Pharmacological Potential of Arthrospira platensis in Mitigating Sub-Chronic Colitis: Redox Homeostasis and Gut Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass and Reagents
2.2. Preparation of A. platensis Aqueous Extract
2.3. Free Radical Scavenging Activities
2.4. Ferrous Ion Chelating Activity
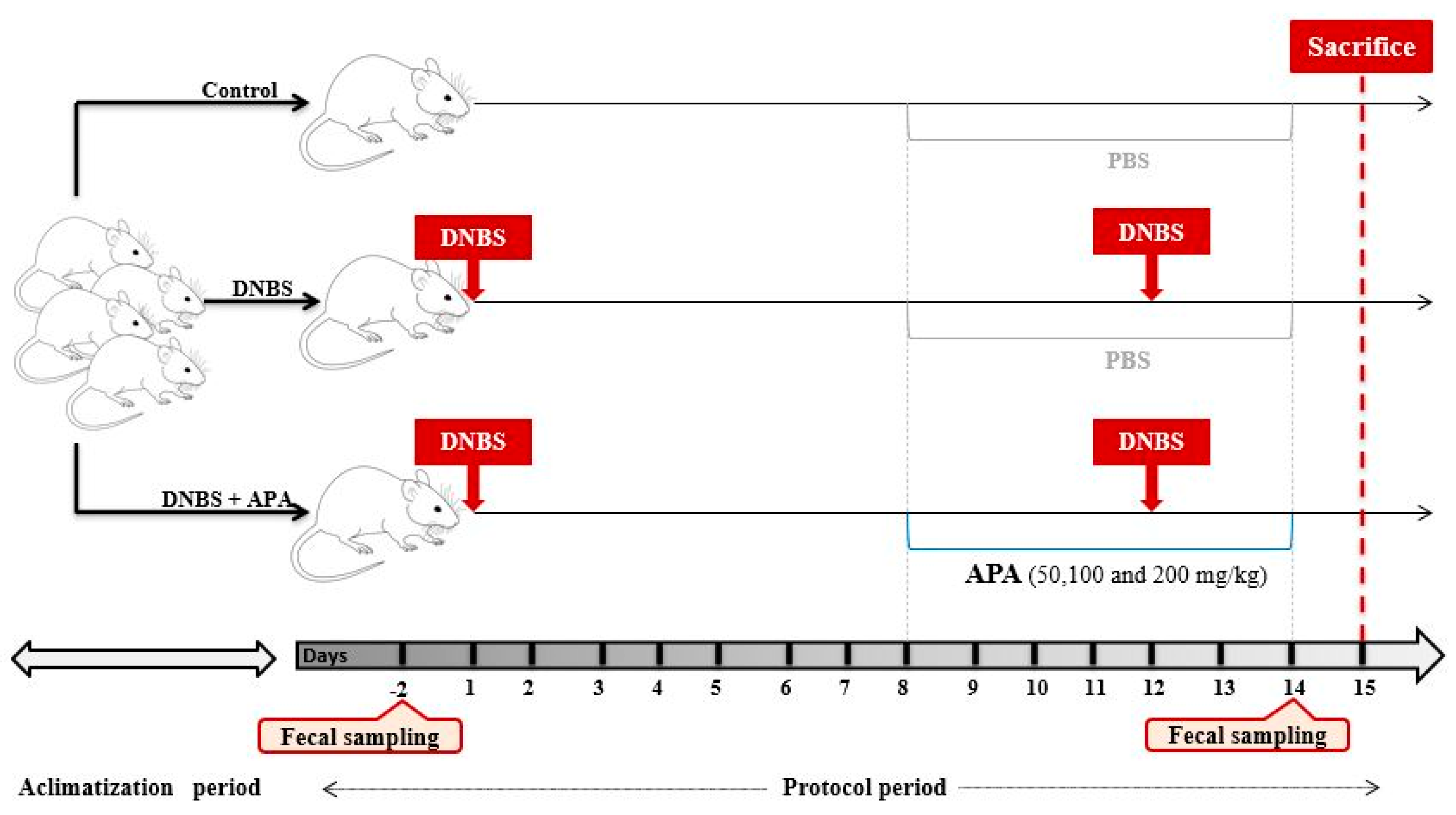
2.5. Animals and Grouping
2.6. Induction and Assessment of Colitis in Mice
2.7. Histological Examination of the Colon
2.8. Evaluation of Polynuclear Neutrophil Infiltration
2.9. Redox Biomarkers Analysis
2.9.1. Nitric Oxide (NO) Levels
2.9.2. Malondialdehyde (MDA) Levels
2.9.3. Catalase (CAT) Activity
2.9.4. Reduced Glutathione (GSH) Levels
2.10. Metagenomic Analysis of the Fecal Microbiota
2.11. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Capacity
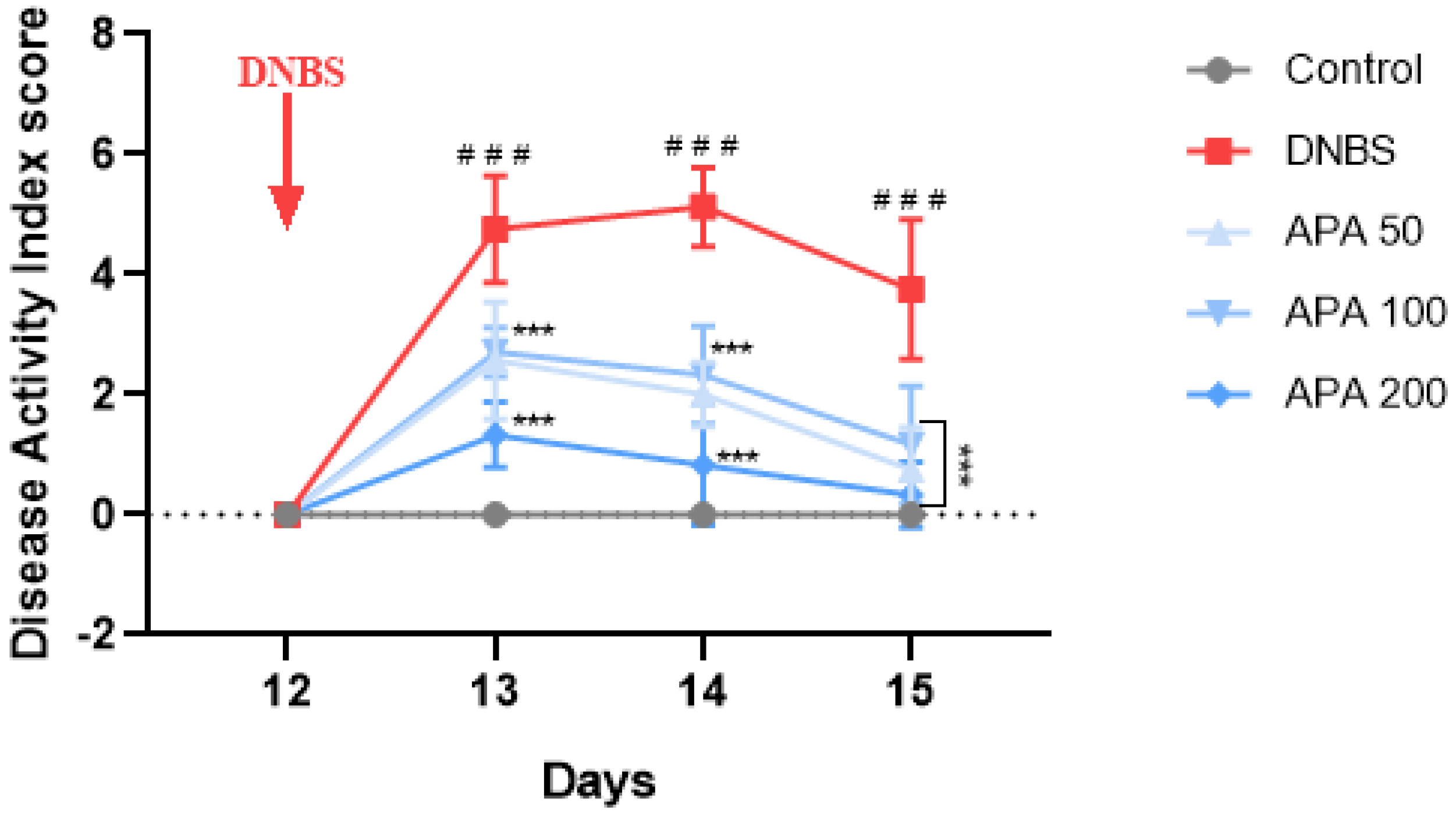
3.2. Effects of APA Extract on the DAI and Colon Morphology
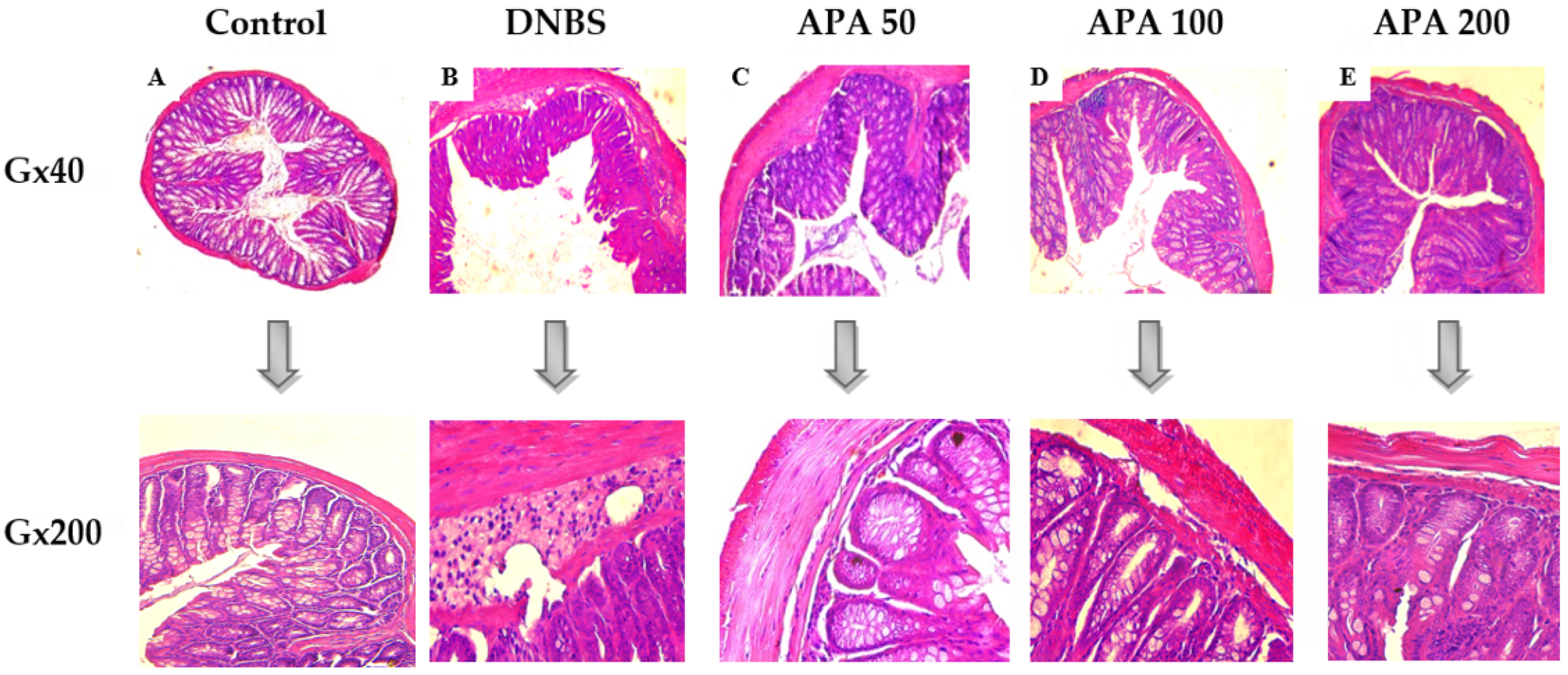
3.3. Effect of APA on Colonic Lesions
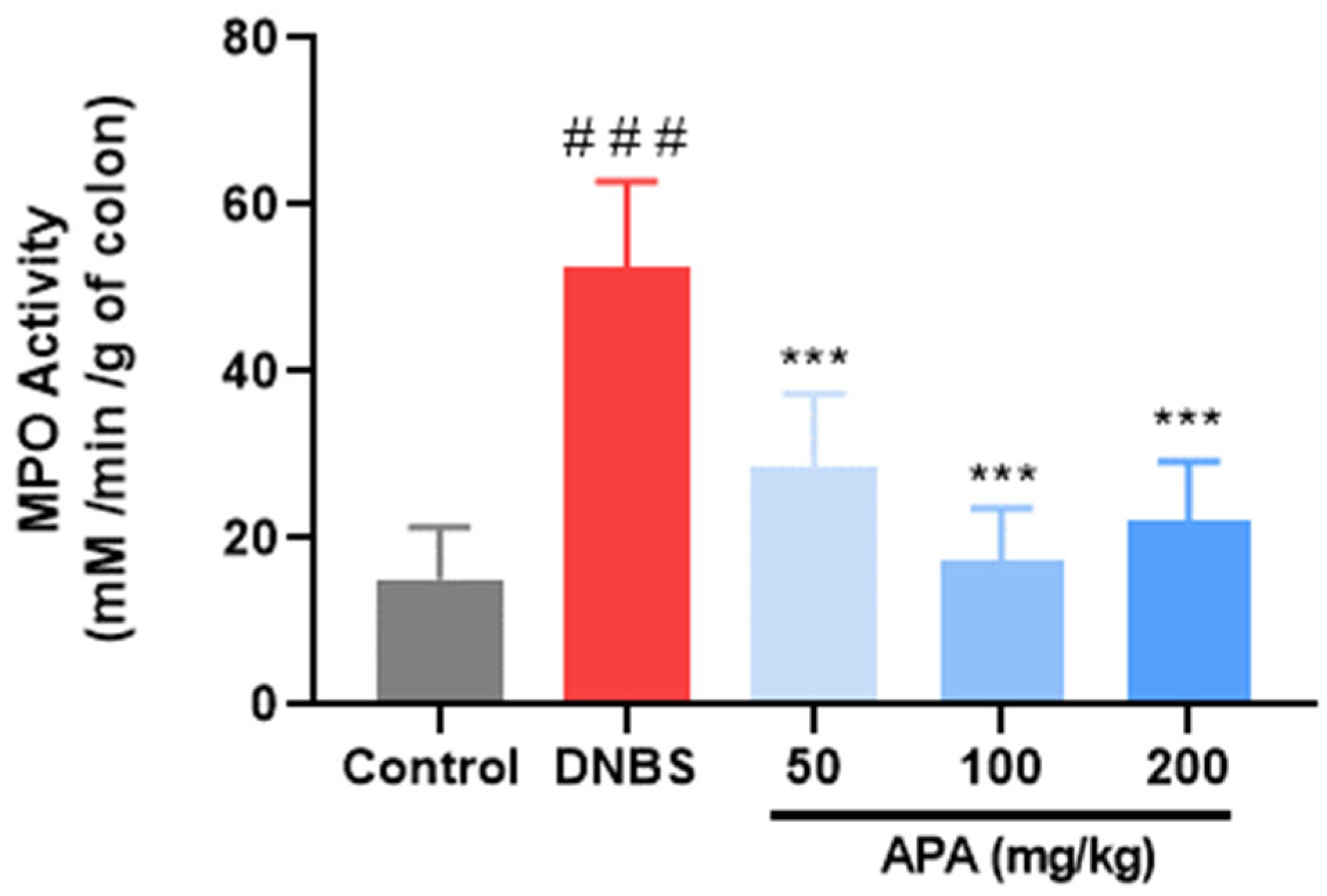
3.4. Effect of APA Extract on Polymorphonuclear Cells Infiltration
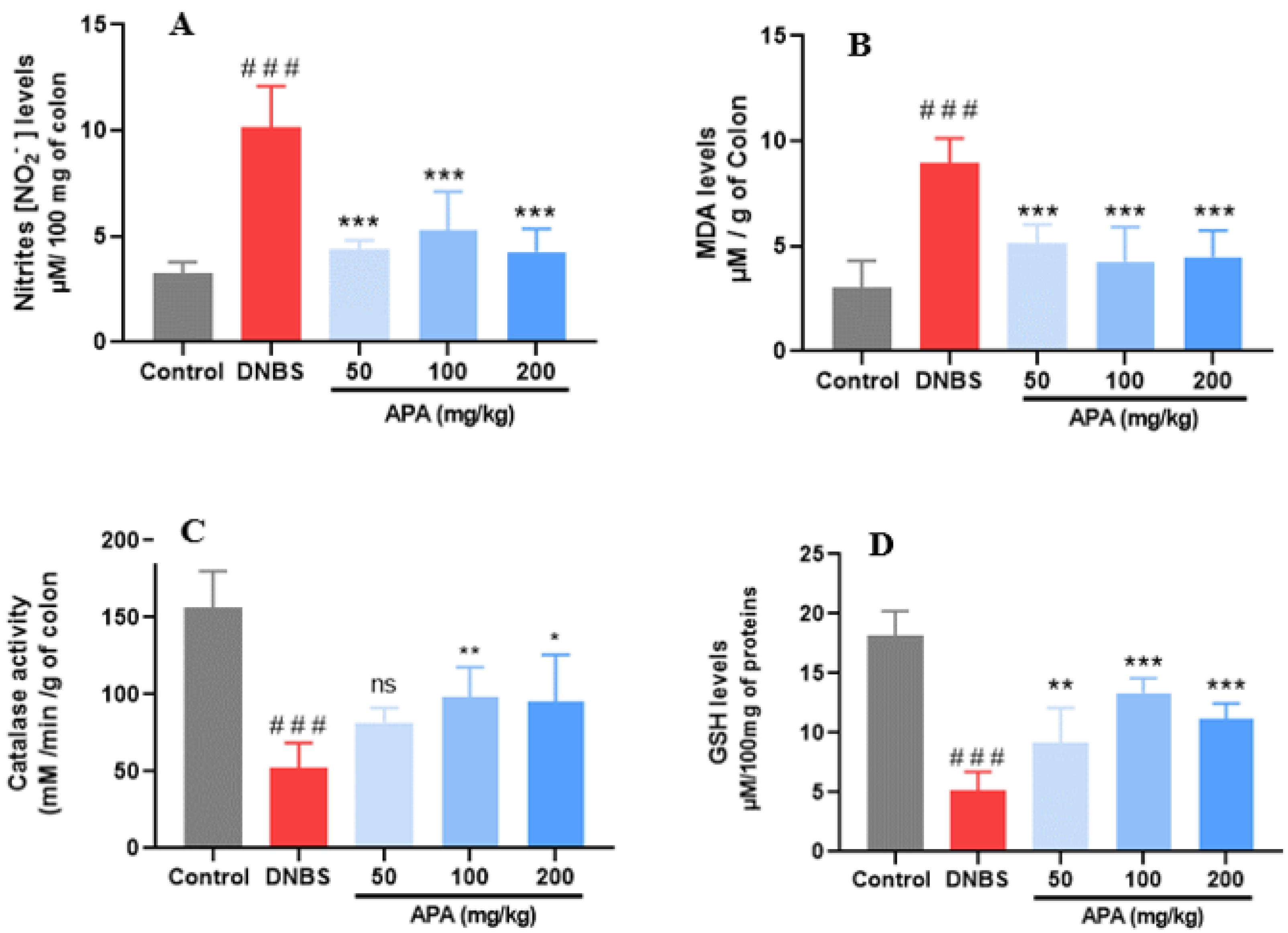
3.5. Effects of APA Extract on Redox Biomarkers
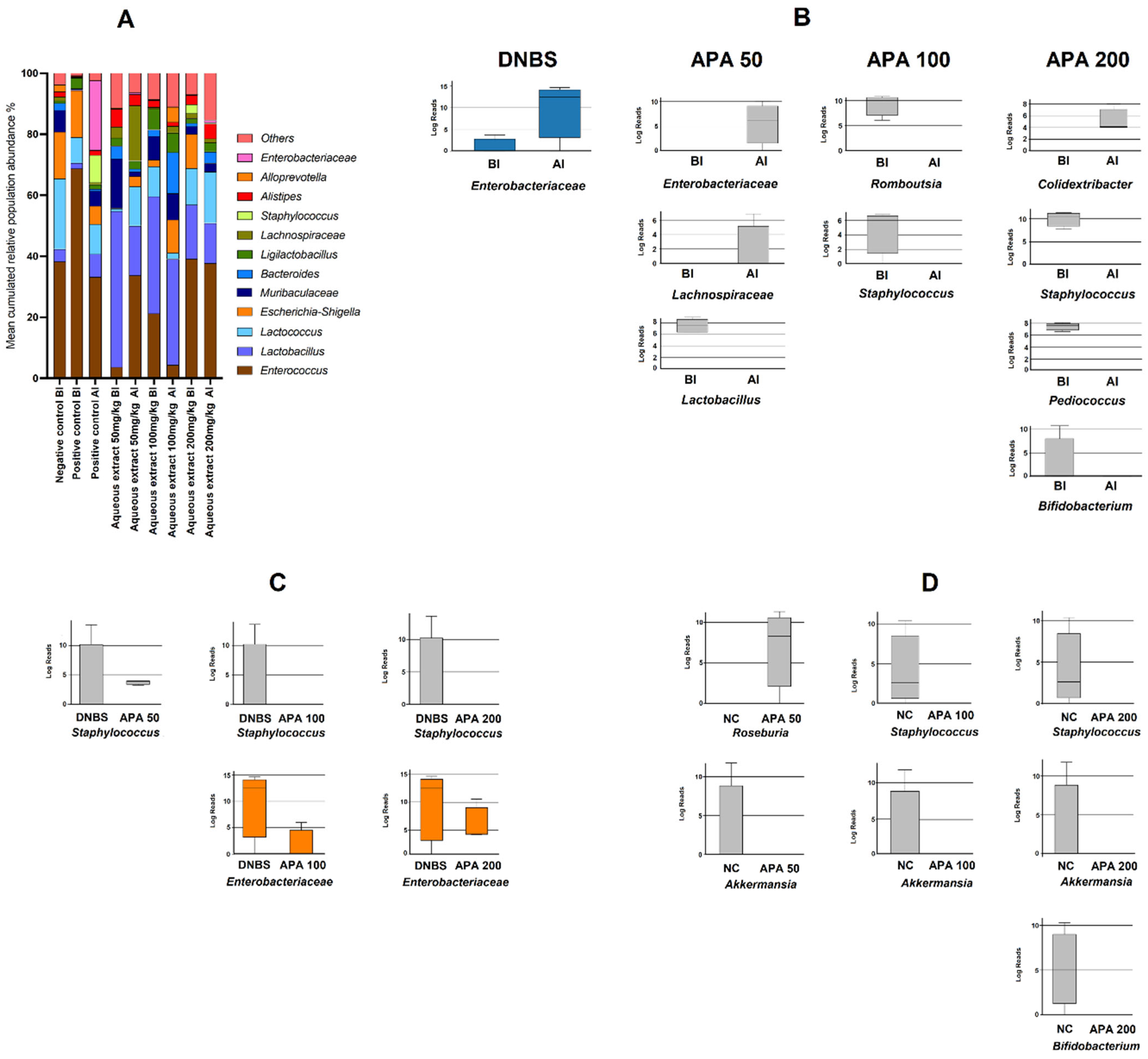
3.6. Metagenomic Profiling of Colonic Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) |
| APA | Arthrospira platensis aqueous extract |
| CD | Crohn’s Disease |
| COX-2 | Cyclooxygenase-2 |
| DAI | Disease Activity Index |
| DNBS | Dinitrobenzene Sulfonic Acid |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DSS | Dextran Sulfate Sodium |
| DTNB | 5,5′-dithiobis 2-nitrobenzoic |
| GSH | Reduced Glutathion |
| HPLC-DAD-ESI-MS | High-Performance Liquid Chromatography coupled with Diode Array Detection and Electrospray Ionization Mass Spectrometry |
| HTAB | Hexadecyl–Trimethyl–Ammonium Bromide |
