1. Introduction
Alismatis Rhizoma (AR) is the dried tuber of
Alisma plantago-aquqtica L., a traditional diuretic used in China with a long history of use for treating edema, as seen in formulations like Wulingsan, Baizhusan, and Zexie Decoction. The active ingredients of AR are primarily terpenoids [
1]. The 2020 edition of the Pharmacopoeia of the People’s Republic of China (hereinafter referred to as the Chinese Pharmacopoeia) includes two specifications, i.e., AR and salt-processed AR (SAR). Research indicates that both the ethyl acetate and n-butanol fractions of AR exhibit significant diuretic effects, with dual actions of promoting and inhibiting diuresis. The more polar components in AR may have anti-diuretic properties [
2]. The total triterpene extract (TTE) and triterpene component compatibility (TCC—a mixture of alisol B 23-acetate, alisol B, alisol A 24-acetate, alisol A, and alisol C 23-acetate) also exhibit significant diuretic effects in saline-loaded rats [
3], possibly by enhancing sodium and chloride symporters in the distal tubule, thereby altering the transport rates of sodium, potassium, and chloride ions. This effect is primarily attributed to triterpenes [
4].
The Depei Bencao records that raw or wine stir-fried products of AR are used for strengthening the spleen, while salt-processed products are used for nourishing Yin and promoting diuresis. Previous laboratory pharmacological experiments also demonstrated that the diuretic effects of AR were enhanced after salt processing. Both AR and SAR showed therapeutic effects in a rat model of edema due to kidney Yin deficiency, with SAR outperforming AR in restoring related indices. Transcriptomics suggested that the SAR group was more effective than the AR group in regulating inflammation-related targets, potentially explaining efficacy of SAR [
5,
6]. However, the specific ingredients responsible for this enhancement have not been reported. Most triterpenoid components showed a decreasing trend after AR was salt-processed [
