Natural Products Targeting the Androgen Receptor Signaling Pathway: Therapeutic Potential and Mechanisms
Abstract
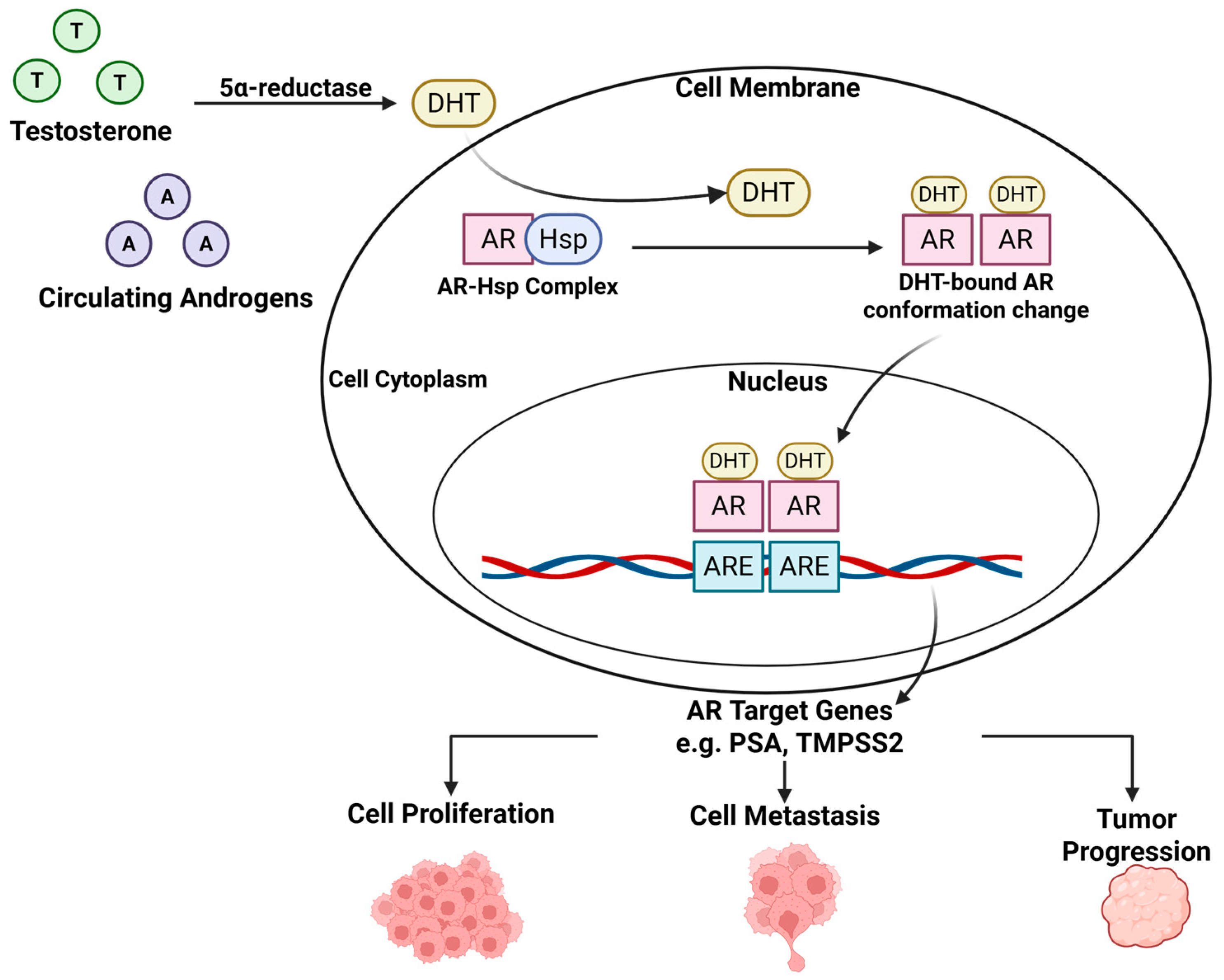
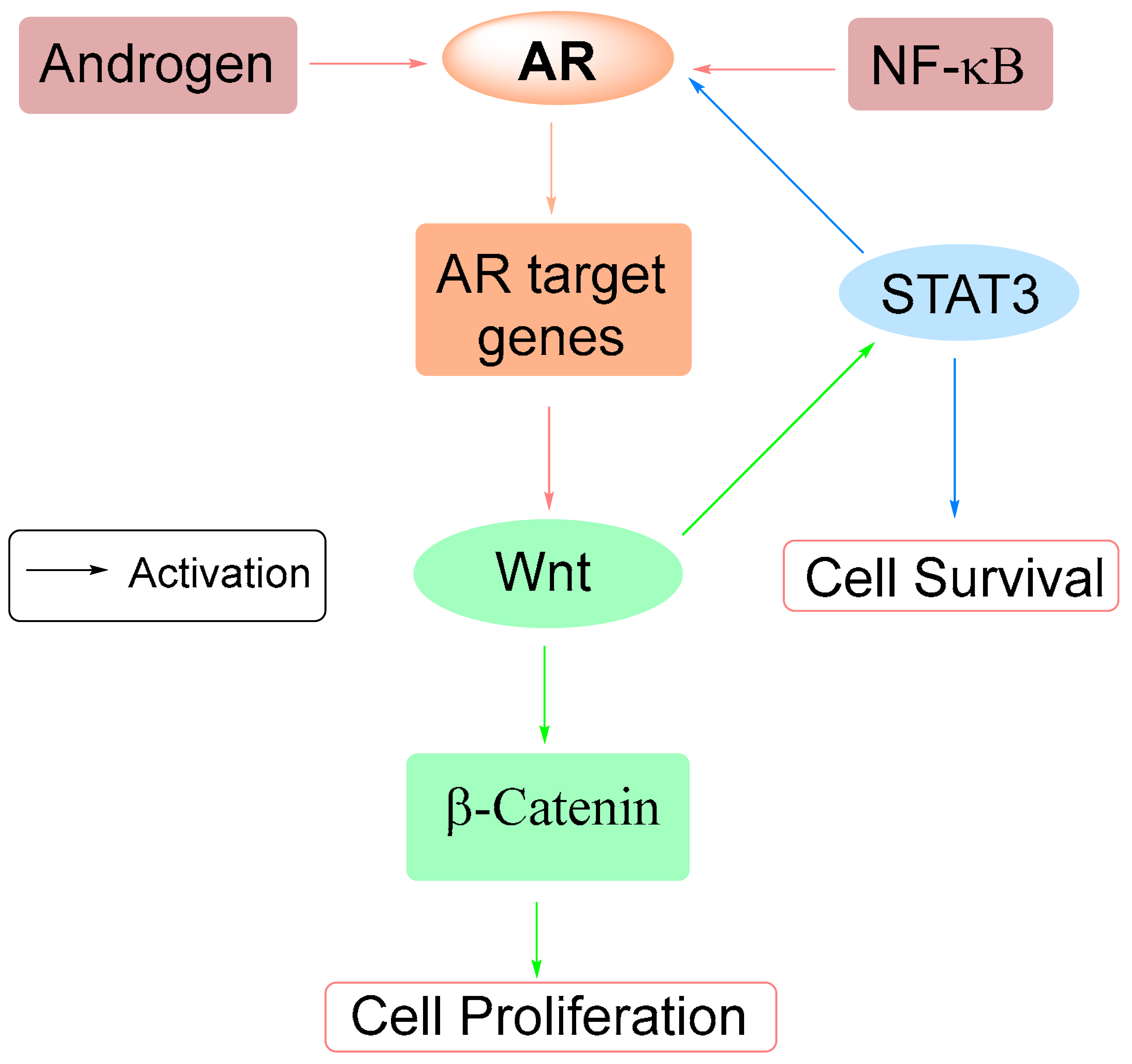
1. Androgen Receptor Signaling Pathway: A Primary Driver of Prostate Cancer
2. Natural Products as Potential Treatments for Prostate Cancer
3. Natural Products Inhibiting Testosterone Production
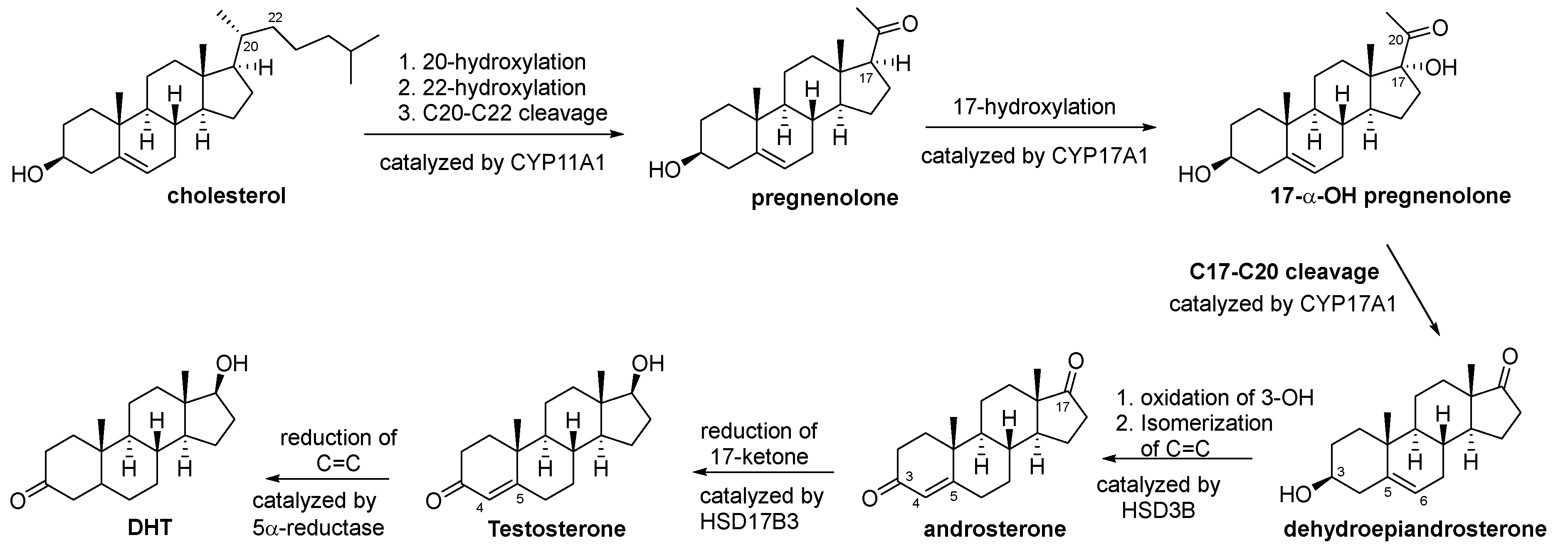
3.1. Biosynthesis of Testosterone and DHT
3.2. Natural Products Inhibiting the Biosynthesis of Testosterone
4. Natural Products Inhibiting 5α Reductase
5. Natural Products as Androgen Receptor Antagonists
5.1. Interacting with LBD
5.2. Interacting with the N-Terminal Domain (NTD)
6. Natural Products Degrading AR
7. Natural Products Suppressing AR Signaling via Other Pathways
8. Promising Scaffolds and Translational Potential
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF-1 | Activation function-1 |
| AKBA | Acetyl-11-keto-β-boswellic |
| AR | Androgen receptor |
| DHT | Dihydrotestosterone |
| DIM | 3,3′-Diindolylmethane |
| DUB | Deubiquitinase |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-3-gallate |
| GA | Glycyrrhetinic acid |
| HSP | Heat shock protein |
| LBD | Ligand binding domain |
| NBBS | N-butylbenzene-sulfonamide |
| NTD | N-terminal domain |
| PSA | Prostate specific antigen |
| SAR | Structure-Activity Relationship |
| SINTI | Sintokamide A |
| TMPRSS2 | Transmembrane protease serine 2 |
References
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Chen, Q.-H. Comprehensive insights into androgen receptor structure, signaling, and targeted therapeutics in prostate cancer. In Anti-Prostate Cancer Agents, 1st ed.; Chen, Q.-H., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2025; pp. 135–157. [Google Scholar]
- Elshan, N.G.R.D.; Rettig, M.B.; Jung, M.E. Molecules targeting the androgen receptor (AR) signaling axis beyond the AR-Ligand binding domain. Med. Res. Rev. 2019, 39, 910–960. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Fizazi, K.; Neal, S.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in nometastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef]
- Singla, R.K.; Sharma, P.; Dubey, A.K.; Gundamaraju, R.; Kumar, D.; Kumar, S.; Madaan, R.; Shri, R.; Tsagkaris, C.; Parisi, S.; et al. Natural product-based studies for the management of castration-resistant prostate cancer: Computational to clinical studies. Front. Pharmacol. 2021, 12, 732266. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, T.; Zhang, J. Recent advances in dietary androgen receptor inhibitors. Med. Res. Rev. 2024, 44, 1446–1500. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizie, A.; Limonta, P. Natural compounds in prostate cancer prevention and treatment: Mechanisms of action and molecular targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef]
- Payne, A.H.; O’Shaughnessy, P.J. Structure, function and regulation of steroidogenic enzymes in the Leydig cell. In The Leydig Cell; Cache River Press: Vienna, IL, USA, 1996; pp. 259–286. [Google Scholar]
- Zamansoltani, F.; Nassiri-Asi, M.; Sarookhani, M.-R.; Jahani-Hashemi, H.; Zangivand, A.-A. Antiandrogenic activities of Glycyrrhiza glabra in male rats. Int. J. Androl. 2009, 32, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Wakabayashi, K. Inhibitory effect of glycyrrhetinic acid on testosterone production in rat gonads. Endocrinol. Jpn. 1988, 35, 333–342. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nishii, O.; Okamura, T.; Yaginuma, T. Effect of Paeoniflorin, Glycyrrhizin and Glycyrrhetic Acid on Ovarian Androgen Production. Am. J. Chin. Med. 1991, 19, 73–78. [Google Scholar] [CrossRef]
