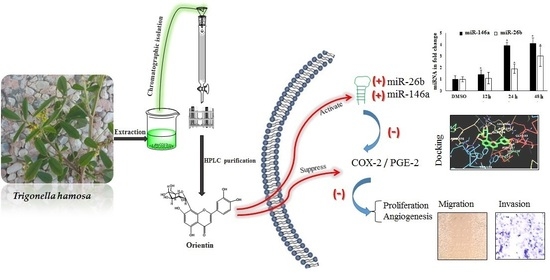
Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a
Abstract
:1. Introduction
2. Results
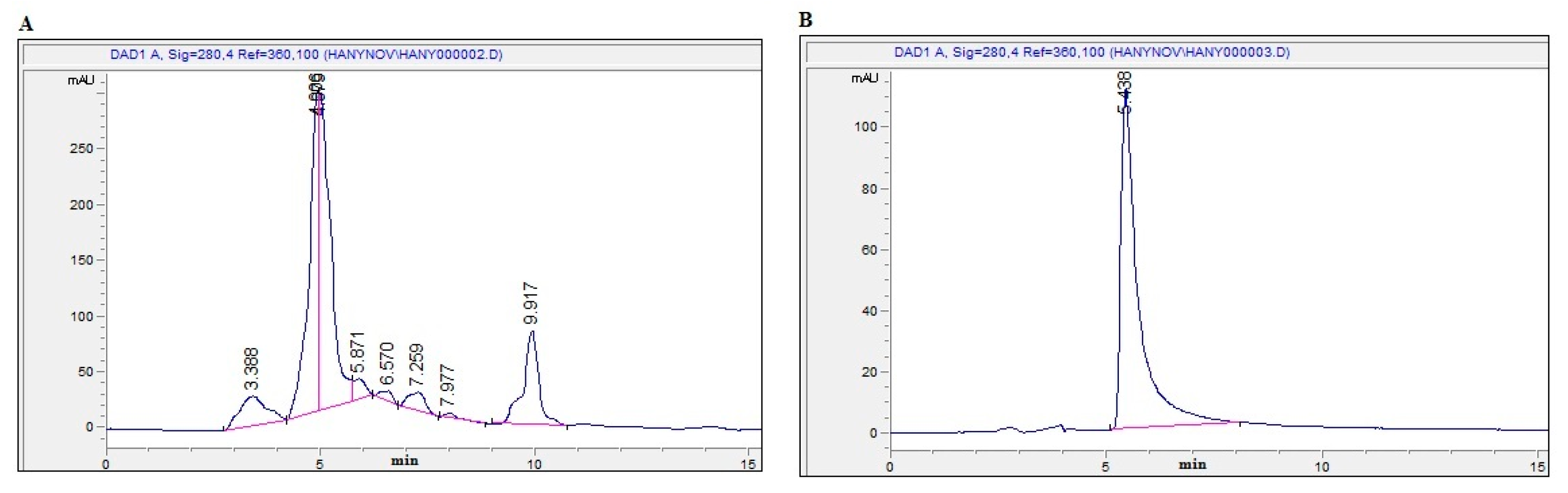
2.1. Bioassay-Guided Investigation
2.2. Isolation and Identification of OT
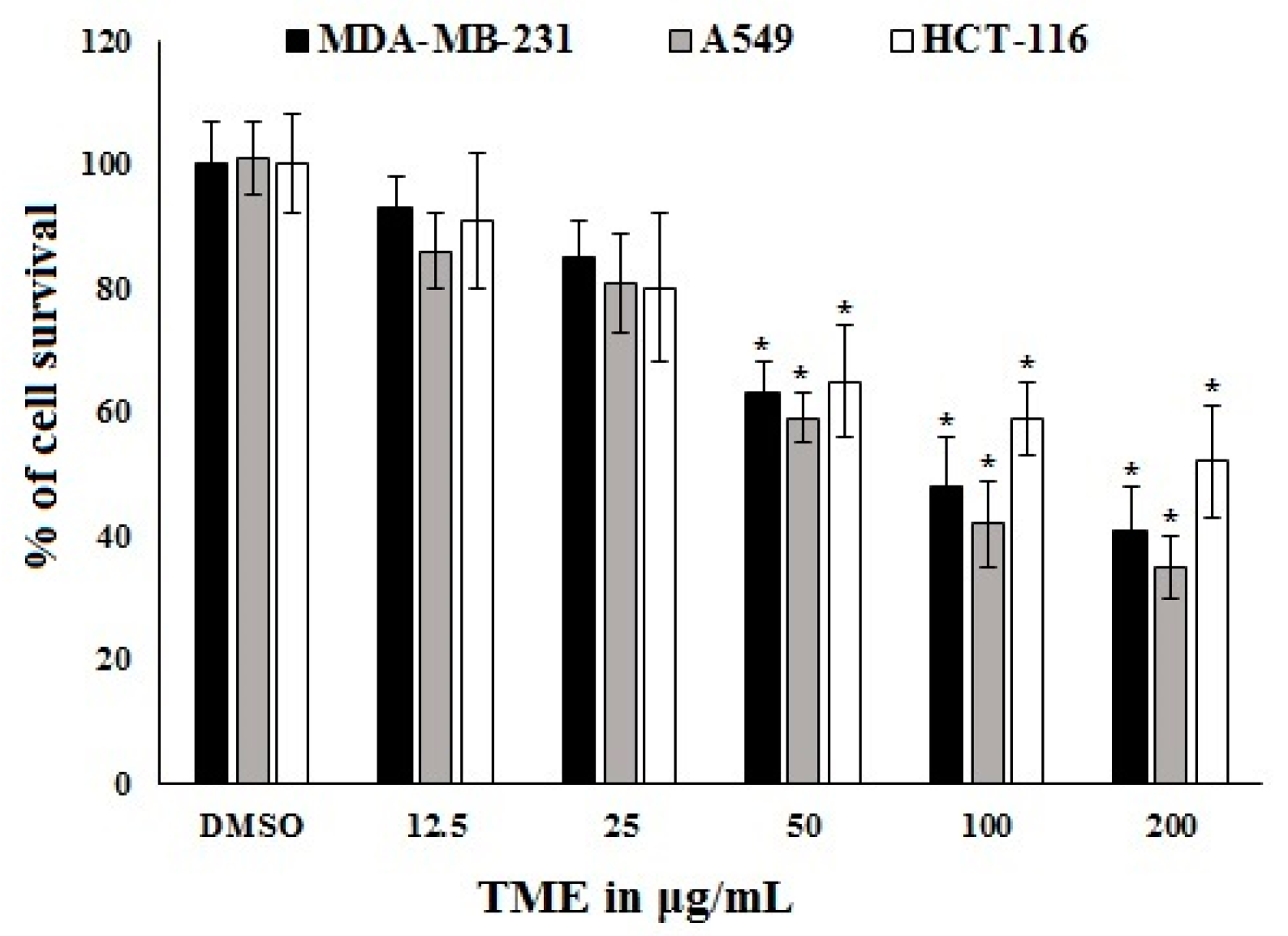
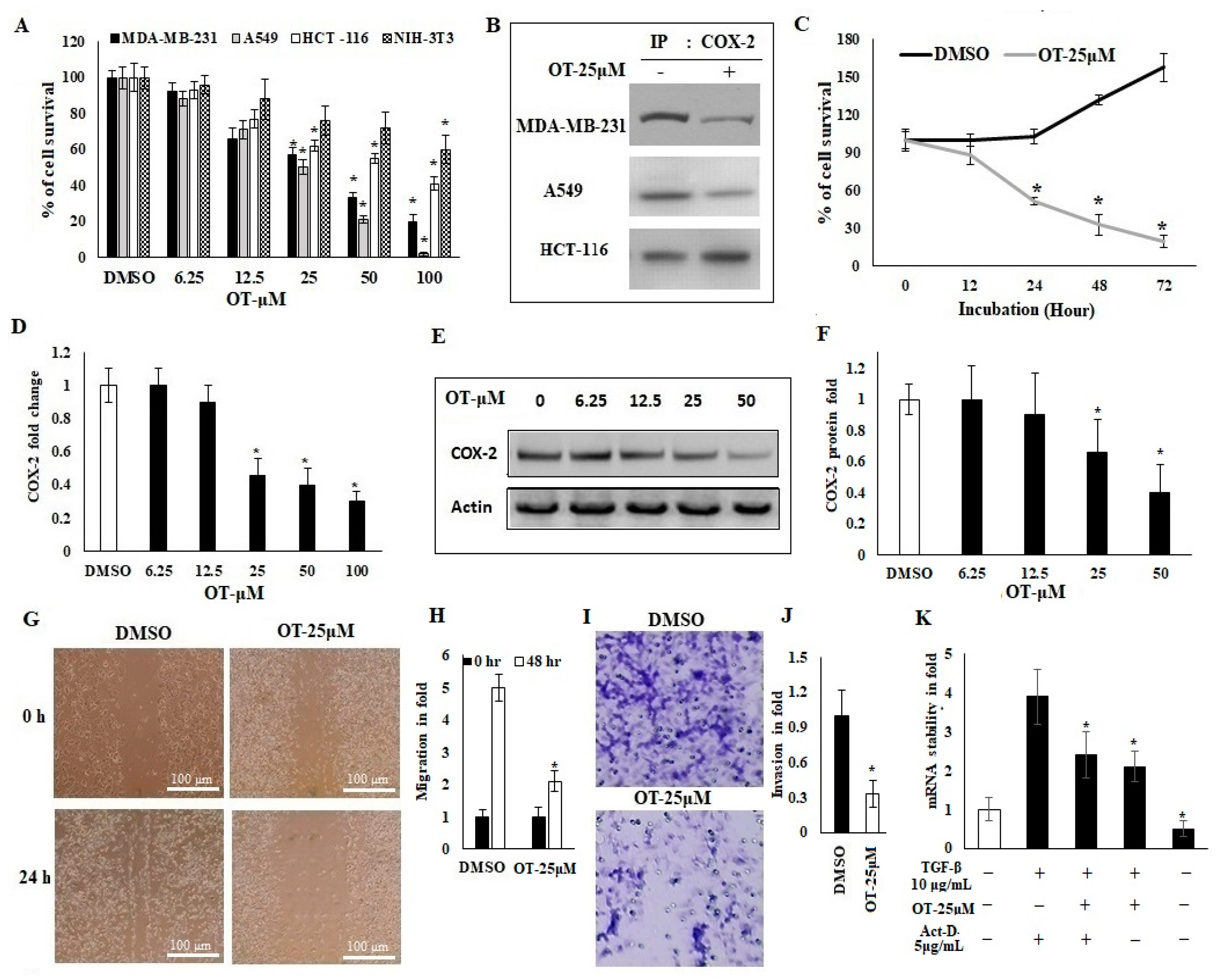
2.3. Antiproliferative Activity of OT and Its Effect on COX-2 Expression in A549 Cell Lines
2.4. OT Destabilized the COX-2 mRNA in A549 Cell Lines
2.5. In Silico Binding of OT against Human COX-2 Protein
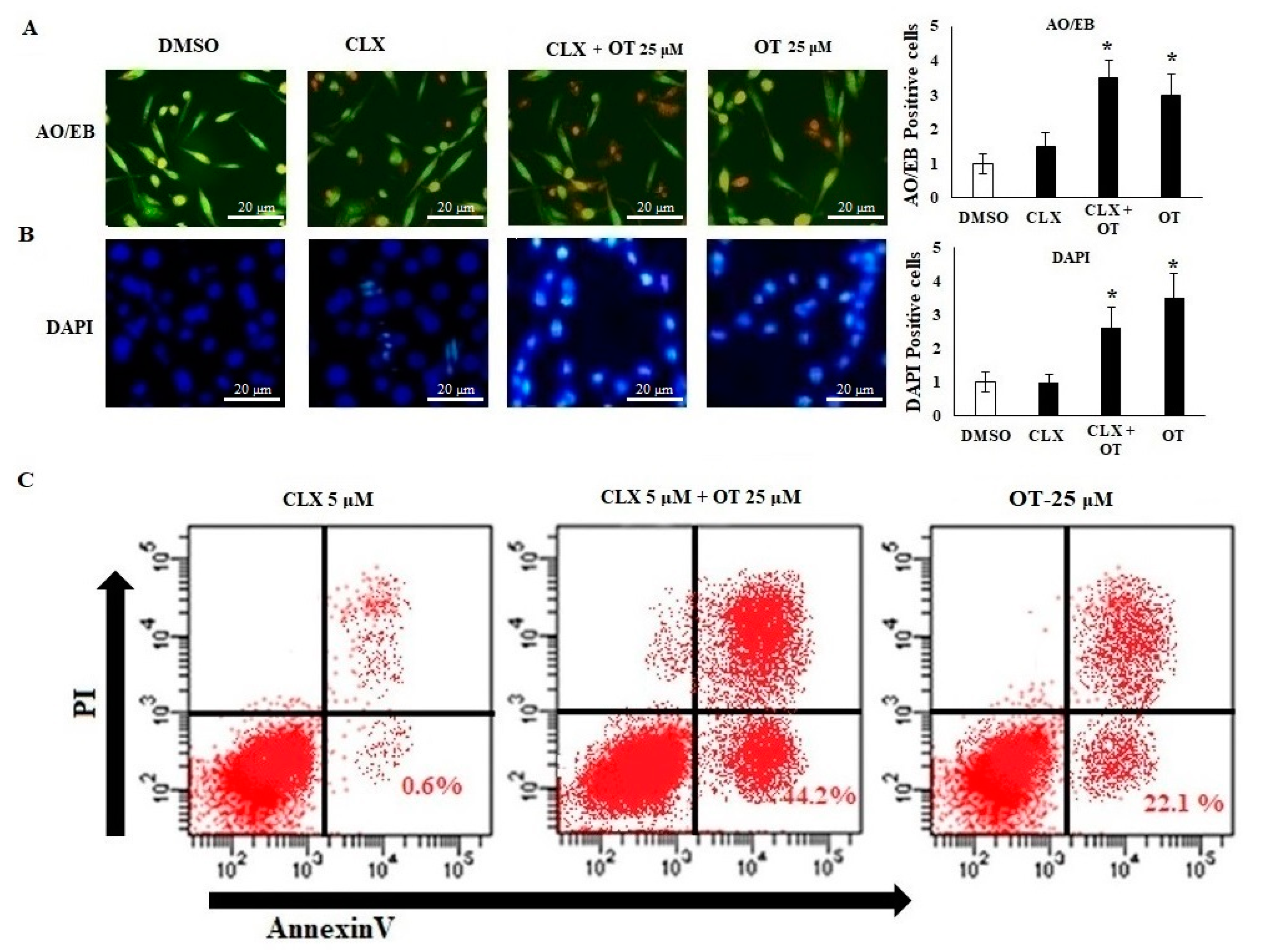
2.6. Effect of OT on Apoptotic Markers and DNA Damage Using CLX-Induced A549 Cells
2.7. Effect of OT on Apoptotic Cell Modifications in A549 Cell Lines
2.8. Interaction of OT with COX-2 via miR-26b and miR-146a
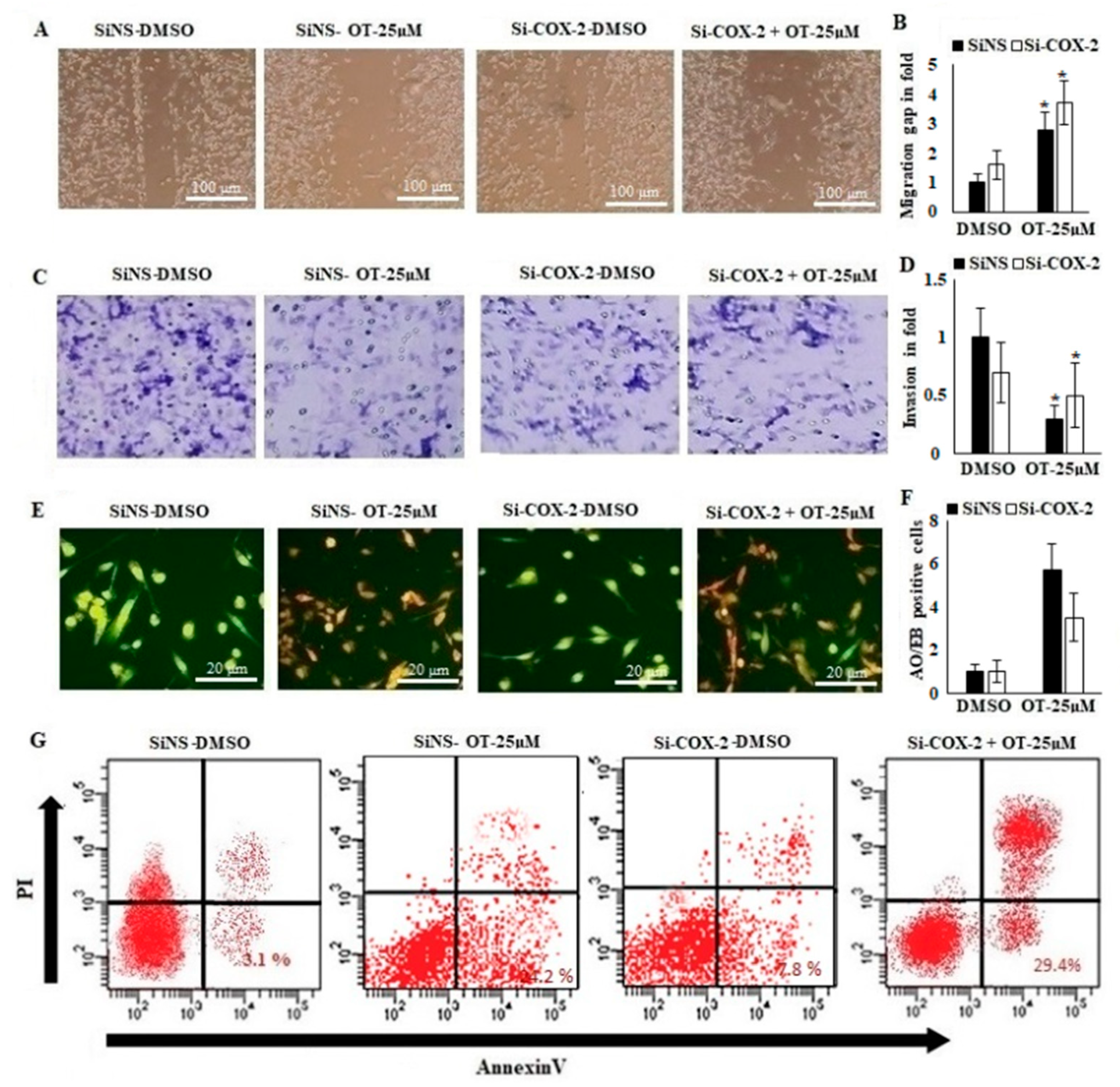
2.9. Effect of OT on Migration, Invasion and Apoptotic Modifications in COX-2-Silenced A549 Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures and Chemicals
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Ligand Preparation and In Silico Docking Analysis
4.5. Cell Culture
4.6. Cell Proliferation and CCK-8 Assay
4.7. Immunoprecipitation
4.8. Cell Transfection of Short Interference COX-2 in A549 Cancer Cell Lines
4.9. Cell Migration Assay (Wound Healing Assay)
4.10. Cell Invasion Assay (Matrigel–Transwell Assay)
4.11. Acridine Orange/Ethidium Bromide (AO/EB) Dual Staining
4.12. 4′,6-Diamidino-2-phenylindole Dihydrochloride (DAPI) Staining
4.13. Flow Cytometric Analysis
4.14. Determination of PGE-2 Production
4.15. Effect of OT on COX-2 mRNA Stability Using Act-D Model
4.16. RNA Isolation and Quantitative Real-Time PCR
4.17. Western Blot Analysis
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, H.E.; Mohamed, M.E.; Morsy, M.A.; Kandeel, M. Flavonoid and phenolic compounds from Carissa macrocarpa: Molecular docking and cytotoxicity studies. Pharmacogn. Mag. 2018, 14, 304. [Google Scholar] [CrossRef]
- Migahid, A. Flora of Saudi Arabia; King Saud University Press: Riyadh, Saudi Arabia, 1987. [Google Scholar]
- AI-Said, M.S. Traditional medicinal plants of Saudi Arabia. Am. J. Chin. Med. 1993, 21, 291–298. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Elaiwi, A.M.; Athar, M.T.; Tariq, M.; Al Eid, A.; Al-Asmary, S.M. A review of hepatoprotective plants used in Saudi traditional medicine. Evid.-Based Complement. Alternat. Med. 2014, 2014, 890842. [Google Scholar]