7]. The 2020 edition of the Chinese Pharmacopoeia uses alisol C 23-acetate and alisol B 23-acetate as the index components for determining the content of AR and SAR, but these are insufficient to represent the quality control components of AR and SAR. Studies have reported significant diuretic activity of alisol A 24-acetate, alisol B, and other compounds, though whether these ingredients are related to the enhanced efficacy of SAR remains unreported [
8].
Chinese medicine quality markers (Q-markers), a new concept proposed by Academician Liu Changxiao in 2016 to improve the quality control of Chinese medicine products, are chemical substances present in Chinese herbal medicines and Chinese medicine products inherently or formed during processing and preparation that are closely related to the functional properties of Chinese medicines and can be used as markers to reflect the safety and efficacy of Chinese medicines, rather than chemical components generated by their metabolic transformation through intra-biological processes [
9,
10]. The establishment of a Q-marker for traditional Chinese medicine (TCM) is of great significance for quality control and improvement of the quality standards of TCM, which can effectively improve the overall quality evaluation of TCM beverages [
11,
12,
13]. At present, the quality evaluation studies on SAR mainly focus on the trait, alisol B 23-acetate content, and so forth, while the Q-marker has not been explored to date [
14].
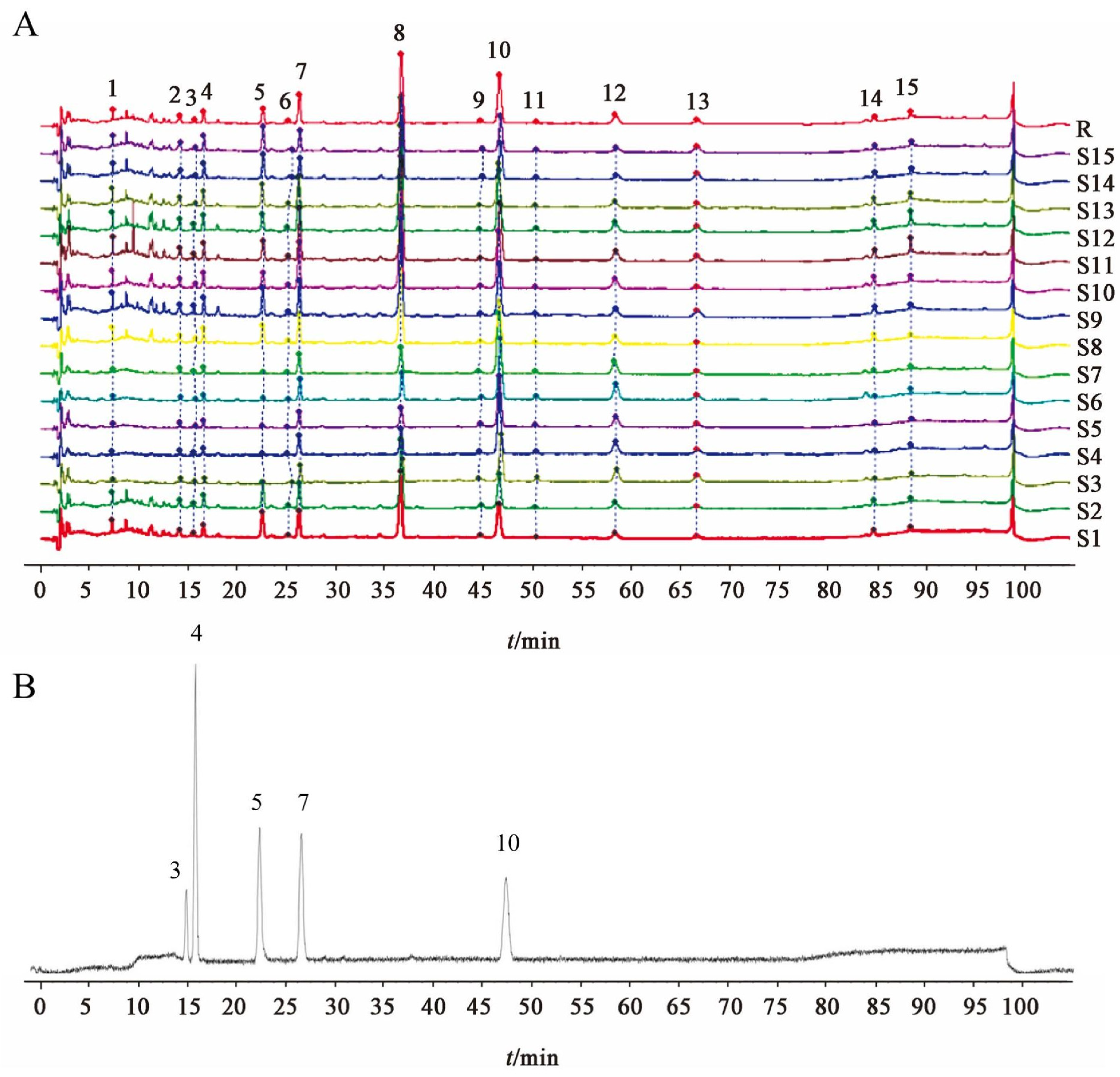
The fingerprint has the characteristics of integrity and dynamics, which can objectively reflect the ingredients in TCM. Moreover, it can reveal the characteristics, traceability, and measurability of ingredients. Network analysis can comprehensively analyze the relationship between ingredients and the efficacy of prepared slices from the drug–disease–target–pathway network to reflect the effectiveness of ingredients and the rationality of compatibility [
15]. Based on this, the present study used fingerprint combined with network analysis research mode to determine the Q-markers of the diuretic enhancement of SAR, aiming to provide a scientific basis for quality control and clinical application of SAR.
4. Discussion
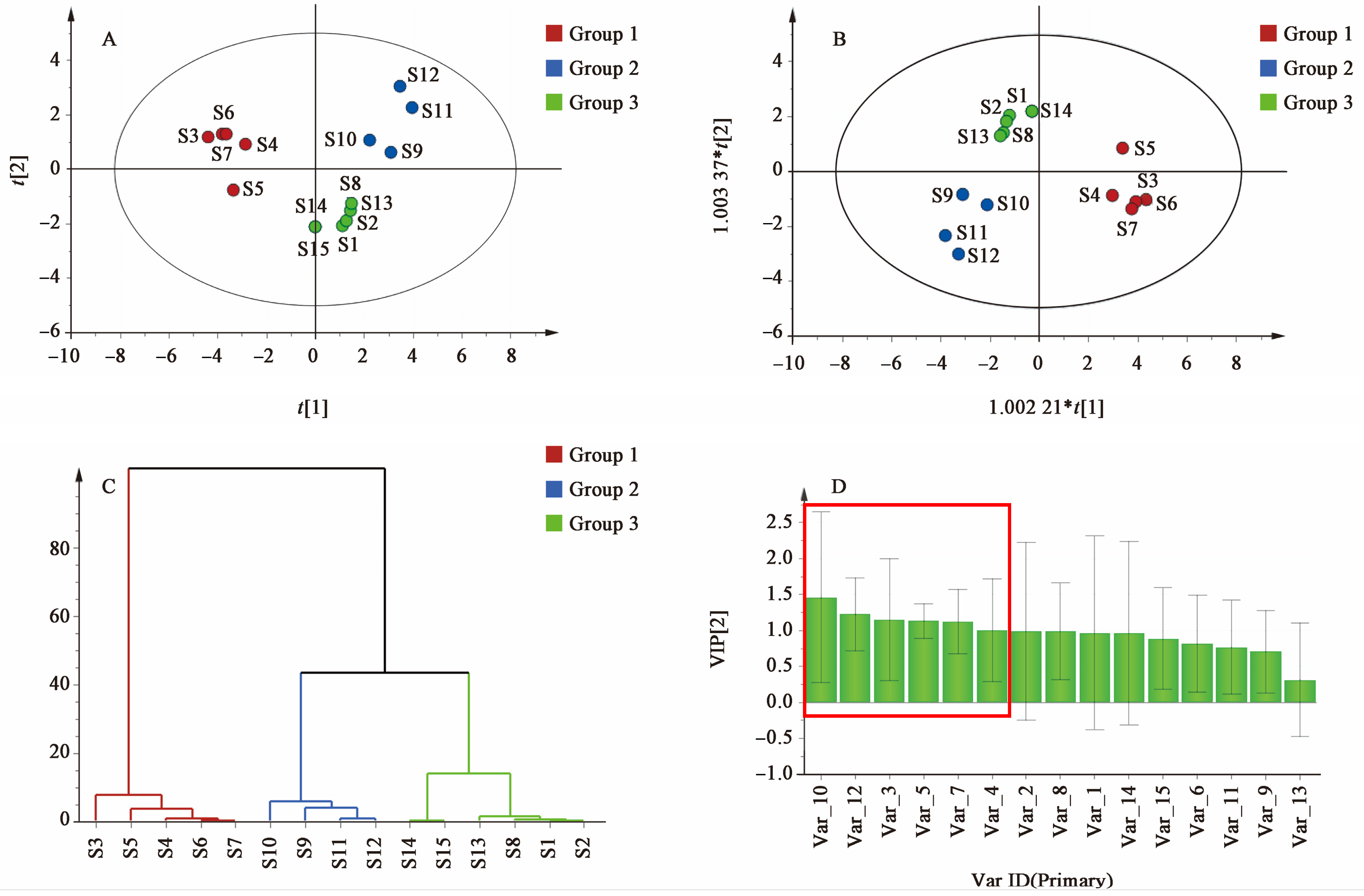
