Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Primary and Secondary Outcomes
2.3. Assays
2.4. Statistical Analyses
3. Results
3.1. Patient Demographic Characteristics
3.2. Group Differences
3.3. TOS/TAC Ratio Correlations
3.4. Oxidant—Antioxidant Independent Associations
3.5. Predictors of Mortality
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso de Vega, J.M.; Díaz, J.; Serrano, E.; Carbonell, L.F. Plasma redox status relates to severity in critically ill patients. Crit. Care Med. 2000, 28, 1812–1814. [Google Scholar] [CrossRef] [PubMed]
- Goodyear-Bruch, C.; Pierce, J.D. Oxidative stress in critically ill patients. Am. J. Crit. Care 2002, 11, 543, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef] [Green Version]
- Karapetsa, M.; Pitsika, M.; Goutzourelas, N.; Stagos, D.; Tousia Becker, A.; Zakynthinos, E. Oxidative status in ICU patients with septic shock. Food Chem. Toxicol. 2013, 61, 106–111. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, E.; Kaushik, S.; Kumar Srivastava, V.; Mehta, S.K.; Jyoti, A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scand. J. Immunol. 2018, 87, e12653. [Google Scholar] [CrossRef] [Green Version]
- Girardot, T.; Rimmelé, T.; Venet, F.; Monneret, G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis Int. J. Program. Cell Death 2017, 22, 295–305. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Martín Giménez, V.M.; de Las Heras, N.; Ferder, L.; Lahera, V.; Reiter, R.J.; Manucha, W. Potential Effects of Melatonin and Micronutrients on Mitochondrial Dysfunction during a Cytokine Storm Typical of Oxidative/Inflammatory Diseases. Disease 2021, 9, 30. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [Green Version]
- Sygitowicz, G.; Sitkiewicz, D. Molecular mechanisms of organ damage in sepsis: An overview. Braz. J. Infect. Dis. 2020, 24, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef]
- Gelain, D.P.; de Bittencourt Pasquali, M.A.; Comim, C.M.; Grunwald, M.S.; Ritter, C.; Tomasi, C.D.; Alves, S.C.; Quevedo, J.; Dal-Pizzol, F.; Moreira, J.C.F. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock 2011, 35, 466–470. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Sepsis Definitions Task Force Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef] [Green Version]
- Matusik, P.; Prokopowicz, Z.; Norek, B.; Olszanecka-Glinianowicz, M.; Chudek, J.; Malecka-Tendera, E. Oxidative/Antioxidative status in obese and sport trained children: A comparative study. BioMed Res. Int. 2015, 2015, 315747. [Google Scholar] [CrossRef]