- Youssef, S. Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plant Res. 2013, 7, 2501–2517. [Google Scholar]
- Mandaville, J.P. Flora of Eastern Saudi Arabia; Routledge: London, UK, 2013. [Google Scholar]
- Ibrahim, H.-I.M.; Darrag, H.M.; Alhajhoj, M.R.; Khalil, H.E. Biomolecule from Trigonella stellata from Saudi Flora to Suppress Osteoporosis via Osteostromal Regulations. Plants 2020, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Mandegary, A.; Pournamdari, M.; Sharififar, F.; Pournourmohammadi, S.; Fardiar, R.; Shooli, S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem. Toxicol. 2012, 50, 2503–2507. [Google Scholar] [CrossRef]
- Kosta, S.; Tiwari, A. Screening and assessment of anti-diabetic and reactive oxygen scavenging (ros), effects of herbs in streptozotacin induced mice. Pharmacol. Online 2009, 3, 695–704. [Google Scholar]
- Kenny, O.; Smyth, T.; Hewage, C.; Brunton, N. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Javan, M.; Semnanian, S.; Barat, E.; Kamalinejad, M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J. Ethnopharmacol. 2001, 75, 283–286. [Google Scholar] [CrossRef]
- Khole, S.; Chatterjee, S.; Variyar, P.; Sharma, A.; Devasagayam, T.; Ghaskadbi, S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods 2014, 6, 270–279. [Google Scholar] [CrossRef]
- Raju, J.; Bird, R. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-α levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int. J. Obes. 2006, 30, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindhu, G.; Shyni, G.; Pushpan, C.K.; Nambisan, B.; Helen, A. Evaluation of anti-arthritic potential of Trigonella foenum graecum L. (Fenugreek) mucilage against rheumatoid arthritis. Prostaglandins Other Lipid Mediat. 2018, 138, 48–53. [Google Scholar]
- Mobashar, A.; Shabbir, A.; Shahzad, M. Evaluation of Immunomodulatory and Antiarthritic Potential of Trigonella gharuensis Extracts. Evid.-Based Complement. Altern. Med. 2020, 2020, 8836080. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.I. Steroidal saponins from the seeds of Trigonella hamosa L. Nat. Prod. Commun. 2007, 2, 1934578X0700200207. [Google Scholar] [CrossRef]
