Combination of the First-in-Class Imipridone ONC201 and Standard Anticancer Therapies as a Rational Approach for Therapeutic Benefit
Abstract
1. Introduction
2. Mechanism of Action of ONC201 as a Single Agent in Different Cancers
3. Combination Strategies Using ONC201 and/or Its Analogs Against Various Cancers
3.1. Colorectal Cancer
3.2. Pancreatic Cancer
3.3. Glioblastoma
3.4. Lung Cancer
3.5. Endometrial Cancer
3.6. Ovarian Cancer
3.7. Triple-Negative Breast Cancer
3.8. Prostate Cancer
3.9. Gastric Adenocarcinoma
4. Limitations of/Current Challenges with ONC201-Based Anticancer Therapy
5. ONC201 Analogs
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, J.; Kline, C.L.; Zhou, L.; Khazak, V.; El-Deiry, W.S. Anti-tumor effects of ONC201 in combination with VEGF-inhibitors significantly impacts colorectal cancer growth and survival in vivo through complementary non-overlapping mechanisms. J. Exp. Clin. Cancer Res. 2018, 37, 11, Erratum in J. Exp. Clin. Cancer Res. 2024, 43, 257. [Google Scholar] [CrossRef]
- Weissenrieder, J.S.; Reed, J.L.; Green, M.V.; Moldovan, G.-L.; Koubek, E.J.; Neighbors, J.D.; Hohl, R.J. The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells. Pharmacology 2020, 105, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual Inactivation of Akt and ERK by TIC10 Signals Foxo3a Nuclear Translocation, TRAIL Gene Induction, and Potent Antitumor Effects. Sci. Transl. Med. 2013, 5, 171ra17. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Kline, C.L.B.; Prabhu, V.V.; Wagner, J.; Ishizawa, J.; Madhukar, N.; Lev, A.; Baumeister, M.; Zhou, L.; Lulla, A.; et al. Discovery and Clinical Introduction of First-in-Class Imipridone ONC201. Oncotarget 2016, 7, 74380–74392. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Lee, A.; Maranto, C.; Allen, J.E.; Lanier, R.; Sethna, P.; Prabhu, V.V. Exth-104. Role of CLPP and Mitochondrial Metabolism in the Anti-Cancer Effects of Imipridone ONC201 and ONC206 in Glioblastoma and DIPG. Neuro-Oncology 2022, 24, vii234. [Google Scholar] [CrossRef]
- Graves, P.R.; Aponte-Collazo, L.J.; Fennell, E.M.J.; Graves, A.C.; Hale, A.E.; Dicheva, N.; Herring, L.E.; Gilbert, T.S.K.; East, M.P.; McDonald, I.M.; et al. Mitochondrial Protease ClpP Is a Target for the Anticancer Compounds ONC201 and Related Analogues. ACS Chem. Biol. 2019, 14, 1020–1029. [Google Scholar] [CrossRef]
- Wang, S.; Dougan, D.A. The Direct Molecular Target for Imipridone ONC201 Is Finally Established. Cancer Cell 2019, 35, 707–708. [Google Scholar] [CrossRef]
- Ishizawa, J.; Zarabi, S.F.; Davis, R.E.; Halgas, O.; Nii, T.; Jitkova, Y.; Zhao, R.; St-Germain, J.; Heese, L.E.; Egan, G.; et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell 2019, 35, 721–737.e9. [Google Scholar] [CrossRef]
- Allen, J.E.; Crowder, R.; El-Deiry, W.S. First-In-Class Small Molecule ONC201 Induces DR5 and Cell Death in Tumor but Not Normal Cells to Provide a Wide Therapeutic Index as an Anti-Cancer Agent. PLoS ONE 2015, 10, e0143082. [Google Scholar] [CrossRef]
- Kline, C.L.B.; Ralff, M.D.; Lulla, A.R.; Wagner, J.M.; Abbosh, P.H.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia 2018, 20, 80–91. [Google Scholar] [CrossRef]
- Kline, C.L.; Van den Heuvel, A.P.; Allen, J.E.; Prabhu, V.V.; Dicker, D.T.; El-Deiry, W.S. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci. Signal. 2016, 9, ra18. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Morrow, S.; Rahman Kawakibi, A.; Zhou, L.; Ralff, M.; Ray, J.; Jhaveri, A.; Ferrarini, I.; Lee, Y.P.; Arker, C.; et al. ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia 2020, 22, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Ozer, B.H.; Boris, L.; Brown, D.; Theeler, B. Imipridones and dopamine receptor antagonism in the therapeutic management of gliomas. Adv. Oncol. 2024, 4, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ralff, M.D.; Kline, C.L.B.; Küçükkase, O.C.; Wagner, J.; Lim, B.; Dicker, D.T.; Prabhu, V.V.; Oster, W.; El-Deiry, W.S. ONC201 demonstrates antitumor effects in both triple-negative and non-triple-negative breast cancers through TRAIL-dependent and TRAIL-Independent mechanisms. Mol. Cancer Ther. 2017, 16, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Greer, Y.E.; Porat-Shliom, N.; Nagashima, K.; Stuelten, C.; Crooks, D.; Koparde, V.N.; Gilbert, S.F.; Islam, C.; Ubaldini, A.; Ji, Y.; et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 2018, 9, 18454–18479. [Google Scholar] [CrossRef]
