Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications
Abstract
1. Introduction
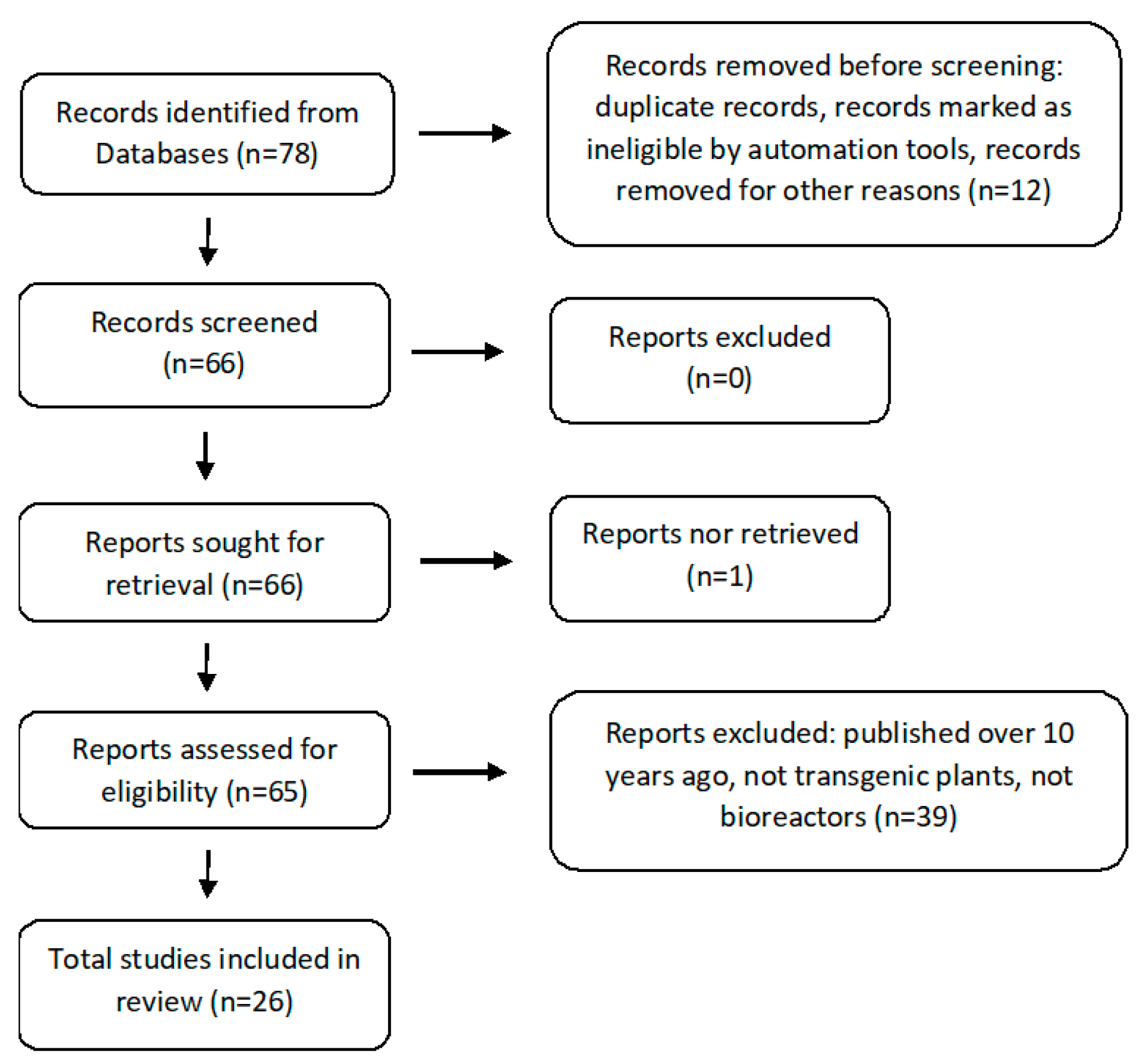
2. Criteria for the Selection of Experimental Articles in the Analyzed Subject
3. Transgenic Plants—Brief History and Potential Industrial Use
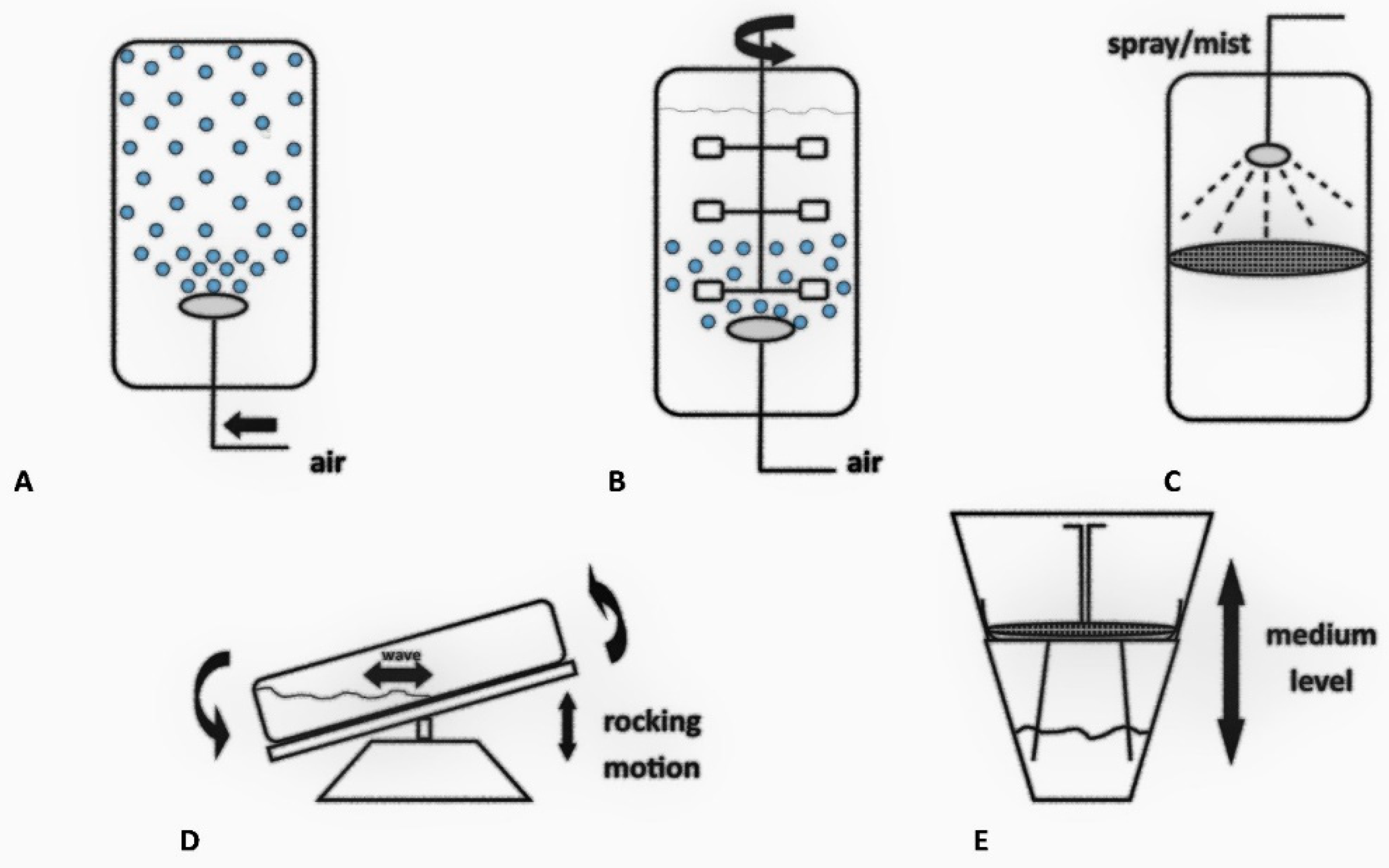
4. Plant Tissue and Organ Cultures in Bioreactors
4.1. Bubble Column Bioreactors
4.2. Stirred Tank Bioreactors
4.3. Nutrient Mist or Sprinkle Bioreactors
4.4. Wave-Mixed Bioreactors
4.5. Temporary Immersion System
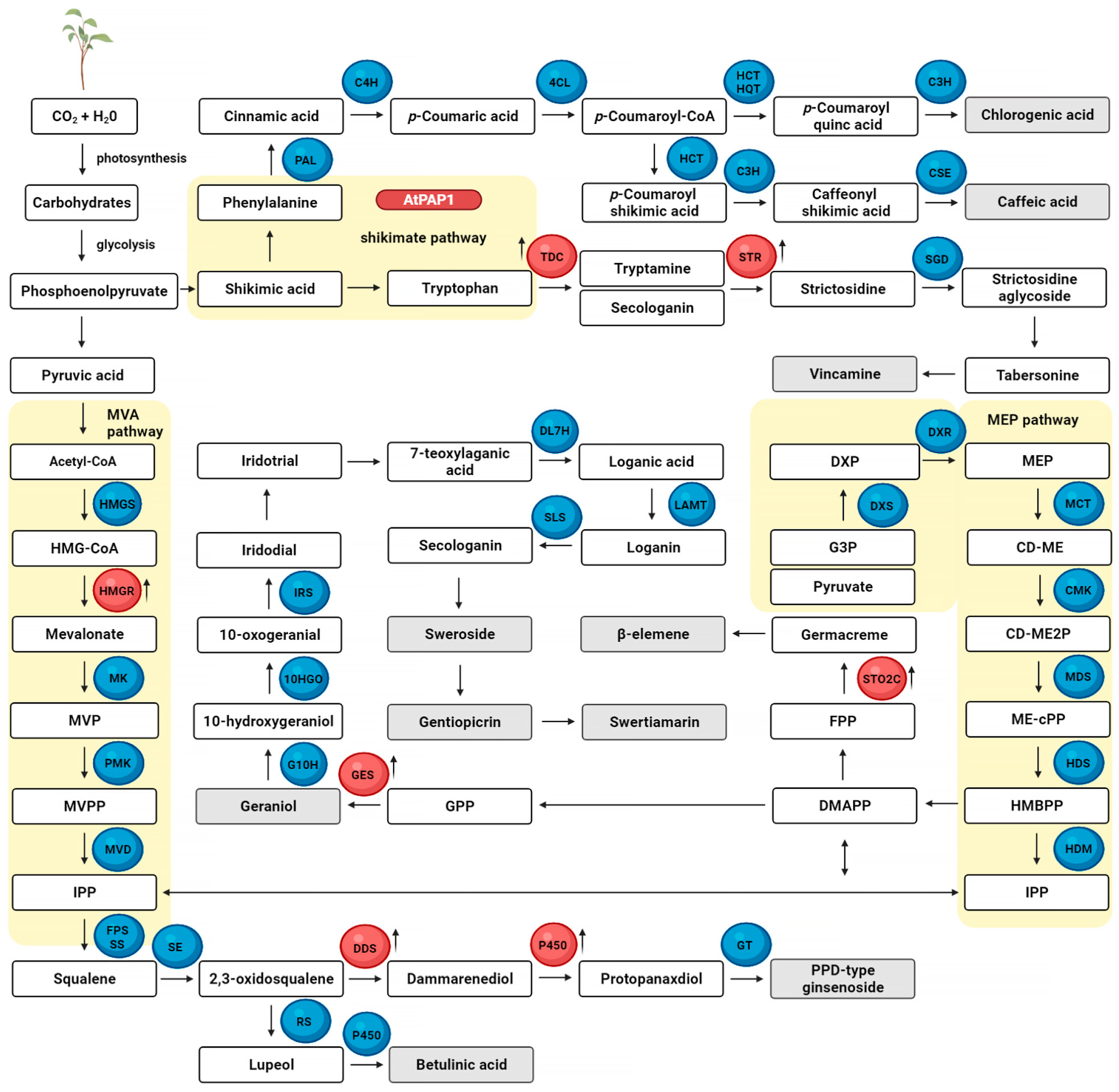
5. Transgenic Plants Manipulated in Metabolic Pathways as a Source of Bioactive Secondary Metabolites Grown in a Bioreactor
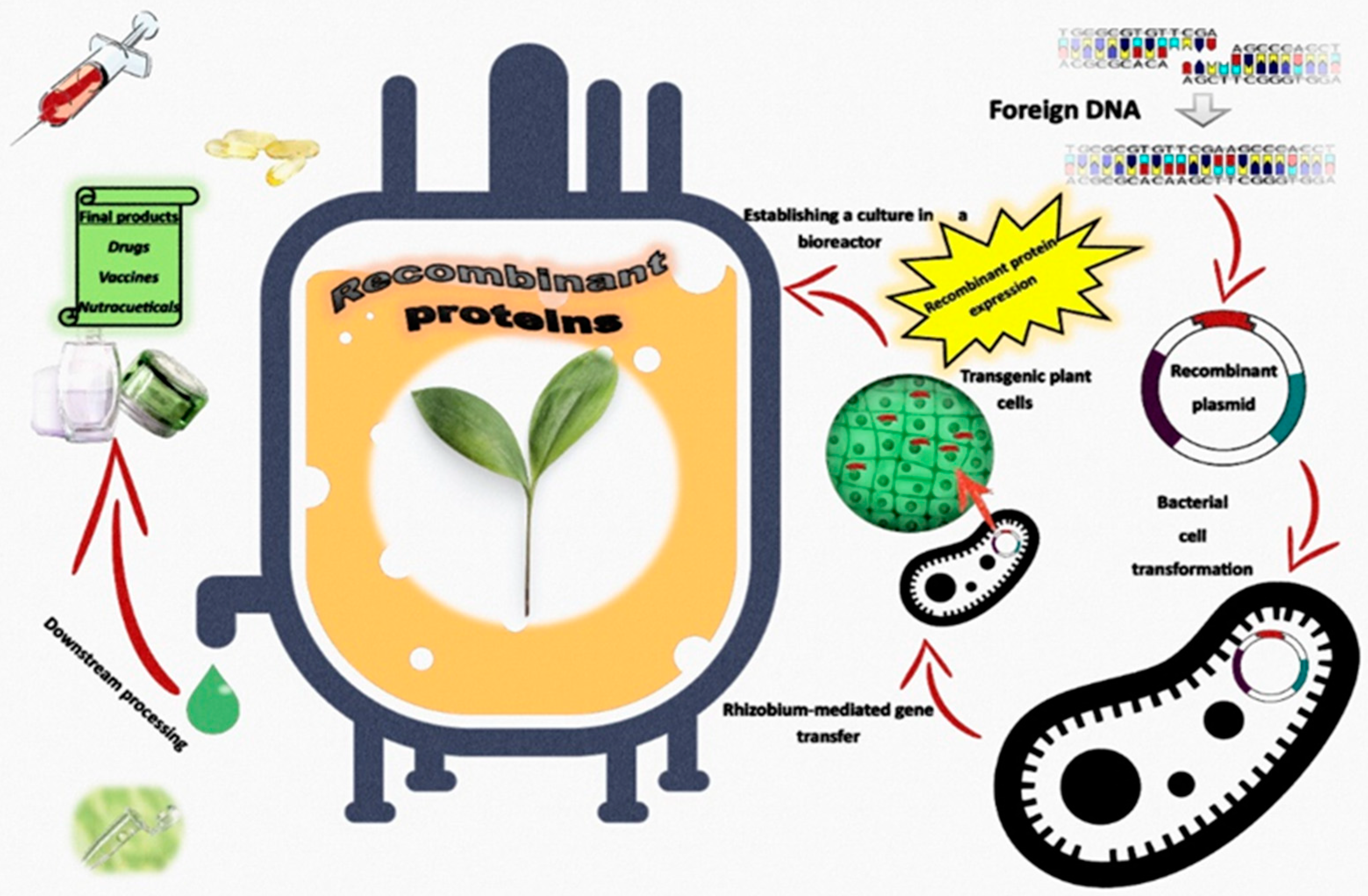
6. Transgenic Plants as Green Biofactories for the Production of Recombinant Proteins Grown in Bioreactors
7. Recent Patents Relating to Bioreactors for the Culture of Plant Cultures
8. Conclusions
9. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvet-Mir, L.; Salpeteur, M. Humans, plants, and networks: A critical review. Environ. Soc. Adv. Res. 2016, 7, 107–128. [Google Scholar] [CrossRef]
- DelSesto, M. People–plant interactions and the ecological self. Plants People Planet. 2020, 2, 201–211. [Google Scholar] [CrossRef]
- Henkhaus, N.; Bartlett, M.; Gang, D.; Grumet, R.; Jordon-Thaden, I.; Lorence, A.; Lyons, E.; Miller, S.; Murray, S.; Nelson, A.; et al. Plant science decadal vision 2020–2030: Reimagining the potential of plants for a healthy and sustainable future. Plant Direct. 2020, 4, e00252. [Google Scholar] [CrossRef] [PubMed]
- Salam, S.A.; Javed, M.S.; Toor, M.D.; Adnan, M.; Awais, M.; Din, M.M.U.; Saeed, M.S.; ur Rehman, F.; Tampubolon, K. Influence of Industrial Waste Water on Soil and Plants: A Review. Curr. Res. Agric. Farming. 2020, 1, 19–23. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction Techniques of Phenolic Compounds and Other Bioactive Compounds From Medicinal and Aromatic Plants. Eng. Tools Beverage Ind. 2019, 3, 293–314. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Espinosa-Lea, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. (Amst) 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Malik, S.; de Costa, F.; Yousefzadi, M.; Mirjalili, M.H.; Arroo, R.; Bhambra, A.S.; Strnad, M.; Bonfill, M.; Fett-Neto, A.G. Specialized Plant Metabolism Characteristics and Impact on Target Molecule Biotechnological Production. Mol. Biotechnol. 2018, 60, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. Hairy Root Culture an Alternative for Bioactive Compound Production from Medicinal Plants. Curr. Pharm. Biotechnol. 2020, 22, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.M.; Ritala, A.; Cardon, F. Hairy Root Cultures—A Versatile Tool With Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological Exploration of Transformed Root Culture for Value-Added Products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef]
- Merlin, M.; Gecchele, E.; Capaldi, S.; Pezzotti, M.; Avesani, L. Comparative evaluation of recombinant protein production in different biofactories: The green perspective. Biomed Res. Int. 2014, 2014, 136419. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Alireza, T.; Nader, R.E. Molecular Farming in Plants. In Plants for the Future; El-Shemy, H., Ed.; IntechOpen Limited: London, UK, 2015. [Google Scholar] [CrossRef][Green Version]
- Georgiev, M.I.; Eibl, R.; Zhong, J.J. Hosting the plant cells in vitro: Recent trends in bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Tavakoli Dinanai, E.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Mamun, N.H.A.; Egertsdotter, U.; Aidun, C.K. Bioreactor technology for clonal propagation of plants and metabolite production. Front. Biol. 2015, 10, 177–193. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.B.; Park, J.S.; Park, Y., II; Song, I.J.; Lee, H.J.; Cho, H.S.; Jeon, J.H.; Kim, H.S. Development of systems for the production of plant-derived biopharmaceuticals. Plants 2020, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Maghari, B.M.; Ardekani, A.M. Genetically modified foods and social concerns. Avicenna J. Med. Biotechnol. 2011, 3, 109–117. [Google Scholar]
- Oliver, M.J. Why we need GMO crops in agriculture. Mo. Med. 2014, 111, 492–507. [Google Scholar]
- Cano, A.; Morgado, C. The role of biotechnology in agricultural production and food supply. Cienc. Investig. Agrar. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Tutelyan, V.A. Chapter 2—World Production of Genetically Engineered Crops. In Genetically Modified Food Sources; Academic Press: Cambridge, MA, USA, 2013; pp. 17–23. [Google Scholar]
- ISAAA. ISAAA Brief 55-2019: Executive Summary Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier. Int. Serv. Acquis. Agri-Biotech Appl. 2019. Available online: https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp (accessed on 13 October 2021).
- ISAAA. Pocket K No. 16: Biotech Crop Highlights in 2019. Available online: https://www.isaaa.org/resources/publications/pocketk/16/ (accessed on 13 October 2021).
- James, C. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. 2017. Available online: https://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf (accessed on 7 September 2021).
- Fedoroff, N.V. Food in a future of 10 billion. Agric. Food Secur. 2015, 4, 11. [Google Scholar] [CrossRef]
- Raman, R. The impact of Genetically Modified (GM) crops in modern agriculture: A review. GM Crop. Food. 2017, 8, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, P.A. Anomalies in the Early Stages of Plant Transgenesis: Interests and Interpretations Surrounding the First Transgenic Plants. Hist. Ciencias Saude—Mang. 2013, 20, 1453–1471. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.A.; Binns, A.N.; Matzke, A.J.M.; Chilton, M.D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell 1983, 32, 1033–1043. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, B.; Citovsky, V. The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. Int. J. Dev. Biol. 2013, 57, 467–481. [Google Scholar] [CrossRef]
