Geniposide Inhibits Oral Squamous Cell Carcinoma by Regulating PI3K-Akt Signaling-Mediated Apoptosis: A Multi-Method Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cell Strain
2.1.2. Drugs and Reagents
2.1.3. Instruments
2.2. Methods
2.2.1. Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation
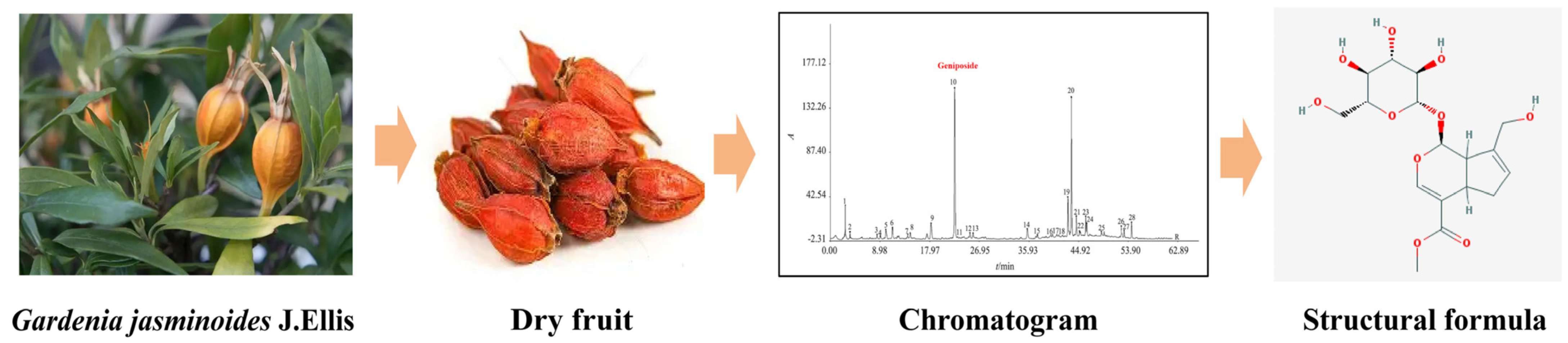
Geniposide’s Molecular Structure and Target Gene Screening
OSCC Target Gene Acquisition
Drug–Disease Cross-Target Screening and Drug–Target–Disease Network Construction
Protein–Protein Interaction (PPI) Network Construction
GO and KEGG Analyses
Molecular Docking
Molecular Dynamics Simulation
2.2.2. Cell Experiments
Cell Culture and Drug Preparation
MTT Assay
Acridine Orange/Ethidium Bromide (AO/EB) Staining
Western Blot
Statistical Analysis
3. Results
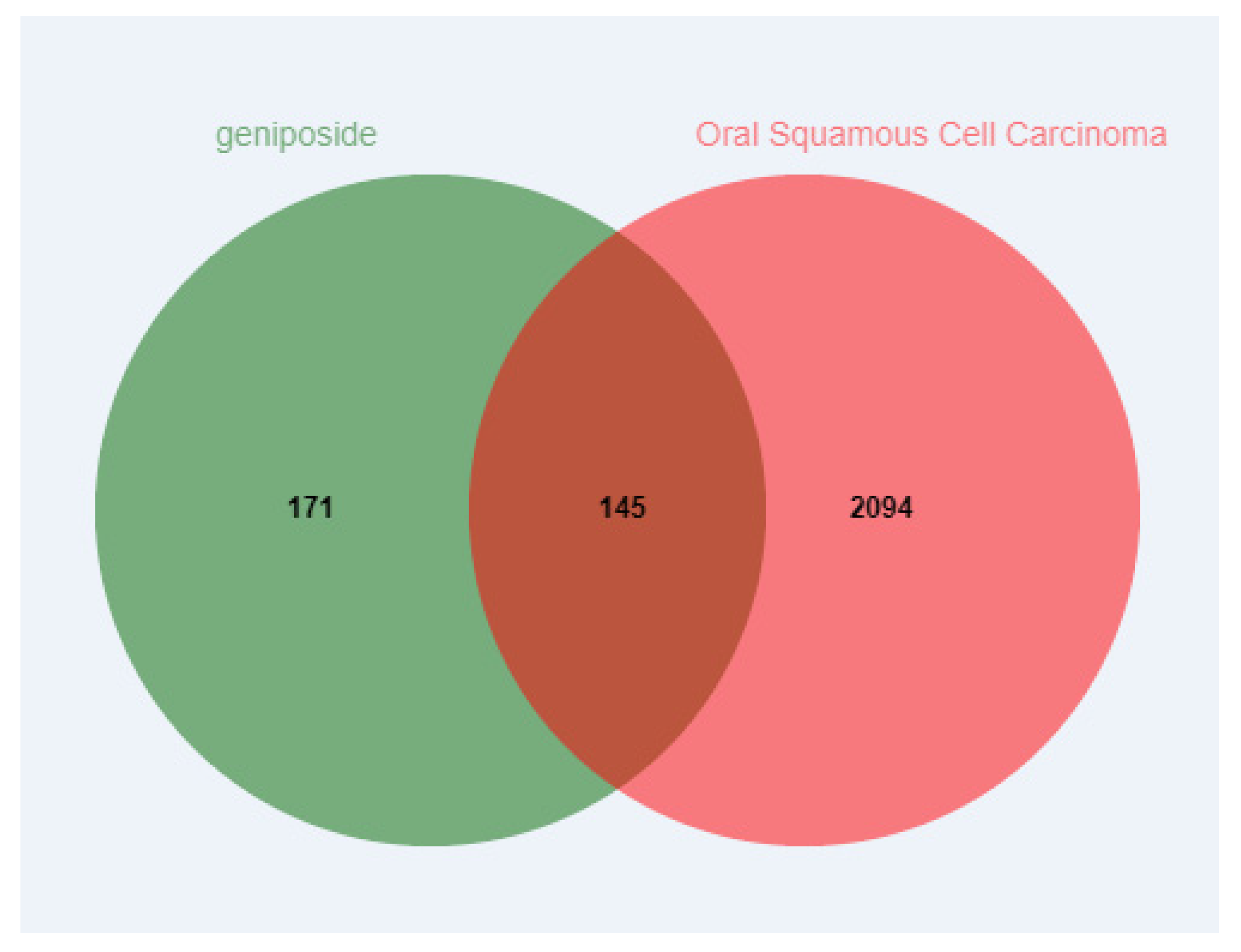
3.1. Prediction of Potential Targets of Geniposide and OSCC
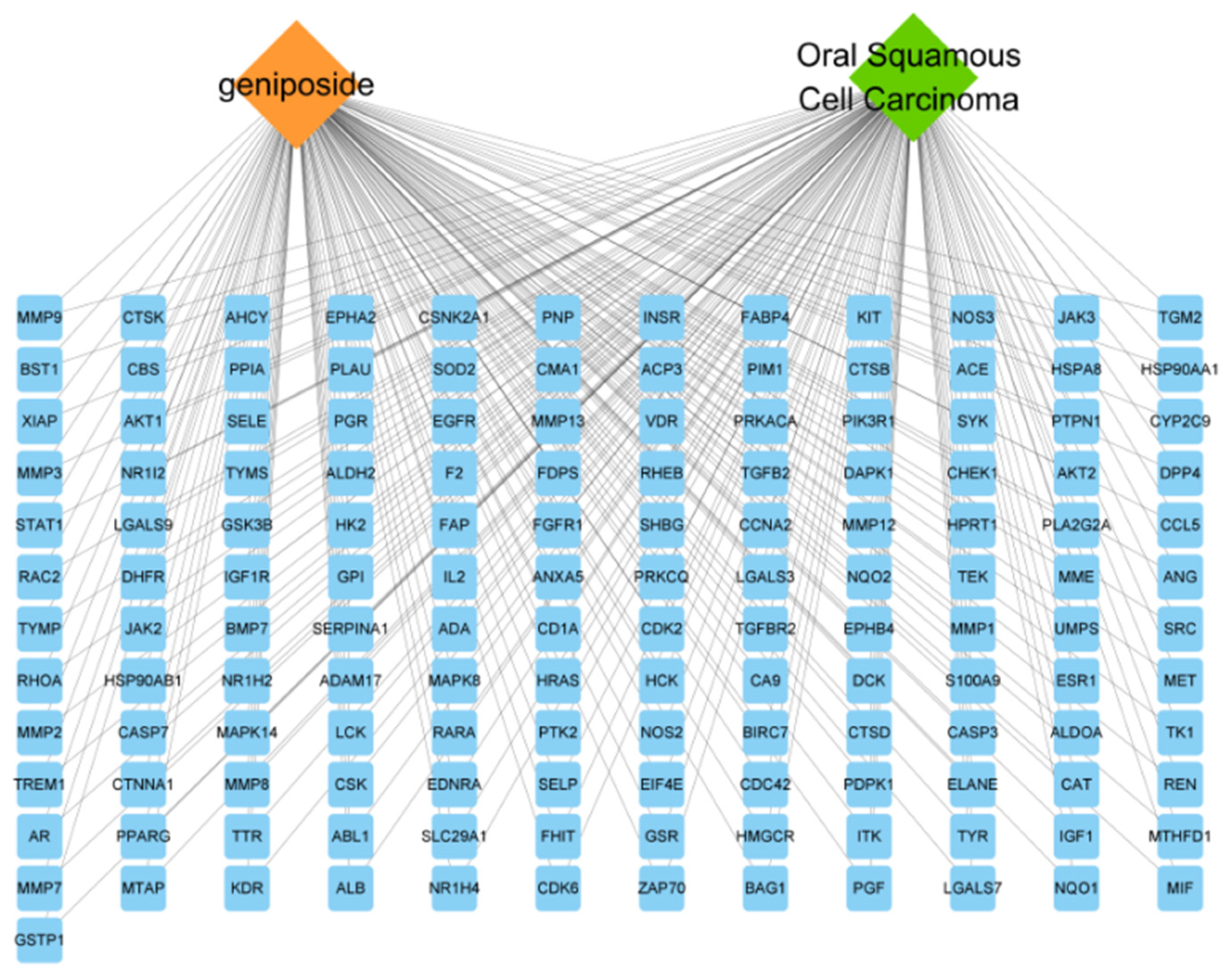
3.2. “Drug–Target–Disease” Network Analysis
3.3. PPI Network Construction and Core Target Screening
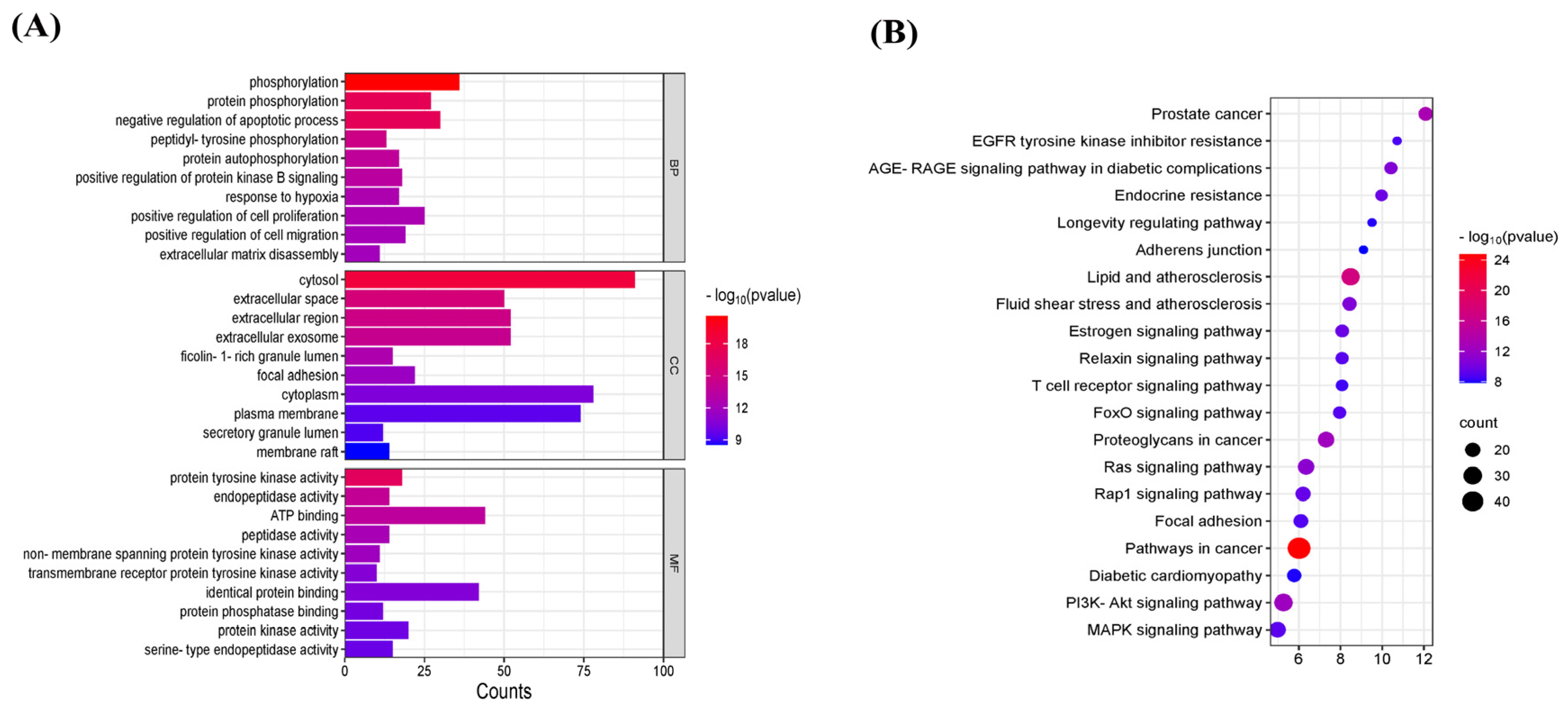
3.4. GO Analysis and KEGG Signaling Pathway Analysis
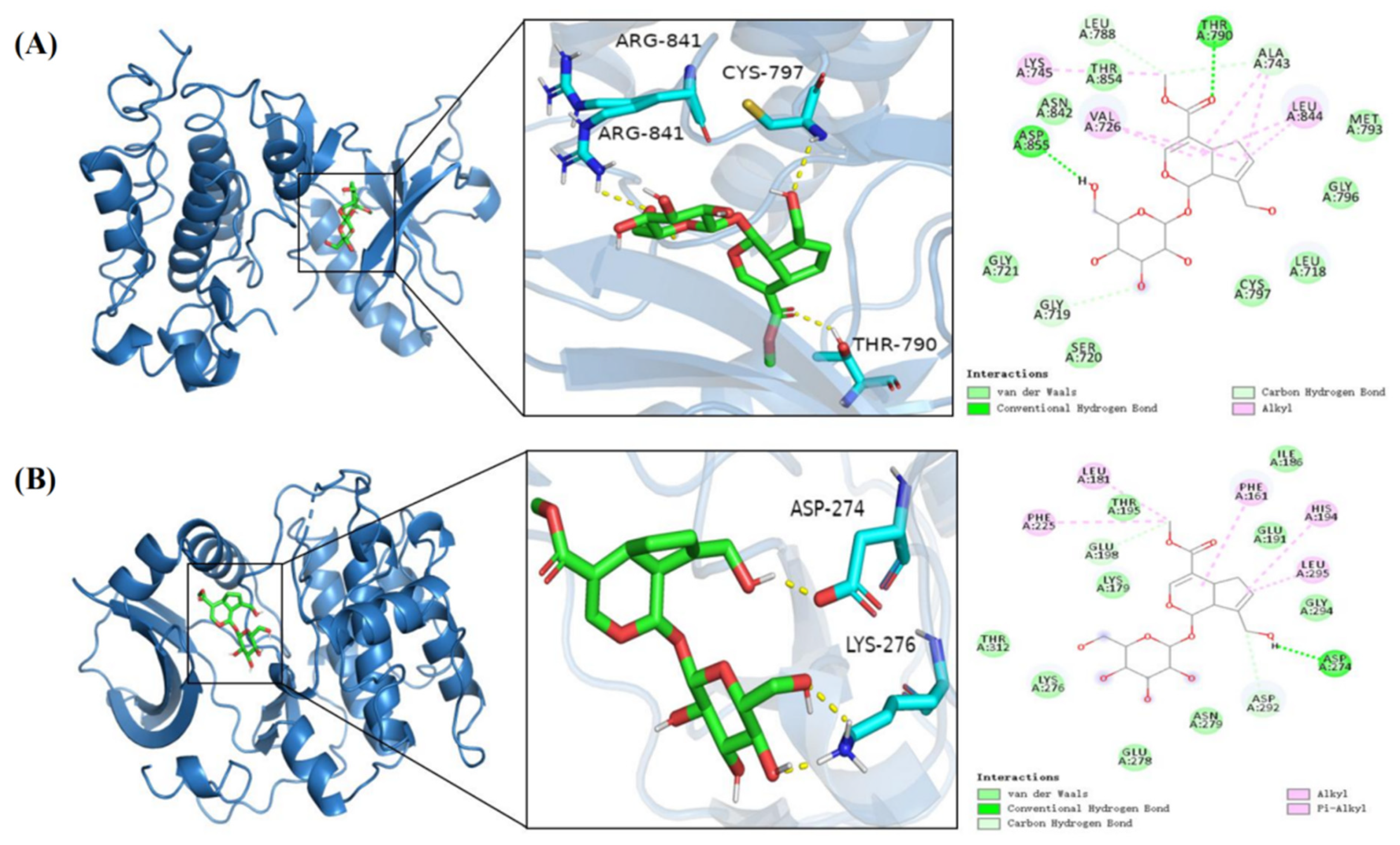
3.5. Molecular Docking
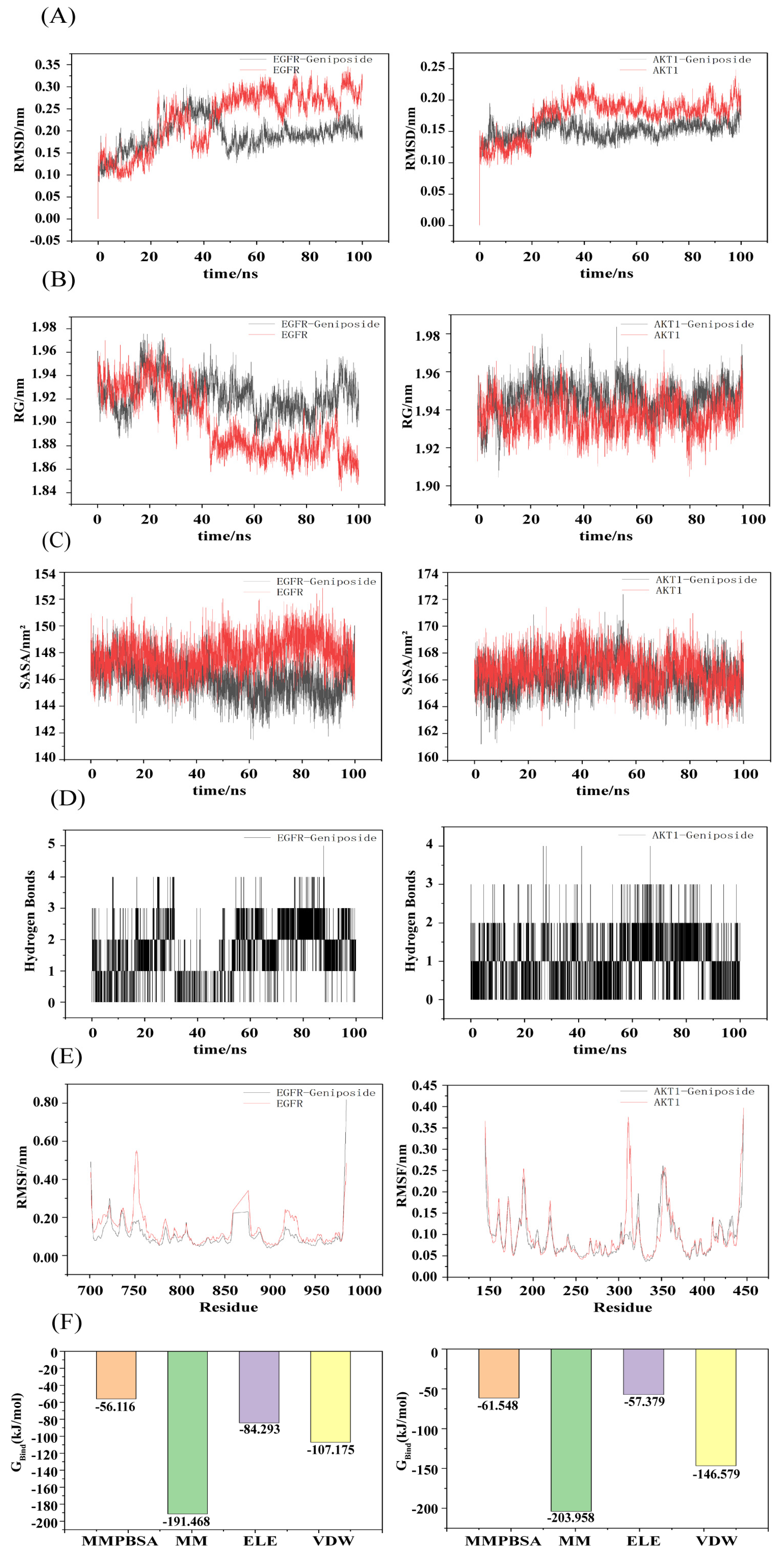
3.6. Molecular Dynamics Simulation
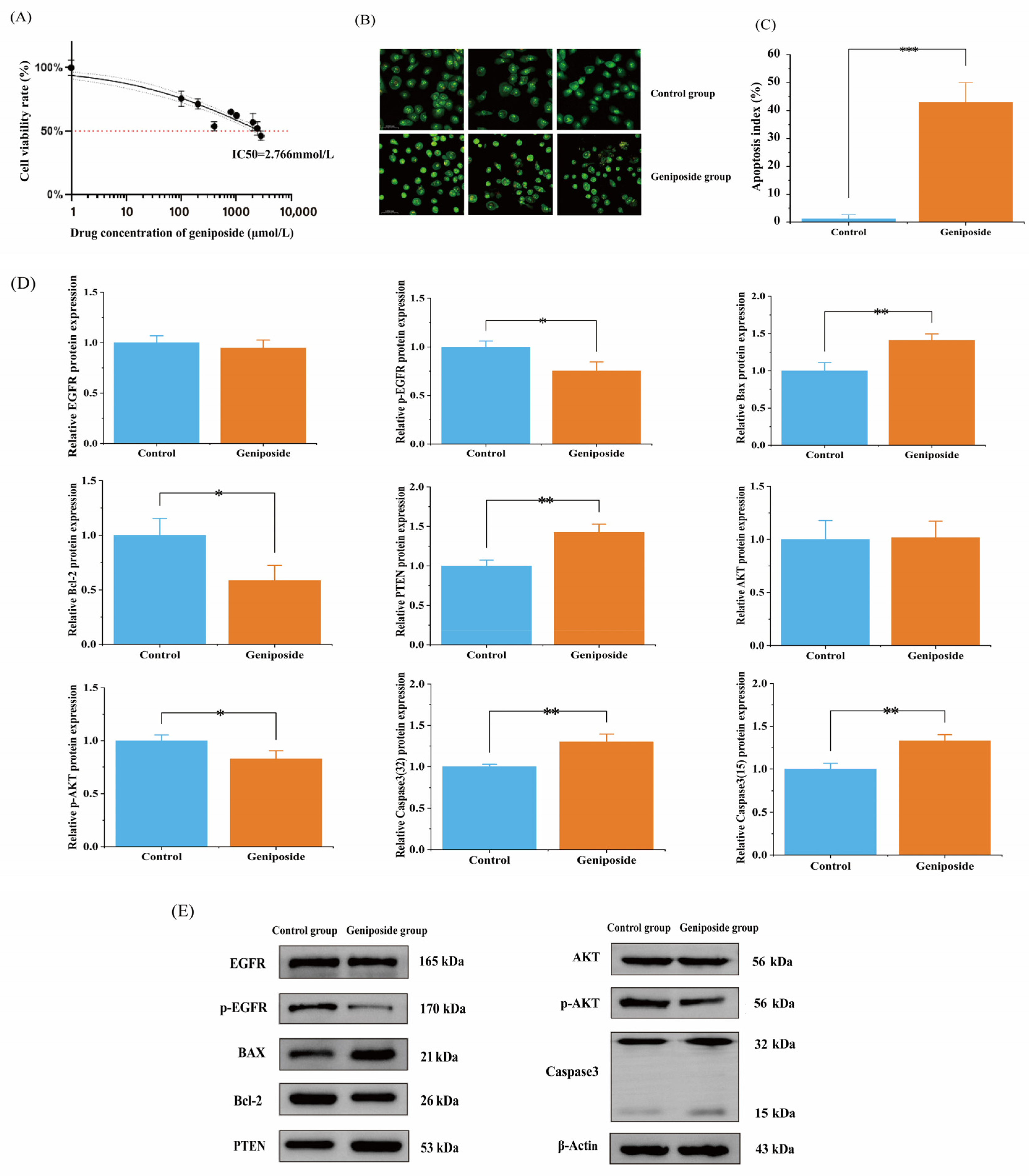
3.7. Geniposide’s Impact on OSCC Cell Activity
3.8. Geniposide’s Impact on Apoptosis
3.9. Western Blot
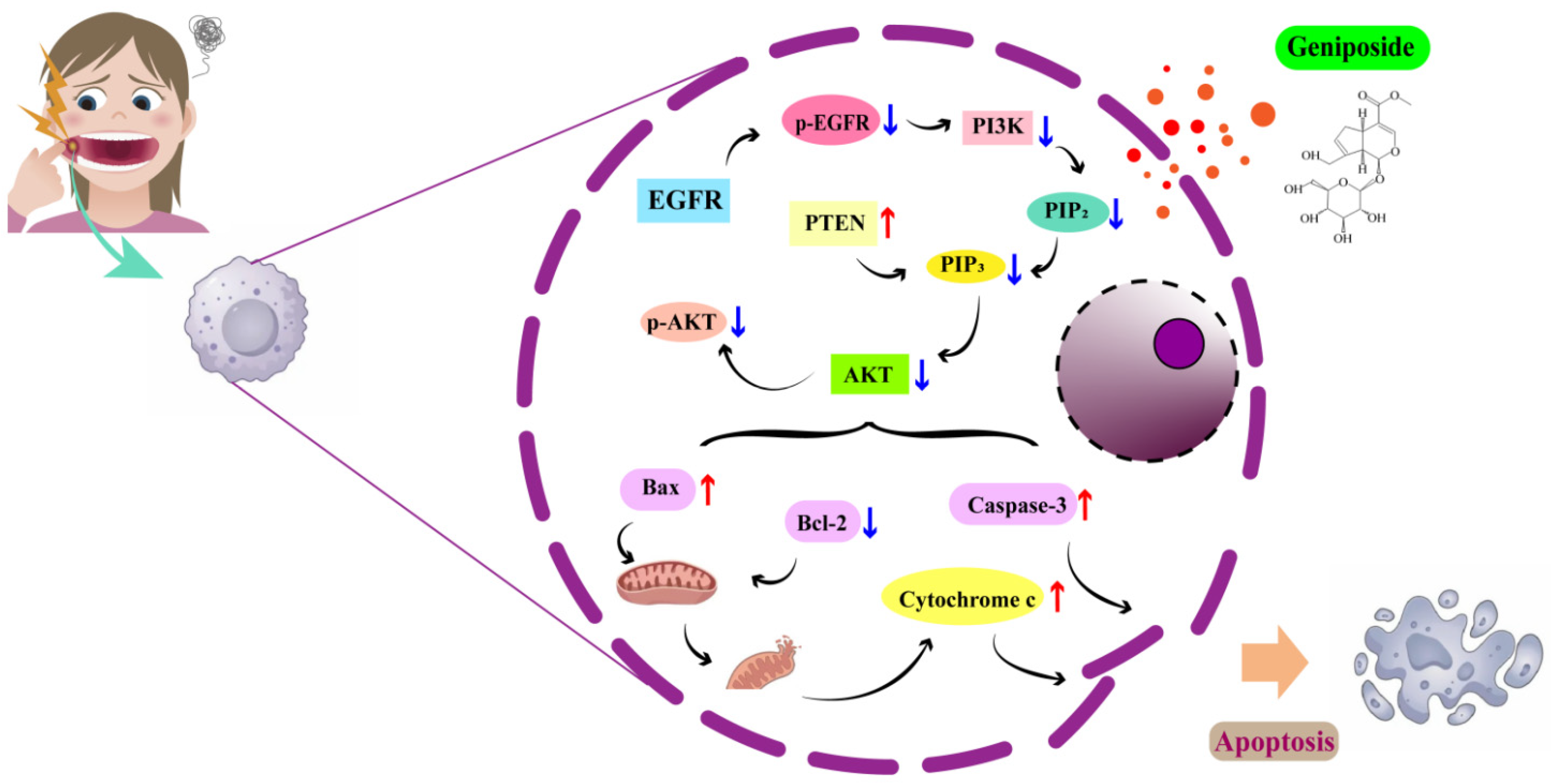