| IBD | Inflammatory Bowel Disease |
| IC50 | Half maximal inhibitory concentration |
| IL-6 | Interleukin 6 |
| MDA | Malondialdehyde. |
| MPO | Myeloperoxydase |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric Oxide |
| PGE2 | Prostaglandin E2 |
| PMS | Post-Mitochondrial Supernatant. |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SCFA | short-chain fatty acid |
| TBARS | Thiobarbituric acid reactive substances |
| TNF-α | Tumor Necrosis Factor- Alpha |
| UC | Ulcerative Colitis |
References
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jarmakiewicz-Czaja, S.; Zielińska, M.; Sokal, A.; Filip, R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes 2022, 13, 2388. [Google Scholar] [CrossRef] [PubMed]
- Bribi, N.; Yanat, B.; Ouahmed-Boudaoud, H. Immunopathogenesis of Ulcerative Colitis and Crohn’s Disease. Int. J. Adv. Res. Microbiol. Immunol. 2019, 1, 45–48. [Google Scholar]
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Bribi, N.; Algieri, F.; Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Utrilla, M.; Contreras, M.d.M.; Maiza, F.; Segura Carretero, A.; Rodriguez-Cabezas, M.; et al. Intestinal Anti-Inflammatory Effects of Total Alkaloid Extract from Fumaria capreolata in the DNBS Model of Mice Colitis and Intestinal Epithelial CMT93 Cells. Phytomedicine 2016, 23, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Quera, R.; Núñez, P.; Sicilia, B.; Flores, L.; Gomollón, F. Corticosteroids in Inflammatory Bowel Disease: Are They Still a Therapeutic Option? Gastroenterol. Hepatol. Engl. Ed. 2023, 46, 716–726. [Google Scholar] [CrossRef]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef]
- Mishra, R.; Dhawan, P.; Srivastava, A.S.; Singh, A.B. Inflammatory Bowel Disease: Therapeutic Limitations and Prospective of the Stem Cell Therapy. World J. Stem Cells 2020, 12, 1050–1066. [Google Scholar] [CrossRef]