- Lawlor, D.W. Genetic engineering to improve plant performance under drought: Physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L., Eds.; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, M.K. Plants as bioreactors: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 811–832. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef]
- Paul, J.Y.; Khanna, H.; Kleidon, J.; Hoang, P.; Geijskes, J.; Daniells, J.; Zaplin, E.; Rosenberg, Y.; James, A.; Mlalazi, B.; et al. Golden bananas in the field: Elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 2017, 15, 520–532. [Google Scholar] [CrossRef]
- Zhang, C.; Wohlhueter, R.; Zhang, H. Genetically modified foods: A critical review of their promise and problems. Food Sci. Hum. Wellness 2016, 5, 116–123. [Google Scholar] [CrossRef]
- Liao, P.; Chen, X.; Wang, M.; Bach, T.J.; Chye, M.L. Improved fruit α-tocopherol, carotenoid, squalene and phytosterol contents through manipulation of Brassica juncea 3-HYDROXY-3-METHYLGLUTARYL-COA SYNTHASE1 in transgenic tomato. Plant Biotechnol. J. 2018, 16, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Towler, M.; Weathers, P.J. Platforms for Plant-Based Protein Production. In Bioprocessing of Plant In Vitro Systems. Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern Trends in the In Vitro Production and Use of Callus, Suspension Cells and Root Cultures of Medicinal Plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Reuter, L.; Nuorti, N.; Joensuu, J.J.; Rischer, H.; Ritala, A. Tobacco BY-2 media component optimization for a cost-efficient recombinant protein production. Front. Plant Sci. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a tool for the efficient production of selected secondary metabolites from in vitro plant cultures. Plants 2020, 9, 132. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y.; Chooi, Y.H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Genetically modified (GM) crops: Milestones and new advances in crop improvement. Theor. Appl. Genet. 2016, 129, 1639–1655. [Google Scholar] [CrossRef]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Cortés, A.J.; This, D. Identification of an ERECTA gene and its drought adaptation associations with wild and cultivated common bean. Plant Sci. 2016, 242, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; Chavarro, M.C.; Madriñán, S.; This, D.; Blair, M.W. Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet. 2012, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; This, D.; Chavarro, C.; Madriñán, S.; Blair, M.W. Nucleotide diversity patterns at the drought-related DREB2 encoding genes in wild and cultivated common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.A.; Cortés, A.J.; Sedlacek, J.; Karrenberg, S.; van Kleunen, M.; Wipf, S.; Hoch, H.; Bossdorf, O.; Rixen, C. The snow and the willows: Earlier spring snowmelt reduces performance in the low-lying alpine shrub Salix herbacea. J. Ecol. 2016, 104, 1041–1050. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Hoch, G.; Cortés, A.J.; Sedlacek, J.; Wipf, S.; Rixen, C. Increased spring freezing vulnerability for alpine shrubs under early snowmelt. Oecologia 2014, 175, 219–229. [Google Scholar] [CrossRef]
- Rani, S.J.; Usha, R. Transgenic plants: Types, benefits, public concerns and future. J. Pharm. Res. 2013, 6, 879–883. [Google Scholar]
- Prakash, D.; Verma, S.; Bhatia, R.; Tiwary, B.N. Risks and Precautions of Genetically Modified Organisms. Int. Sch. Res. Not. 2011. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Thomas, J.A. Biopharmaceuticals derived from genetically modified plants. QJM 2004, 97, 705–716. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and microalgae derived peptides are advantageously employed as bioactive compounds in cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Kumar, R.; Singh, K.; Gehlot, A.; Bhushan, S.; Kumar, S. In vitro adventitious roots: A non-disruptive technology for the production of phytoconstituents on the industrial scale. Crit. Rev. Biotechnol. 2021, 41, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Krolicka, A.; Zabiegala, B.; Luczkiewicz, M. Elicitation strategies for the improvement of essential oil content in Rhododendron tomentosum (Ledum palustre) bioreactor-grown microshoots. Ind. Crops Prod. 2018, 123, 461–469. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, S.; Banerjee, S. Ocimum basilicum suspension culture as resource for bioactive triterpenoids: Yield enrichment by elicitation and bioreactor cultivation. Plant Cell. Tissue Organ Cult. 2019, 137, 65–75. [Google Scholar] [CrossRef]
- Zarei, A.; Behdarvandi, B.; Tavakouli Dinani, E.; Maccarone, J. Cannabis sativa L. photoautotrophic micropropagation: A powerful tool for industrial scale in vitro propagation. Vitr. Cell. Dev. Biol. Plant 2021. [Google Scholar] [CrossRef]
- Udomsin, O.; Yusakul, G.; Kitisripanya, T.; Juengwatanatrakul, T.; Putalun, W. The Deoxymiroestrol and Isoflavonoid Production and Their Elicitation of Cell Suspension Cultures of Pueraria candollei var. mirifica: From Shake Flask to Bioreactor. Appl. Biochem. Biotechnol. 2020, 190, 57–72. [Google Scholar] [CrossRef]
- Routien, J.B.; Nickell, L.G. Cultivation of plant tissue. USA Pat. 2 1956, 747, 334. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhong, J.J. Bioreactor Engineering. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 131–161. [Google Scholar] [CrossRef]