- Salah-Eldin, A.-E.; Mahalel, U.A.; Hamed, A.I. Protective role of trigonella hamosa saponins against diabetic perturbations and complications in rats. Nat. Prod. Commun. 2007, 2, 1934578X0700200805. [Google Scholar] [CrossRef] [Green Version]
- Al-Mazroa, S.; Al-Wahaibi, L.; Mousa, A.; Al-Khathlan, H. Essential oil of some seasonal flowering plants grown in Saudi Arabia. Arab. J. Chem. 2015, 8, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Qari, S.H.; Fahmy, N.M. Evaluation of some biological activities of Trigonella hamosa aerial parts. J. Pharmacogn. Phytother. 2017, 9, 165–172. [Google Scholar]
- Shahat, A.A.; Ibrahim, A.Y.; Alsaid, M.S. Antioxidant capacity and polyphenolic content of seven Saudi Arabian medicinal herbs traditionally used in Saudi Arabia. Indian J. Tradit. Knowl. 2015, 1, 28–35. [Google Scholar]
- Gonzalez-Gallego, J.; Sánchez-Campos, S.; Tunon, M. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A. 1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, O.; Shukla, A.; Singh Rajpurohit, C.; Vasudev, P.G.; Luqman, S.; Kumar Srivastava, S.; Bhushan Pant, A.; Khan, F. Structure-activity relationship studies on holy basil (Ocimum sanctum L.) based flavonoid orientin and its analogue for cytotoxic activity in liver cancer cell line HepG2. Comb. Chem. High. Throughput Screen. 2016, 19, 656–666. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Wang, S.; Tian, Q.; Zhu, D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015, 10, 2627–2633. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Gachechiladze, M.A.; Ludwig, C.H.; Laurie, M.T.; Hong, J.Y.; Nathaniel, D.; Prabhu, A.V.; Fernandopulle, M.S.; Patel, R.; Abshari, M. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron 2019, 104, 239–255.e212. [Google Scholar] [CrossRef]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-glucosides derived from flax straw extracts reduce human breast cancer cell growth in vitro and induce apoptosis. Front. Pharmacol. 2016, 7, 282. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Pham, T.-H.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar] [CrossRef]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1, 2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef]