SAR, serving as the most commonly used processed variety, underscores the importance of establishing quality standards for its effective development and use. Q-markers offer valuable references for quality control of SAR. By utilizing fingerprinting technology, this study identified common peaks in SAR from different origins, screened compounds with significant contributions through chemical pattern recognition, and analyzed the diuretic mechanisms of these compounds via network analysis and molecular docking. This comprehensive evaluation of Q-markers for SAR aims to establish quality standards, provide data support for clinical applications, and ultimately better serve patients with renal edema.
The fingerprint analysis of 15 batches of SAR identified 15 compounds with high content across different manufacturers and origins. This suggests that the content of these ingredients significantly affects the efficacy of SAR. VIP analysis highlighted six key compounds (peaks 3, 4, 5, 7, 10, and 12) with VIP values of >1.0 as potential Q-markers for SAR. These compounds include alisol F, alisol C 23-acetate, alisol A, alisol A 24-acetate, alisol B 23-acetate, and an alisol O isomer. Pharmacological research supports the significant activity of these compounds. Alisol F, alisol A, and alisol A 24-acetate are known for their anti-inflammatory effects by inhibiting inflammatory cytokines [
21,
22], making them crucial in treating nephrotic edema. Alisol C 23-acetate and alisol B 23-acetate, which are used as index components in the content determination of AR, also demonstrate anti-inflammatory, diuretic, and lipid-lowering activities [
3,
23]. Specifically, alisol B 23-acetate has been shown to activate renal farnesoid X receptor (FXR), providing renoprotection against ischemia–reperfusion injury and alleviating renal fibrosis through mechanisms such as gut microbiome modulation and blood pressure regulation [
24,
25]. This indicates its significant potential in treating renal diseases. Although alisol O exhibits nephrotoxicity [
24], the activity of its isomer remains unexplored, warranting further investigation to determine if it also possesses nephrotoxic effects.