- Bribi, N.; Mohamed Sofiane, M.; Ouahmed-Boudaoud, H. Intestinal Anti-Inflammatory Effects of Linum usitatissimum Alkaloid on Experimental Ulcerative Colitis in BALB/c Mice. Curr. Bioact. Compd. 2023, 19, 69–74. [Google Scholar] [CrossRef]
- DeVoss, J.; Diehl, L. Murine Models of Inflammatory Bowel Disease (IBD): Challenges of Modeling Human Disease. Toxicol. Pathol. 2014, 42, 99–110. [Google Scholar] [CrossRef]
- Wen, C.; Chen, D.; Zhong, R.; Peng, X. Animal Models of Inflammatory Bowel Disease: Category and Evaluation Indexes. Gastroenterol. Rep. 2024, 12, goae021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Eilam, Y.; Khattib, H.; Pintel, N.; Avni, D. Microalgae—Sustainable Source for Alternative Proteins and Functional Ingredients Promoting Gut and Liver Health. Glob. Chall. 2023, 7, 2200177. [Google Scholar] [CrossRef]
- Papadaki, S.; Tricha, N.; Panagiotopoulou, M.; Krokida, M. Innovative Bioactive Products with Medicinal Value from Microalgae and Their Overall Process Optimization through the Implementation of Life Cycle Analysis—An Overview. Mar. Drugs 2024, 22, 152. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Spirulina in Health Care Management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Habib, A.; Hasan, M. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feed for Domestic Animals and Fish. Int. J. Sci. Res. Eng. Manag. 2024, 8, 1–5. [Google Scholar]
- Dillon, J.C.; Phuc, A.P.; Dubacq, J.P. Nutritional Value of the Alga Spirulina. World Rev. Nutr. Diet. 1995, 77, 32–46. [Google Scholar] [CrossRef]
- Ben Mya, O.; Souici, S.; Guenfoud, M. Comparative Analysis of Nutritional and Bioactive Components in Algerian and Egyptian Spirulina from Tamanrasset and Khatatba Regions. Biomass Convers. Biorefinery 2024, 15, 4465–4475. [Google Scholar] [CrossRef]