- Finkbeiner, T.; Manz, C.; Raorane, M.L.; Metzger, C.; Schmidt-Speicher, L.; Shen, N.; Ahrens, R.; Maisch, J.; Nick, P.; Guber, A.E. A modular microfluidic bioreactor to investigate plant cell–cell interactions. Protoplasma 2022, 259, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.; Chen, T.; Zhao, X. Development of Novel Bioreactor Control Systems Based on Smart Sensors and Actuators. Front. Bioeng. Biotechnol. 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N. Bioreactors Design, Types, Influencing Factors and Potential Application in Dentistry. A Literature Review. Curr. Stem Cell Res. Ther. 2019, 14, 351–366. [Google Scholar] [CrossRef]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; Van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clement, C.; Pereau, D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Biochem. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Joshiba, G.J.; Kumar, V.V. Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: A review. Environ. Chem. Lett. 2021, 19, 329–343. [Google Scholar] [CrossRef]
- Terrier, B.; Courtois, D.; Hénault, N.; Cuvier, A.; Bastin, M.; Aknin, A.; Dubreuil, J.; Pétiard, V. Two new disposable bioreactors for plant cell culture: The wave and undertow bioreactor and the slug bubble bioreactor. Biotechnol. Bioeng. 2007, 96, 914–923. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design of bioreactors suitable for plant cell and tissue cultures. Phytochem. Rev. 2008, 7, 593–598. [Google Scholar] [CrossRef]
- Pinto, D.; da Silva, C.L.; Cabral, J. Scalable Expansion of Mesenchymal Stem/Stromal Cells in Bioreactors: A Focus on Hydrodynamic Characterization. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Yudharaj, P.; Shankar, M.; Sowjanya, R.; Sireesha, B.; Naik, E.A.; Priyadarshini, R.J. Importance and uses of medicinal plants–An overview. Int. J. Preclin. Pharm. Res. 2016, 7, 67–73. [Google Scholar]
- Gaosheng, H.; Jingming, J. Production of Useful Secondary Metabolites Through Regulation of Biosynthetic Pathway in Cell and Tissue Suspension Culture of Medicinal Plants. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L., Eds.; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef]
- Tiago, O.; Maicon, N.; Ivan, R.C.; Diego, N.F.; Vinícius, J.S.; Mauricio, F.; de Alan, J.P.; de Velci, Q.S. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: A. review. African. J. Agric. Res. 2017, 12, 71–84. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant. 2019, 12, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar] [CrossRef]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Chitra, J.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A. review. Int. J. Pharm. Sci. Res. 2019, 10, 494. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Phcog. Rev. 2007, 1, 69–79. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. In Secondary Metabolites-Sources and Applications; Vijayakumar, R., Raja, S., Eds.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tan, K.; Li, P. Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front. Plant Sci. 2016, 7, 1647. [Google Scholar] [CrossRef]
- Barh, D.; Azevedo, V. Omics Technologies and Bio-Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Genetically Engineered Crops. Past Experience and Future Prospects. Genetically Engineered Crops: Experiences and Prospects; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Miralpeix, B.; Rischer, H.; Hakkinen, S.; Ritala, A.; Seppanen-Laakso, T.; Oksman-Caldentey, K.-M.; Capell, T.; Christou, P. Metabolic Engineering of Plant Secondary Products: Which Way Forward? Curr. Pharm. Des. 2013, 19, 5622–5639. [Google Scholar] [CrossRef]
- Aftab, T.; Rehman, K. Medicinal and Aromatic Plants. In Expanding their Horizons through Omics; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, O.S.; Jung, H.Y.; Kang, S.M.; Lee, B.H.; Karigar, C.; Prasad, T.; Bahk, J.D.; Choi, M.S. Overexpression of hyoscyamine 6β-hydroxylase(h6h) gene and enhanced production of tropane alkaloids in Scopolia parviflora hairy root lines. J. Microbiol. Biotechnol. 2005, 15, 91–98. [Google Scholar]
- Riad, M.; Hithe, C.C. Scopolamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Picot, L.; Wielanek, M.; Skała, E.; Śliwiński, T. An extract of transgenic Senna obtusifolia L. Hairy roots with overexpression of PgSS1 gene in combination with chemotherapeutic agent induces apoptosis in the leukemia cell line. Biomolecules 2020, 10, 510. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor sup-pression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, O.R.; Kim, Y.J.; Lee, J.H.; Kim, J.H.; Jung, D.Y.; In, J.G.; Lee, B.S.; Yang, D.C. Overexpression of PgSQS1 increases ginsenoside production and negatively affects ginseng growth rate in Panax ginseng. J. Ginseng Res. 2010, 34, 98–103. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginseno-sides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Bassolino, L.; Zhang, Y.; Schoonbeek, H.J.; Kiferle, C.; Perata, P.; Martin, C. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol. 2013, 200, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Kowalczyk, T.; Rijo, P.; Białas, A.J.; Wielanek, M.; Wysokińska, H.; Garcia, C.; Toma, M.; Śliwiński, T.; Skała, E. Over-Expression of AtPAP1 Transcriptional Factor Enhances Phenolic Acid Production in Transgenic Roots of Leonurus sibiricus L. and Their Biological Activities. Mol. Biotechnol. 2018, 60, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising thera-peutic applications. Biotechnol. Rep. (Amst) 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sun, J.; Peebles, C.A.M. Engineering overexpression of ORCA3 and strictosidine glucosidase in Catharanthus roseus hairy roots increases alkaloid production. Protoplasma 2016, 253, 1255–1264. [Google Scholar] [CrossRef]
- Expósito, O.; Bonfill, M.; Moyano, E.; Onrubia, M.; Mirjalili, M.H.; Cusidó, R.M.; Palazón, J. Biotechnological production of taxol and related taxoids: Current state and prospects. Anticancer Agents Med. Chem. 2009, 9, 109–121. [Google Scholar] [CrossRef]
- Marsh, S. Taxane pharmacogenetics. Per. Med. 2006, 3, 33–43. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Zhou, Z.; Tan, H.; Li, Q.; Chen, J.; Gao, S.; Wang, Y.; Chen, W.; Zhang, L. CRISPR/Cas9-mediated efficient targeted mutagenesis of RAS in Salvia miltiorrhiza. Phytochemistry 2018, 148, 63–70. [Google Scholar] [CrossRef]