- Akdoğan, M.; Tamer, M.N.; Cüre, M.C.; Köroglu, B.K.; Delibas, N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother. Res. 2007, 21, 444–447. [Google Scholar] [CrossRef]
- Sadeghi Ataabadi, M.; Alaee, S.; Bagheri, M.J.; Bahmanpoor, S. Role of essential oil of Mentha spicata (spearmint) in addressing reverse hormonal and folliculogenesis disturbances in a polycystic ovarian syndrome in a rat model. Adv. Pharm. Bull. 2017, 7, 651–654. [Google Scholar] [CrossRef]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Li, X.; Liu, J.; Hong, T.; Zhu, Q.; Huang, P.; Ge, R.-S. Suppression of rat and human androgen biosynthetic enzymes by apigenin: Possible use for the treatment of prostate cancer. Fitoterapia 2016, 111, 66–72. [Google Scholar] [CrossRef]
- Ye, D.; Li, M.; Zhang, Y.; Wang, X.; Liu, H.; Wu, W.; Ma, W.; Quan, K.; Ng, E.H.Y.; Wu, X.; et al. Cryptotanshinone regulates androgen synthesis through the ERK/c-Fos/CYP17 pathway in porcine granulosa cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 5985703. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, L.; Wang, Y.; Zhang, H.; Xu, D.; Zhao, X.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar] [CrossRef]
- Yepuru, M.; Wu, Z.; Kulkarni, A.; Yin, F.; Barrett, C.M.; Kim, J.; Steiner, M.S.; Miller, D.D.; Dalton, J.T.; Narayanan, R. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin. Cancer Res. 2013, 19, 5613–5625. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, W.; Zhu, Y.; Yang, J.C.; Nadininty, N.; Gaikwad, N.W.; Evans, C.P.; Gao, A.C. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015, 75, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Doonan, B.P.; Radwan, F.F.; Haque, A. Ganoderic Acid DM: An Alternative Agent for the Treatment of Advanced Prostate Cancer. Open Prostate Cancer 2010, 3, 78–85. [Google Scholar] [CrossRef]
- Liu, J.; Kurashiki, K.; Shimizu, K.; Kondo, R. Structure–activity relationship for inhibition of 5α-reductase by triterpenoids isolated from Ganoderma lucidum. Bioorg. Med. Chem. 2006, 14, 8654–8660. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Kondo, R. Ganoderic acid TR, a new lanostanoid with 5α-reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Nat. Prod. Commun. 2006, 1, 345–350. [Google Scholar] [CrossRef]
- Liao, S.; Hiipakka, R.A. Selective inhibition of steroid 5 alpha-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 1995, 214, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Suphrom, N.; Pumthong, G.; Khorana, N.; Waranuch, N.; Limpeanchob, N.; Ingkaninan, K. Anti-androgenic effect of sesquiterpenes isolated from the rhizomes of Curcuma aeruginosa Roxb. Fitoterapia 2012, 83, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Bae, J.-S.; Kim, Y.-U. 5α-reductase inhibitory components as antiandrogens from herbal medicine. J. Acupunct. Meridian Stud. 2010, 3, 116–118. [Google Scholar] [CrossRef]