Edema is commonly associated with various pathological conditions such as nephritis and nephrotic syndrome, leading to sodium retention and microcirculation disorders. Inflammation in the body is a significant predisposing factor for edema [
25], as it can lead to renal edema through mechanisms such as renal neutrophil influx and capillary leakage [
26]. Therefore, reducing inflammation and enhancing the body’s immune response are crucial for treating edema and may be key to the diuretic effects of certain drugs. Research has demonstrated that alisol F and 25-anhydroalisol F inhibit the production of nitric oxide (NO), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β) by interfering with the MAPK, STAT3, and NF-κB signaling pathways in cells [
21]. MAPK, a crucial upstream signaling molecule for NF-κB, is implicated in the core targets of SAR for edema treatment. As an essential pathway of intracellular signal transduction, MAPK is involved in regulating cell growth, oxidative stress, inflammatory responses, and other cellular processes. Chae, Song [
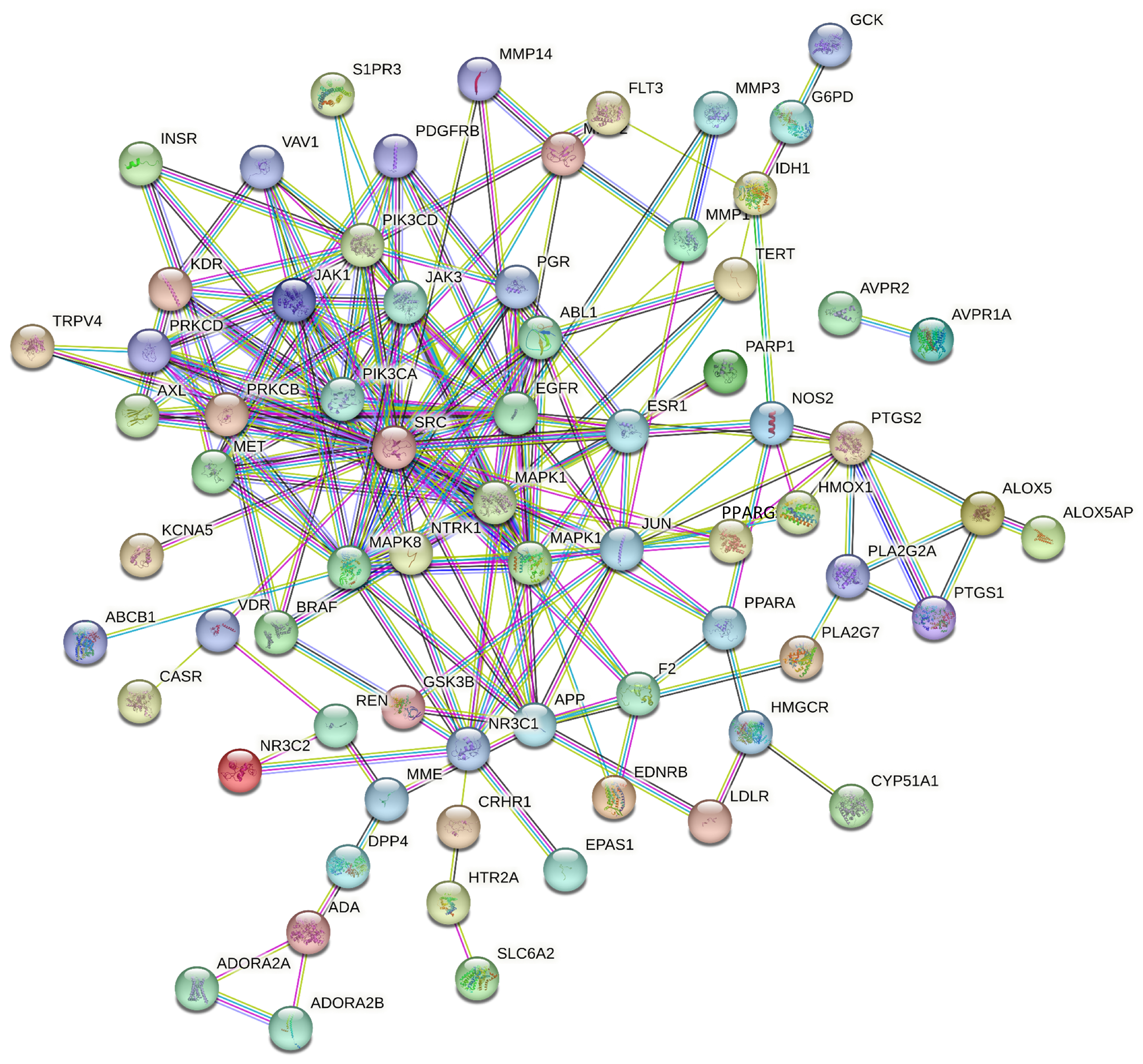
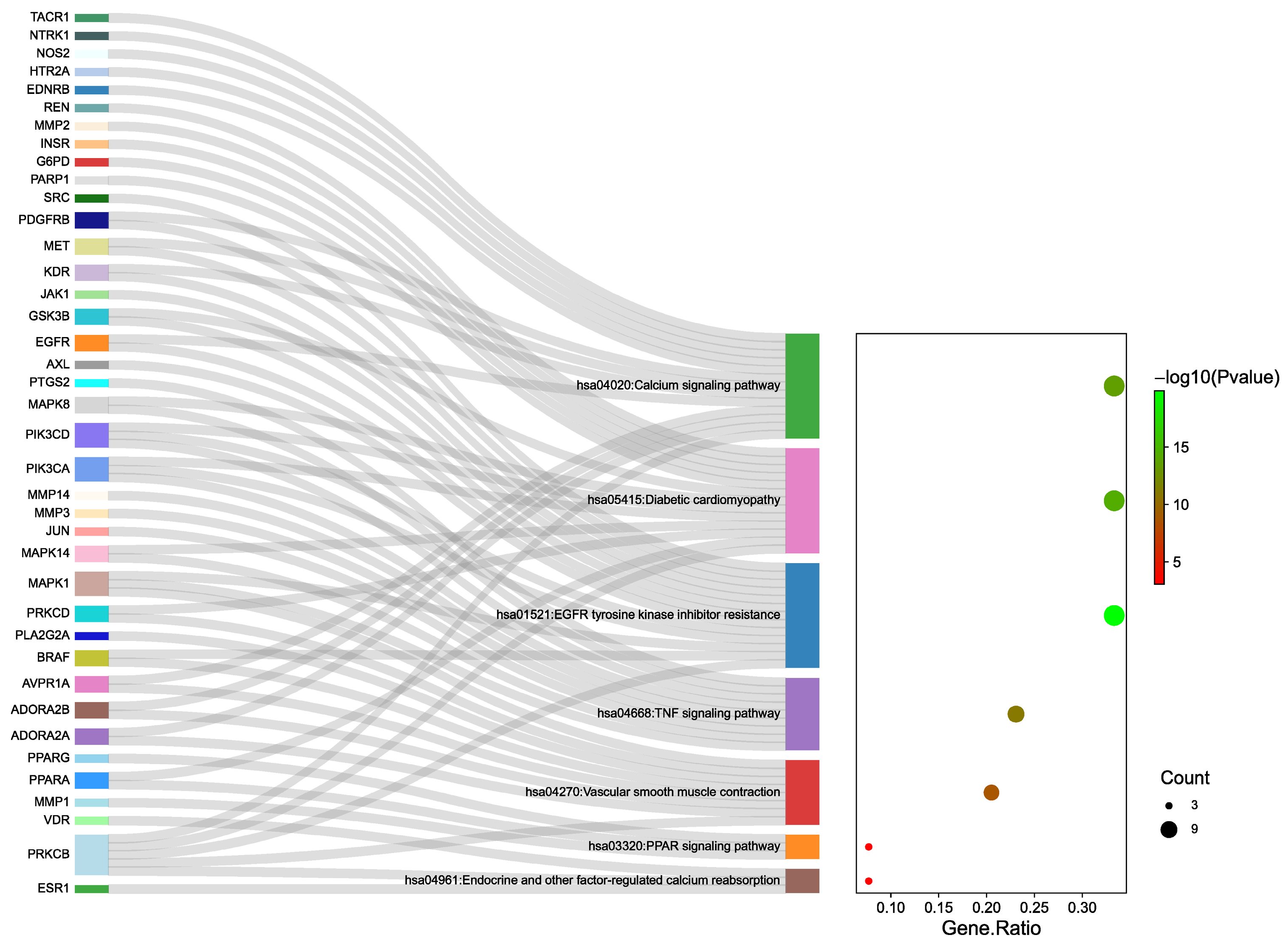
27] found that inhibiting MAPK signaling phosphorylation and activating degranulation modulated the expression of inflammatory mediators. Pathway enrichment analysis further highlighted MAPK as a significant target within the TNF signaling pathway, with MAPK1, MAPK14, and other related proteins playing key roles in this pathway.