- Li, B.; Cui, G.; Shen, G.; Zhan, Z.; Huang, L.; Chen, J.; Qi, X. Targeted mutagenesis in the medicinal plant Salvia miltiorrhiza. Sci. Rep. 2017, 7, 43320. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.; Yugay, Y.; Avramenko, T.; Grigorchuk, V.; Gorpenchenko, T.; Grischenko, O.; Bulgakov, V. CRISPR/Cas9-Mediated Knockout of HOS1 Reveals Its Role in the Regulation of Secondary Metabolism in Arabidopsis thaliana. Plants 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.H.; Adhikari, P.B.; Park, S.B.; Han, J.Y.; Choi, Y.E. Production of the dammarene sapogenin (protopanaxadiol) in transgenic tobacco plants and cultured cells by heterologous expression of PgDDS and CYP716A47. Plant Cell Rep. 2015, 34, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Kowalczyk, T.; Picot, L.; Michalska-Hejduk, D.; Bijak, M.; Białas, A.J.; Wielanek, M.; Śliwiński, T.; Skała, E. Growth of Leonurus sibiricus L. roots with over-expression of AtPAP1 transcriptional factor in closed bioreactor, production of bioactive phenolic compounds and evaluation of their biological activity. Ind. Crops Prod. 2018, 122, 732–739. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Rijo, P.; Domínguez-Martín, E.; Falcó, I.; Sánchez, G.; Śliwiński, T. Enhanced Accumulation of Betulinic Acid in Transgenic Hairy Roots of Senna obtusifolia Growing in the Sprinkle Bioreactor and Evaluation of Their Biological Properties in Various Biological Models. Chem. Biodivers. 2021, 18, e2100455. [Google Scholar] [CrossRef]
- Ritala, A.; Dong, L.; Imseng, N.; Seppänen-Laakso, T.; Vasilev, N.; Van der Krol, S.; Rischer, H.; Maaheimo, H.; Virkki, A.; Brändli, J.; et al. Evaluation of tobacco (Nicotiana tabacum L. cv. Petit Havana SR1) hairy roots for the production of geraniol, the first committed step in terpenoid indole alkaloid pathway. J. Biotechnol. 2014, 176, 20–28. [Google Scholar] [CrossRef]
- Mišić, D.; Šiler, B.; Skorić, M.; Djurickovic, M.S.; Nestorović Živković, J.; Jovanović, V.; Giba, Z. Secoiridoid glycosides production by Centaurium maritimum (L.) Fritch hairy root cultures in temporary immersion bioreactor. Process Biochem. 2013, 48, 1587–1591. [Google Scholar] [CrossRef]
- Wang, G.R.; Wang, H. Cell suspension culture of Rhizoma zedoariae in a two-stage perfusion bioreactor system for β-elemene production. Vitr. Cell. Dev. Biol. Plant 2019, 55, 209–220. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, A.; Khan, S.A.; Shanker, K.; Mathur, A.K. Over-expression of Catharanthus roseus tryptophan decarboxylase and strictosidine synthase in rol gene integrated transgenic cell suspensions of Vinca minor. Protoplasma 2015, 252, 373–381. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.J.; Shui, M.; Wan, J.B.; Gao, J.L. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evid. Based. Complement. Alternat. Med. 2016, 2016, 5738694. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G. Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review). Int. J. Oncol. 2016, 4, 1772–1782. [Google Scholar] [CrossRef]
- Muhamad Fadzil, N.S.; Sekar, M.; Gan, S.H.; Bonam, S.R.; Wu, Y.S.; Vaijanathappa, J.; Ravi, S.; Lum, P.T.; Dhadde, S.B. Chemistry, Pharmacology and Therapeutic Potential of Swertiamarin—A Promising Natural Lead for New Drug Discovery and Development. Drug Des. Devel. Ther. 2021, 15, 2721–2746. [Google Scholar] [CrossRef]
- Xie, Q.; Li, F.; Fang, L.; Liu, W.; Gu, C. The Antitumor Efficacy of β-Elemene by Changing Tumor Inflammatory Environment and Tumor Microenvironment. Biomed. Res. Int. 2020, 2020, 6892961. [Google Scholar] [CrossRef]
- Zhai, B.; Zeng, Y.; Zeng, Z.; Zhang, N.; Li, C.; Zeng, Y.; You, Y.; Wang, S.; Chen, X.; Sui, X.; et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int. J. Nanomed. 2018, 13, 6279–6296. [Google Scholar] [CrossRef]
- Yin, H.; Sun, Y.H. Vincamine-producing endophytic fungus isolated from Vinca minor. Phytomedicine 2011, 18, 802–805. [Google Scholar] [CrossRef]
- Al-Rashed, S.; Baker, A.; Ahmad, S.S.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Khan, M.S. Vincamine, a safe natural alkaloid, represents a novel anticancer agent. Bioorg. Chem. 2021, 107, 104626. [Google Scholar] [CrossRef]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet. 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Lico, C.; Santi, L.; Twyman, R.M.; Pezzotti, M.; Avesani, L. The use of plants for the production of therapeutic human peptides. Plant Cell Rep. 2012, 31, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, K.; Makhzoum, A.; Trémouillaux-Guiller, J. Molecular farming on rescue of pharma industry for next generations. Crit. Rev. Biotechnol. 2016, 36, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.C.; Drake, P.M.W.; Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yeh, M.K. Introductory Chapter: Biopharmaceuticals. In Biopharmaceuticals; Yeh, M.K., Chen, Y.C., Eds.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef]
- Dirisala, V.R.; Nair, R.R.; Srirama, K.; Reddy, P.N.; Rao, K.R.S.S.; Satya Sampath Kumar, N.; Parvatam, G. Recombinant pharmaceutical protein production in plants: Unraveling the therapeutic potential of molecular pharming. Acta Physiol. Plant. 2017, 39, 18. [Google Scholar] [CrossRef]
- Ahmad, A.; Pereira, E.O.; Conley, A.J.; Richman, A.S.; Menassa, R. Green biofactories: Recombinant protein production in plants. Recent Pat. Biotechnol. 2010, 4, 242–259. [Google Scholar] [CrossRef]
- Rech, E.; Vianna, G.; Murad, A.; Cunha, N.; Lacorte, C.; Araujo, A.; Brigido, M.; Michael, W.; Fontes, A.; Barry, O.; et al. Recombinant proteins in plants. BMC Proc. 2014, 8, 1. [Google Scholar] [CrossRef]
- Che, Q.; Lai, H. Gene delivery into plant cells for recombinant protein production. Biomed Res. Int. 2015, 2015, 932161. [Google Scholar] [CrossRef]
- Tusé, D.; Nandi, S.; McDonald, K.A.; Buyel, J.F. The Emergency Response Capacity of Plant-Based Biopharmaceutical Manufacturing-What It Is and What It Could Be. Front. Plant Sci. 2020, 11, 594019. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.S.; Sun, S.S.M. Plant seeds as bioreactors for recombinant protein production. Biotechnol. Adv. 2009, 27, 1015–1022. [Google Scholar] [CrossRef]