- Yam, J.; Kreuter, M.; Drewe, J. Piper cubeba targets multiple aspects of the androgen-signaling pathway. A potential phytotherapy against prostate cancer growth? Planta Med. 2008, 74, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shiono, J.; Shimizu, K.; Kukita, A.; Kukita, T.; Kondo, R. Ganoderic acid DM: Anti-androgenic osteoclastogenesis inhibitor. Bioorg. Med. Chem. Lett. 2009, 19, 2154–2157. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef]
- Le, H.T.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003, 278, 21136–21145. [Google Scholar] [CrossRef]
- Hwang, C.; Sethi, S.; Heilbrun, L.A.; Gupta, N.S.; Chitale, D.A.; Sakr, W.A.; Menon, M.; Peabody, J.O.; Smith, D.W.; Sarkar, F.H.; et al. Anti-androgenic activity of absorption-enhanced 3, 3′-diindolylmethane in prostatectomy patients. Am. J. Transl. Res. 2016, 8, 166–176. [Google Scholar] [PubMed]
- Shi, W.-F.; Leong, M.; Cho, E.; Farrell, J.; Chen, H.-C.; Tian, J.; Zhang, D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS ONE 2009, 4, e7398. [Google Scholar] [CrossRef]
- Chakraborty, S. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: In silico approach to understand biological actions. Mol. bioSyst. 2016, 12, 1702–1709. [Google Scholar] [CrossRef]
- Xu, D.; Chen, Q.; Liu, Y.; Wen, X. Baicalein suppresses the androgen receptor (AR)-mediated prostate cancer progression via inhibiting the AR N-C dimerization and AR-coactivators interaction. Oncotarget 2017, 8, 105561–105573. [Google Scholar] [CrossRef]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef]
- Papaioannou, M.; Soderholm, A.A.; Hong, W.; Dai, Y.; Roediger, J.; Roell, D.; Thiele, M.; Nyronen, T.H.; Baniahmad, A. Computational and functional analysis of the androgen receptor antagonist atraric acid and its derivatives. Anti-Cancer Agents Med. Chem. 2013, 13, 801–810. [Google Scholar] [CrossRef]
- Papaioannou, M.; Schleich, S.; Roell, D.; Schubert, U.; Tanner, T.; Claessens, F.; Matusch, R.; Baniahmad, A. NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Investig. New Drugs 2010, 28, 729–743. [Google Scholar] [CrossRef]
- Liu, S.; Yamauchi, H. Hinokitiol, a metal chelator derived from natural plants, suppresses cell growth and disrupts androgen receptor signaling in prostate carcinoma cell lines. Biochem. Biophys. Res. Commun. 2006, 351, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ikezoe, T.; Zheng, Z.; Taguchi, H.; Koeffler, H.P.; Zhu, W.-G. Saw Palmetto induces growth arrest and apoptosis of androgen-dependent prostate cancer LNCaP cells via inactivation of STAT 3 and androgen receptor signaling. Int. J. Oncol. 2007, 31, 593–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, D.; Lin, T.-H.; Li, S.; Da, J.; Wen, X.-Q.; Ding, J.; Chang, C.; Yeh, S. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 2012, 316, 11–22. [Google Scholar] [CrossRef]
- Khan, N.; Asim, M.; Afag, F.; Zaid, M.A.; Mukhtar, H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008, 68, 8555–8563. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, C.; Wang, Z.; Lee, H.J.; Hu, H.; Malewicz, B.; Lee, H.J.; Lee, J.H.; Baek, N.I.; Jeong, J.H.; et al. A novel class of pyranocoumarin anti-androgen receptor signaling compounds. Mol. Cancer Ther. 2007, 6, 907–917. [Google Scholar] [CrossRef]