- Nuhu, A. Spirulina (Arthrospira): An Important Source of Nutritional and Medicinal Compounds. J. Mar. Biol. 2013, 1, 325636. [Google Scholar] [CrossRef]
- Gumbo, J.; Nesamvuni, C. A Review: Spirulina a Source of Bioactive Compounds and Nutrition. J. Chem. Pharm. Sci. 2017, 10, 1317. [Google Scholar]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Wang, J.; Su, L.; Zhang, L.; Zeng, J.; Chen, Q.; Deng, R.; Wang, Z.; Kuang, W.; Jin, X.; Gui, S.; et al. Spirulina platensis Aqueous Extracts Ameliorate Colonic Mucosal Damage and Modulate Gut Microbiota Disorder in Mice with Ulcerative Colitis by Inhibiting Inflammation and Oxidative Stress. J. Zhejiang Univ. Sci. B 2022, 23, 481–501. [Google Scholar] [CrossRef]
- Tolpeznikaite, E.; Bartkevics, V.; Ruzauskas, M.; Pilkaityte, R.; Viskelis, P.; Urbonaviciene, D.; Zavistanaviciute, P.; Zokaityte, E.; Ruibys, R.; Bartkiene, E. Characterization of Macro-and Microalgae Extracts Bioactive Compounds and Micro-and Macroelements Transition from Algae to Extract. Foods 2021, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Chai, T.; Mohan, M.; Ong, H.; Wong, F. Antioxidant, Iron-Chelating and Anti-Glucosidase Activities of Typha Domingensis Pers (Typhaceae). Trop. J. Pharm. Res. 2014, 13, 67–72. [Google Scholar] [CrossRef]
- Martín, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.-J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermúdez-Humarán, L.G.; Langella, P. The Commensal Bacterium Faecalibacterium prausnitzii Is Protective in DNBS-Induced Chronic Moderate and Severe Colitis Models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Merakeb, M.S.; Bribi, N.; Ferhat, R.; Aziez, M.; Yanat, B. Alkaloids Extract from Linum usitatissimum Attenuates 12-OTetradecanoylphorbol-13-Acetate (TPA)-Induced Inflammation and Oxidative Stress in Mouse Skin. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2023, 21, 179–187. [Google Scholar] [CrossRef]