- Masomi-Bornwasser, J.; Kurz, E.; Frenz, C.; Schmitt, J.; Wesp, D.M.A.; König, J.; Lotz, J.; Ringel, F.; Kerz, T.; Krenzlin, H.; et al. The Influence of Oxidative Stress on Neurological Outcomes in Spontaneous Intracerebral Hemorrhage. Biomolecules 2021, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Deutschman, C.S. Elevated malondialdehyde levels in sepsis—Something to “stress” about? Crit. Care 2014, 18, 125. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Abreu-González, P.; López, R.O.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Palmero, S.; et al. Serum total antioxidant capacity during the first week of sepsis and mortality. J. Crit. Care 2018, 47, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.; Shiesh, S.; Chi, C.; Tu, Y.; Hor, L.; Shieh, C.; Chen, M. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit. Care 2006, 10, R36. [Google Scholar] [CrossRef] [Green Version]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Pérez-Cejas, A.; Abreu-González, P.; Ortiz-López, R.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; et al. Association between DNA and RNA oxidative damage and mortality in septic patients. J. Crit. Care 2019, 54, 94–98. [Google Scholar] [CrossRef]
- Skenderi, K.P.; Tsironi, M.; Lazaropoulou, C.; Anastasiou, C.A.; Matalas, A.-L.; Kanavaki, I.; Thalmann, M.; Goussetis, E.; Papassotiriou, I.; Chrousos, G.P. Changes in free radical generation and antioxidant capacity during ultramarathon foot race. Eur. J. Clin. Investig. 2008, 38, 159–165. [Google Scholar] [CrossRef]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef]
- Gizi, A.; Papassotiriou, I.; Apostolakou, F.; Lazaropoulou, C.; Papastamataki, M.; Kanavaki, I.; Kalotychou, V.; Goussetis, E.; Kattamis, A.; Rombos, I.; et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells. Mol. Dis. 2011, 46, 220–225. [Google Scholar] [CrossRef]
- Miliaraki, M.; Briassoulis, P.; Ilia, S.; Polonifi, A.; Mantzourani, M.; Briassouli, E.; Vardas, K.; Nanas, S.; Pistiki, A.; Theodorakopoulou, M.; et al. Survivin and caspases serum protein levels and survivin variants mRNA expression in sepsis. Sci. Rep. 2021, 11, 1049. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef]
- Almalki, W.H. The sepsis induced defective aggravation of immune cells: A translational science underling chemico-biological interactions from altered bioenergetics and/or cellular metabolism to organ dysfunction. Mol. Cell. Biochem. 2021, 476, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef] [PubMed]
- Toro-Pérez, J.; Rodrigo, R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. 2021, 26, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Andrades, M.; Ritter, C.; Moreira, J.C.F.; Dal-Pizzol, F. Oxidative parameters differences during non-lethal and lethal sepsis development. J. Surg. Res. 2005, 125, 68–72. [Google Scholar] [CrossRef]
- Hsiao, S.-Y.; Kung, C.-T.; Su, C.-M.; Lai, Y.-R.; Huang, C.-C.; Tsai, N.-W.; Wang, H.-C.; Cheng, B.-C.; Su, Y.-J.; Lin, W.-C.; et al. Impact of oxidative stress on treatment outcomes in adult patients with sepsis: A prospective study. Medicine 2020, 99, e20872. [Google Scholar] [CrossRef]
