ray photoelectron spectra. Effect of delocalization on Fe3s spectra splitting. J. Alloys Compd. 2015, 647, 947–955. [Google Scholar] [CrossRef]
- Sun, D.; McLaughlan, J.; Zhang, L.; Falzon, B.G.; Mariotti, D.; Maguire, P.D.; Sun, D. Atmospheric Pressure Plasma-Synthesized Gold Nanoparticle/Carbon Nanotube Hybrids for Photothermal Conversion. Langmuir 2019, 35, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Woo, L.Y.; Martin, L.P.; Glass, R.S.; Wang, W.; Jung, S.; Gorte, R.J.; Murray, E.P.; Novak, R.F.; Visser, J.H. Effect of Electrode Composition and Microstructure on Impedancemetric Nitric Oxide Sensors Based on YSZ Electrolyte. J. Electrochem. Soc. 2008, 155, J32–J40. [Google Scholar] [CrossRef]
- Martin, L.P.; Woo, L.Y.; Glass, R.S. Impedancemetric NOx Sensing Using YSZ Electrolyte and YSZ/Cr2O3 Composite Electrodes. J. Electrochem. Soc. 2007, 154, J97–J104. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Brosha, E.L.; Mukundan, R.; Nelson, M.A.; Toracco, D.; Garzon, F.H. Effect of yttria-stabilized zirconia sintering temperature on mixed potential sensor performance. Solid State Ion. 2010, 181, 947–953. [Google Scholar] [CrossRef]
- Patra, A.S.; Kumar, N.V.; Barpuzary, D.; De, M.; Qureshi, M. Strontium doped lanthanum manganites for efficient and robust photocatalytic water oxidation coupled with graphene oxide. Mater. Lett. 2014, 131, 125–127. [Google Scholar] [CrossRef]
- Sakai, N.; Yamaji, K.; Horita, T.; Xiong, Y.P.; Kishimoto, H.; Brito, M.E.; Yokokawa, H. Erratum to “Effect of water on electrochemical oxygen reduction at the interface between fluorite-type oxide-ion conductors and various types of electrodes”. Solid State Ion. 2005, 176, 2327–2333. [Google Scholar] [CrossRef]
- Rheaume, J.M.; Pisano, A.P. A review of recent progress in sensing of gas concentration by impedance change. Ionics 2011, 17, 99–108. [Google Scholar] [CrossRef]
- Tan, C.; Dutta, G.; Yin, H.; Siddiqui, S.; Arumugam, P.U. Detection of neurochemicals with enhanced sensitivity and selectivity via hybrid multiwall carbon nanotube-ultrananocrystalline diamond microelectrodes. Sens. Actuators B Chem. 2018, 258, 193–203. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. (Eds.) Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Schipani, F.; Miller, D.R.; Ponce, M.A.; Aldao, C.M.; Akbar, S.A.; Morris, P.A. Electrical Characterization of Semiconductor Oxide-Based Gas Sensors Using Impedance Spectroscopy: A Review. Rev. Adv. Sci. Eng. 2016, 5, 86–105. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hamano, H.; Mori, T.; Yoshikawa, Y.; Nagao, M. Specific Adsorption Behavior of Water on a Y2O3 Surface. Langmuir 2000, 16, 6937–6947. [Google Scholar] [CrossRef]