- Aziez, M.; Suharoschi, R.; Merakeb, M.S.; Pop, O.L.; Ciont, C.; Ranga, F.; Ferhat, R.; Affenai, S.; Vodnar, D.C.; Cozma, A.; et al. Phenolic Profiling and Bioactive Properties of Arthrospira platensis Extract in Alleviating Acute and Sub-Chronic Colitis. Int. J. Mol. Sci. 2025, 26, 5692. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.U.; Saleem, M.; Raza, S.A.; Bashir, S.; Muhammad, T.; Asghar, S.; Qamar, M.U.; Shah, T.A.; Bin Jardan, Y.A.; Mekonnen, A.B.; et al. Anti-Ulcerative Colitis Effects of Chemically Characterized Extracts from Calliandra Haematocephala in Acetic Acid-Induced Ulcerative Colitis. Front. Chem. 2024, 12, 1291230. [Google Scholar] [CrossRef]
- Aziez, M.; Bribi, N.; Mohamed Sofiane, M.; Riad, F.; Affenai, S. Intestinal Anti-Inflammatory and Antioxidant Potential of Arthrospira platensis Aqueous Extract on DNBS-Induced Colitis in BALB/c Mice. Curr. Chem. Biol. 2024, 19, 238–248. [Google Scholar] [CrossRef]
- Avula, S.K.; Khan, A.; Halim, S.A.; Rehman, N.U.; Karim, N.; Khan, I.; Csuk, R.; Das, B.; Al-Harrasi, A. Synthesis and Antidepressant-like Effects of New 5-Epi-Incensole and 5-Epi-Incensole Acetate in Chronic Unpredictable Mild Stress Model of Depression; Behavioural and Biochemical Correlates. Biomed. Pharmacother. 2022, 156, 113960. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Arora, I.; Rastogi, S.; Akhtar, M.; Singh, S.; Samim, M. Collagen Nanoparticle-Mediated Brain Silymarin Delivery: An Approach for Treating Cerebral Ischemia and Reperfusion-Induced Brain Injury. Front. Neurosci. 2020, 14, 538404. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, C.; Martín-Reyes, F.; Taminiau, B.; Ho-Plágaro, A.; Camargo, R.; Fernandez-Garcia, F.; Pinazo-Bandera, J.; Toro-Ortiz, J.P.; Gonzalo, M.; López-Gómez, C.; et al. The Metagenomic Composition and Effects of Fecal-Microbe-Derived Extracellular Vesicles on Intestinal Permeability Depend on the Patient’s Disease. Int. J. Mol. Sci. 2023, 24, 4971. [Google Scholar] [CrossRef]