- Fanelli, M.F.; Chinen, L.T.D.; Begnami, M.D.; Costa, W.L., Jr.; Fregnami, J.H.T.; Soares, F.A.; Montagnini, A.L. The influence of transforming growth factor-α, cyclooxygenase-2, matrix metalloproteinase (MMP)-7, MMP-9 and CXCR4 proteins involved in epithelial–mesenchymal transition on overall survival of patients with gastric cancer. Histopathology 2012, 61, 153–161. [Google Scholar] [CrossRef]
- Ma, X.; Holt, D.; Kundu, N.; Reader, J.; Goloubeva, O.; Take, Y.; Fulton, A.M. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2013, 2, e22647. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Imanishi, Y.; Ozawa, H.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Habu, N.; Otsuka, K.; Sato, Y.; Sekimizu, M. Selective EP2 and Cox-2 inhibition suppresses cell migration by reversing epithelial-to-mesenchymal transition and Cox-2 overexpression and E-cadherin downregulation are implicated in neck metastasis of hypopharyngeal cancer. Am. J. Transl. Res. 2020, 12, 1096. [Google Scholar]
- Peddareddigari, V.G.; Wang, D.; DuBois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.E.; Dixon, D.A. Posttranscriptional regulation of cyclooxygenase 2 expression in colorectal cancer. Curr. Colorectal. Cancer Rep. 2010, 6, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, T.; Su, J.; Huang, C.; Yu, D.; Dai, S.; Huang, X.; Chen, B.; Zhou, M. Isoorientin induces apoptosis, decreases invasiveness, and downregulates VEGF secretion by activating AMPK signaling in pancreatic cancer cells. OncoTargets Ther. 2016, 9, 7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Qu, Z.; Zhao, Y.; Yang, L.; Gao, P. Orientin ameliorates LPS-induced inflammatory responses through the inhibitory of the NF-κB pathway and NLRP3 inflammasome. Evid.-Based Complement. Alternat. Med. 2017, 2017, 2495496. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.-I.M.; Ismail, M.B.; Ammar, R.B.; Ahmed, E.A. Thidiazuron suppresses breast cancer via targeting miR-132 and dysregulation of the PI3K–Akt signaling pathway mediated by the miR-202-5p–PTEN axis. Biochem. Cell Biol. 2021, 99, 374–384. [Google Scholar] [CrossRef]
- Cornett, A.L.; Lutz, C.S. Regulation of COX-2 expression by miR-146a in lung cancer cells. RNA 2014, 20, 1419–1430. [Google Scholar] [CrossRef] [Green Version]