- Petrovic, J.; Turnic, T.N.; Zivkovic, V.; Andjic, M.; Draginic, N.; Stojanovic, A.; Milinkovic, I.; Bolevich, S.; Jevdjic, J.; Jakovljevic, V. Correlation of Redox Status with Procalcitonin and C-reactive Protein in Septic Patients. Oxid. Med. Cell. Longev. 2020, 2020, 5147364. [Google Scholar] [CrossRef]
- Bjugstad, K.B.; Rael, L.T.; Levy, S.; Carrick, M.; Mains, C.W.; Slone, D.S.; Bar-Or, D. Oxidation-Reduction Potential as a Biomarker for Severity and Acute Outcome in Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2016, 2016, 6974257. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Almeida, T.; Abreu-González, P.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Jiménez, A. Association between serum total antioxidant capacity and mortality in severe septic patients. J. Crit. Care 2015, 30, e7–e12. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Almeida, T.; Abreu-González, P.; Ramos, L.; Argueso, M.; Riaño-Ruiz, M.; Solé-Violán, J.; Jiménez, A. Total antioxidant capacity is associated with mortality of patients with severe traumatic brain injury. BMC Neurol. 2015, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity. Brain Sci. 2020, 10, E110. [Google Scholar] [CrossRef] [Green Version]
- Asci, A.; Surmeli-Onay, O.; Erkekoglu, P.; Yigit, S.; Yurdakok, M.; Kocer-Gumusel, B. Oxidant and antioxidant status in neonatal proven and clinical sepsis according to selenium status. Pediatr. Int. 2015, 57, 1131–1137. [Google Scholar] [CrossRef]
- Keskin, D.; Kiziltunc, A. Reduction of Total Antioxidant Capacity after Femoral Fracture. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 293–295. [Google Scholar] [PubMed]
- Servia, L.; Serrano, J.C.E.; Pamplona, R.; Badia, M.; Montserrat, N.; Portero-Otin, M.; Trujillano, J. Location-dependent effects of trauma on oxidative stress in humans. PLoS ONE 2018, 13, e0205519. [Google Scholar] [CrossRef]
- Obi, J.; Pastores, S.M.; Ramanathan, L.V.; Yang, J.; Halpern, N.A. Treating sepsis with vitamin C, thiamine, and hydrocortisone: Exploring the quest for the magic elixir. J. Crit. Care 2020, 57, 231–239. [Google Scholar] [CrossRef]
- Belsky, J.B.; Wira, C.R.; Jacob, V.; Sather, J.E.; Lee, P.J. A review of micronutrients in sepsis: The role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr. Res. Rev. 2018, 31, 281–290. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, E2097. [Google Scholar] [CrossRef]
- Briassoulis, G.; Ilia, S. Vitamin D Deficiency in Sepsis: “Body Humors” Imbalance or Sepsis “Epiphenomenon”? Crit. Care Med. 2017, 45, 376–377. [Google Scholar] [CrossRef] [Green Version]
- Briassoulis, P.; Ilia, S.; Briassouli, E.; Miliaraki, M.; Briassoulis, G. The lonely glutamine tree in the middle of the infinite critically ill forest. Crit. Care Lond. Engl. 2021, 25, 342. [Google Scholar] [CrossRef]