- Rather, I.A.; Bajpai, V.K.; Ching, L.L.; Majumder, R.; Nam, G.-J.; Indugu, N.; Singh, P.; Kumar, S.; Hajrah, N.H.; Sabir, J.S.M.; et al. Effect of a Bioactive Product SEL001 from Lactobacillus sakei Probio65 on Gut Microbiota and Its Anti-Colitis Effects in a TNBS-Induced Colitis Mouse Model. Saudi J. Biol. Sci. 2020, 27, 261–270. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Beaman, J.L.; Guthrie, J.; Ehmann, A.; Benson, K.F. Antioxidant and Anti-Inflammatory Properties of an Aqueous Cyanophyta Extract Derived from Arthrospira platensis: Contribution to Bioactivities by the Non-Phycocyanin Aqueous Fraction. J. Med. Food 2015, 18, 535–541. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a Novel Protein from Spirulina platensis—In Vivo Toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Shalaby, E.; Shanab, S. Comparison of DPPH and ABTS Assays for Determining Antioxidant Potential of Water and Methanol Extracts of Spirulina platensis. Indian J. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Bellahcen, T.O.; AAmiri, A.; Touam, I.; Hmimid, F.; Amrani, A.E.; Cherif, A.; Cherki, M. Evaluation of Moroccan Microalgae: Spirulina platensis as a Potential Source of Natural Antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef]
- Coskun, Z.K.; Kerem, M.; Gurbuz, N.; Omeroglu, S.; Pasaoglu, H.; Demirtas, C.; Lortlar, N.; Salman, B.; Pasaoglu, O.T.; Turgut, H.B. The Study of Biochemical and Histopathological Effects of Spirulina in Rats with TNBS-Induced Colitis. Bratisl. Lek. Listy 2011, 112, 235–243. [Google Scholar]