In addition, this study suggests that SAR may also exert anti-inflammatory and immune-modulating effects through targets such as SRC and EGFR, along with their associated pathways. SRC is known to regulate various signaling pathways related to biological activities, including gene transcription, immune responses, and cell adhesion. Inhibiting SRC activity can reduce the release of pro-inflammatory cytokines, thereby ameliorating inflammatory responses [
28]. EGFR, a receptor for epidermal growth factor and transforming growth factor α, interacts with active ingredients in SAR to block the binding of pro-inflammatory factors, thereby exerting anti-inflammatory effects and enhancing the body’s immune function [
29]. Moreover, KEGG enrichment analysis indicated that the EGFR tyrosine kinase inhibitor resistance pathway might be involved in the diuretic effects of SAR. EGFR tyrosine kinase inhibitors mediate multiple signal transduction pathways, transmitting extracellular signals into the cell and playing a crucial regulatory role in the proliferation, differentiation, and apoptosis of normal and tumor cells. This process can enhance the immune response and significantly improve the body’s disease resistance [
30]. Research has shown that compounds like alisol F, alisol A, and alisol B 23-acetate can regulate MAPK activation [
31,
32], thereby inhibiting MAPK expression and activity [
21,
33,
34]. The SRC/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is also critical in the pathogenesis of renal fibrosis [
35]. Both alisol A 24-acetate and alisol B 23-acetate have been found to inhibit the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway [
36]. Since SRC regulates PI3K/Akt signaling, with inhibitors like PP1 and LY294002 inhibiting Akt phosphorylation, it is hypothesized that the components in SAR may act on these inflammatory targets. By exerting anti-inflammatory effects, these components may improve renal inflammation and consequently enhance the balance of water and sodium in the kidneys, thereby achieving diuretic effects.
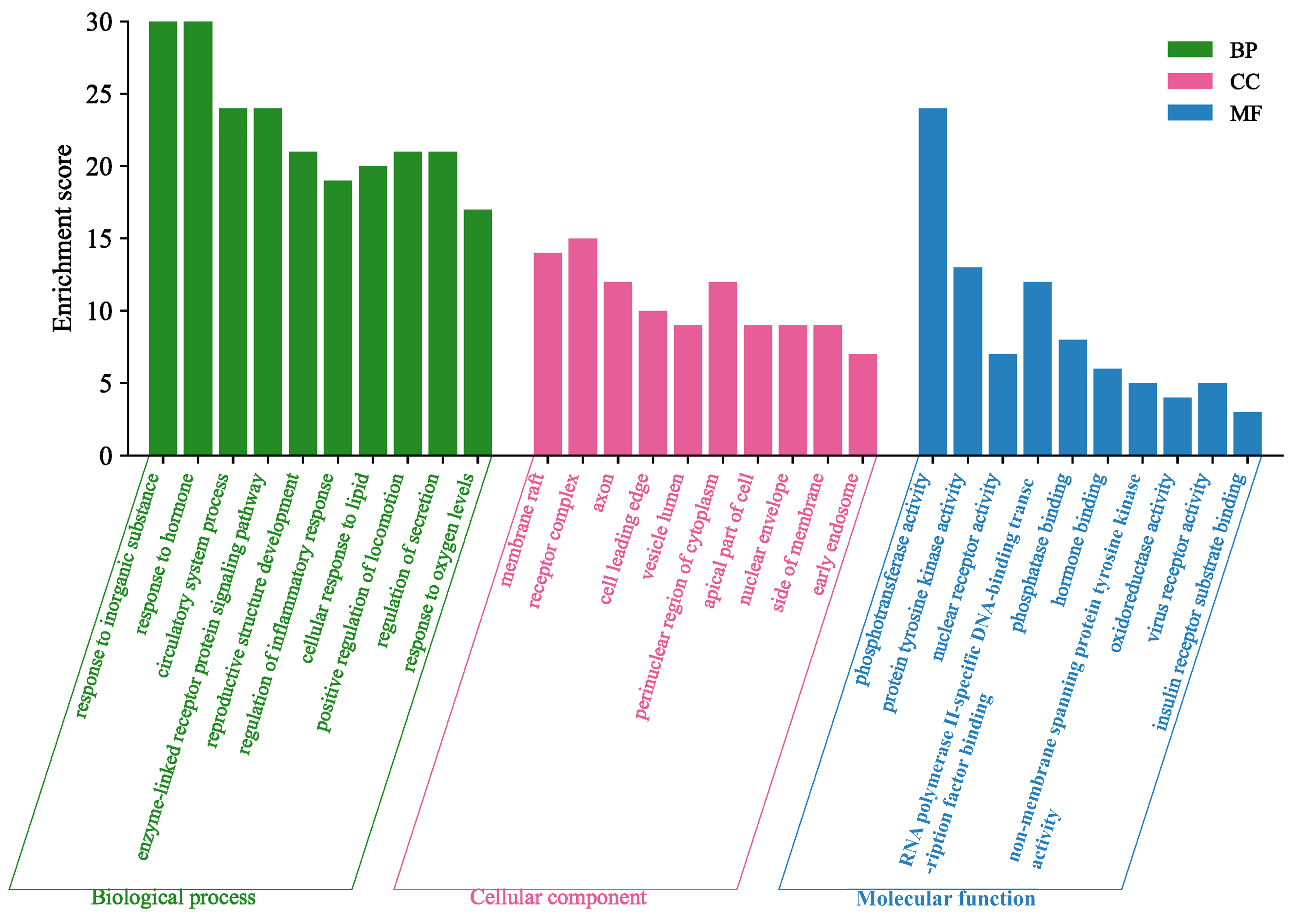