- Kumaraswamy, E.; Wendt, K.L.; Augustine, L.A.; Stecklein, S.R.; Sibala, E.C.; Li, D.; Gunewardena, S.; Jensen, R.A. BRCA1 regulation of epidermal growth factor receptor (EGFR) expression in human breast cancer cells involves microRNA-146a and is critical for its tumor suppressor function. Oncogene 2015, 34, 4333–4346. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xiao, J.; Lu, X.; Liu, T.; Jin, X.; Xiao, Y.; He, X. PVT1 (rs13281615) and miR-146a (rs2910164) polymorphisms affect the prognosis of colon cancer by regulating COX2 expression and cell apoptosis. J. Cell. Physiol. 2019, 234, 17538–17548. [Google Scholar] [CrossRef]
- Poli, M.S.S.; Khajeniazi, S.; Behnampour, N.; Kalani, M.R.; Moradi, A.; Marjani, A. MicroRNA-146a as a prognostic biomarker for esophageal squamous cell carcinoma. Cancer Manag. Res. 2020, 12, 973. [Google Scholar] [CrossRef] [Green Version]
- Iacona, J.R.; Monteleone, N.J.; Lutz, C.S. miR-146a suppresses 5-lipoxygenase activating protein (FLAP) expression and Leukotriene B4 production in lung cancer cells. Oncotarget 2018, 9, 26751. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Duan, M.; Tong, J.; Xu, J. MiR-26b suppresses tumor cell proliferation, migration and invasion by directly targeting COX-2 in lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4728–4737. [Google Scholar] [PubMed]
- Rahmati-Yamchi, M.; Ghareghomi, S.; Haddadchi, G.; Milani, M.; Aghazadeh, M.; Daroushnejad, H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol. Biol. Rep. 2014, 41, 6247–6252. [Google Scholar] [CrossRef]
- El Bairi, K.; Ouzir, M.; Agnieszka, N.; Khalki, L. Anticancer potential of Trigonella foenum graecum: Cellular and molecular targets. Biomed. Pharmacother. 2017, 90, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer-review paper. Ann. Agric. Environ. Med. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Yadav, U.C.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chu, C.-J.; Li, X.-L.; Yao, S.; Yan, B.; Ren, H.-L.; Xu, N.-Y.; Liang, Z.-T.; Zhao, Z.-Z. Isolation and identification of antioxidant compounds in Vaccinium bracteatum Thunb. by UHPLC-Q-TOF LC/MS and their kidney damage protection. J. Funct. Foods 2014, 11, 62–70. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Shi, Q.; Jiang, Z.; Yang, J.; Cheng, Y.; Pang, Y.; Zheng, N.; Chen, J.; Chen, W.; Jia, L. A flavonoid glycoside compound from Murraya paniculata (L.) interrupts metastatic characteristics of A549 cells by regulating STAT3/NF-κB/COX-2 and EGFR signaling pathways. AAPS J. 2017, 19, 1779–1790. [Google Scholar] [CrossRef]