- Ishizawa, J.; Kojima, K.; Chachad, D.; Ruvolo, P.; Ruvolo, V.; Jacamo, R.O.; Borthakur, G.; Mu, H.; Zeng, Z.; Tabe, Y.; et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal. 2016, 9, ra17. [Google Scholar] [CrossRef]
- Greer, Y.E.; Lipkowitz, S. ONC201: Stressing tumors to death. Sci. Signal. 2016, 9, fs1. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Bâ, M.; Shu, C.; Halatsch, M.-E.; Westhoff, M.-A.; Bruce, J.N.; Canoll, P.; Siegelin, M.D. TIC10/ONC201 Synergizes with Bcl-2/Bcl-xL Inhibition in Glioblastoma by Suppression of Mcl-1 and Its Binding Partners in Vitro and in Vivo. Oncotarget 2015, 6, 36456–36471. [Google Scholar] [CrossRef]
- Anderson, P.M.; Gortz, J. Phase 2 Study of DRD2 Antagonist/ClpP Agonist ONC201 in Neuroendocrine Tumors. J. Clin. Oncol. 2021, 39, 3002. [Google Scholar] [CrossRef]
- Atkins, S.L.P.; Greer, Y.E.; Jenkins, S.; Gatti-Mays, M.E.; Houston, N.; Lee, S.; Lee, M.-J.; Rastogi, S.; Sato, N.; Burks, C.; et al. A Single-Arm, Open-Label Phase II Study of ONC201 in Recurrent/Refractory Metastatic Breast Cancer and Advanced Endometrial Carcinoma. Oncologist 2023, 28, 919–e972. [Google Scholar] [CrossRef]
- Ralff, M.D.; Lulla, A.R.; Wagner, J.; El-Deiry, W.S. ONC201: A new treatment option being tested clinically for recurrent glioblastoma. Transl. Cancer Res. 2017, 6, S1239–S1243. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin. Cancer Res. 2017, 23, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Arrillaga-Romany, I.; Chi, A.S.; Allen, J.E.; Oster, W.; Wen, P.Y.; Batchelor, T.T. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget 2017, 8, 79298–79304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, Y.; Lu, P.-H.; Peng, Y.; Yuan, Q.; Gu, X.; Jin, Y.; Chen, M.-B.; Bai, X. Identification of DNA-PKcs as a Primary Resistance Factor of TIC10 in Hepatocellular Carcinoma Cells. Oncotarget 2017, 8, 28385–28394. [Google Scholar] [CrossRef]
- Lev, A.; Lulla, A.R.; Ross, B.C.; Ralff, M.D.; Makhov, P.B.; Dicker, D.T.; El-Deiry, W.S. ONC201 Targets AR and AR-V7 Signaling, Reduces PSA, and Synergizes with Everolimus in Prostate Cancer. Mol. Cancer Res. 2018, 16, 754–766. [Google Scholar] [CrossRef]
- Pathak, S.O.; Manohar, S.M. ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells. Biomolecules 2025, 15, 463. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ran, L.; Zhang, Z.; Jiang, R. The Preclinical Evaluation of TIC10/ONC201 as an Anti-Pancreatic Cancer Agent. Biochem. Biophys. Res. Commun. 2016, 476, 260–266. [Google Scholar] [CrossRef]
- Ray, J.; Ralff, M.; Dicker, D.; El-Deiry, W. Abstract 262: Anti-Tumorigenic Effect of ONC201 Is Enhanced by Combination Treatment with TRAIL or a DR5 Agonist in Endometrial Cancer in Vitro. Cancer Res. 2019, 79, 262. [Google Scholar] [CrossRef]
- Ralff, M.D.; Jhaveri, A.; Ray, J.E.; Zhou, L.; Lev, A.; Campbell, K.S.; Dicker, D.T.; Ross, E.A.; El-Deiry, W.S. TRAIL Receptor Agonists Convert the Response of Breast Cancer Cells to ONC201 from Anti-Proliferative to Apoptotic. Oncotarget 2020, 11, 3753–3769. [Google Scholar] [CrossRef]
- Bentayebi, K.; Suwan, K.; Ibrahimi, A.; Sara, L.; Ouadghiri, M.; Aanniz, T.; Amzazi, S.; Belyamani, L.; Hajitou, A.; Eljaoudi, R. Preclinical Evaluation of panobinostat and ONC201 for the treatment of diffuse intrinsic pontine glioma (DIPG). Brain Disord. 2024, 13, 100113. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.M.; Joshi, K.S. Promising Anticancer Activity of Multitarget Cyclin Dependent Kinase Inhibitors against Human Colorectal Carcinoma Cells. Curr. Mol. Pharmacol. 2022, 15, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef] [PubMed]
- Frei, E., III; Eder, J.P. Principles of Dose, Schedule, and Combination Therapy. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada, 2003; Chapter 44. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12635/ (accessed on 4 January 2025).
- Manohar, S.M. Cyclin-dependent kinases as potential targets for colorectal cancer: Past, present and future. Future Med. Chem. 2022, 14, 1087–1105. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Jemal, A. Colorectal Cancer Statistics, 2014. CA. Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer (IARC). Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 29 July 2025).