The GO enrichment analysis indicated that SAR exerted its diuretic effects by modulating inflammatory responses, hormone signaling, and circulatory processes. There appears to be a synergistic interaction among various signaling pathways, as shown by the KEGG pathway enrichment data, which highlighted the involvement of pathways such as EGFR tyrosine kinase inhibitor resistance, peroxisome proliferator-activated receptor (PPAR) signaling, and TNF signaling. Additionally, KEGG analysis revealed that the diuretic effects of SAR may be mediated through pathways like the calcium signaling pathway, endocrine and other factor-regulated calcium reabsorption, and vascular smooth muscle contraction. Research on the diuretic effects of AR in saline-loaded rats demonstrated significant diuretic effects by enhancing the excretion of Na
+, K
+, and Cl
− ions [
37], suggesting that the diuretic effects of AR are closely related to water and sodium ion channels. Studies have shown that calcium signaling pathways may be involved in the activation of diuretic hormones [
38]. In vivo experiments with olive leaf extract treatment revealed increased expression of calcium-sensitive receptor mRNA, decreased expression of aquaporin (AQP)-2 mRNA, and increased expression of AQP-2-targeted MicroRNA (miRNA)-137, suggesting that olive leaf extract antagonizes the effects of vasopressin by stimulating calcium-sensitive receptors [
39]. In earlier studies by this research group, it was also found that AR and SAR reduced the transcription levels of renal AQP-1 and AQP-2 mRNA, alleviating edema symptoms in rats with kidney Yin deficiency [
40]. This effect may be achieved by stimulating calcium-sensitive receptors. The endocrine and other factor-regulated calcium reabsorption pathway plays a key role in regulating intracellular calcium ion concentration, which serves as a secondary messenger in the antidiuretic hormone response [
41], directly affecting renal water reabsorption. It is speculated that SAR may act on the calcium signaling pathway, thereby affecting the expression of water and sodium transport proteins and regulating the balance of water metabolism. However, the key targets within these pathways still require experimental validation, and more detailed mechanisms need further investigation.