- Briassouli, E.; Briassoulis, G. Glutamine randomized studies in early life: The unsolved riddle of experimental and clinical studies. Clin. Dev. Immunol. 2012, 2012, 749189. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-F.; Lu, L.; Dai, C.-M.; Wang, D.; Yang, Y.-H.; Yang, Y.-W.; Liu, Y.-S. Analysis of the diagnostic efficiency of serum oxidative stress parameters in patients with breast cancer at various clinical stages. Clin. Biochem. 2016, 49, 692–698. [Google Scholar] [CrossRef]
- Eren, E.; Abuhandan, M.; Solmaz, A.; Taşkın, A. Serum paraoxonase/arylesterase activity and oxidative stress status in children with metabolic syndrome. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 163–168. [Google Scholar] [CrossRef]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J.; et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowicka, G.; Dyląg, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chełchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxid. Med. Cell. Longev. 2017, 2017, 5621989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, E.; Özer, Ö.F.; Erek Toprak, A.; Erman, H.; Torun, E.; Kesgin Ayhan, S.; Caglar, H.G.; Selek, S.; Kocyigit, A. Oxidative Stress Status in Childhood Obesity: A Potential Risk Predictor. Med. Sci. Monit. 2016, 22, 3673–3679. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, C.; Yu, N.; Si, L.; Zhu, L.; Zeng, A.; Liu, Z.; Wang, X. INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway. Biomed. Pharmacother. 2019, 111, 151–161. [Google Scholar] [CrossRef]
- Gu, M.; Mei, X.-L.; Zhao, Y.-N. Sepsis and Cerebral Dysfunction: BBB Damage, Neuroinflammation, Oxidative Stress, Apoptosis and Autophagy as Key Mediators and the Potential Therapeutic Approaches. Neurotox. Res. 2021, 39, 489–503. [Google Scholar] [CrossRef]
- Vardas, K.; Apostolou, K.; Briassouli, E.; Goukos, D.; Psarra, K.; Botoula, E.; Tsagarakis, S.; Magira, E.; Routsi, C.; Nanas, S.; et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. BioMed Res. Int. 2014, 2014, 803561. [Google Scholar] [CrossRef]
- Vardas, K.; Ilia, S.; Sertedaki, A.; Charmandari, E.; Briassouli, E.; Goukos, D.; Apostolou, K.; Psarra, K.; Botoula, E.; Tsagarakis, S.; et al. Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Med. Exp. 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Briassouli, E.; Tzanoudaki, M.; Goukos, D.; Routsi, C.; Nanas, S.; Vardas, K.; Apostolou, K.; Kanariou, M.; Daikos, G.; Briassoulis, G. Glutamine may repress the weak LPS and enhance the strong heat shock induction of monocyte and lymphocyte HSP72 proteins but may not modulate the HSP72 mRNA in patients with sepsis or trauma. BioMed Res. Int. 2015, 2015, 806042. [Google Scholar] [CrossRef] [Green Version]