- Neuss, H.; Huang, X.; Hetfeld, B.K.; Deva, R.; Henklein, P.; Nigam, S.; Mall, J.W.; Schwenk, W.; Dubiel, W. The ubiquitin-and proteasome-dependent degradation of COX-2 is regulated by the COP9 signalosome and differentially influenced by coxibs. J. Mol. Med. 2007, 85, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Saha, C.; Hegde, P.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album-mediated COX-2 inhibition implicates destabilization of COX-2 mRNA. PLoS ONE 2015, 10, e0114965. [Google Scholar] [CrossRef] [Green Version]
- Świątek, P.; Gębczak, K.; Gębarowski, T.; Urniaz, R. Biological evaluation and molecular docking studies of dimethylpyridine derivatives. Molecules 2019, 24, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambandam, B.; Islam, V.I.H.; Raman, P.; Bhattacharjee, M.; Balasubramanian, A.; Thiyagarajan, D. Coal fly ash nanoparticles induced cytotoxicity and oxidative DNA damage and apoptosis in Chang liver cells. Afr. J. Pharm. Pharmacol. 2014, 8, 801–808. [Google Scholar]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar]
- Kumar, V.S.; Kumaresan, S.; Tamizh, M.M.; Islam, M.I.H.; Thirugnanasambantham, K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019, 61, 152830. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Dominguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Park, S.J.; Yun, K.-J.; Cho, Y.-W.; Park, H.-J.; Lee, K.-T. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur. J. Pharmacol. 2008, 584, 175–184. [Google Scholar] [CrossRef]
- Park, J.S.; Jun, H.J.; Cho, M.J.; Cho, K.H.; Lee, J.S.; Zo, J.I.; Pyo, H. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin. Cancer Res. 2006, 12, 4989–4999. [Google Scholar] [CrossRef] [Green Version]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, Q.A.; Thi, T.H.N.; Pham, M.Q.; Delfino, D.; Do, T.T. Antiproliferative and antiinflammatory coxib–combretastatin hybrids suppress cell cycle progression and induce apoptosis of MCF7 breast cancer cells. Mol. Divers. 2021, 25, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Guruswamy, S.; Rao, C.V. Synergistic effects of lovastatin and celecoxib on caveolin-1 and its down-stream signaling molecules: Implications for colon cancer prevention. Int. J. Oncol. 2009, 35, 1037–1043. [Google Scholar] [PubMed] [Green Version]