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral. Sci. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Andisheh-Tadbir, A.; Mansouri, Z.M.; Khademi, B.; Bayat, P.; Sedarat, H.; Tabesh, A.; Khorami, E.T. Evaluation of recurrence, mortality and treatment complications of oral squamous cell carcinoma in public health centers in Shiraz during 2010 to 2020. BMC Oral. Health 2023, 23, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhang, R.Q.; Rahman, K.; Cao, Z.X.; Zhang, H.; Peng, C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid.-Based Complement. Altern. Med. 2019, 2019, 4925682. [Google Scholar] [CrossRef]
- Bai, G.; Chen, B.; Xiao, X.; Li, Y.; Liu, X.; Zhao, D.; Zhang, L.; Zhao, D. Geniposide inhibits cell proliferation and migration in human oral squamous carcinoma cells via AMPK and JNK signaling pathways. Exp. Ther. Med. 2022, 24, 706. [Google Scholar] [CrossRef]
- Cheng, Z.; Xu, H.; Wang, X.; Liu, Z. Lactobacillus raises in vitro anticancer effect of geniposide in HSC-3 human oral squamous cell carcinoma cells. Exp. Ther. Med. 2017, 14, 4586–4594. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Y.; Liu, X.; Liu, L.; Zhu, L.; Chen, G.; Ye, Q.; He, C.; Xiao, X.; Li, J. Geniposide Suppresses Tumor Progression Through DUOX1-Mediated Ferroptosis in Hepatocellular Carcinoma. Am. J. Chin. Med. 2025, 53, 1573–1589. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, J.; Liu, Y.; Liu, X.; Lu, Q.; Zeng, Z.; Zhu, L.; Tong, X.; Xu, Q. Geniposide inhibits proliferation and induces apoptosis of diffuse large B-cell lymphoma cells by inactivating the HCP5/miR-27b-3p/MET axis. Int. J. Med. Sci. 2020, 17, 2735–2743. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. Plant-Derived Anticancer Agents: Lessons from the Pharmacology of Geniposide and Its Aglycone, Genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Wei, M.; Wu, Y.; Liu, H.; Xie, C. Genipin Induces Autophagy and Suppresses Cell Growth of Oral Squamous Cell Carcinoma via PI3K/AKT/MTOR Pathway. Drug Des. Dev. Ther. 2020, 14, 395–405. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Bauer, P.; Hess, B.; Lindahl, E. GROMACS 2022.5 Manual, version 2022.5; Zenodo: Geneva, Switzerland, 2023. [CrossRef]
- Buglak, A.A.; Samokhvalov, A.V.; Zherdev, A.V.; Dzantiev, B.B. Methods and Applications of In Silico Aptamer Design and Modeling. Int. J. Mol. Sci. 2020, 21, 8420. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Pérez, A.; Marchán, I.; Svozil, D.; Sponer, J.; Cheatham, T.E., 3rd; Laughton, C.A.; Orozco, M. Refinement of the AMBER Force Field for Nucleic Acids: Improving the Description of α/γ Conformers. Biophys. J. 2007, 92, 3817–3829. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 2. Explicit Solvent Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, X.; Sang, J.; Zhang, Y.; Zhang, H.; Lu, F.; Liu, F. An acid-stable β-glucosidase from Aspergillus aculeatus: Gene expression, biochemical characterization and molecular dynamics simulation. Int. J. Biol. Macromol. 2018, 119, 462–469. [Google Scholar] [CrossRef]