- Briassouli, E.; Goukos, D.; Daikos, G.; Apostolou, K.; Routsi, C.; Nanas, S.; Briassoulis, G. Glutamine suppresses Hsp72 not Hsp90α and is not inducing Th1, Th2, or Th17 cytokine responses in human septic PBMCs. Nutrition 2014, 30, 1185–1194. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Lagarde, D.; Jeanson, Y.; Portais, J.-C.; Galinier, A.; Ader, I.; Casteilla, L. The emerging roles of lactate as a redox substrate and signaling molecule in adipose tissues. J. Physiol. Biochem. 2020, 76, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, K.F.; Almalki, A.A.; Alsanie, W.F.; Alzahrani, K.J.; Kabrah, S.M.; Elshopakey, G.E.; Alghamdi, A.A.A.; Lokman, M.S.; Sberi, H.A.; Bauomy, A.A.; et al. Protocatechuic acid attenuates lipopolysaccharide-induced septic lung injury in mice: The possible role through suppressing oxidative stress, inflammation and apoptosis. J. Food Biochem. 2021, 45, e13915. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, J.; Kang, Q.; He, X.; Guo, D. Protective Effect of Hesperidin Against Sepsis-Induced Lung Injury by Inducing the Heat-Stable Protein 70 (Hsp70)/Toll-Like Receptor 4 (TLR4)/ Myeloid Differentiation Primary Response 88 (MyD88) Pathway. Med. Sci. Monit. 2019, 25, 107–114. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Domínguez-Rodriguez, A.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Ferreres, J.; Cabrera, J.; Igeño, J.C.; et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit. Care 2013, 17, R290. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, P.; Pistiki, A.; Theodorakopoulou, M.; Christodoulopoulou, T.; Damoraki, G.; Goukos, D.; Briassouli, E.; Dimopoulou, I.; Armaganidis, A.; Nanas, S.; et al. Immunoparalysis: Clinical and immunological associations in SIRS and severe sepsis patients. Cytokine 2017, 92, 83–92. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis Int. J. Program. Cell Death 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. JAD 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef] [Green Version]
- Modanloo, M.; Shokrzadeh, M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-carnitine. Iran. J. Kidney Dis. 2019, 13, 74–86. [Google Scholar]
- Qi, Z.; Wang, R.; Liao, R.; Xue, S.; Wang, Y. Neferine Ameliorates Sepsis-Induced Myocardial Dysfunction Through Anti-Apoptotic and Antioxidative Effects by Regulating the PI3K/AKT/mTOR Signaling Pathway. Front. Pharmacol. 2021, 12, 706251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cai, S.; Jin, Y.; Wu, F.; He, J.; Wu, X.; Tan, Y.; Wang, Y. Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-Dependent Mitophagy. Oxid. Med. Cell. Longev. 2021, 2021, 2989974. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Feng, J.; Wu, R.; Yang, Y.; Dai, C.; Li, J.; Liao, Y.; Xiang, M.; Wang, D.; Du, X.-B. Total Oxidant/Antioxidant Status in Sera of Patients with Esophageal Cancer. Med. Sci. Monit. 2017, 23, 3789–3794. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Control (H) (n = 89) | Trauma/Surgery (I) (n =112) | Sepsis (S) (n = 145) | p # |
|---|---|---|---|---|
| Age (years), mean ± SD | 40.3 ± 14.4 | 40.8 ± 15.7 | 62.2 ± 15.6 | 0.001 |