- Desai, P.N.; Shrivastava, N.; Padh, H. Production of heterologous proteins in plants: Strategies for optimal expression. Biotechnol. Adv. 2010, 28, 427–435. [Google Scholar] [CrossRef]
- Lim, C.Y.; Lee, K.J.; Oh, D.B.; Ko, K. Effect of the developmental stage and tissue position on the expression and glycosylation of recombinant glycoprotein GA733-FcK in transgenic plants. Front. Plant Sci. 2015, 5, 778. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Shin, Y.K.; Park, S.W.; Ko, K. Effect of nitrogen deficiency on recombinant protein production and dimerization and growth in transgenic plants. Hortic. Environ. Biotechnol. 2016, 57, 299–307. [Google Scholar] [CrossRef]
- Lu, Z.; Lee, K.J.; Shao, Y.; Lee, J.H.; So, Y.; Choo, Y.K.; Oh, D.B.; Hwang, K.A.; Oh, S.H.; Han, Y.S.; et al. Expression of GA733-Fc fusion protein as a vaccine candidate for colorectal cancer in transgenic plants. J. Biomed. Biotechnol. 2012, 2012, 364240. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.; Castilho, A.; Grass, J.; Vorauer-Uhl, K.; Sterovsky, T.; Altmann, F.; Steinkellner, H. Expression of functionally active sialylated human erythropoietin in plants. Biotechnol. J. 2013, 8, 371–382. [Google Scholar] [CrossRef]
- Thomas, D.R.; Walmsley, A.M. Improved expression of recombinant plant-made hEGF. Plant Cell Rep. 2014, 33, 1801–1814. [Google Scholar] [CrossRef]
- Luchakivskaya, Y.; Kishchenko, O.; Gerasymenko, I.; Olevinskaya, Z.; Simonenko, Y.; Spivak, M.; Kuchuk, M. High-level expression of human interferon alpha-2b in transgenic carrot (Daucus carota L.) plants. Plant Cell Rep. 2011, 30, 407–415. [Google Scholar] [CrossRef]
- Mercx, S.; Smargiasso, N.; Chaumont, F.; De Pauw, E.; Boutry, M.; Navarre, C. Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 Cells by a Multiplex CRISPR/Cas9 Strategy Results in Glycoproteins without Plant-Specific Glycans. Front. Plant Sci. 2017, 8, 403. [Google Scholar] [CrossRef]
- Matsuo, K. CRISPR/Cas9-mediated knockout of the DCL2 and DCL4 genes in Nicotiana benthamiana and its productivity of recombinant proteins. Plant Cell Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Macharoen, K.; McDonald, K.A.; Nandi, S. A method to simplify bioreactor processing for recombinant protein production in rice cell suspension cultures. MethodsX 2020, 7, 101139. [Google Scholar] [CrossRef] [PubMed]
- Macharoen, K.; Du, M.; Jung, S.; McDonald, K.A.; Nandi, S. Production of recombinant butyrylcholinesterase from transgenic rice cell suspension cultures in a pilot-scale bioreactor. Biotechnol. Bioeng. 2021, 118, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Macharoen, K.; Li, Q.; Márquez-Escobar, V.A.; Corbin, J.M.; Lebrilla, C.B.; Nandi, S.; McDonald, K.A. Effects of kifunensine on production and n-glycosylation modification of butyrylcholinesterase in a transgenic rice cell culture bioreactor. Int. J. Mol. Sci. 2020, 21, 6896. [Google Scholar] [CrossRef]
- Corbin, J.M.; Hashimoto, B.I.; Karuppanan, K.; Kyser, Z.R.; Wu, L.; Roberts, B.A.; Noe, A.R.; Rodriguez, R.L.; McDonald, K.A.; Nandi, S. Semicontinuous bioreactor production of recombinant butyrylcholinesterase in transgenic rice cell suspension cultures. Front. Plant Sci. 2016, 7, 412. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Cheon, S.H.; Nam, H.J.; Choi, H.Y.; Kim, D. Process characterization of hCTLA4Ig production in transgenic rice cell cultures using a 3-L bioreactor. Appl. Biochem. Biotechnol. 2013, 171, 1276–1288. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Yang, Y.S.; Cheon, S.H.; Nam, H.J.; Jin, G.H.; Kim, D. Bioreactor engineering using disposable technology for enhanced production of hCTLA4Ig in transgenic rice cell cultures. Biotechnol. Bioeng. 2013, 110, 2412–2424. [Google Scholar] [CrossRef]
- Park, C.I.; Lee, S.J.; Kang, S.H.; Jung, H.S.; Kim, D.; Lim, S.M. Fed-batch cultivation of transgenic rice cells for the production of hCTLA4Ig using concentrated amino acids. Process Biochem. 2010, 45, 67–74. [Google Scholar] [CrossRef]
- Myoung, H.J.; Choi, H.Y.; Nam, H.J.; Kim, D.I. In situ Recovery of hGM-CSF in Transgenic Rice Cell Suspension Cultures. KSBB J. 2015, 30, 103–108. [Google Scholar] [CrossRef][Green Version]
- López, E.G.; Ramírez, E.G.R.; Gúzman, O.G.; Calva, G.C.; Ariza-Castolo, A.; Pérez-Vargas, J.; Rodríguez, H.G.M. MALDI-TOF characterization of hGH1 produced by hairy root cultures of Brassica oleracea var. italica grown in an airlift with mesh bioreactor. Biotechnol. Prog. 2014, 30, 161–171. [Google Scholar] [CrossRef]
- Michoux, F.; Ahmad, N.; Hennig, A.; Nixon, P.J.; Warzecha, H. Production of leafy biomass using temporary immersion bioreactors: An alternative platform to express proteins in transplastomic plants with drastic phenotypes. Planta 2013, 237, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Huang, L.F.; Ho, S.L.; Liao, C.Y.; Liu, H.Y.; Lai, Y.H.; Yu, S.M.; Lu, C.A. Production of mouse granulocyte-macrophage colony-stimulating factor by gateway technology and transgenic rice cell culture. Biotechnol. Bioeng. 2012, 109, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klöckner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Büchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Macharoen, K.; McDonald, K.A.; Nandi, S. Simplified bioreactor processes for recombinant butyrylcholinesterase production in transgenic rice cell suspension cultures. Biochem. Eng. J. 2020, 163, 107751. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, S.; Chouksey, A.; Panwar, B.S.; Verma, P.C.; Roy, S.; Singh, P.K.; Saxena, G.; Tuli, R. Expression of Rabies Glycoprotein and Ricin Toxin B Chain (RGP–RTB) Fusion Protein in Tomato Hairy Roots: A Step Towards Oral Vaccination for Rabies. Mol. Biotechnol. 2015, 57, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Michoux, F.; Ahmad, N.; Mccarthy, J.; Nixon, P.J. Contained and high-level production of recombinant protein in plant chloroplasts using a temporary immersion bioreactor. Plant Biotechnol, J. 2011, 9, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Li, Y.T.; Lu, C.F.; Huang, L.F. Enhancement of recombinant human serum albumin in transgenic rice cell culture system by cultivation strategy. N. Biotechnol. 2015, 32, 328–334. [Google Scholar] [CrossRef]