- Ahmed, B.; Ashfaq, U.A.; Mirza, M.U. Medicinal plant phytochemicals and their inhibitory activities against pancreatic lipase: Molecular docking combined with molecular dynamics simulation approach. Nat. Prod. Res. 2018, 32, 1123–1129. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, X.; Duan, H.; Shen, Y.; Gu, K.; Huang, K.; Wang, Y.; Shu, M.; Zhang, R.; Lin, Z. Molecular dynamics simulation-driven focused virtual screening and experimental validation of Fisetin as an inhibitor of Helicobacter pylori HtrA protease. Mol. Divers. 2025, 1–16. [Google Scholar] [CrossRef]
- Li, C.; Wen, R.; Liu, D.; Yan, L.; Gong, Q.; Yu, H. Assessment of the Potential of Sarcandra glabra (Thunb.) Nakai. in Treating Ethanol-Induced Gastric Ulcer in Rats Based on Metabolomics and Network Analysis. Front. Pharmacol. 2022, 13, 810344. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.; Mitro, A.; Hoque, H.; Hasan, N.; Jewel, G.N.A. Identification of potential novel therapeutic drug target against Elizabethkingia anophelis by integrative pan and subtractive genomic analysis: An in silico approach. Comput. Biol. Med. 2023, 165, 107436. [Google Scholar] [CrossRef] [PubMed]
- Sakano, T.; Mahamood, I.; Yamashita, T.; Fujitani, H. Molecular dynamics analysis to evaluate docking pose prediction. Biophys. Physicobiology 2016, 13, 181–194. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, Z.; Zhang, J.; Wu, D.; Li, H.; Geng, F. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 2023, 405 Pt A, 134824. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, S.; Luo, S.; Yu, F.; Sun, K. Traditional Chinese Medicine Monomers and Their Derivatives as a Promising Therapeutic Tool for Hepatocellular Carcinoma by Activation of Mitophagy. Drug Des. Dev. Ther. 2025, 19, 7069–7087. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, N.; Tan, H.; Guo, W.; Chen, F.; Zhong, Z.; Man, K.; Tsao, S.W.; Lao, L.; Feng, Y. Direct inhibition of the TLR4/MyD88 pathway by geniposide suppresses HIF-1α-independent VEGF expression and angiogenesis in hepatocellular carcinoma. Br. J. Pharmacol. 2020, 177, 3240–3257. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.-L.; Sun, P.; Yi, R.; Liu, H.; Feng, X.; Park, K.-Y.; Zhao, X. Lactobacillus casei Strain Shirota Enhances the In Vitro Antiproliferative Effect of Geniposide in Human Oral Squamous Carcinoma HSC-3 Cells. Molecules 2018, 23, 1069. [Google Scholar] [CrossRef]
- Camaya, I.; Donnelly, S.; O’BRien, B. Targeting the PI3K/Akt signaling pathway in pancreatic β-cells to enhance their survival and function: An emerging therapeutic strategy for type 1 diabetes. J. Diabetes 2022, 14, 247–260. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Sun, Z. Spectrum of EGFR aberrations and potential clinical implications: Insights from integrative pan-cancer analysis. Cancer Commun. 2020, 40, 43–59. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Wangzhou, K.; Lu, Z.; Lai, Z.; Fu, W.; Liu, C.; Tan, Y.; Hao, C. Upregulated circ_0002141 facilitates oral squamous cell carcinoma progression via the miR-1231/EGFR axis. Oral. Dis. 2023, 29, 902–912. [Google Scholar] [CrossRef]