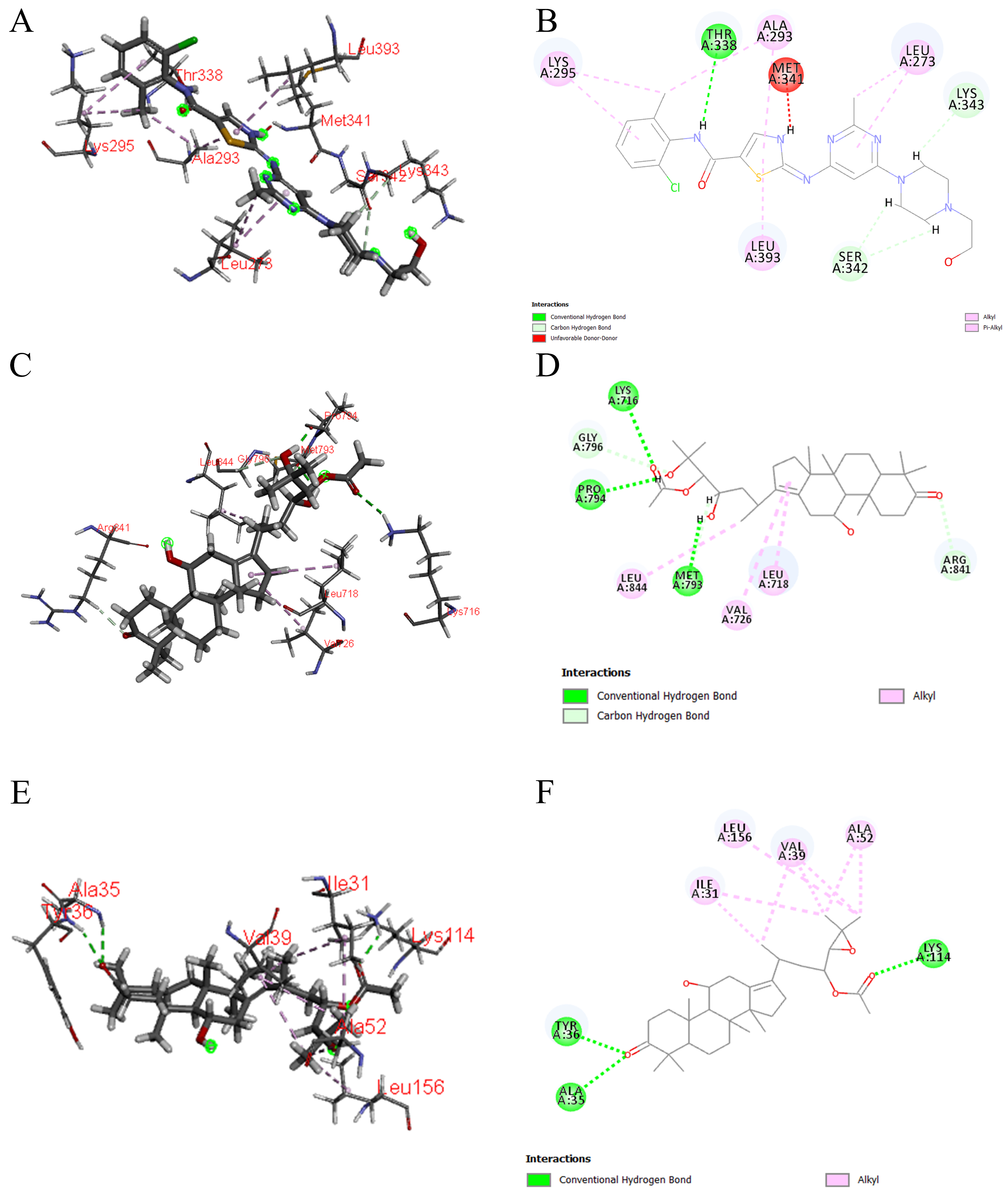
Moreover, molecular docking results indicated that the six Q-markers effectively bind to core targets, suggesting that the active components of SAR might exert their diuretic effects by participating in EGFR tyrosine kinase inhibitor resistance, regulated by EGFR. Consequently, the Q-markers identified through fingerprinting combined with chemical pattern recognition analysis were validated to reflect their biological activity, as confirmed by network analysis and molecular docking studies. This study provides a solid foundation for the quality control of SAR.
Despite the comprehensive identification of potential Q-markers and the proposed multi-target mechanisms underlying the diuretic effects of SAR, this study has several limitations. Primarily, the network pharmacology and pathway enrichment analyses are predictive in nature and lack experimental validation, particularly through in vivo models, to confirm the actual involvement of targets such as MAPK, SRC, EGFR, and calcium signaling pathways in the diuretic action of SAR. Moreover, molecular docking results, while indicative of binding potential, may not fully reflect the actual binding affinity or functional impact of the compounds on the target proteins due to limitations in simulating physiological conditions. Additionally, the current approach does not account for potential synergistic effects among the multiple active compounds in SAR, which could be critical for its overall efficacy. Future studies should prioritize in vivo validation using animal models of renal edema to verify the predicted targets and pathways, incorporate binding affinity assays (e.g., surface plasmon resonance) to confirm compound–target interactions, and explore combinatory effects of the Q-markers to assess synergism. Furthermore, detailed mechanistic studies, including gene knockout or pharmacological inhibition of key targets, would help elucidate the precise contributions of each pathway to the diuretic activity of SAR.