- Raz, S. Characterization of adsorbed water layers on Y2O3-doped ZrO2. Solid State Ion. 2001, 143, 181–204. [Google Scholar] [CrossRef]
- Chaopradith, D.T.; Scanlon, D.O.; Catlow, C.R.A. Adsorption of Water on Yttria-Stabilized Zirconia. J. Phys. Chem. C 2015, 119, 22526–22533. [Google Scholar] [CrossRef]
- Ueda, T.; Nagano, T.; Okawa, H.; Takahashi, S. Zirconia-based amperometric sensor using La–Sr-based perovskite-type oxide sensing electrode for detection of NO2. Electrochem. Commun. 2009, 11, 1654–1656. [Google Scholar] [CrossRef]
- Suehiro, S.; Okawa, H.; Takahashi, S. Synthesis and NOx sensing evaluation of hollow/porous La0.8Sr0.2MnO3 microspheres. RSC Adv. 2016, 6, 53919–53924. [Google Scholar] [CrossRef]
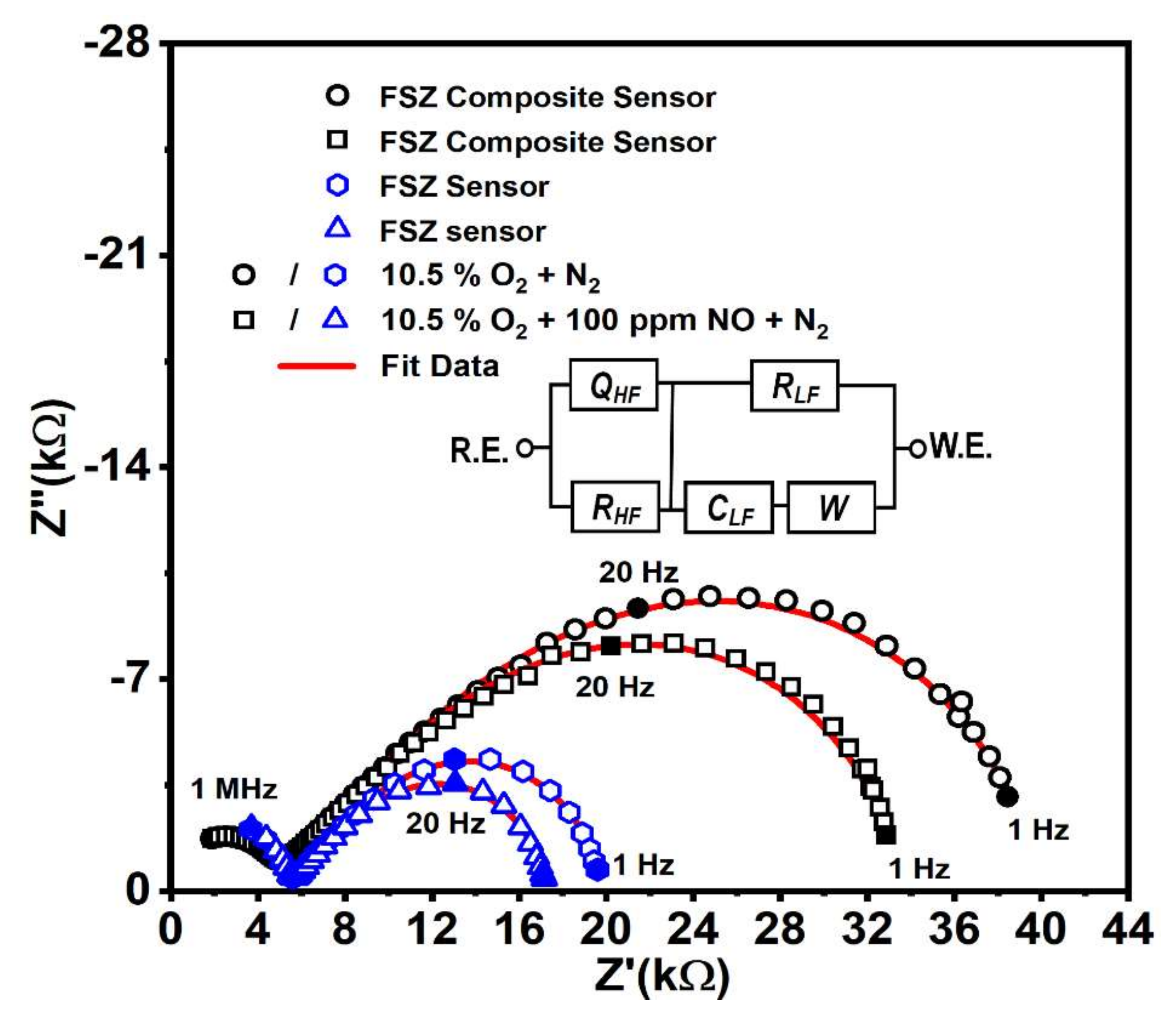
| Circuit Parameters | ||||||
|---|---|---|---|---|---|---|
| The FSZ Composite Electrolyte with the LSM-Au Electrode | ||||||
| NOx | Electrolyte Impedance | Electrode-Electrolyte Interface | ||||
| RHF (kΩ) | QHF (nF.sn−1) | n | CLF (μF) | RLF (kΩ) | AW (kΩ.s−0.5) | |
| 0 ppm | 4.97 ± 0.75 | 3.17 ± 0.01 | 0.75 ± 0.5 | 0.62 ± 0.0 | 34.7 ± 0.5 | 240.6 ± 0.04 |
| 100 ppm | 4.95 ± 0.8 | 3.17 ± 0.09 | 0.75 ± 0.5 | 0.40 ± 0.0 | 28.5 ± 0.9 | 239.5 ± 0.05 |
| The FSZ electrolyte with the LSM-Au electrode | ||||||
| NOx | RHF (kΩ) | QHF (nF.sn−1) | n | CLF (μF) | RLF (kΩ) | AW (kΩ.s−0.5) |
| 0 ppm | 5.43 ± 0.54 | 0.215 ± 0.00 | 0.84 ± 0.5 | 0.61 ± 0.0 | 14.3 ± 0.7 | 118.1 ± 0.04 |
| 100 ppm | 5.46 ± 0.59 | 0.176 ± 0.00 | 0.85 ± 0.5 | 0.46 ± 0.0 | 11.7 ± 0.8 | 122.1 ± 0.06 |
| The capacitance calculated for Electrolytes from Equation (4) | ||||||
| NOx | FSZ composite electrolyte | FSZ electrolyte | ||||
| CHF (fF) | CHF (fF) | |||||
| 0 ppm | 40.9 | 13.6 | ||||
| 100 ppm | 42.2 | 14.3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, N.; Dutta, G.; Kharashi, K.; Murray, E.P. Investigation of an Impedimetric LaSrMnO3-Au/Y2O3-ZrO2-Al2O3 Composite NOx Sensor. Materials 2022, 15, 1165. https://doi.org/10.3390/ma15031165
Pal N, Dutta G, Kharashi K, Murray EP. Investigation of an Impedimetric LaSrMnO3-Au/Y2O3-ZrO2-Al2O3 Composite NOx Sensor. Materials. 2022; 15(3):1165. https://doi.org/10.3390/ma15031165
Chicago/Turabian StylePal, Nabamita, Gaurab Dutta, Khawlah Kharashi, and Erica P. Murray. 2022. "Investigation of an Impedimetric LaSrMnO3-Au/Y2O3-ZrO2-Al2O3 Composite NOx Sensor" Materials 15, no. 3: 1165. https://doi.org/10.3390/ma15031165