| Sex (Female/Male), (%) | 39/50 (44/56) | 23/89 (20/80) | 60/85 (41/59) | 0.478 |
| ICU LOS (days), mean ± SD | 21 ± 20.8 | 25 ± 21.9 | 0.636 | |
| Mortality ICU, n (%) | 9 (8) | 42 (30) † | 0.001 | |
| APACHE score, mean ± SD | 15 ± 5.6 | 22.4 ± 8.1 † | 0.001 | |
| SOFA score, mean ± SD | 8.3 ± 2.7 | 10 ± 3 † | 0.001 | |
| SAPS score, mean ± SD | 48.8 ± 9.9 | 71.5 ± 13.3 † | 0.001 | |
| WBC × 103 (cells/μL), median (IQR) | 12.7 (9.5–16.7) | 13.9 (8.6–20) | 0.037 | |
| Lactate (mg/dL), median (IQR) | 4.3 (2–13) | 4.7 (1.9–23) | 0.013 | |
| Glucose (mg/dL), median (IQR) | 147 (129–189) | 195 (134–267) † | 0.003 | |
| Albumin (mg/dL), median (IQR) | 3.1 (2.7–3.5) | 2.6 (2.3–2.9) † | 0.001 | |
| Urea (mg/dL), median (IQR) | 28 (20–39) | 83 (48.2–133) † | 0.001 | |
| Creatinine (mg/dL), median (IQR) | 0.81 (0.7–1.1) | 1.72 (1.1–2.9) † | 0.001 | |
| CRP (mg/dL) median (IQR) | 9.9 (4.1–37.4) | 25.6 (13–89) † | 0.001 | |
| Procalcitonin (ng/mL), median (IQR) | 0.77 (0.44–1.82) | 5 (1.14–28.8) † | 0.011 |
| Characteristic | Control (H) (n = 89) | Trauma/Surgery (I) (n =112) | Sepsis (S) (n = 145) | p # |
|---|---|---|---|---|
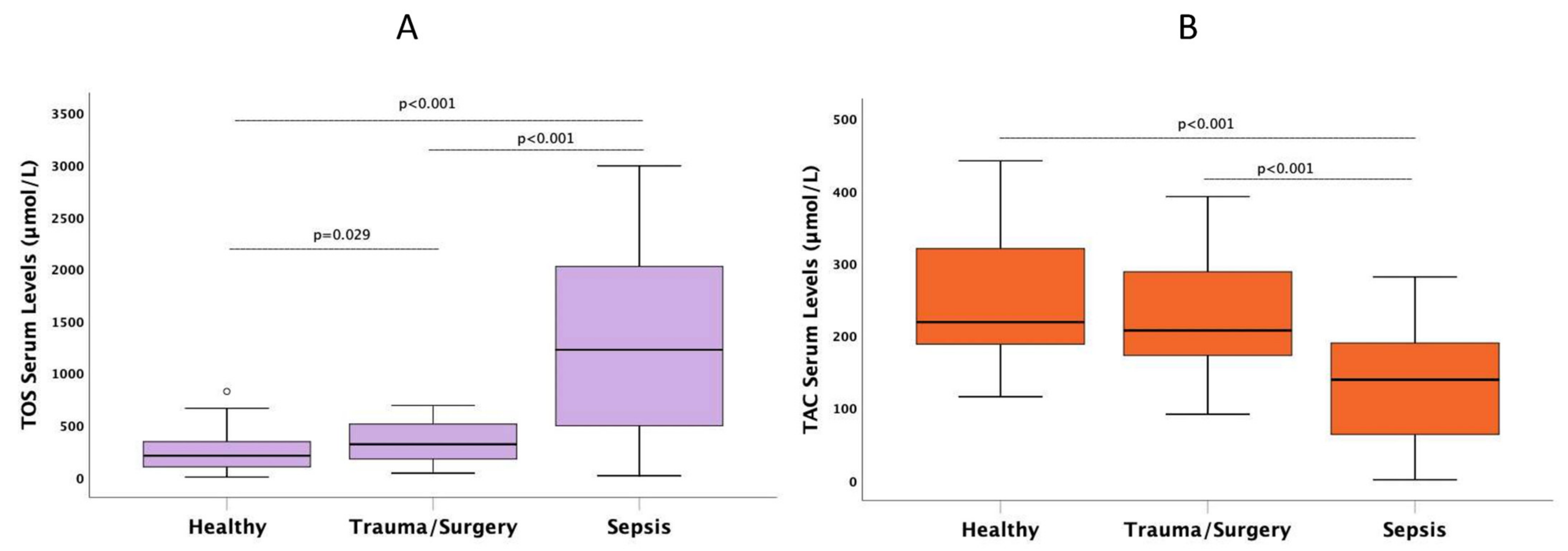
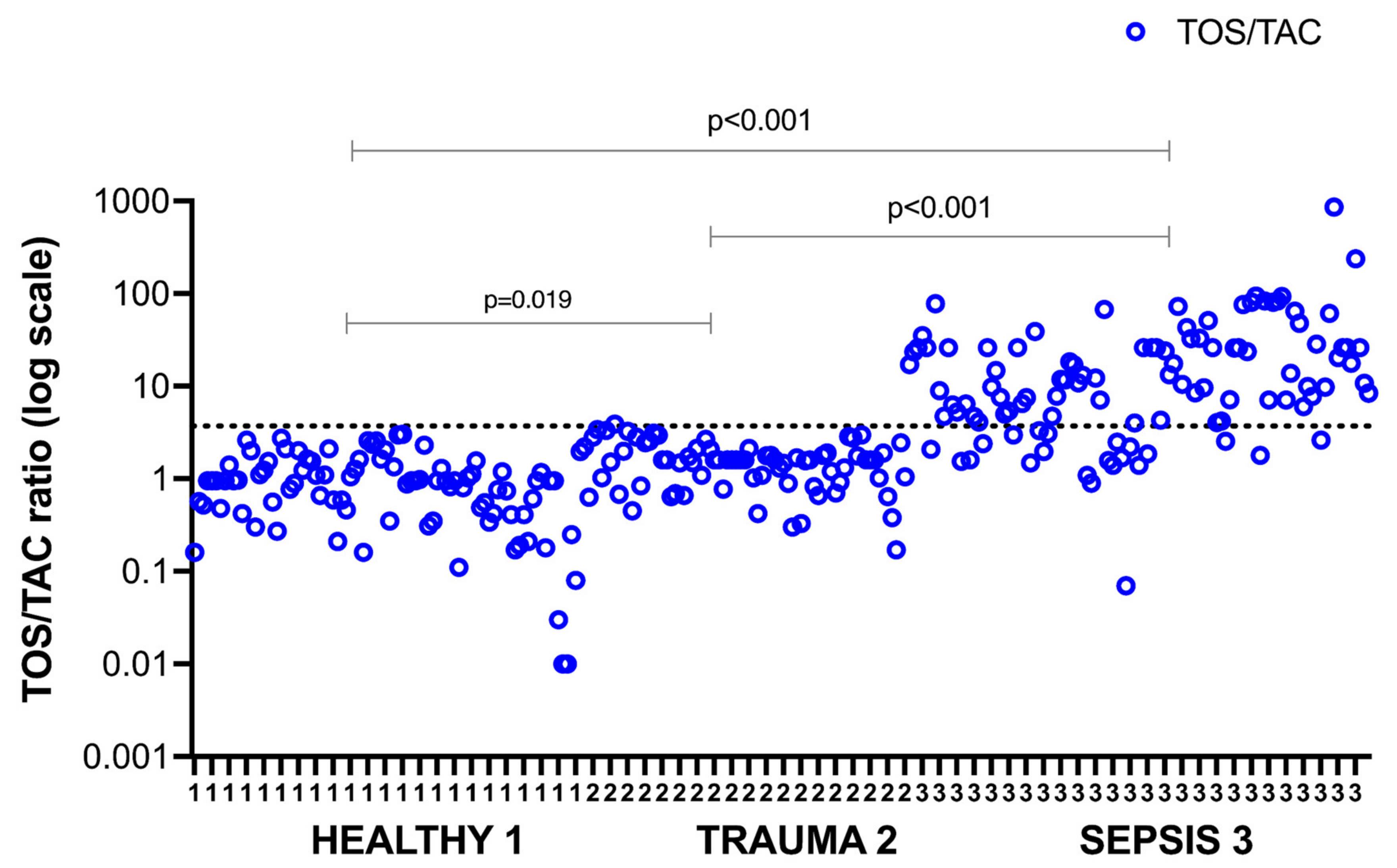
| TOS/TAC, median (IQR) | 0.82 (0.40–1.40) ** | 1.49 (0.81–2.20) * | 8.90 (4.05–24.9) † | 0.001 |
| TOS (μmol/L), median (IQR) | 206 (99–340) ** | 315.6 (175–510) * | 1222 (493–2022) † | 0.001 |
| TAC (μmol/L), median (IQR) | 218 (188–320) ** | 207 (172–288.5) | 138.8 (63.5–190) † | 0.001 |
| IL-6 (pg/mL), median (IQR) | 3.5 (1.24–14.8) ** | 77 (19–157) * | 86 (27–399) † | 0.001 |
| IL-8 (pg/mL), median (IQR) | 53.5 (25.5–154.7) | 50.5 (28–96) | 126.4 (56.4–233) | 0.083 |
| IL-10 (pg/mL), median (IQR) | 3.7 (0.4–11.5) ** | 10.2 (0.01–25) | 16.7 (5.2–68.7) † | 0.001 |
| IL-27 (pg/mL), median (IQR) | 0.4 (0.21–0.6) ** | 0.27 (0.2–0.7) | 0.51 (0.25–0.9) † | 0.009 |
| IL-17 (pg/mL), median (IQR) | 0.77 (0.2–33) | 0.75 (0.2–7.9) | 2.4 (0.2–8.5) | 0.711 |
| Hsp72 (ng/mL), median (IQR) | 0.2 (0.1–0.4) ** | 0.22 (0.12–0.4) | 0.67 (0.2–1.6) ** | 0.001 |
| Hsp90 (ng/mL), median (IQR) | 43.8 (13.6–76.6) ** | 45.5 (26.2–106) | 75.7 (38.2–183) ** | 0.001 |
| IFN-γ (IU/mL), median (IQR) | 5.3 (0.53–9.7) | 4.6 (0.13–7.9) | 8.9 (3.2–15.2) ** | 0.007 |
| TNF-α (pg/mL), median (IQR) | 4.36 (2.1–12.7) | 7.8 (3.6–259) ** | 25.4 (6.1–332.5) | 0.016 |
| Zinc (μg/dl), median (IQR) | 80 (72–93) ** | 49.5 (35–56.2) | 49.5 (37–60.7) ** | 0.001 |
| Glutamine (μmol/L), mean ± SE | 460 ± 238 | 478 ± 153 | 492 ± 162 | 0.602 |
| Glutathione (μmol/L), median (IQR) | 750 (550–800) ** | 650 (550–900) | 825 (650–1050) ** | 0.029 |
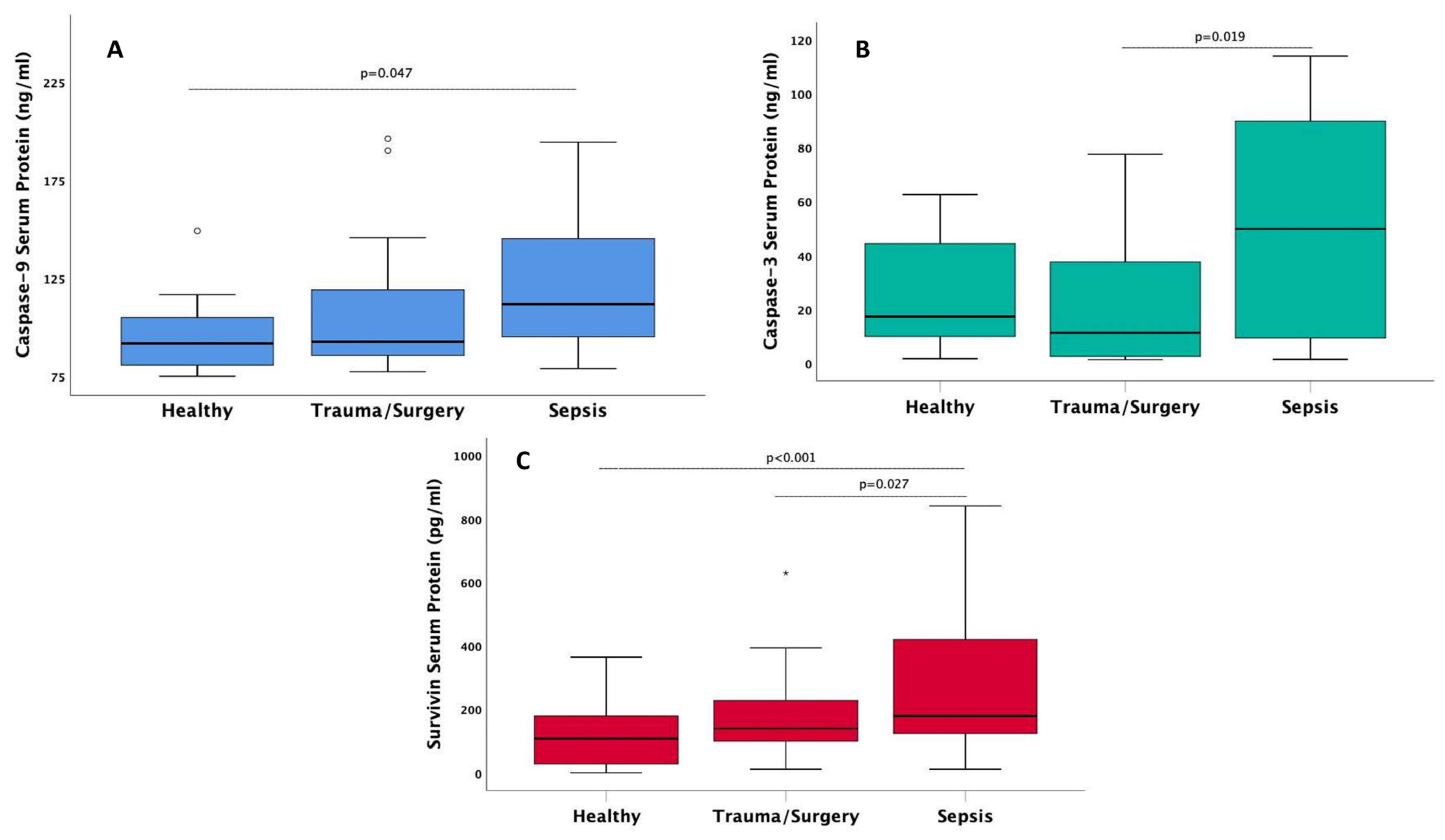
| Survivin protein (pg/mL), median (IQR) | 108 (28.6–180) ** | 140 (99.9–228) | 179 (125–420) ** | 0.001 |
| Caspase-3 (ng/mL), median (IQR) | 17.3 (9.9–44.4) | 11.3 (2.6–37.6) | 49.8 (9.3–90) ** | 0.021 |