- Tuyaerts, S.; Van Nuffel, A.M.T.; Naert, E.; Van Dam, P.A.; Vuylsteke, P.; De Caluwé, A.; Aspeslagh, S.; Dirix, P.; Lippens, L.; De Jaeghere, E.; et al. PRIMMO Study Protocol: A Phase II Study Combining PD-1 Blockade, Radiation and Immunomodulation to Tackle Cervical and Uterine Cancer. BMC Cancer 2019, 19, 506. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem–like Cells in an Akt/Foxo3a/TRAIL–Dependent Manner. Cancer Res. 2015, 75, 1423–1432. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, Y. Raw Lacquer Extract from Toxicodendron Vernicifluum in Combination with ONC201 Enhances the Inhibitory Effects on Colorectal Cancer Cell Activity. Turk. J. Gastroenterol. 2023, 34, 211–220. [Google Scholar] [CrossRef]
- Khan, N.; Jajeh, F.; Eberhardt, E.L.; Miller, D.D.; Albrecht, D.M.; Van Doorn, R.; Hruby, M.D.; Maresh, M.E.; Clipson, L.; Mukhtar, H.; et al. Fisetin and 5-fluorouracil: Effective Combination for PIK3CA-mutant Colorectal Cancer. Int. J. Cancer 2019, 145, 3022–3032. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, W.; Fang, D.; Jin, Y. mTOR Inhibition Sensitizes ONC201-Induced Anti-Colorectal Cancer Cell Activity. Biochem. Biophys. Res. Commun. 2016, 478, 1515–1520. [Google Scholar] [CrossRef]
- Rathos, M.J.; Joshi, K.; Khanwalkar, H.; Manohar, S.M.; Joshi, K.S. Molecular Evidence for Increased Antitumor Activity of Gemcitabine in Combination with a Cyclin-Dependent Kinase Inhibitor, P276-00 in Pancreatic Cancers. J. Transl. Med. 2012, 10, 161. [Google Scholar] [CrossRef]
- Gaidhani, R.H.; Balasubramaniam, G. An Epidemiological Review of Pancreatic Cancer with Special Reference to India. Indian J. Med. Sci. 2021, 73, 99–109. [Google Scholar] [CrossRef]
- Huang, J.-H.; Guo, W.; Liu, Z. Discussion on Gemcitabine Combined with Targeted Drugs in the Treatment of Pancreatic Cancer. World J. Gastroenterol. 2023, 29, 579–581. [Google Scholar] [CrossRef]
- Greenhill, C. Gemcitabine Confirmed as the First-Line Therapy for Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 3. [Google Scholar] [CrossRef]
- Gong, J.; Tuli, R.; Shinde, A.; Hendifar, A.E. Meta-Analyses of Treatment Standards for Pancreatic Cancer. Mol. Clin. Oncol. 2016, 4, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.; Jansen, G.; Giovannetti, E. Drug Resistance in Pancreatic Cancer: Impact of Altered Energy Metabolism. Crit. Rev. Oncol. Hematol. 2017, 114, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef] [PubMed]
- Sally, Á.; McGowan, R.; Finn, K.; Moran, B.M. Current and Future Therapies for Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 2417. [Google Scholar] [CrossRef]
- Lev, A.; Lulla, A.R.; Wagner, J.; Ralff, M.D.; Kiehl, J.B.; Zhou, Y.; Benes, C.H.; Prabhu, V.V.; Oster, W.; Astsaturov, I.; et al. Anti-Pancreatic Cancer Activity of ONC212 Involves the Unfolded Protein Response (UPR) and Is Reduced by IGF1-R and GRP78/BIP. Oncotarget 2017, 8, 81776–81793. [Google Scholar] [CrossRef]
- Jhaveri, A.V.; Zhou, L.; Ralff, M.D.; Lee, Y.S.; Navaraj, A.; Carneiro, B.A.; Safran, H.; Prabhu, V.V.; Ross, E.A.; Lee, S.; et al. Combination of ONC201 and TLY012 Induces Selective, Synergistic Apoptosis in Vitro and Significantly Delays PDAC Xenograft Growth in Vivo. Cancer Biol. Ther. 2021, 22, 607–618. [Google Scholar] [CrossRef]
- Pandit, B.; Royzen, M. Recent Development of Prodrugs of Gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sethi, B.; Staller, D.W.; Shrestha, P.; Mahato, R.I. Gemcitabine Elaidate and ONC201 Combination Therapy for Inhibiting Pancreatic Cancer in a KRAS Mutated Syngeneic Mouse Model. Cell Death Discov. 2024, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Tummala, T.; Cruz, A.D.L.; Uruchurtu, A.; Liguori, N.R.; Abbas, A.E.; Zhang, L.; Azzoli, C.G.; Zhou, L.; El-Deiry, W.S. Abstract 2674: Synergistic Combinations of Lurbinectedin with Irinotecan and ONC212 in Pancreatic Cancer. Cancer Res. 2023, 83, 2674. [Google Scholar] [CrossRef]
- Zhou, W.; Wahl, D.R. Metabolic Abnormalities in Glioblastoma and Metabolic Strategies to Overcome Treatment Resistance. Cancers 2019, 11, 1231. [Google Scholar] [CrossRef]