- Dellal, H.; Boulahtouf, A.; Alaterre, E.; Cuenant, A.; Grimaldi, M.; Bourguet, W.; Gongora, C.; Balaguerm, P.; Pourquier, P. High content screening using new U2OS reporter cell models identified harmol hydrochloride as a selective and competitive antagonist of the androgen receptor. Cells 2020, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.O.; Bolton, E.C.; Huang, Y.; Feau, C.; Guy, R.K.; Yamamoto, K.R.; Hann, B.; Diamond, M.I. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 7233–7238. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-Y.; Yuan, X.-F.; Jin, L.; Li, J.; Luo, C.; Ye, W.-C.; Jiang, R.-W. A bufadienolide derived androgen receptor antagonist with inhibitory activities against prostate cancer cells. Chem. Biol. Interact. 2014, 207, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-F.; Tian, H.-Y.; Li, J.; Jin, L.; Jiang, S.-T.; Liu, K.W.; Luo, C.; Middleton, D.A.; Esmann, M.; Ye, W.-C.; et al. Synthesis of bufalin derivatives with inhibitory activity against prostate cancer cells. Nat. Prod. Res. 2014, 28, 843–847. [Google Scholar] [CrossRef]
- Li, G.; Petiwala, S.M.; Yan, M.; Won, J.H.; Petukhov, P.A.; Johnson, J.J. Gartanin, an isoprenylated xanthone from the mangosteen fruit (Garcinia mangostana), is an androgen receptor degradation enhancer. Mol. Nutr. Food Res. 2016, 60, 1458–1469. [Google Scholar] [CrossRef]
- Bovee, T.F.H.; Schoonen, W.G.E.J.; Hamers, A.R.M.; Bento, M.J.; Peijnenburg, A.A.C.M. Screening of synthetic and plant-derived compounds for (anti)estrogenic and (anti)androgenic activities. Anal. Bioanal. Chem. 2008, 390, 1111–1119. [Google Scholar] [CrossRef]
- Burris, T.P.; Montrose, C.; Houck, K.A.; Osborne, H.E.; Bocchinfuso, W.P.; Yaden, B.C.; Cheng, C.C.; Zink, R.W.; Barr, R.J.; Hepler, C.D.; et al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol. Pharmacol. 2005, 67, 948–954. [Google Scholar] [CrossRef]
- Miao, L.; Ma, S.; Fan, G.; Wang, H.; Wang, Y.; Chai, L. Bakuchiol inhibits the androgen induced-proliferation of prostate cancer cell line LNCaP through suppression of AR transcription activity. Tianjin J. Tradit. Chin. Med. 2013, 30, 291–293. [Google Scholar]
- Myung, J.-K.; Banuelos, C.A.; Fernandez, J.G.; Mawji, N.R.; Wang, J.; Tien, A.H.; Yang, Y.C.; Tavakoli, I.; Haile, S.; Watt, K.; et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J. Clin. Investig. 2013, 123, 2948–2960. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Banuelos, C.A.; Mawji, N.R.; Wang, J.; Kato, M.; Haile, S.; McEwan, I.J.; Plymate, S.; Sadar, M.D. Targeting androgen receptor activation function-1 with EPI to overcome resistance mechanisms in castration-resistant prostate cancer. Clin. Cancer Res. 2016, 22, 4466–4477. [Google Scholar] [CrossRef]
- De Mol, E.; Fenwick, R.B.; Phang, C.T.W.; Buzon, V.; Szulc, E.; de la Fuente, A.; Escobedo, A.; Garcia, J.; Bertoncini, C.W.; Estebanez-Perpina, E.; et al. EPI-001, A compound active against castration-resistant prostate cancer, targets transactivation unit 5 of the androgen receptor. ACS Chem. Biol. 2016, 11, 2499–2505. [Google Scholar] [CrossRef]
- Andersen, R.J.; Mawji, N.R.; Wang, J.; Haile, S.; Myung, J.-K.; Watt, K.; Tam, T.; Yang, Y.C.; Barneulos, C.A.; Williams, D.E.; et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 2010, 17, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Meimetis, L.G.; Williams, D.E.; Mawji, N.R.; Banuelos, C.A.; Lal, A.A.; Park, J.J.; Tien, A.H.; Fernandez, J.G.; de Voodg, N.J.; Sadar, M.D.; et al. Niphatenones, glycerol ethers from the sponge Niphates digitalis block androgen receptor transcriptional activity in prostate cancer cells: Structure elucidation, synthesis, and biological activity. J. Med. Chem. 2012, 55, 503–514. [Google Scholar] [CrossRef]