| Caspase-9 (ng/mL), median (IQR) | 91.8 (80.7–105) ** | 92.7 (86–119) | 112 (95.3–146) | 0.014 |
| Survivin-WT isoform (copies/μL), median (IQR) | 0.005 (0.001–0.015) ** | 0.006 (0.001–0.019) | 0.046 (0.005–0.2) ** | 0.001 |
| Survivin-2B isoform (copies/μL), median (IQR) | 5.7 (2.4–11.3) | 10.9 (6.7–18.2) | 20.6 9.2–35.4) ** | 0.001 |
| Survivin-ΔΕx3 isoform (copies/μL), median (IQR) | 0.005 (0.003–0.016) | 0.011 (0.003–0.034) | 0.1 (0.012–0.36) ** | 0.001 |
| Survivin-3B isoform (copies/μL), median (IQR) | 0.1 (0.01–0.25) | 0.26 (0.03–0.57) ** | 0.11 (0.02–0.27) ** | 0.029 |
| TOS/TAC | TOS | TAC | |
|---|---|---|---|
| Spearman’s r (p-Value) | - | ||
| Age | −0.16 (0.116) | 0.44 (0.671) | −0.19 (0.083) |
| SOFA score | 0.22 (0.001) | 0.22 (0.003) | −0.16 (0.027) |
| APACHE score | 0.216 (0.001) | 0.22 (0.006) | −0.17 (0.029) |
| SAPS score | 0.366 (0.001) | 0.37 (0.001) | −0.37 (0.001) |
| CRP | 0.215 (0.008) | 0.17 (0.03) | −0.23 (0.003) |
| Procalcitonin | 0.56 (0.002) | 0.5 (0.007) | −0.5 (0.008) |
| IL-6 | 0.38 (0.001) | 0.35 (0.001) | −0.28 (0.001) |
| IL-10 | 0.28 (0.001) | 0.224 (0.001) | −0.18 (0.007) |
| IL-27 | 0.21 (0.009) | 0.226 (0.005) | −0.08 (0.3) |
| IFN-γ | 0.31 (0.001) | 0.34 (0.001) | −0.08 (0.35) |
| Hsp72 | 0.3 (0.001) | 0.25 (0.001) | −0.32 (0.001) |
| Hsp90 | 0.2 (0.001) | 0.18 (0.004) | −0.13 (0.042) |
| Survivin protein | 0.19 (0.009) | 0.16 (0.026) | −0.17 (0.019) |
| Caspase-3 | 0.24 (0.088) | 0.17 (0.35) | −0.2 (0.13) |
| Caspase-9 | 0.16 (0.232) | 0.13 (0.35) | −0.1 (0.4) |
| Survivin-WT | 0.32 (0.001) | 0.3 (0.002) | −0.14 (0.14) |
| Survivin-2B | 0.34 (0.001) | 0.34 (0.001) | −0.19 (0.018) |
| Survivin-ΔΕx3 | 0.4 (0.001) | 0.4 (0.001) | −0.2 (0.012) |
| Survivin-3B | −0.10 (0.28) | −0.12 (0.21) | −0.27 (0.76) |
| Zinc (Zn) | −0.39 (0.001) | −0.37 (0.001) | 0.22 (0.03) |
| Asymptotic 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Test Result Variable(s) | Area | Std. Error | Asymptotic Sig. | Lower Bound | Upper Bound |
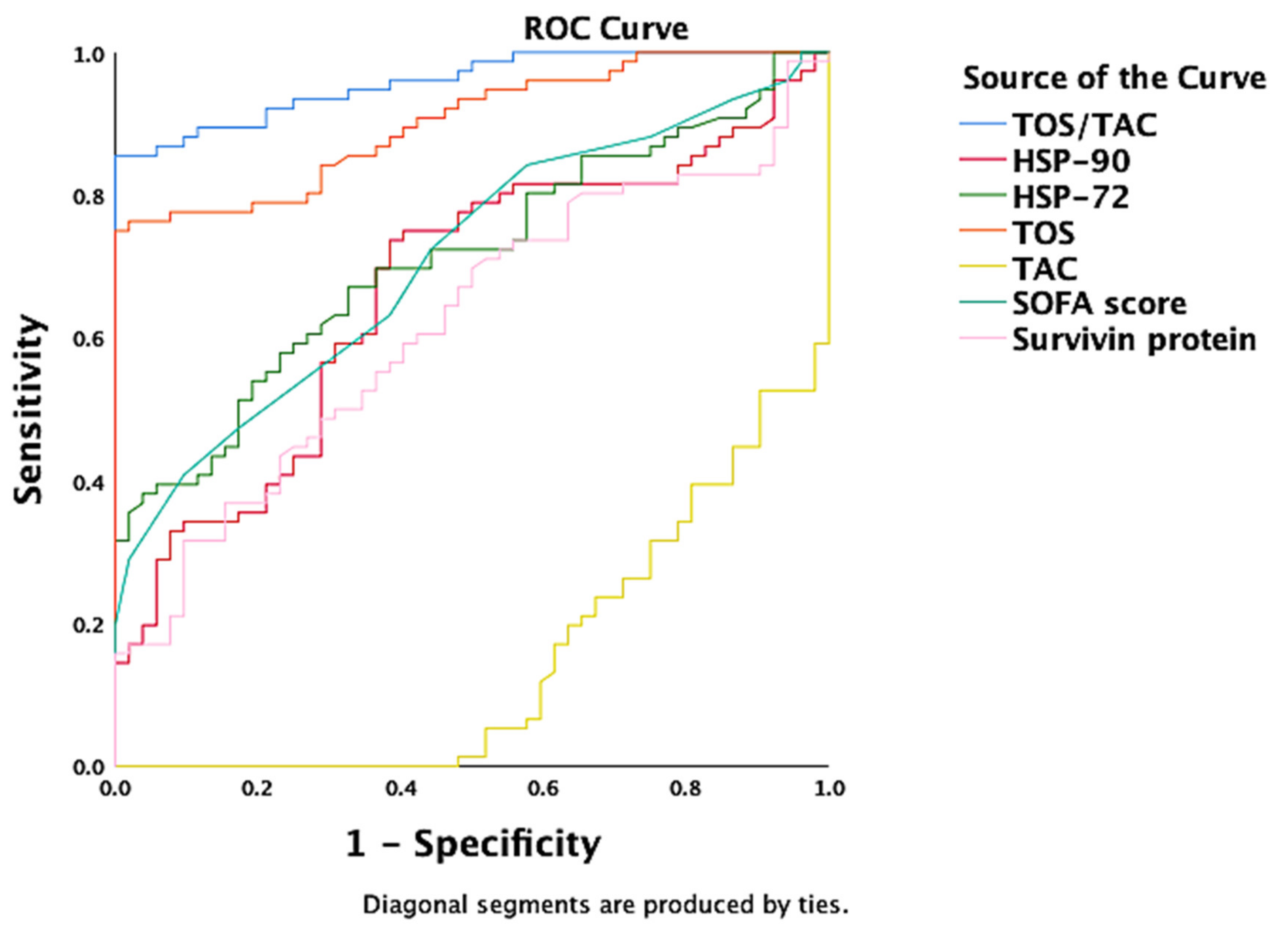
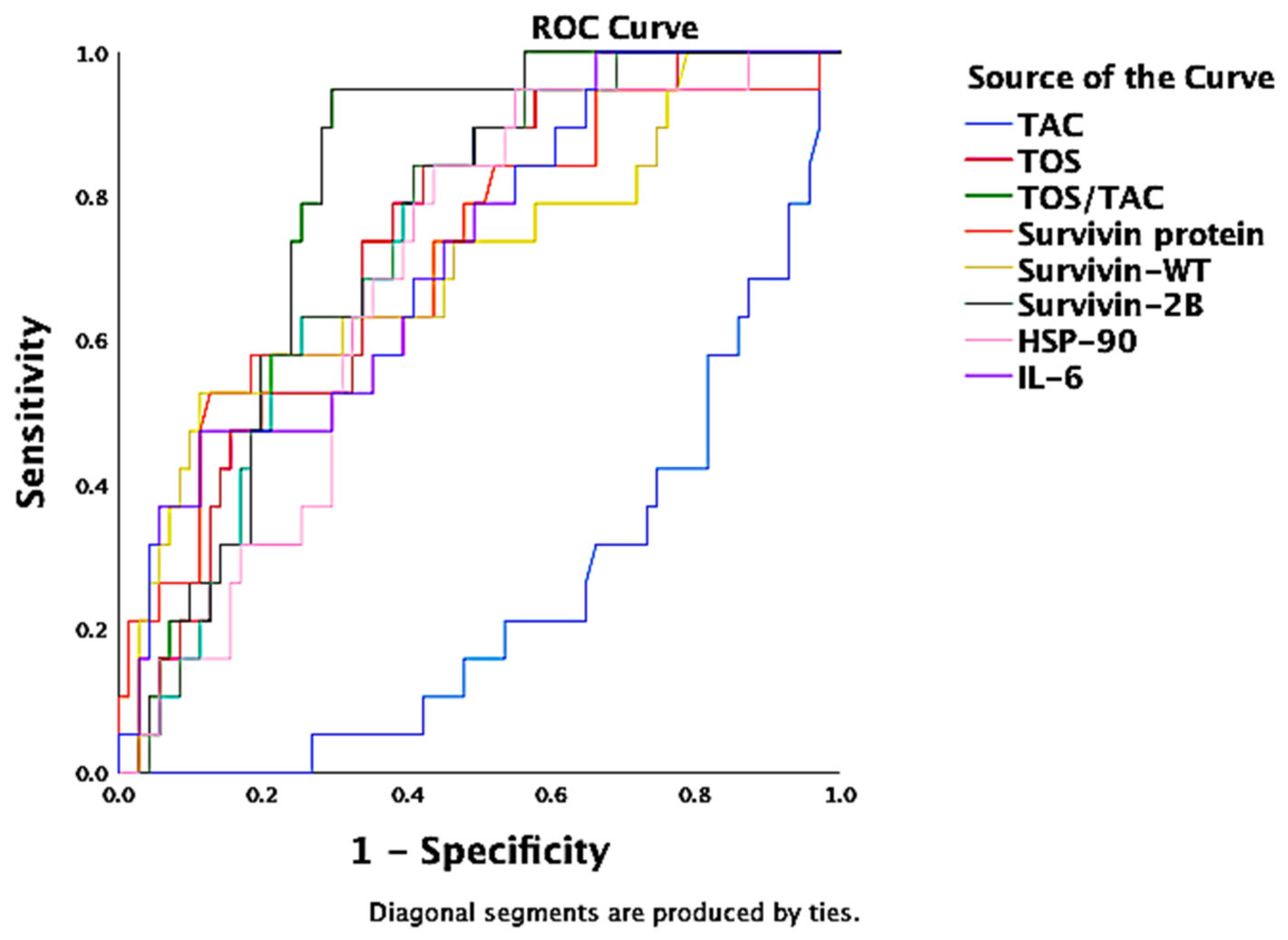
| TOS/TAC | 0.958 | 0.015 | <0.000 | 0.928 | 0.988 |
| Hsp90 | 0.666 | 0.048 | 0.001 | 0.572 | 0.761 |
| Hsp72 | 0.712 | 0.045 | <0.000 | 0.625 | 0.800 |
| TOS | 0.901 | 0.026 | <0.000 | 0.851 | 0.952 |
| SOFA score | 0.711 | 0.045 | <0.000 | 0.623 | 0.799 |
| Survivin protein | 0.618 | 0.049 | 0.024 | 0.521 | 0.715 |
| TAC | −0.852 | 0.033 | <0.000 | 0.084 | 0.213 |
| Laboratory Assay | Survival | Mortality | p # |
|---|---|---|---|
| TOS/TAC ratio, median (IQR) | 1.52 (0.7–3) | 7.5 (3.2–26) | 0.001 |
| TOS, median (IQR) | 318 (172.5–568) | 1243 (505–2104) | 0.001 |
| TAC, median (IQR) | 197 (160–257) | 142 (72–258) | 0.001 |
| IL-6 (pg/mL), median (IQR) | 32 (3.7–130) | 64 (26–310) | 0.002 |
| IL-8 (pg/mL), median (IQR) | 60.6 (30–168) | 77.8 (51.4–126) | 0.785 |
| IL-10 (pg/mL), median (IQR) | 9 (1.2–20) | 13.9 (1.5–55) | 0.148 |