- Imai, R.; Sasaki, H. Glioblastoma That Does Not Improve with Standard Treatment: Standard and Personalized Treatment Making The Most of Limited Modalities. Brain Nerve Shinkei Kenkyu no Shinpo 2022, 74, 677–684. [Google Scholar] [CrossRef]
- Abedi, A.A.; Grunnet, K.; Christensen, I.J.; Michaelsen, S.R.; Muhic, A.; Møller, S.; Hasselbalch, B.; Poulsen, H.S.; Urup, T. A Prognostic Model for Glioblastoma Patients Treated with Standard Therapy Based on a Prospective Cohort of Consecutive Non-Selected Patients From a Single Institution. Front. Oncol. 2021, 11, 597587. [Google Scholar] [CrossRef]
- Bou-Gharios, J.; Noël, G.; Burckel, H. Preclinical and Clinical Advances to Overcome Hypoxia in Glioblastoma Multiforme. Cell Death Dis. 2024, 15, 503. [Google Scholar] [CrossRef]
- Sadowski, K.; Jażdżewska, A.; Kozłowski, J.; Zacny, A.; Lorenc, T.; Olejarz, W. Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5774. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, X.; Gao, P.; Han, X.; Zhao, P.; Xie, F.; Liu, M. Advancing Glioblastoma Treatment by Targeting Metabolism. Neoplasia 2024, 51, 100985. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, J.; Safran, H.P.; El-Deiry, W.S. Abstract 5448: Synergistic Antitumor Effect of ONC201, Radiotherapy and Temozolomide in Glioblastoma Mouse Orthotopic Models. Cancer Res. 2022, 82, 5448. [Google Scholar] [CrossRef]
- Chen, J.; Roady, T.J.; Zhou, L.; El-Deiry, W.S. Abstract B027: Synergy between ONC201 and temozolomide on ATF4 integrated stress response in glioblastoma. Mol. Cancer Ther. 2023, 22, B027. [Google Scholar] [CrossRef]
- Pruss, M.; Dwucet, A.; Tanriover, M.; Hlavac, M.; Kast, R.E.; Debatin, K.-M.; Wirtz, C.R.; Halatsch, M.-E.; Siegelin, M.D.; Westhoff, M.-A.; et al. Dual Metabolic Reprogramming by ONC201/TIC10 and 2-Deoxyglucose Induces Energy Depletion and Synergistic Anti-Cancer Activity in Glioblastoma. Br. J. Cancer 2020, 122, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Kawakibi, A.R.; Ji, S.; Waszak, S.M.; Sweha, S.R.; Mota, M.; Pun, M.; Deogharkar, A.; Chung, C.; Tarapore, R.S.; et al. Clinical Efficacy of ONC201 in H3K27M-Mutant Diffuse Midline Gliomas Is Driven by Disruption of Integrated Metabolic and Epigenetic Pathways. Cancer Discov. 2023, 13, 2370–2393. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.R.; Duchatel, R.J.; Staudt, D.E.; Persson, M.L.; Mannan, A.; Yadavilli, S.; Parackal, S.; Game, S.; Chong, W.C.; Jayasekara, W.S.N.; et al. ONC201 in Combination with Paxalisib for the Treatment of H3K27-Altered Diffuse Midline Glioma. Cancer Res. 2023, 83, 2421–2437. [Google Scholar] [CrossRef] [PubMed]
- Suhasini, G.; Surender Reddy, K.; Madhulekha, R.; Ugandhar, T. Emerging Epidemic: Non-Smoker Lung Cancer in India’s Urban Centres. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 285–292. [Google Scholar] [CrossRef]
- Choughule, A.; D′Souza, H. Liquid Biopsy in Lung Cancer-Hope or Hype? Cancer Res. Stat. Treat. 2019, 2, 221. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Vidaurre, I.; Cai, R.; Sha, W.; Schally, A.V. Inhibition of Experimental Small-Cell and Non-Small-Cell Lung Cancers by Novel Antagonists of Growth Hormone-Releasing Hormone. Int. J. Cancer 2018, 142, 2394–2404. [Google Scholar] [CrossRef]
- McNamee, N.; da Silva, I.P.; Nagrial, A.; Gao, B. Small-Cell Lung Cancer—An Update on Targeted and Immunotherapies. Int. J. Mol. Sci. 2023, 24, 8129. [Google Scholar] [CrossRef]
- Besse, B.; Paz-Ares, L.G.; Peters, S.; Cappuzzo, F.; Reck, M.; Calles, A.; Califano, R.; Lopez-Vilariño, J.A.; Veramendi, S.; Kahatt, C.M.; et al. A Phase III Study of Lurbinectedin Alone or in Combination with Irinotecan vs. Investigator’s Choice (Topotecan or Irinotecan) in Patients with Relapsed Small Cell Lung Cancer (SCLC; LAGOON Trial). J. Clin. Oncol. 2023, 41, TPS8613. [Google Scholar] [CrossRef]
- Lee, Y.S.; Zhou, L.; Azzoli, C.; El-Deiry, W.S. Abstract 4056: ONC201 in Combination with Carboplatin and Etoposide as a Novel Triple Drug Treatment for Small Cell Lung Cancer (SCLC). Cancer Res. 2022, 82, 4056. [Google Scholar] [CrossRef]