- Reuter, L.J.; Bailey, M.J.; Joensuu, J.J.; Ritala, A. Scale-up of hydrophobin-assisted recombinant protein production in tobacco BY-2 suspension cells. Plant Biotechnol. J. 2014, 12, 402–410. [Google Scholar] [CrossRef]
- Top, O.; Parsons, J.; Bohlender, L.L.; Michelfelder, S.; Kopp, P.; Busch-Steenberg, C.; Hoernstein, S.N.W.; Zipfel, P.F.; Häffner, K.; Reski, R.; et al. Recombinant production of mfhr1, a novel synthetic multitarget complement inhibitor, in moss bioreactors. Front. Plant Sci. 2019, 10, 260. [Google Scholar] [CrossRef]
- Kim, S.R.; Sim, J.S.; Ajjappala, H.; Kim, Y.H.; Hahn, B.S. Expression and large-scale production of the biochemically active human tissue-plasminogen activator in hairy roots of Oriental melon (Cucumis melo). J. Biosci. Bioeng. 2012, 113, 106–111. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Lindberg, A. Predicting growth in response to growth hormone treatment. Growth Horm. IGF Res. 2009, 19, 1–11. [Google Scholar] [CrossRef]
- Buchbinder, E.; Stephen Hodi, F. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J. Clin. Investig. 2015, 125, 3377–3383. [Google Scholar] [CrossRef]
- Waterhouse, P.; Penninger, J.M.; Timms, E.; Wakeham, A.; Shahinian, A.; Lee, K.P.; Thompson, C.B.; Griesser, H.; Mak, T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995, 270, 985–988. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Zeng, R.Y.; Tan, H.S.W.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef]
- Morrison, T.B.; Weis, J.H.; Weis, J.J. Borrelia burgdorferi Outer Surface Protein a (OspA) Activates and Primes Human Neutrophils. J. Immunol. 1997, 158, 4838–4845. [Google Scholar]
- Lambert, J.M.; Goldmacher, V.S.; Collinson, A.R.; Nadler, L.M.; Bllattler, W.A. An Immunotoxin Prepared with Blocked Ricin: A Natural Plant Toxin Adapted for Therapeutic Use. Cancer Res. 1991, 51, 6236–6242. [Google Scholar]
- Pellizzari, R.; Rossetto, O.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Mechanism of action and therapeutic uses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 259–268. [Google Scholar] [CrossRef]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11 (Suppl. S4), s18–s25. [Google Scholar] [CrossRef]
- Mendez, C.M.; McClain, C.J.; Marsano, L.S. Albumin therapy in clinical practice. Nutr. Clin. Pract. 2005, 20, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Gravanis, I.; Tsirka, S.E. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin. Ther. Targets 2008, 12, 159–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wösten, H.A.B.; Scholtmeijer, K. Applications of hydrophobins: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Moyano, E.; Cusidó, R.M.; Oksman-Caldentey, K.M. Exploring the metabolic stability of engineered hairy roots after 16 years maintenance. Front. Plant Sci. 2016, 7, 1486. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient transient expression of recombinant proteins in plants by the novel pEff vector based on the genome of potato virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science. 2018, 361, 866–869. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
| Plant Species/Family | Type of Culture | Vector/Genetic Construct | Type of Metabolites | Bioreactor | Medium | Effect/Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Nicotiana tabacum L./Solanaceae | suspension culture | Panax ginseng dammarenediol-II synthase (PgDDS) and Cytochrome P450 716A47 (CYP716A47) under the control of the CaMV35 promoter | triterpenoid saponins (Protopanaxadiol (PPD), Dammarenediol-II) | 5 L balloon-type bioreactor 2 L of Murashige & Skoog (MS) medium (working volume) | 2 L of MS medium (working volume) | enhanced production of Dammarenediol-II (166.92 µg/g dry weight (DW), 1.6 mg/L) Protopanaxadiol (980.85l µg/g DW, 9.4 mg/L) | [128] |
| Leonurus sibiricus L./Lamiaceae | roots | anthocyanin pigment 1 (AtPAP1) transcription factor from Arabidopsis thaliana/pCAMBIA1305.1-AtPAP1 vector | phenolic acids | 5 L sprinkle bioreactor | 2.5 L Schenk & Hilde-brandt (SH) medium with 3% (w/v) sucrose | the greatest increase in DW (20.83 g/L) and highest yields of phenolic acids (chlorogenic acid 448 mg/L and caffeic acids 302 mg/L) | [129] |
| Senna obtusifolia (L.) H.S.Irwin & Barneby/Fabaceae | roots | Panax ginseng squalene synthase 1 gene (PgSS1)/pGFPGUSPlus-PgSS1 vector | pentacyclic triterpene (betulinic acid) | 10 L sprinkle bioreactor | 2 L of MS liquid media with 3% (w/v) sucrose | -an increase in the content of betulinic acid (38.125 mg/g DW), compared to the SOA41 hairy root line (4.213 mg/g DW) | [130] |
| Nicotiana tabacum L. cv. Petit Havana SR1/Solanaceae | hairy roots | a plastid targeted geraniol synthase gene originally isolated from Valeriana officinalis L. (VoGES)/pBIN2.4VoGES1 vector under the control of 35S promoter | terpenoid indole alkaloid (geraniol) | 20-L wave-mixed bioreactor | 2 L modified Gamborg’s B5 liquid medium | -scale production batch was successfully completed, yielding milligram quantities of geraniol. | [131] |
| Centauries maritimum (L.) Fitch/Gentianaceae | hairy roots | plasmid with GUS construct integrated into TL region of pRiA4 plasmid/GUS construct contains uidA sequence under the 70S promoter (enhancer-doubled 35S CaMV promoter), followed by NOS polyadenilation sequence. | secoiridoid glycosides (swertiamarin (SM), gentiopicrin (GP), and sweroside (SW)) | RITA® temporary immersion bioreactors (TIBs) | 200 of liquid MS medium | -about 2–4 times higher biomass production rate and up to 8 times higher total secoiridoid glycosides production. | [132] |