- Banuelos, C.A.; Lal, A.; Tien, A.H.; Shah, N.; Yang, Y.C.; Mawji, N.R.; Meimetis, L.G.; Park, J.; Jian, K.; Andersen, R.J.; et al. Characterization of niphatenones that inhibit androgen receptor N-terminal domain. PLoS ONE 2014, 9, e107991. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.E.; McEwan, I.J. A sting in the tail: The N-terminal domain of the androgen receptor as a drug target. Asian J. Androl. 2016, 18, 687–694. [Google Scholar] [CrossRef]
- Martinez-Ariza, G.; Hulme, C. Recent advances in allosteric androgen receptor inhibitors for the potential treatment of castration-resistant prostate cancer. Pharm. Pat. Anal. 2015, 4, 387–402. [Google Scholar] [CrossRef]
- Banuelos, C.A.; Travakoli, I.; Tien, A.H.; Caley, D.P.; Mawji, N.; Li, Z.; Wang, J.; Yang, Y.C.; Imamura, Y.; Yan, L.; et al. Sintokamide A is a novel antagonist of androgen receptor that uniquely binds activation function-1 in its amino-terminal domain. J. Biol. Chem. 2016, 291, 22231–22243. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Munoz, E.; Ashong, D. Insight into recent advances in degrading androgen receptor for castration-resistant prostate cancer. Cancers 2024, 16, 663. [Google Scholar] [CrossRef]
- Deep, G.; Oberlies, N.H.; Kroll, D.J.; Agarwal, R. Isosilybin B causes androgen receptor degradation in human prostate carcinoma cells via PI3K-Akt-Mdm2-mediated pathway. Oncogene 2008, 27, 3986–3998. [Google Scholar] [CrossRef]
- Lin, H.-K.; Yeh, S.; Kang, H.-Y.; Chang, C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7200–7205. [Google Scholar] [CrossRef]
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002, 21, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Feng, T.H.; Lin, C.M.; Chang, P.L.; Juang, H.H. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J. Androl. 2008, 29, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.T.; Figg, W.D. The potential role of curcumin in prostate cancer: The importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016, 5, S1107. [Google Scholar] [CrossRef]
- Chiu, F.-L.; Lin, J.-K. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 2008, 68, 61–71. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, Q.; Bedner, E.; Deptala, A.; Wang, X. Effects of the flavonoid baicalin and its metabolite bascalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif. 2001, 34, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.; Posakony, J.; Coleman, I.; Montgomery, B.; Simon, J.; Nelson, P.S. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin. Cancer Res. 2005, 11, 3905–3914. [Google Scholar] [CrossRef]
- Gibbs, A.; Schwartzman, J.; Deng, V.; Alumkal, J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc. Natl. Acad. Sci. USA 2009, 106, 16663–16668. [Google Scholar] [CrossRef]
- Kim, S.-H.; Singh, S.V. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1946–1954. [Google Scholar] [CrossRef]
- Khurana, N.; Talwar, S.; Chandra, R.K.; Sharma, P.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells. Int. J. Oncol. 2016, 49, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Karlsson, A.I.; Bonner, M.Y.; Arbiser, J.L.; Singh, S.V. Honokiol inhibits androgen receptor activity in prostate cancer cells. Prostate 2014, 74, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol. Cancer Ther. 2008, 7, 3195–3202. [Google Scholar] [CrossRef]