- Sinto, M.S.; Thomas, S.; Kannan, S. Combinatorial treatment with Gefitinib and Bay11-7085 sensitizes primary Gefitinib-resistant OSCC cells by influencing the EGFR- NFκB signaling axis. Med. Oncol. 2021, 38, 110. [Google Scholar] [CrossRef]
- Brandmaier, A.; Hou, S.-Q.; Shen, W.H. Cell Cycle Control by PTEN. J. Mol. Biol. 2017, 429, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Vanaja, K.G.; Boyer, J.A.; Gadal, S.; Solomon, H.; Chandarlapaty, S.; Levchenko, A.; Rosen, N. Regulation of PTEN translation by PI3K signaling maintains pathway homeostasis. Mol. Cell 2021, 81, 708–723.e5. [Google Scholar] [CrossRef] [PubMed]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Kerdjoudj, M.; De La Torre, R.A.; Arnouk, H. Characterization of DJ-1, PTEN, and p-Akt as Prognostic Biomarkers in the Progression of Oral Squamous Cell Carcinoma. Cureus 2023, 15, e34436. [Google Scholar] [CrossRef]
- Hsu, A.H.; Lum, M.A.; Shim, K.-S.; Frederick, P.J.; Morrison, C.D.; Chen, B.; Lele, S.M.; Sheinin, Y.M.; Daikoku, T.; Dey, S.K.; et al. Crosstalk between PKCα and PI3K/AKT Signaling Is Tumor Suppressive in the Endometrium. Cell Rep. 2018, 24, 655–669. [Google Scholar] [CrossRef]
- Roy, N.K.; Monisha, J.; Padmavathi, G.; Lalhruaitluanga, H.; Kumar, N.S.; Singh, A.K.; Bordoloi, D.; Baruah, M.N.; Ahmed, G.N.; Longkumar, I.; et al. Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules 2019, 9, 253. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, S.; Liao, J.; Wang, R.; Yu, X.; Li, M. Isoliquiritigenin Inhibits Oral Squamous Cell Carcinoma and Overcomes Chemoresistance by Destruction of Survivin. Am. J. Chin. Med. 2023, 51, 2221–2241. [Google Scholar] [CrossRef] [PubMed]
- Thalij, K.M.; You, H.W.; Aher, K.B.; Bhavar, G.B.; Kumbhar, S.T.; Habeeb, M. Advances in Lipid-Based Nanomedicine: Pathway Specific siRNA Therapy and Optimizing Delivery for Hepatocellular Carcinoma. Int. J. Nanomed. 2025, 20, 10541–10566. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, J.; Cui, C.; Yang, F.; Li, K.; Gao, B.; Fu, S.; Yang, X. Folate-Modified Smart Responsive Nanosystems for Enhancing Anti-Tumor Therapy Through Calcium Overload and Chemotherapy. Int. J. Nanomed. 2025, 20, 10233–10249. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Mustafa, M.A.; Kumar, A.; Alamir, H.T.A.; Kumar, A.; Khudair, S.A.; Faisal, A.; Alubiady, M.H.S.; Jalal, S.S.; Shafik, S.S.; et al. Stealth Nanocarriers in Cancer Therapy: A Comprehensive Review of Design, Functionality, and Clinical Applications. AAPS PharmSciTech 2024, 25, 140. [Google Scholar] [CrossRef]
- Basu, B.; Garala, K.K.; Patel, R.; Dutta, A.; Ash, D.; Prajapati, B.; Singh, S.; Jha, S.K. Advanced Targeted Drug Delivery of Bioactive Nanomaterials in the Management of Cancer. Curr. Med. Chem. 2025, 32, 2711–2730. [Google Scholar] [CrossRef]
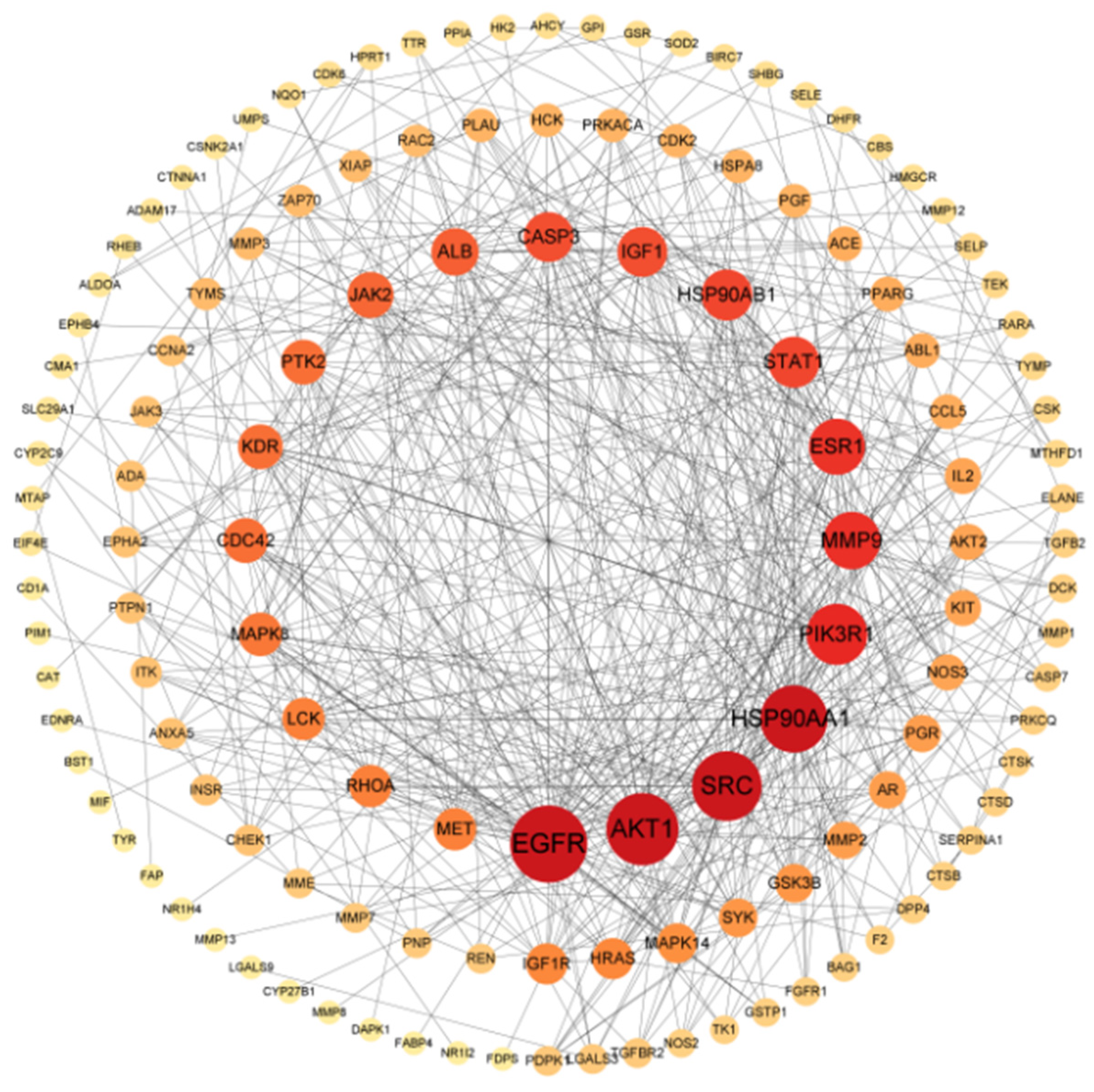
| Rank | Name | Degree |
|---|---|---|
| 1 | EGFR | 44 |
| 2 | AKT1 | 40 |
| 3 | SRC | 38 |