- Calles, A.; Navarro, A.; Doger De Speville Uribe, B.G.; Colomé, E.Á.; De Miguel, M.; Álvarez, R.; Arregui, M.; Moreno, V.; Rocha, P.; Calvo, E.; et al. Lurbinectedin Plus Pembrolizumab in Relapsed SCLC: The Phase I/II LUPER Study. J. Thorac. Oncol. 2025, 20, 969–982. [Google Scholar] [CrossRef]
- Rathos, M.J.; Khanwalkar, H.; Joshi, K.; Manohar, S.M.; Joshi, K.S. Potentiation of in Vitro and in Vivo antitumor Efficacy of Doxorubicin by Cyclin-Dependent Kinase Inhibitor P276-00 in Human Non-Small Cell Lung Cancer Cells. BMC Cancer 2013, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, C.; Lin, J.; Chen, S.; Weng, L.; He, Z. Efficacy Analysis of Immunotherapy-based Combinations for Patients with EGFR-mutant Advanced Non-small Cell Lung Cancer after TKI Failure. Oncol. Lett. 2024, 28, 504. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.D.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Lee, J.; Im, S.; Chung, C. Navigating the Complexity of Resistance in Lung Cancer Therapy: Mechanisms, Organoid Models, and Strategies for Overcoming Treatment Failure. Cancers 2024, 16, 3996. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, J.; Li, Z.; Jiang, Y.; Zhou, Y. Small Molecular TRAIL Inducer ONC201 Induces Death in Lung Cancer Cells: A Preclinical Study. PLoS ONE 2016, 11, e0162133. [Google Scholar] [CrossRef]
- Kataki, A.C.; Baruah, U.; Maheshwari, A.; Medhi, P.; Kataki, K.J. Endometrial Cancer. In Fundamentals in Gynaecologic Malignancy; Kataki, A., Barmon, D., Eds.; Springer: Singapore, 2023; pp. 247–278. [Google Scholar]
- Rousset-Rouviere, S.; Rochigneux, P.; Chrétien, A.-S.; Fattori, S.; Gorvel, L.; Provansal, M.; Lambaudie, E.; Olive, D.; Sabatier, R. Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology. Biomedicines 2021, 9, 632. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Karpel, H.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Biomarker-Driven Therapy in Endometrial Cancer. Int. J. Gynecol. Cancer 2023, 33, 343–350. [Google Scholar] [CrossRef]
- Maiorano, M.F.P.; Messina, C.; Maiello, E.; Cormio, G.; Maiorano, B.A. Immune Checkpoint Inhibitors plus Chemotherapy in First-line Endometrial Cancer Treatment: Still the Era of Microsatellites? BJOG Int. J. Obstet. Gynaecol. 2024, 131, 1157–1159. [Google Scholar] [CrossRef]
- Hill, E.K.; Dizon, D.S. Medical Therapy of Endometrial Cancer: Current Status and Promising Novel Treatments. Drugs 2012, 72, 705–713. [Google Scholar] [CrossRef]
- Ray, J.E.; Ralff, M.D.; Jhaveri, A.; Zhou, L.; Dicker, D.T.; Ross, E.A.; El-Deiry, W.S. Antitumorigenic Effect of Combination Treatment with ONC201 and TRAIL in Endometrial Cancer in Vitro and in Vivo. Cancer Biol. Ther. 2021, 22, 554–563. [Google Scholar] [CrossRef]
- Paraghamian, S.E.; Qiu, J.; Hawkins, G.M.; Zhao, Z.; Sun, W.; Fan, Y.; Zhang, X.; Suo, H.; Hao, T.; Prabhu, V.V.; et al. A Novel Dopamine Receptor D2 Antagonist (ONC206) Potentiates the Effects of Olaparib in Endometrial Cancer. Cancer Biol. Ther. 2023, 24, 2202104. [Google Scholar] [CrossRef] [PubMed]
- de Zafra, C.L.Z.; Ashkenazi, A.; Darbonne, W.C.; Cheu, M.; Totpal, K.; Ortega, S.; Flores, H.; Walker, M.D.; Kabakoff, B.; Lum, B.L.; et al. Antitherapeutic Antibody-Mediated Hepatotoxicity of Recombinant Human Apo2l/TRAIL in the Cynomolgus Monkey. Cell Death Dis. 2016, 7, e2338. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Sahin, I.; Zhang, S.; Schwermann, M.P. Abstract 551: MDM2 Inhibition in Combination with Imipridone ONC201 Treatment as a Synergistic Combination in Solid Tumors. Cancer Res. 2023, 83, 551. [Google Scholar] [CrossRef]
- Badola, A.; Singh, P.; Anand, S.; Sekar, V.; Verma, J. 321P Epidemiology and Survival Analysis of Epithelial Ovarian Cancer: Results from Comprehensive Care Center in North India. Ann. Oncol. 2023, 34, S1596. [Google Scholar] [CrossRef]
- Singh, L.; Sehrawat, A.; N, S.; Philips, A.O.; Panda, S.S.; Cyriac, S.L.; Rathnam, K.; SV, S.; Moharana, L.; Samantaray, L.; et al. Unveiling Primary Platinum Resistant Ovarian Cancer: Demographic and Clinical Perspectives from Multicenter Cancer Registry in India. J. Clin. Oncol. 2024, 42, e17572. [Google Scholar] [CrossRef]