| Curcumae zedoariae L./Zingiberaceae. (Christm.) Roscoe (Rhizoma) | cell suspensions |
3-hydroxy-3-methylglutaryl–coenzyme A reductase (HMGR), Farnesyl-diphosphate synthase (FDS), Germacrene A synthetase GAS as well as terpene synthase (ST02C) driven by CaMV35S promoter, were separately introduced into the Agrobacterium GV3101 | sesquiterpenes (β-elemene) | 2 L stir-tank and airlift bioreactor | liquid MS medium containing 28 g L−1 sucrose, 0.5 mg L−1 6-BA, 1.0 mg L−1 naphthylacetic acid (NAA), 1.0 mg L−1 2,4-D | -highest β-elemene content of 0.22% (w/v) was detected in ST02C-transformed lines | [133] |
| Vinca minor L./Apocynaceae | cell suspensions | Tryptophan decarboxylase (TDC) and strictosidine synthase (STR) genes | monomeric eburnamine-type indole alkaloid vincamine | 5-L stirred tank bioreactor | MS medium with 2% sucrose | -only PVG3 line registered a twofold increase in total alkaloid content (2.1 ± 0.1% DW) and showed vincamine presence (0.003 ± 0.001% DW) which was further enhanced at the bioreactor level (2.7 ± 0.3 and 0.005 ± 0.001% DW, respectively) | [134] |
| Name of the Species/Family | Type of Culture | Vector/Genetic Construct Elements | Recombinant Protein | Type of Bioreactor | Medium/ Elicitors Used | Effect/Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Oryza sativa L./ Poaceae | suspension culture | metabolically-regulated promoter, rice alpha-amylase 3D (RAmy3D) | recombinant human butyrylcholinesterase (BChE), | 5-L stirred-tank bioreactor | -half-strength sucrose of the culture medium | -the method significantly improved the maximum accumulation level, purity, and productivity of the recombinant protein | [168] |
| Oryza sativa L./ Poaceae | suspension culture | metabolically-regulated promoter, rice alpha-amylase 3D (RAmy3D) | recombinant human butyrylcholinesterase (BChE), | 40-L stainless-steel stirred tank bioreactors (STB) bioreactor | -NB + S medium contains 30 g sucrose/L, while NB + 0.5xS contains 15 g sucrose/L |
-maximum total active rrBChE production level of 46–58 µg/g fresh weight (FW) in four cycles over 82 days -overall volumetric oxygen mass transfer coefficient (kLa) in the pilot-scale STB to be equivalent to the lab-scale STB volumetric productivity to 85 µg/g FW and 387 µg/L/day | [169] |
| Oryza sativa L./ Poaceae | suspension culture | α-amylase 3D (RAmy3D) promoter | N-glycosylation of recombinant human butyrylcholinesterase (BChE) | 5 L bioreactor | -using normal sugar-free (NB-S) media with no kifunensine treatment | -total active rrBChE production level of 79 ± 2 µg/g FW or 7.5 ± 0.4 mg/L in the presence of kifunensine | [170] |
| Oryza sativa L./ Poaceae | suspension culture | alpha amylase 3D (RAmy3D) | tetrameric form of recombinant butyrylcholinesterase (BChE | 5 L bioreactor | -fresh liquid NB + S medium | -maximum yield of 1.6 mg BChE/L of culture during the second expression phase | [171] |
| Oryza sativa L./ Poaceae | suspension culture | RAmy3D promoter | human cytotoxic T-lymphocyte antigen 4-immunoglobulin (hCTLA4Ig) | 3-L multi-bioreactor | -AA medium (1.4 L), except the volume of amino acid solution | -total protein concentration was at levels from 301.0 to 782.8 mg/L | [172] |
| Oryza sativa L./ Poaceae | suspension culture | RAmy3D promoter | human cytotoxic T-lymphocyte antigen 4-immunoglobulin (hCTLA4Ig) | stirred-tank reactors (5-L STR) | -AA medium (2.1 L) except 10% (v/v) amino acid mixture | -the results in both disposable bioreactors presented similar values of the maximum cell density (11.9 g DCW/L and 12.6 g DCW/L), the doubling time (4.8 and 5.0 days) and the maximum hCTLA4Ig concentration (43.7 and 43.3 mg/L). | [173] |
| Oryza sativa L./ Poaceae | suspension culture | RAmy3D promoter | human cytotoxic T-lymphocyte antigen 4-immunoglobulin (hCTLA4Ig) | 7-L bioreactor, 15-L stirred-tank bioreactor | AA medium (2.3 L) | -maximum hCTLA4Ig level was 76.5 mg/L at day 10 | [174] |
| Oryza sativa L./ Poaceae | suspension culture | RAmy3D promoter | human granulocyte-macrophage colony-stimulating factor (hGM-CSF) | 2-L bioreactor, 5-L stirred-tank bioreactor | -N6 medium 0.2 mg/L kinetin, 2 mg/L, 2mg/L 2,4-D, 30 g/L sucrose | -induction using sugar free media produced 33% more hGM-CSF -using buffer exchange when CM-Sepharose was used as a cationic exchange resin, optimal pH for binding was 4.8 and adsorption yield was 77%. -DEAE-Sepharose was used as an anionic exchange resin, it was 5.5 (74%). -without buffer exchange, optimal pH was 4.6 (84%). | [175] |
| Brassica oleracea var. italica (broccoli)/ Brassicaceae | hairy roots | pCAMBIA1105.1 binary vector | isoform 1 of the human growth hormone (hGH1) | 1.5-L mesh airlift bioreactor | -1.25 L of Schenk & Hildebrandt (SH) medium supplemented with sucrose 30 g/L and (NH4)2SO4 300 mg/L. | -the production of hGH1 was 5.1 ± 0.42 µg/g dry weight (DW) for flask cultures and 7.8 ± 0.3 µg/g DW for the bioreactor, with a capacity of 0.68 ± 0.05 and 1, 5 ± 0.06 µg/g DW days | [176] |
| Nicotiana tabacumcv Petit Havana/ Solanaceae | suspension culture/leaves | pOA:YFP4411 | protein A (OspA) from Borrelia burgdorferi | immersion bioreactors (TIBs) using AlkaBurstTM | -1-L and 0.3-L Murashige & Skoog (MS) media | -OspA expression up to 7.6% TSP with a maximum OspA yield of about 108 mg | [177] |
| Oryza sativa L./ Poaceae | suspension culture | a-amylase gene aAmy8 promotor/Gateway-compatible binary T-DNA destination vector | mouse granulocyte-macrophage colony stimulating factor (mGM-CSF) | 2-L bioreactor | -1.5 L of N6 medium | -the highest yield of rmGM-CSF was 24.6 mg/L | [178] |
| Nicotiana tabacum L./ Solanaceae | suspension culture BY-2 | binary vector pTRAkc-MTAD | human monoclonal antibody M12 | 200-L Orbitally-Shaken Disposable Bioreactor, 20-L Nalgene polycarbonate carboy vessels | -MSN medium | -final cell fresh weights of 300–387 g/L and M12 yields of 20 mg/L -resulting in an overall M12 recovery of 75–85% and a purity of >95% | [179] |