| 4 | HSP90AA1 | 36 |
| 5 | PIK3R1 | 31 |
| 6 | MMP9 | 28 |
| 7 | ESR1 | 27 |
| 8 | STAT1 | 23 |
| 8 | HSP90AB1 | 23 |
| 9 | CASP3 | 22 |
| 9 | IGF1 | 22 |
| 10 | ALB | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, J.; Hua, H.; Wei, P.; Chen, X.; Peng, Y.; Liu, L.; Yu, D.; You, X.; Yang, S. Geniposide Inhibits Oral Squamous Cell Carcinoma by Regulating PI3K-Akt Signaling-Mediated Apoptosis: A Multi-Method Validation Study. Curr. Issues Mol. Biol. 2025, 47, 786. https://doi.org/10.3390/cimb47090786
Wang X, Wang J, Hua H, Wei P, Chen X, Peng Y, Liu L, Yu D, You X, Yang S. Geniposide Inhibits Oral Squamous Cell Carcinoma by Regulating PI3K-Akt Signaling-Mediated Apoptosis: A Multi-Method Validation Study. Current Issues in Molecular Biology. 2025; 47(9):786. https://doi.org/10.3390/cimb47090786
Chicago/Turabian StyleWang, Xue, Jianbo Wang, Hua Hua, Ping Wei, Xue Chen, Yusheng Peng, Li Liu, Dongmei Yu, Xiaozhou You, and Siye Yang. 2025. "Geniposide Inhibits Oral Squamous Cell Carcinoma by Regulating PI3K-Akt Signaling-Mediated Apoptosis: A Multi-Method Validation Study" Current Issues in Molecular Biology 47, no. 9: 786. https://doi.org/10.3390/cimb47090786
APA StyleWang, X., Wang, J., Hua, H., Wei, P., Chen, X., Peng, Y., Liu, L., Yu, D., You, X., & Yang, S. (2025). Geniposide Inhibits Oral Squamous Cell Carcinoma by Regulating PI3K-Akt Signaling-Mediated Apoptosis: A Multi-Method Validation Study. Current Issues in Molecular Biology, 47(9), 786. https://doi.org/10.3390/cimb47090786