- Bektic, J.; Berger, A.P.; Pfeil, K.; Dobler, G.; Bartsch, G.; Klocker, H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor β. Eur. Urol. 2004, 45, 245–251. [Google Scholar] [CrossRef]
- Reiner, T.; Parrondo, R.; de las Pozas, A.; Palenzuela, D.; Perez-Stable, C. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: Possible role for inhibition of deubiquitinase activity. PLoS ONE 2013, 8, e56234. [Google Scholar] [CrossRef]
- de Las Pozas, A.; Reiner, T.; de Cesare, V.; Trost, M.; Perez-Stable, C. Inhibiting multiple deubiquitinases to reduce androgen receptor expression in prostate cancer cells. Sci. Rep. 2018, 8, 13146. [Google Scholar] [CrossRef]
- Cha, T.-L.; Qiu, L.; Chen, C.-T.; Wen, Y.; Hung, M.-C. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005, 65, 2287–2295. [Google Scholar] [CrossRef]
- Li, J.; Cao, B.; Liu, X.; Fu, X.; Xiong, Z.; Chen, L.; Sartor, O.; Dong, Y.; Zhang, H. Berberine suppresses androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2011, 10, 1346–1356. [Google Scholar] [CrossRef]
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M.; et al. Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci. 2016, 107, 1022–1028. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kwak, J.; Choi, H.-K.; Choi, K.-C.; Kim, S.; Lee, J.; Jun, W.; Park, H.-J.; Yoon, H.-G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [CrossRef]
- Hsu, J.C.; Zhang, J.; Dev, A.; Wing, A.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol inhibition of androgen receptor expression and downregulation of androgen responsiveness in human prostate cancer cells. Carcinogenesis 2005, 26, 1896–1904. [Google Scholar] [CrossRef]
- Xing, N.; Chen, Y.; Mitchell, S.H.; Young, C.Y. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 2001, 22, 409–414. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Jia, X.; Zhang, Y.; Du, Y.; Li, H.; Ma, L.; Huang, J. miR-21 promotes growth, invasion and migration of lung cancer cells by AKT’P-AKT/cleaved-caspase 3/MMP-9 signaling pathway. Int. J. Clin. Exp. Pathol. 2020, 13, 692–700. [Google Scholar]
- Grant, P.; Ramasamy, S. An update on plant derived anti-androgens. Int. J. Endocrinol. Metab. 2012, 10, 497–502. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, G.; Choi, H.Y. Effect of curcumin on the interaction between androgen receptor and Wnt/β-catenin in LNCaP xenografts. Korean J. Urol. 2015, 56, 656–665. [Google Scholar] [CrossRef]
- Dorai, T.; Dutcher, J.P.; Dempster, D.W.; Wiernik, P.H. Therapeutic potential of curcumin in prostate cancer—V: Interference with the osteomimetic properties of hormone refractory C4-2B prostate cancer cells. Prostate 2004, 60, 1–17. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Naiki, T.; Kato, H.; Iida, K.; Etani, T.; Nagayasu, Y.; Suzuki, S.; Yamashita, Y.; Inaguma, S.; Onishi, M.; et al. Recruitment of MiR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis 2020, 8, 1145–1157. [Google Scholar] [CrossRef]
- Malagarie-Cazenave, S.; Olea-Herrero, N.; Vara, D.; Diaz-Laviada, I. Capsaicin, a component of red peppers, induces expression of androgen receptor via PI3K and MAPK pathways in prostate LNCaP cells. FEBS Lett. 2009, 583, 141–147. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L. Capsaicin causes inactivation and degradation of the androgen receptor by inducing the restoration of miR-449a in prostate cancer. Oncol. Rep. 2015, 34, 1027–1034. [Google Scholar] [CrossRef]