- Morsy, M.A.; Gupta, S.; Nair, A.B.; Venugopala, K.N.; Greish, K.; El-Daly, M. Protective Effect of Spirulina platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients 2019, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhu, S.; Feng, G.; Wu, L.; Feng, Y.; Guo, T.; Yang, Y.; Wu, H.; Zeng, M. Microalgae Aqueous Extracts Exert Intestinal Protective Effects in Caco-2 Cells and Dextran Sodium Sulphate-Induced Mouse Colitis. Food Funct. 2020, 11, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.A.d.O.; Sales-Campos, H.; Yuen, V.G.; Machado, J.R.; Viana, G.S.d.B.; Oliveira, C.J.F.; McNeill, J.H. Arthrospira (Spirulina) Platensis Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice by Suppressing Key Pro-Inflammatory Cytokines. Korean J. Gastroenterol. 2020, 76, 150–158. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial Activity of Arthrospira (Former Spirulina) and Dunaliella Related to Recognized Antimicrobial Bioactive Compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef] [PubMed]
- Masoudi-Sobhanzadeh, Y.; Pourseif, M.M.; Khalili-Sani, A.; Jafari, B.; Salemi, A.; Omidi, Y. Deciphering Anti-Biofilm Property of Arthrospira platensis-Origin Peptides against Staphylococcus aureus. Comput. Biol. Med. 2023, 160, 106975. [Google Scholar] [CrossRef]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Gutiérrez Casbas, A.; Yilmaz, B.; Wawrzyniak, M.; Francés, R. Dysbiotic Microbiota Interactions in Crohn’s Disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, Oral FMT for Long-Term Maintenance Therapy in Ulcerative Colitis: Results of a Single-Center, Prospective, Randomized Pilot Study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Butyrate in Inflammatory Bowel Disease Therapy. Gastroenterology 2020, 158, 1511. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
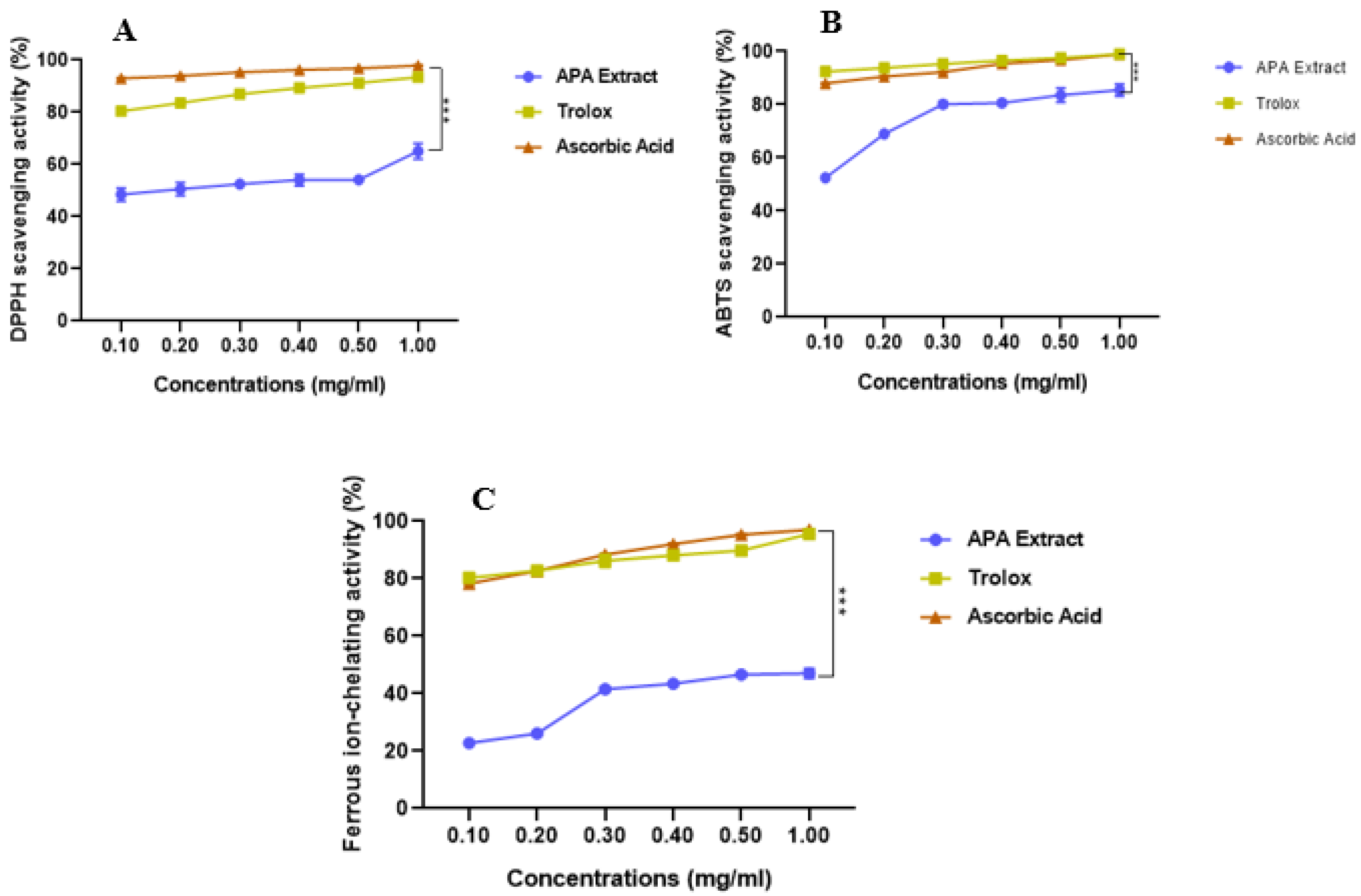

| IC50 (mg/mL) | |||
|---|---|---|---|