- Pawłowska, A.; Rekowska, A.; Kuryło, W.; Pańczyszyn, A.; Kotarski, J.; Wertel, I. Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 10859. [Google Scholar] [CrossRef]
- Lage, H.; Denkert, C. Resistance to Chemotherapy in Ovarian Carcinoma. In Targeted Therapies in Cancer; Dietel, M., Ed.; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2007; Volume 176, pp. 51–60. ISBN 978-3-540-46090-9. [Google Scholar]
- Moufarrij, S.; O’Cearbhaill, R.E. Novel Therapeutics in Ovarian Cancer: Expanding the Toolbox. Curr. Oncol. 2023, 31, 97–114. [Google Scholar] [CrossRef]
- Petrić-Miše, B. First-Line Treatment of Advanced Ovarian Cancer: An Expert Update. Libri Oncol. Croat. J. Oncol. 2020, 48, 77–84. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Fang, Z.; Pierce, S.R.; West, L.; Staley, A.; Tucker, K.; Yin, Y.; Sun, W.; Kong, W.; et al. Anti-Tumor and Anti-Invasive Effects of ONC201 on Ovarian Cancer Cells and a Transgenic Mouse Model of Serous Ovarian Cancer. Front. Oncol. 2022, 12, 789450. [Google Scholar] [CrossRef]
- Rumman, M.; Buck, S.; Polin, L.; Dzinic, S.; Boerner, J.; Winer, I.S. ONC201 Induces the Unfolded Protein Response (UPR) in High- and Low-grade Ovarian Carcinoma Cell Lines and Leads to Cell Death Regardless of Platinum Sensitivity. Cancer Med. 2021, 10, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Ghandali, M.; Selvi, K.M.; Huntington, K.E.; George, A.; Ochsner, A.; Carneiro, B.A.; Dizon, D.S.; El-Deiry, W.S. Abstract 5512: TIC10/ONC201 in Combination with Ceralasertib Exhibits Potent Synergy in High Grade Serous Ovarian Cancer Cells. Cancer Res. 2023, 83, 5512. [Google Scholar] [CrossRef]
- Di Cristofano, F.R.; Fong, M.W.; Huntington, K.E.; Carneiro, B.A.; Zhou, L.; El-Deiry, W.S. Synergistic activity of ABT-263 and ONC201/TIC10 against solid tumor cell lines is associated with suppression of anti-apoptotic Mcl-1, BAG3, pAkt, and upregulation of pro-apoptotic Noxa and Bax cleavage during apoptosis. Am. J. Cancer Res. 2023, 13, 307–325. [Google Scholar] [PubMed]
- Wang, L.; Wang, X.; Zhu, X.; Zhong, L.; Jiang, Q.; Wang, Y.; Tang, Q.; Li, Q.; Zhang, C.; Wang, H.; et al. Drug Resistance in Ovarian Cancer: From Mechanism to Clinical Trial. Mol. Cancer 2024, 23, 66. [Google Scholar] [CrossRef]
- Roy, N.; Mathew, A. Triple Negative Breast Cancer in India: What Is the Real Incidence? Indian J. Med. Paediatr. Oncol. 2023, 44, 442–444. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz Van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Gadhia, P.K.; Joshi, N.; Vaniawala, S. Triple-Negative Breast Cancer in India: Exploring Age Distribution and Laterality Patterns. Int. J. Mol. Immun. Oncol. 2024, 9, 62–65. [Google Scholar] [CrossRef]
- Mir, M.A. Therapeutic Potential of Cell Cycle Kinases in Breast Cancer; Springer Nature: Singapore, 2023; pp. 232–252. [Google Scholar]
- Riaz, F. New Strategies for the Management of Triple-Negative Breast Cancer. Curr. Opin. Obstet. Gynecol. 2024, 36, 40–44. [Google Scholar] [CrossRef]
- Ahmadimoghari, F.; Zhang, Y.; Mavrommatis, I.; Thakur, S.; Starling, C.; Roxanis, I.; Haider, S.; Natrajan, R. Abstract B059: Epigenetically Defined Sub-Clonal Heterogeneity Drives Therapy Resistance in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, B059. [Google Scholar] [CrossRef]
- Da Silva Fernandes, T.; Gillard, B.M.; Dai, T.; Martin, J.C.; Chaudhry, K.A.; Dugas, S.M.; Fisher, A.A.; Sharma, P.; Wu, R.; Attwood, K.M.; et al. Inosine Monophosphate Dehydrogenase 2 (IMPDH2) Modulates Response to Therapy and Chemo-Resistance in Triple Negative Breast Cancer. Sci. Rep. 2025, 15, 1061. [Google Scholar] [CrossRef]
- Ralff, M.D.; Wagner, J.; El-Deiry, W.S. Abstract LB-082: ONC201 Sensitizes Resistant Breast Cancer Cells to TRAIL through Death Receptor 5 Upregulation. Cancer Res. 2018, 78, LB-082. [Google Scholar] [CrossRef]