| Oryza sativa L./ Poaceae | suspension culture | RAmy3D promoter | recombinant human butyrylcholinesterase (BChE) | 5-L stirred-tank bioreactor | -3 L of NB + Smedium | -maximum total active rrBChE (77 μg/g FW) and 1.6-fold increase of total active rrBChE specific productivity (86 μg/g DW/day) compared to the two-stage batch cultures. | [180] |
| Solanum lycopersicum L./ Solanaceae | hairy roots | CaMV35S promoter | recombinant protein containing a fusion of rabies glycoprotein and ricin toxin B chain (rgp–rtxB) | 5 L bioreactor Bench-top fermenter (Bioflo-3000) | -the quantity of 2.5 L of 1/2 MS medium with B5 vitamins and 3% sucrose | -biomass yield 197.4 (g/L) -RGP RTB 7.84 (µg/g) -the efficiency of the bioreactor in terms of protein expression remained relatively lower than that of the shake flask, which may be due to callogenesis of the root tissues in the bioreactor. | [181] |
| Nicotiana tabacum L./ Solanaceae | suspension cultures (and calli)/leaves | Plasmid pFMGFP | a vaccine antigen, fragment C of tetanus toxin (TetC)/green fluorescent protein (GFP+) | 2 L bioreactor | MS medium supplemented with 0.1 lM TDZ | -GFP+ yield reached 660 mg/L of bioreactor (33% TSP), and TetC accumulated to about 95 mg/L (8% TSP) | [182] |
| Oryza sativa L./ Poaceae | suspension culture | α-amylase gene promoter, RAmy3Dp/αAmy3p | recombinant human serum albumin (rHSA) | 2-L airlift and a 2-L stirred tank bioreactor | MS medium | -rHSA production has been enriched to 45 mg/L in plant culture | [183] |
| Nicotina tabacum L./ Solanaceae | suspension cell cultures BY-2 | vector pCaMterX enhanced virus 35S promoter | green fluorescent protein-hydrophobin fusion (GFP-HFBI) | 30-L bioreactor, 600-L standard stirred tank bioreactor | MS-medium | -HFB-fusion technology in large-scale tobacco BY-2 suspension cell culture, formation of protein bodies and efficient purification of GFP-HFBI fusion by aqueous two-phase separation (ATPS)
-GFP-HFBI titer reached a level of 0.30 ± 0.018 g/L, corresponding to 16.5% of TSP (total soluble protein) | [184] |
| Physcomitrella patens (Hedw.) Bruch & Schimp/ Funariaceae | whole plant | MFHR1 construct | factor H (FH) and FH-related proteins (FHRs) | 5-L bioreactor | -fresh medium with the addition of 5 μM naphthaleneacetic acid (NAA) | -it was obtained 17 mg of MFHR1 protein | [185] |
| Cucumis melo L./ Cucurbitaceae | hairy roots | binary plasmid p221 that included cauliflower mosaic virus 35S promoter, tobacco etch virus (TEV) leader sequence and 35S terminator | human tissue-plasminogen activator (t-PA) protein | 18 L bioreactor | -MS, Woody Plant Me-dium (WPM), B5 medium | -biomass accumulation 615.4 g/FW in MS medium, 457.6 g/FW in B5 medium and 621.8 g/FW in WPM medium -the maximum content of t-PA 0.46 μg/mg TSP was obtained in the cultures grown on the B5 medium, and then the content of t-PA 0.33 and 0.40 μg/mg TSP in the cultures grown on the MS and WPM medium | [186] |
| Patent/Patent Application No | Assignee | Type of Plant Culture | Year |
|---|---|---|---|
| WO2012044239A1 | - | Tissue cultures | 2012 |
| CN102408991B | Bright Oceans Corp. (Shaanxi, China) | Cells, tissues and organs | 2014 |
| CN103120126B | Nanjing University Nanjing University of Science and Technology | Plant tissue culture | 2014 |
| US20140026260A1 | Worcester Polytechnic Institute | Tissues and organs, whole plants | 2014 |
| WO2015066779A1 | - | Tissue culture | 2015 |
| EP2674479B1 | Eppendorf AG (Hamburg, Germany) | Cell culture | 2015 |
| CN103270946B | Nanjing Biofunction Biological Science & Technology Co Ltd. (Nanjing, China) | Plant tissue culture | 2016 |
| WO2016092098A1 | - | Cell or tissue cultures | 2016 |
| CN104770304B | Nanjing Biofunction Biological Science & Technology Co Ltd. | Plant tissue culture | 2017 |
| EP3069591B1 | Fibria Celulose SA (Sao Paulo, Brazil) | Plant tissue culture | 2018 |
| CN104379722B | Eppendorf AG (Hamburg, Germany) | Cell culture | 2018 |
| USD822223S1 | University of Guelph | Tissue culture | 2018 |
| EP3502229A1 | Evologic Technologies GmbH (Vienna, AT) | Hairy root cultures | 2019 |
| US20190282983A1 | Life Technologies Corp (Carlsbad, CA) | Cell culture | 2019 |
| CN208857314U | PURUIKANG BIOTECHNOLOGY CO Ltd. (Shenzhen, China) | plant cell, organ | 2019 |
| CN111226794A | Beijing Forestry University | Mature somatic embryos | 2020 |
| CN212247083U | Zhejiang University of Technology ZJUT | Cell culture | 2020 |
| ES2763637B2 | Institut Recerca i Tecnologia Agroalimentaries IRTA | Plant tissues, organs, seeds and/or plant cells | 2020 |
| US20200032185A1 | Oklahoma State University | Cell culture | 2020 |
| US20200230568A1 | ABEC Inc. | Cell culture | 2020 |
| US20200339931A1 | Sartorius Stedim Biotech GmbH (Goettingen Germany) | Cell culture | 2020 |
| US20210130765A1 | Ori biotech Ltd. (London, United Kingdom) | Cell culture | 2021 |
| US20210214668A1 | Membio Inc (Mississauga, ON, Canada) | Cell or tissue culture | 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Merecz-Sadowska, A.; Picot, L.; Brčić Karačonji, I.; Wieczfinska, J.; Śliwiński, T.; Sitarek, P. Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Molecules 2022, 27, 795. https://doi.org/10.3390/molecules27030795
Kowalczyk T, Merecz-Sadowska A, Picot L, Brčić Karačonji I, Wieczfinska J, Śliwiński T, Sitarek P. Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Molecules. 2022; 27(3):795. https://doi.org/10.3390/molecules27030795
Chicago/Turabian StyleKowalczyk, Tomasz, Anna Merecz-Sadowska, Laurent Picot, Irena Brčić Karačonji, Joanna Wieczfinska, Tomasz Śliwiński, and Przemysław Sitarek. 2022. "Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications" Molecules 27, no. 3: 795. https://doi.org/10.3390/molecules27030795
APA StyleKowalczyk, T., Merecz-Sadowska, A., Picot, L., Brčić Karačonji, I., Wieczfinska, J., Śliwiński, T., & Sitarek, P. (2022). Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Molecules, 27(3), 795. https://doi.org/10.3390/molecules27030795