| APA | Trolox | Ascorbic Acid | |
| DPPH• scavenging activity | 0.166 ± 0.00 | 0.081 ± 0.00 | 0.067 ± 0.00 |
| ABTS•+ scavenging activity | 0.118 ± 0.00 | 0.068 ± 0.00 | 0.072 ± 0.00 |
| Ferrous ion chelating activity | 0.939 ± 0.05 | 0.081 ± 0.00 | 0.083 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziez, M.; Yanat, B.; Rodriguez-Diaz, C.; Suharoschi, R.; Vulturar, R.; Heghes, S.-C.; Guenaoui, N.; Ali, A.M.; Garcia-Fuentes, E.; Bribi, N. Pharmacological Potential of Arthrospira platensis in Mitigating Sub-Chronic Colitis: Redox Homeostasis and Gut Microbiota Modulation. Curr. Issues Mol. Biol. 2025, 47, 778. https://doi.org/10.3390/cimb47090778
Aziez M, Yanat B, Rodriguez-Diaz C, Suharoschi R, Vulturar R, Heghes S-C, Guenaoui N, Ali AM, Garcia-Fuentes E, Bribi N. Pharmacological Potential of Arthrospira platensis in Mitigating Sub-Chronic Colitis: Redox Homeostasis and Gut Microbiota Modulation. Current Issues in Molecular Biology. 2025; 47(9):778. https://doi.org/10.3390/cimb47090778
Chicago/Turabian StyleAziez, Meriem, Betitera Yanat, Cristina Rodriguez-Diaz, Ramona Suharoschi, Romana Vulturar, Simona-Codruta Heghes, Nawel Guenaoui, Awadh M. Ali, Eduardo Garcia-Fuentes, and Noureddine Bribi. 2025. "Pharmacological Potential of Arthrospira platensis in Mitigating Sub-Chronic Colitis: Redox Homeostasis and Gut Microbiota Modulation" Current Issues in Molecular Biology 47, no. 9: 778. https://doi.org/10.3390/cimb47090778
APA StyleAziez, M., Yanat, B., Rodriguez-Diaz, C., Suharoschi, R., Vulturar, R., Heghes, S.-C., Guenaoui, N., Ali, A. M., Garcia-Fuentes, E., & Bribi, N. (2025). Pharmacological Potential of Arthrospira platensis in Mitigating Sub-Chronic Colitis: Redox Homeostasis and Gut Microbiota Modulation. Current Issues in Molecular Biology, 47(9), 778. https://doi.org/10.3390/cimb47090778