- Mishukov, A.; Odinokova, I.; Mndlyan, E.; Kobyakova, M.; Abdullaev, S.; Zhalimov, V.; Glukhova, X.; Galat, V.; Galat, Y.; Senotov, A.; et al. ONC201-Induced Mitochondrial Dysfunction, Senescence-like Phenotype, and Sensitization of Cultured BT474 Human Breast Cancer Cells to TRAIL. Int. J. Mol. Sci. 2022, 23, 15551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kho, D.; Xu, J.; Gajan, A.; Wu, K.; Wu, G.S. ONC201 Activates ER Stress to Inhibit the Growth of Triple-Negative Breast Cancer Cells. Oncotarget 2017, 8, 21626–21638. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, E.; Nath, A.; Emond, R.; Karimi, K.L.; Grolmusz, V.K.; Cosgrove, P.A.; Bild, A.H. ONC201/TIC10 Enhances Durability of mTOR Inhibitor Everolimus in Metastatic ER+ Breast Cancer. eLife 2023, 12, e85898. [Google Scholar] [CrossRef] [PubMed]
- Ghandali, M.; Huntington, K.E.; Srinivasan, P.; Dizon, D.S.; Graff, S.L.; Carneiro, B.A.; El-Deiry, W.S. Abstract 1066: PARP Inhibitor Rucaparib in Combination with Imipridones ONC201 or ONC212 Demonstrates Preclinical Synergy against BRCA1/2-Deficient Breast, Ovarian, and Prostate Cancer Cells. Cancer Res. 2023, 83, 1066. [Google Scholar] [CrossRef]
- Lim, B.; Peterson, C.B.; Davis, A.; Cho, E.; Pearson, T.; Liu, H.; Hwang, M.; Ueno, N.T.; Lee, J. ONC201 and an MEK Inhibitor Trametinib Synergistically Inhibit the Growth of Triple-Negative Breast Cancer Cells. Biomedicines 2021, 9, 1410. [Google Scholar] [CrossRef]
- Kumar, V.; Jena, D.; Zahiruddin, Q.S.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Prasad, G.V.S.; Rajput, P.; Syed, R.; et al. Prostate Cancer Burden in South Asia: A Systematic Analysis of Global Burden of Disease Data (1990–2021). Int. J. Urol. 2025, 32, 277–284. [Google Scholar] [CrossRef]
- Sankarapillai, J.; Krishnan, S.; Ramamoorthy, T.; Sudarshan, K.L.; Mathur, P. Descriptive Epidemiology of Prostate Cancer in India, 2012–2019: Insights from the National Cancer Registry Programme. Indian J. Urol. 2024, 40, 167–173. [Google Scholar] [CrossRef]
- Manohar, S.M. Insights into molecular drivers of prostate cancer. In Horizons in Cancer Research; Watanabe, H.S., Ed.; Nova Science Publishers: New York, NY, USA, 2020; Volume 76, pp. 1–84. [Google Scholar]
- Thakur, N.; Singh, P.; Bagri, A.; Srivastava, S.; Dwivedi, V.; Singh, A.; Jaiswal, S.K.; Dholpuria, S. Therapy Resistance in Prostate Cancer: Mechanism, Signaling and Reversal Strategies. Explor. Target. Anti-Tumor Ther. 2024, 5, 1110–1134. [Google Scholar] [CrossRef]
- Ferraldeschi, R.; Welti, J.; Luo, J.; Attard, G.; De Bono, J.S. Targeting the Androgen Receptor Pathway in Castration-Resistant Prostate Cancer: Progresses and Prospects. Oncogene 2014, 34, 1745–1757. [Google Scholar] [CrossRef]
- Wu, J.L.; Zhou, L.; Zhang, L.; Huntington, K.E.; Carneiro, B.; El-Deiry, W.S. Abstract 3947: Antitumor Efficacy of Combination Treatment with ONC201 and Enzalutamide or Darolutamide in Metastatic Castration-Resistant Prostate Cancer. Cancer Res. 2022, 82, 3947. [Google Scholar] [CrossRef]
- Wagner, J.; Kline, C.L.; Zhou, L.; Campbell, K.S.; MacFarlane, A.W.; Olszanski, A.J.; Cai, K.Q.; Hensley, H.H.; Ross, E.A.; Ralff, M.D.; et al. Dose Intensification of TRAIL-Inducing ONC201 Inhibits Metastasis and Promotes Intratumoral NK Cell Recruitment. J. Clin. Investig. 2018, 128, 2325–2338. [Google Scholar] [CrossRef]
- Xia, Y.; Schwermann, M.P.; George, A.; Ochsner, A.; Carneiro, B.A.; El-Deiry, W.S. Abstract 1070: The Anti-Tumor Efficacy of Combining Oral ATR Kinase Inhibitor Ceralasertib with TIC10/ONC201, an Oral Akt/ERK Inhibitor, TRAIL Pathway and Integrated Stress Response Inducer, in Prostate Cancer Treatment. Cancer Res. 2023, 83, 1070. [Google Scholar] [CrossRef]
- Baumeister, M.D.; Küçükkase, O.C.; Prabhu, V.V.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Abstract 3212: ONC201 Shows Efficacy in BRCA-Deficient Cancer Cells and Synergy with PARP Inhibitors in Glioblastoma, Breast, Prostate, and Ovarian Cancers. Cancer Res. 2017, 77, 3212. [Google Scholar] [CrossRef]