- Shamaladevi, N.; Lyn, D.A.; Shaaban, K.A.; Zhang, L.; Villate, S.; Rohr, J.; Lokeshwar, B.L. Ericifolin: A novel antitumor compound from allspice that silences androgen receptor in prostate cancer. Carcinogenesis 2013, 34, 1822–1832. [Google Scholar] [CrossRef]
- Otto-Duessel, M.; Tew, B.Y.; Vonderfecht, S.; Moore, R.; Jones, J.O. Identification of neuron selective androgen receptor inhibitors. World J. Biol. Chem. 2017, 8, 138–150. [Google Scholar] [CrossRef]
- Isharwal, S.; Modi, S.; Arora, N.; Uhlrich, C., 3rd; Giri, B.; Barlass, U.; Soubra, A.; Chugh, R.; Dehn, S.M.; Dudeja, V.; et al. Minnelide inhibits androgen dependent, castration resistant prostate cancer growth by decreasing expression of androgen receptor full length and splice variants. Prostate 2017, 77, 584–596. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.-D.; Liao, K.; Liu, Y.; Wan, Z.-J.; Dong, Y.-F.; Cao, Q.-Q.; Zhu, Q.; Gu, X. Alteration of Androgen Receptor Protein Stability by Triptolide in LNCaP Cells. Medicina 2018, 54, 39. [Google Scholar] [CrossRef]
- Han, Y.; Huang, W.; Liu, J.; Liu, D.; Cui, Y.; Huang, R.; Yan, J.; Lei, M. Triptolide inhibits the AR signaling pathway to suppress the proliferation of enzalutamide resistant prostate cancer cells. Theranostics 2017, 7, 1914–1927. [Google Scholar] [CrossRef]
- Dai, Y.; DeSano, J.; Tang, W.; Meng, X.; Meng, Y.; Burstein, E.; Lawrence, T.S.; Xu, L. Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS ONE 2010, 5, e14153. [Google Scholar] [CrossRef]
- Jiang, C.; Lee, H.-J.; Li, G.-X.; Guo, J.; Malewicz, B.; Zhao, Y.; Lee, E.-O.; Lee, H.-J.; Lee, J.-H.; Kim, M.-S.; et al. Potent antiandrogen and androgen receptor activities of an Angelica gigas–containing herbal formulation: Identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res. 2006, 66, 453–463. [Google Scholar] [CrossRef]
- Song, G.-Y.; Lee, J.-H.; Cho, M.; Park, B.-S.; Kim, D.-E.; Oh, S. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of β-catenin. Mol. Pharmacol. 2007, 72, 1599–1606. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.; Muthuirulan, P. Androgen signaling: Promising target for prospective cancer therapy. J. Drug Des. Devel. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Yuan, H.-Q.; Kong, F.; Wang, X.-L.; Young, C.Y.F.; Hu, X.-Y.; Lou, H.-X. Inhibitory effect of acetyl-11-keto-β-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem. Pharmacol. 2008, 75, 2112–2121. [Google Scholar] [CrossRef]
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.R.; Lephart, E.D.; Handa, R.J. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 2004, 70, 1188–1195. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hsieh, C.-Y.; Huang, K.-E.; Chang, C.; Kang, H.-Y. Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer 2012, 131, 1423–1434. [Google Scholar] [CrossRef]
- Yan, L.; Banuelos, C.A.; Mawji, N.R.; Patrick, B.O.; Sadar, M.D.; Andersen, R.J. Structure-activity relationships for the marine natural product sintokamides: Androgen receptor N-terminus antagonists of interest for treatment of metastatic castration-resistant prostate cancer. J. Nat. Prod. 2021, 84, 797–813. [Google Scholar] [CrossRef]
- Laccetti, A.L.; Chatta, G.S.; Iannotti, N.; Kyriakopoulos, C.; Villaluna, K.; Le Moigne, R.; Cesano, A. Phase 1/2 study of EPI-7386 in combination with enzalutamide (enz) compared with enz alone in subjects with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. 6), 179. [Google Scholar] [CrossRef]