- Jeon, Y.-W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2013, 29, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Qiu, R.; Chen, J.; Sima, J.; Shen, X.; Liu, D.; Shen, J. NS398 induces apoptosis in non-small cell lung cancer cells. J. Cancer Res. Clin. Oncol. 2012, 138, 119–124. [Google Scholar] [CrossRef]
- Ji, Y.; He, Y.; Liu, L.; Zhong, X. MiRNA-26b regulates the expression of cyclooxygenase-2 in desferrioxamine-treated CNE cells. FEBS Lett. 2010, 584, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Thirugnanasambantham, K.; Muralidaran, S.; Mandal, A.K.A. Molecular cloning, computational and expression analysis of anthocyanidin reductase in tea (Camellia sinensis). Appl. Biochem. Biotechnol. 2014, 174, 130–145. [Google Scholar] [CrossRef]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.-I.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef]
- Abdullah, A.; Maged, M.; Hairul-Islam, M.I.; Osama, I.A.; Maha, H.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019, 14, e0215981. [Google Scholar] [CrossRef]
- Hanieh, H.; Ibrahim, H.-I.M.; Mohammed, M.; Alwassil, O.I.; Abukhalil, M.H.; Farhan, M. Activation of aryl hydrocarbon receptor signaling by gallic acid suppresses progression of human breast cancer in vitro and in vivo. Phytomedicine 2021, 96, 153817. [Google Scholar] [CrossRef]
- Yi, Q.-Y.; Zhang, W.-Y.; He, M.; Du, F.; Wang, X.-Z.; Wang, Y.-J.; Gu, Y.-Y.; Bai, L.; Liu, Y.-J. Anticancer and antibacterial activity in vitro evaluation of iridium (III) polypyridyl complexes. J. Biol. Inorg. Chem. 2019, 24, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Șoica, C.; Babaev, M.; Petrova, A.; Khusnutdinova, E.; Poptsov, A.; Macașoi, I.; Drăghici, G.; Avram, Ș.; Vlaia, L. 3-Pyridinylidene Derivatives of Chemically Modified Lupane and Ursane Triterpenes as Promising Anticancer Agents by Targeting Apoptosis. Int. J. Mol. Sci. 2021, 22, 10695. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; El-Fakharany, E.M.; Abu-Serie, M.M.; Almehdar, H.A.; Redwan, E.M. Divergent anticancer activity of free and formulated camel milk α-lactalbumin. Cancer Investig. 2017, 35, 610–623. [Google Scholar] [CrossRef] [PubMed]
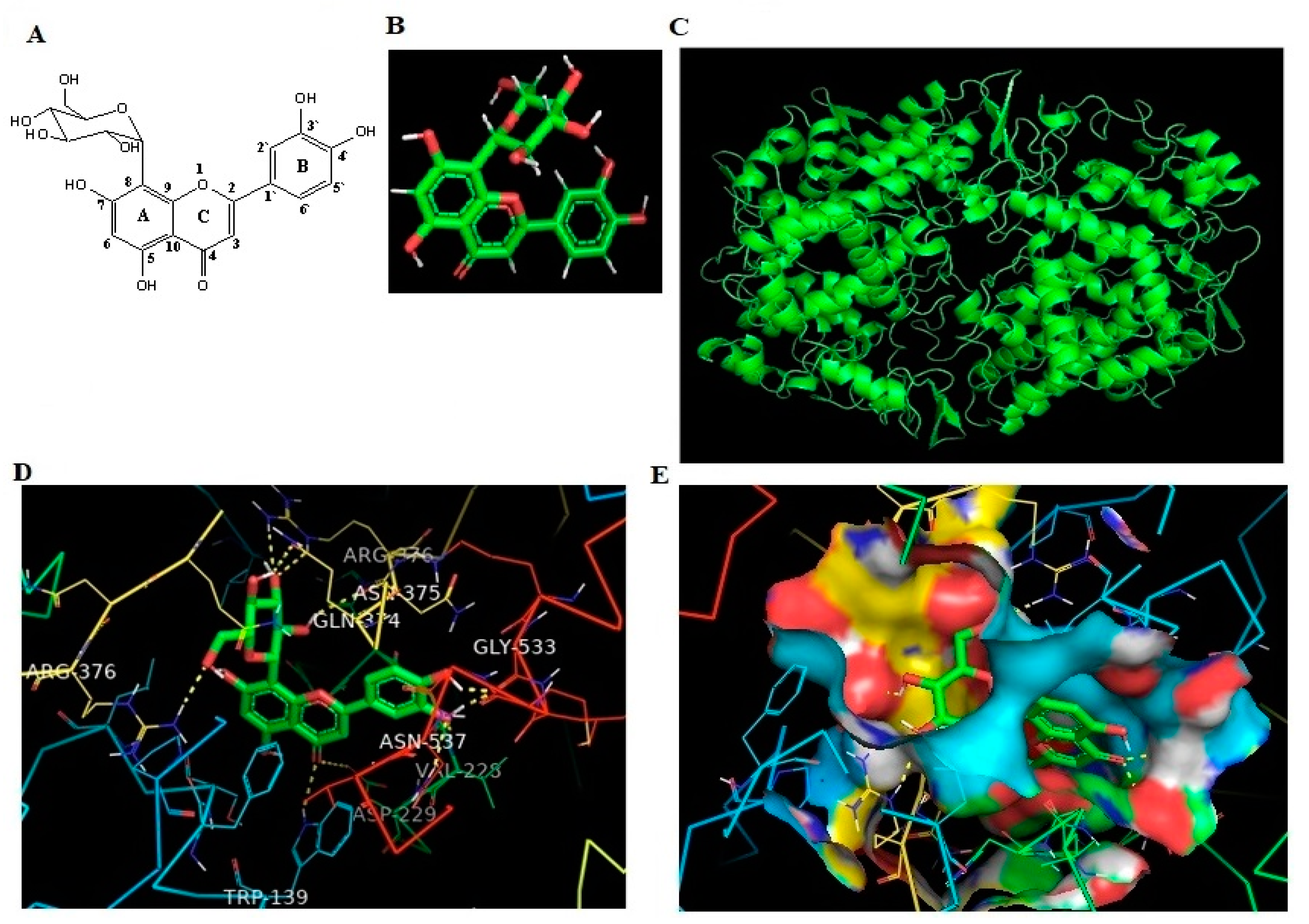


| Protein | Ligand | Binding Energy | Ligand Efficiency | Intermole Energy | Ligand Atoms (Ring) | Docked Amino Acid Residue (Bond Length) |