| IL-27 (pg/mL), median (IQR) | 0.4 (0.2–0.7) | 0.6 (0.26–0.95) | 0.015 |
| Hsp72 (ng/mL), median (IQR) | 0.27 (0.14–0.70) | 0.58 (0.24–1.5) | 0.001 |
| Hsp90 (ng/mL), median (IQR) | 54.8 (25.4–117) | 62.4 (35–146) | 0.105 |
| IFN-γ (IU/mL), median (IQR) | 5.1 (0.6–10.3) | 6.69 (3.15–13.3) | 0.196 |
| TNF (pg/mL), median (IQR) | 7.85 (3. 6–67.7) | 4.62 (4.4–4.8) | 0.543 |
| Zinc (μg/dL), median (IQR) | 56 (41–76) | 55.5 (43–66) | 0.409 |
| Survivin-WT isoform (copies/μL), median (IQR) | 0.007 (0.002–0.03) | 0.022 (0.004–0.27) | 0.019 |
| Survivin-2B isoform (copies/μL), median (IQR) | 0.13 (0.01–0.3) | 0.16 (0.04–0.35) | 0.015 |
| Survivin protein (pg/mL), median (IQR) | 136.7 (84–240) | 179 (130–424) | 0.002 |
| Caspase-3 (ng/mL), median (IQR) | 21.1 (4.5–55.4) | 15.2 (5.1–92.6) | 0.674 |
| Caspase-9 (ng/mL), median (IQR) | 102 (87.5–136.5) | 99 (90.3–123.4) | 0.784 |
| Asymptotic 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Test Result Variable (s) | Area | Std. Error | Asymptotic Sig. | Lower Bound | Upper Bound |
| TOS/TAC | 0.801 | 0.046 | <0.001 | 0.710 | 0.892 |
| TOS | 0.732 | 0.058 | 0.002 | 0.619 | 0.846 |
| Survivin protein | 0.720 | 0.069 | 0.003 | 0.585 | 0.855 |
| Survivin-WT | 0.705 | 0.071 | 0.006 | 0.565 | 0.845 |
| Survivin-2B | 0.735 | 0.055 | 0.002 | 0.627 | 0.844 |
| Hsp90 | 0.687 | 0.060 | 0.013 | 0.569 | 0.805 |
| IL-6 | 0.720 | 0.063 | 0.003 | 0.597 | 0.843 |
| TAC | −0.758 | 0.057 | 0.001 | 0.131 | 0.354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliaraki, M.; Briassoulis, P.; Ilia, S.; Michalakakou, K.; Karakonstantakis, T.; Polonifi, A.; Bastaki, K.; Briassouli, E.; Vardas, K.; Pistiki, A.; et al. Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants 2022, 11, 231. https://doi.org/10.3390/antiox11020231
Miliaraki M, Briassoulis P, Ilia S, Michalakakou K, Karakonstantakis T, Polonifi A, Bastaki K, Briassouli E, Vardas K, Pistiki A, et al. Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants. 2022; 11(2):231. https://doi.org/10.3390/antiox11020231
Chicago/Turabian StyleMiliaraki, Marianna, Panagiotis Briassoulis, Stavroula Ilia, Kalliopi Michalakakou, Theodoros Karakonstantakis, Aikaterini Polonifi, Kalliopi Bastaki, Efrossini Briassouli, Konstantinos Vardas, Aikaterini Pistiki, and et al. 2022. "Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations" Antioxidants 11, no. 2: 231. https://doi.org/10.3390/antiox11020231
APA StyleMiliaraki, M., Briassoulis, P., Ilia, S., Michalakakou, K., Karakonstantakis, T., Polonifi, A., Bastaki, K., Briassouli, E., Vardas, K., Pistiki, A., Theodorakopoulou, M., Tavladaki, T., Spanaki, A.-M., Kondili, E., Dimitriou, H., Venihaki, M., Tsiodras, S., Georgopoulos, D., Mantzourani, M., ... Briassoulis, G. (2022). Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants, 11(2), 231. https://doi.org/10.3390/antiox11020231