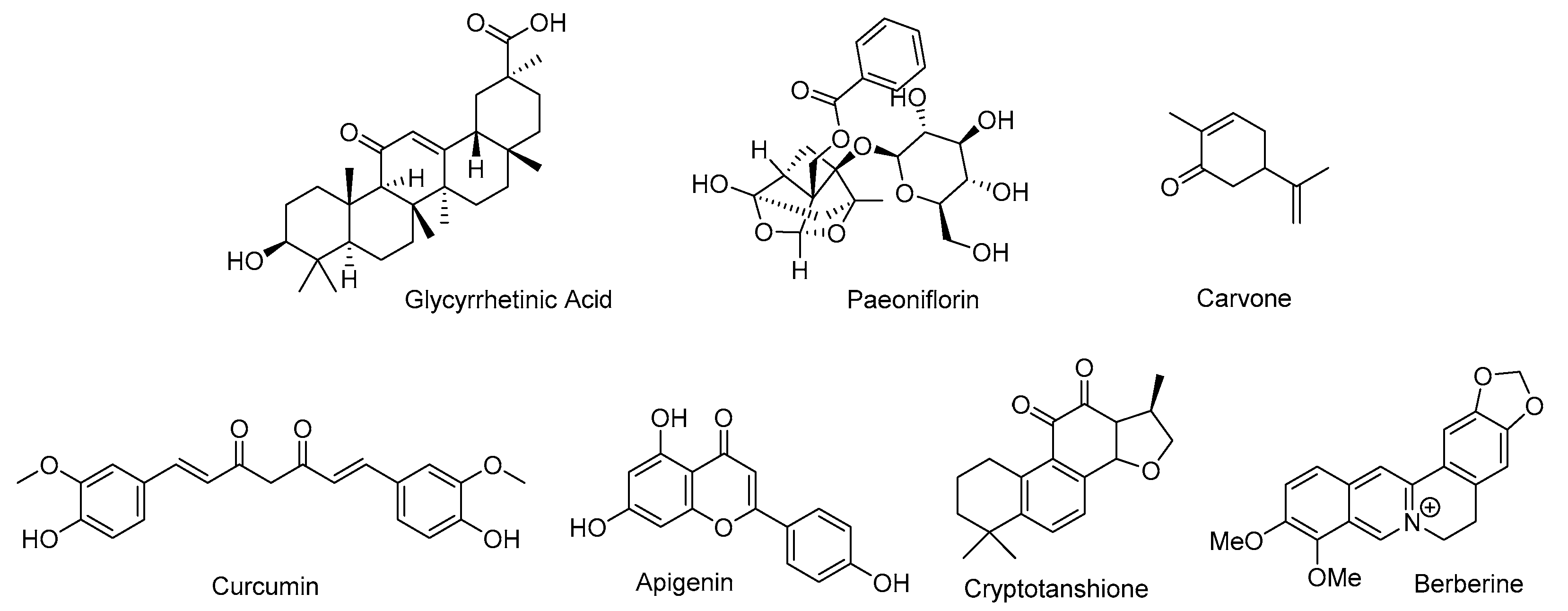
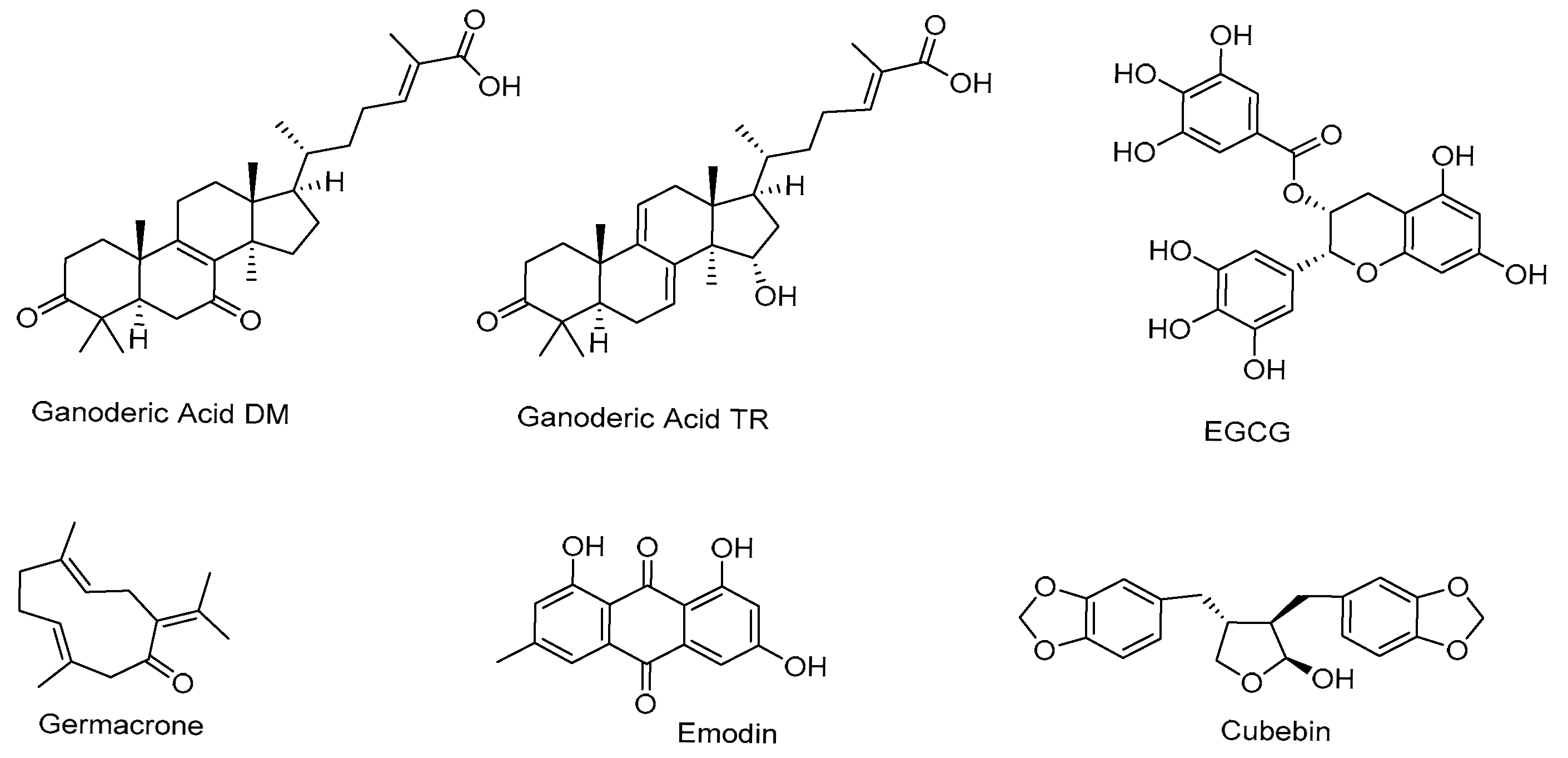
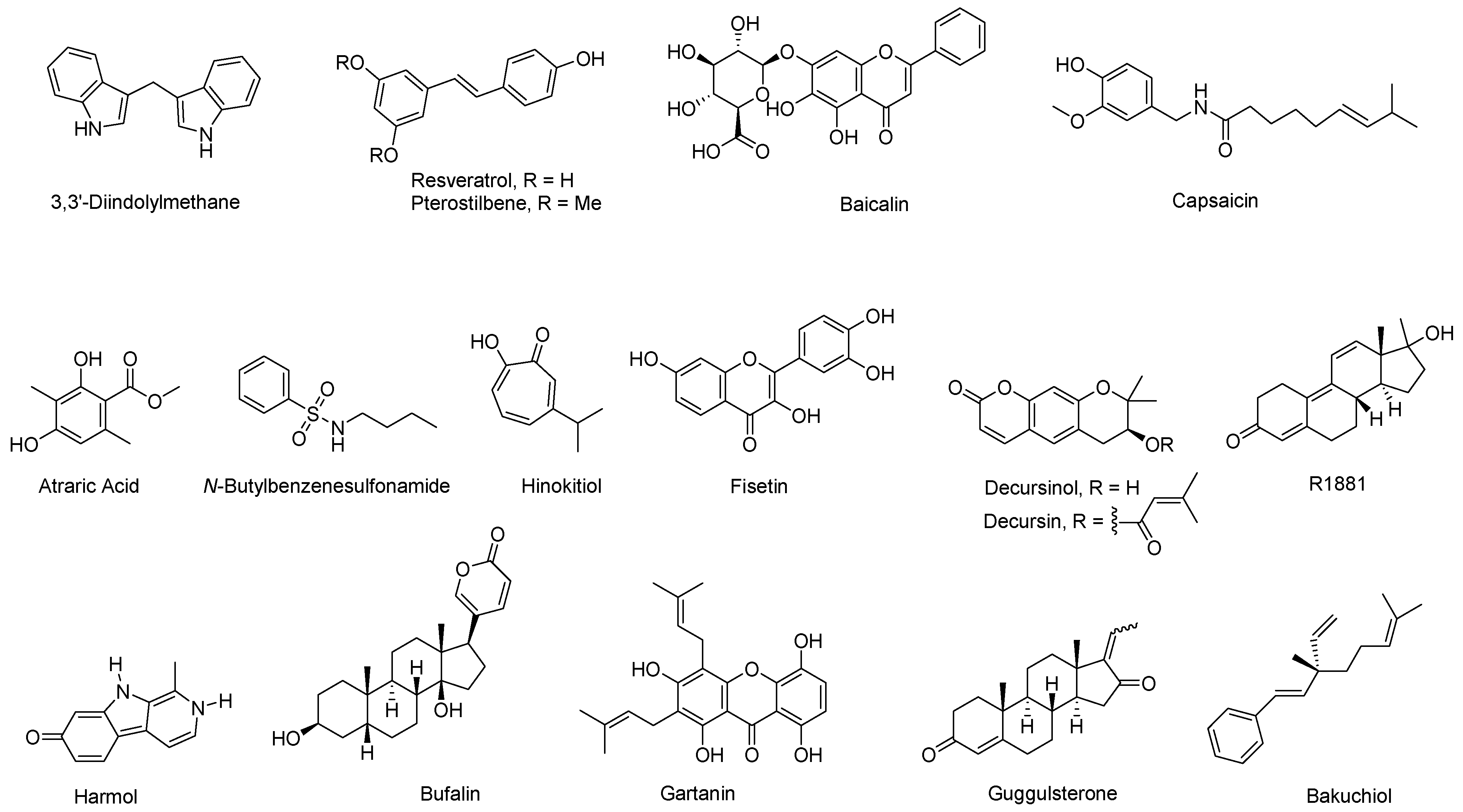
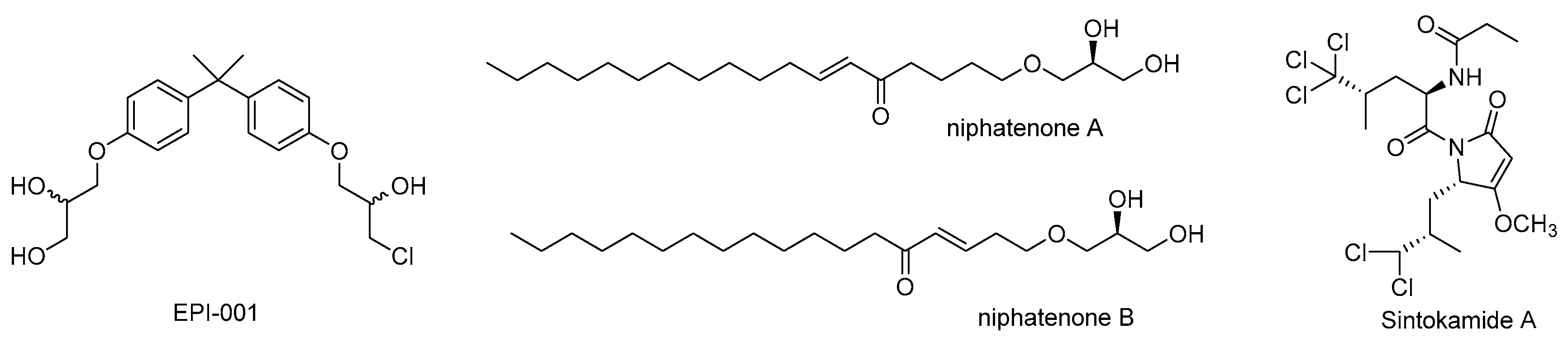
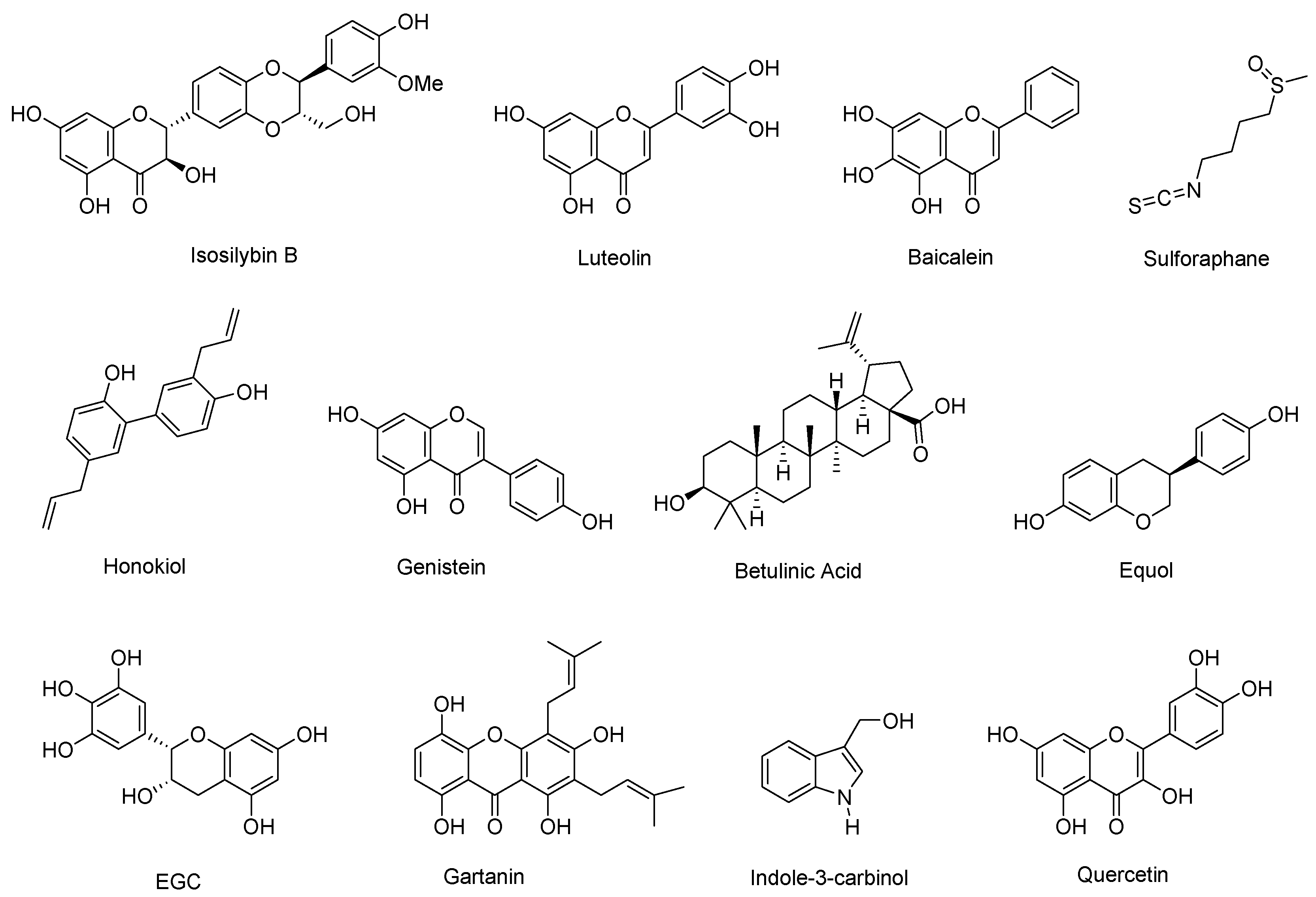

| Natural Product | Primary Molecular Target | Mechanism of Action on AR Signaling | References |
|---|---|---|---|
| Glycyrrhetinic acid | 17,20-lyase, 17β-HSD | Inhibits steroidogenic enzymes Suppresses testosterone biosynthesis | [12,13] |
| Paeoniflorin | Aromatase | Promotes conversion of testosterone to estrogen | [14] |
| Carvone | Endocrine modulation | Reduces testosterone levels in animal and clinical studies | [15,16] |
| Curcumin | StAR, CYP11A1, HSD3B2, AKR1C2 | Inhibits steroidogenesis and promotes androgen inactivation | [17,65,66,67] |
| Apigenin | HSD3B, SYP17A1, HSD17B3 | Inhibits key steroidogenic enzymes | [18] |
| Cryptotanshinone | ERK/c-Fos/CYP17, AR-LBD, LSD1 | Suppresses steroidogenesis Selective AR antagonist Disrupts AR-LSD1 | [19,42,103] |
| Berberine | AKR1C3, AR-Hsp90 complex | Inhibits androgen biosynthesis Destabilizes AR | [20,21,22,80] |
| Ganoderic Acids (DM/TR) | 5α-reductase AR-LBD | Inhibit DHT synthesis Compete with AR binding | [23,24,25,30] |
| EGCG | AR-LBD, NF-κB | Competes with AR ligan Inhibits AR nuclear translocation Modulates miRNAs | [26,31,82,85,86] |
| DIM/BR-DIM | AR-LBD | Competitive antagonist Inhibits AR nuclear translocation | [32,33] |
| Resveratrol/Pterostilbene | AR-LBD | Antagonist, Inhibits AR transcriptional activity | [34,35] |
| Fisetin | AR-LBD, AR stability | Competes with androgens Promotes AR degradation | [10,43] |
| Sulforaphane | HDAC6-Hsp90-AR complex | Destabilizes AR, Enhances ubiquitination | [71,72,73] |
| Luteolin | AR-V7, miR-8080 | Suppresses AR-V7 via miRNA regulation | [89] |
| EPI-001 | AR-NTD (AF-1 domain) | Covalently binds to NTD Disrupts AR transcription | [53,54,55,56] |
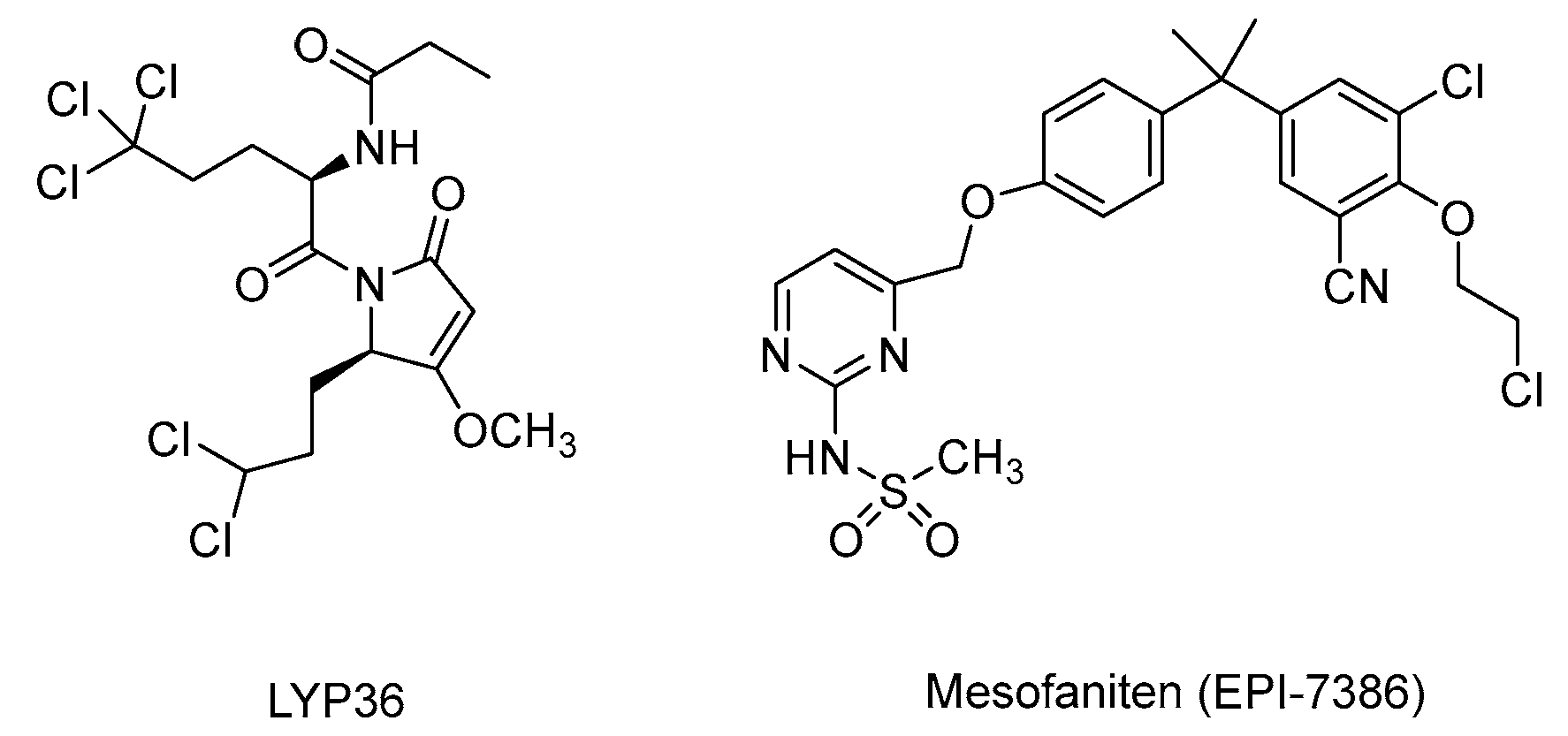
| Sintokamide A/LYP36 | AR-NTD | Selective NTD antagonist | [61,104] |
| Masofaniten (EPI-7386) | AR-NTD | Optimized derivative of EPI-001 Progressed to Phase II clinical trials. | NCT04421222 NCT05075577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Oceguera Nava, E.I.; Ashong, D.; Chen, G.; Chen, Q.-H. Natural Products Targeting the Androgen Receptor Signaling Pathway: Therapeutic Potential and Mechanisms. Curr. Issues Mol. Biol. 2025, 47, 780. https://doi.org/10.3390/cimb47090780
Wu S, Oceguera Nava EI, Ashong D, Chen G, Chen Q-H. Natural Products Targeting the Androgen Receptor Signaling Pathway: Therapeutic Potential and Mechanisms. Current Issues in Molecular Biology. 2025; 47(9):780. https://doi.org/10.3390/cimb47090780
Chicago/Turabian StyleWu, Sitong, Esveidy Isabel Oceguera Nava, Dennis Ashong, Guanglin Chen, and Qiao-Hong Chen. 2025. "Natural Products Targeting the Androgen Receptor Signaling Pathway: Therapeutic Potential and Mechanisms" Current Issues in Molecular Biology 47, no. 9: 780. https://doi.org/10.3390/cimb47090780
APA StyleWu, S., Oceguera Nava, E. I., Ashong, D., Chen, G., & Chen, Q.-H. (2025). Natural Products Targeting the Androgen Receptor Signaling Pathway: Therapeutic Potential and Mechanisms. Current Issues in Molecular Biology, 47(9), 780. https://doi.org/10.3390/cimb47090780