|---|---|---|---|---|---|---|
| COX-2 | OT | −10.43 | −0.33 | −10.37 | C-3 OH (B) | Chain B GLY`533/O (1.7 Å) |
| C-4 OH (B) | Chain B GLY`533/O (1.8 Å) | |||||
| C-4 O (B) | Chain B VAL`228/HN (1.8 Å) | |||||
| C-4 O (B) | Chain B ASN`537/2HD2 (2.4 Å) | |||||
| C-4 O (C) | Chain B ASP`229/OD2 (3.3 Å) | |||||
| C-4 O (C) | Chain A TRP`139/HE1 (2.5 Å) | |||||
| C-2` OH (C) | Chain B ASN`375/O (2.4 Å) | |||||
| C-3` O (C) | Chain B ARG`376/HE (2.5 Å) | |||||
| C-3` O (C) | Chain B ARG`376/2HH2 (2.3 Å) | |||||
| C-4` OH (C) | Chain B GLN`374/OE1 (2.2 Å) | |||||
| C-6` O (C) | Chain A ARG`376/1HH2 (2.2 Å) |
| Protein | Ligand | Binding Energy | Ligand Efficiency | Intermole Energy | Ligand Atoms (Ring) | Docked Amino Acid Residue (Bond Length) |
|---|---|---|---|---|---|---|
| COX-2 | CLX | −9.4 | −0.18 | −8.5 | C-4 O (B) | Chain B THR387/HE (1.9 Å) |
| C-4 O (C) | Chain B THR385/HH2 (2.1 Å) | |||||
| C-2 O (C) | Chain B THR206/HN (2.0 Å) | |||||
| C-3-OH (B) | Chain A ARG 376/OE1 (1.9 Å) | |||||
| C-3 O (C) | Chain B GLY 535/O (1.8 Å) |
| Primer Name | Forward Sequence | Reverse Sequence | Product Size |
|---|---|---|---|
| COX-2 | TGTATGCTACCATCTGGCTTCGG | GTTTGGAACAGTCGCTCGTCATC | 190 |
| BCL-2 | TGTGGATGACTGACTACCTGAACC | CAGCCAGGAGAAATCAAACAGAGG | 186 |
| iNOS | ACAACAGGAACCTACCAGCTCA | GATGTTGTAGCGCTGTGTGTCA | 190 |
| CYP-1A1 | GGCCACTTTGACCCTTACAA | CAGGTAACGGAGGACAGGAA | 236 |
| β-actin | AAGATCCTGACCGAGCGTGG | CAGCACTGTGTTGGCATAGAGG | 225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.E.; Ibrahim, H.-I.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals 2022, 15, 154. https://doi.org/10.3390/ph15020154
Khalil HE, Ibrahim H-IM, Ahmed EA, Emeka PM, Alhaider IA. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals. 2022; 15(2):154. https://doi.org/10.3390/ph15020154
Chicago/Turabian StyleKhalil, Hany Ezzat, Hairul-Islam Mohamed Ibrahim, Emad A. Ahmed, Promise Madu Emeka, and Ibrahim A. Alhaider. 2022. "Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a" Pharmaceuticals 15, no. 2: 154. https://doi.org/10.3390/ph15020154
APA StyleKhalil, H. E., Ibrahim, H.-I. M., Ahmed, E. A., Emeka, P. M., & Alhaider, I. A. (2022). Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals, 15(2), 154. https://doi.org/10.3390/ph15020154