- Werner, M.; Becker, K.F.; Keller, G.; Höfler, H. Gastric Adenocarcinoma: Pathomorphology and Molecular Pathology. J. Cancer Res. Clin. Oncol. 2001, 127, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The Future of Cancer Treatment: Immunomodulation, CARs and Combination Immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Chaudhuri, T.; Bankira, J.; Upadhyay, A.K.; Panda, R.; Pandey, V.; Mitra, S.; Mukherjee, S. Efficacy and Safety of First-Line Palliative Chemotherapy with Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel (FLOT) in HER2-Negative Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma: A Single-Institutional Real-World Experience from Eastern India. South Asian J. Cancer 2024. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, J.; Ruan, S.; Zhang, B.; Zhu, H.; Que, Y.; Ying, S.; Li, X.; Hu, Y.; Song, Z. Overcoming Immunotherapy Resistance in Gastric Cancer: Insights into Mechanisms and Emerging Strategies. Cell Death Dis. 2025, 16, 75. [Google Scholar] [CrossRef]
- Parker, C.; Zhou, L.; Prabhu, V.; Allen Wolfgang Oster, J.; El-Deiry, W. ONC201 Induces Apoptosis in Gastric Adenocarcinoma and Exhibits Synergy with rhTRAIL. J. Am. Coll. Surg. 2020, 231, S276. [Google Scholar] [CrossRef]
- Parker, C.S.; Zhou, L.; Prabhu, V.V.; Lee, S.; Miner, T.J.; Ross, E.A.; El-Deiry, W.S. ONC201/TIC10 plus TLY012 Anti-Cancer Effects via Apoptosis Inhibitor Downregulation, Stimulation of Integrated Stress Response and Death Receptor DR5 in Gastric Adenocarcinoma. Am. J. Cancer Res. 2023, 13, 6290–6312. [Google Scholar]
- Prabhu, V.V.; Lulla, A.R.; Madhukar, N.S.; Ralff, M.D.; Zhao, D.; Kline, C.L.B.; Van Den Heuvel, A.P.J.; Lev, A.; Garnett, M.J.; McDermott, U.; et al. Cancer Stem Cell-Related Gene Expression as a Potential Biomarker of Response for First-in-Class Imipridone ONC201 in Solid Tumors. PLoS ONE 2017, 12, e0180541. [Google Scholar] [CrossRef]
- Jhaveri, A.V.; Zhou, L.; Ferarrini, I.; Lee, Y.; El-Deiry, W.S. Abstract 6225: Addition of TRAIL Receptor Agonists after Treatment with ONC201 or ONC212 Converts Pancreatic Cancer Cells from Anti-Proliferative to Apoptotic in Vitro. Cancer Res. 2020, 80, 6225. [Google Scholar] [CrossRef]
- Ishida, C.T.; Zhang, Y.; Bianchetti, E.; Shu, C.; Nguyen, T.T.T.; Kleiner, G.; Sanchez-Quintero, M.J.; Quinzii, C.M.; Westhoff, M.-A.; Karpel-Massler, G.; et al. Metabolic Reprogramming by Dual AKT/ERK Inhibition through Imipridones Elicits Unique Vulnerabilities in Glioblastoma. Clin. Cancer Res. 2018, 24, 5392–5406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, L.; Zhang, J.; Wu, L.J.; Zhang, S.; George, A.; Hahn, M.; Safran, H.P.; Chen, C.C.; Seyhan, A.A.; et al. Imipridones ONC201/ONC206 + RT/TMZ triple (IRT) therapy reduces intracranial tumor burden, prolongs survival in orthotopic IDH-WT GBM mouse model, and suppresses MGMT. Oncotarget 2025, 16, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Liguori, N.R.; Sanchez Sevilla Uruchurtu, A.; Zhang, L.; Abbas, A.E.; Lee, Y.S.; Zhou, L.; Azzoli, C.G.; El-Deiry, W.S. Preclinical studies with ONC201/TIC10 and lurbinectedin as a novel combination therapy in small cell lung cancer (SCLC). Am. J. Cancer Res. 2022, 12, 729–743. [Google Scholar] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Patel, L.; Mayes, P.A.; Dicker, D.T.; Wu, G.S.; El-Deiry, W.S. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol. Cancer 2015, 14, 99, Erratum in Mol. Cancer 2024, 23, 233. [Google Scholar] [CrossRef]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK Dual Inhibitors: The Way Forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef]
- Greer, Y.E.; Lipkowitz, S. TIC10/ONC201: A Bend in the Road to Clinical Development. Oncoscience 2015, 2, 75–76. [Google Scholar] [CrossRef]
- Cantor, E.; Wierzbicki, K.; Tarapore, R.S.; Ravi, K.; Thomas, C.; Cartaxo, R.; Yadav, V.N.; Ravindran, R.; Bruzek, A.K.; Wadden, J.; et al. Serial H3K27M cell-free tumor DNA (cf-tDNA) tracking predicts ONC201 treatment response and progression in diffuse midline glioma. Neuro-Oncology 2022, 24, 1366–1374. [Google Scholar] [CrossRef]
- Morrow, S.; Nath, K.; Zhang, Y.; Garnett, M.J.; McDermott, U.; Benes, C.H.; Basken, J.; El-Deiry, W.S.; Allen, J.E.; Prabhu, V.V. Abstract 393: Predictive biomarker evaluation and molecular differentiation for imipridones ONC201 and ONC206. Cancer Res. 2021, 81, 393. [Google Scholar] [CrossRef]
- Kawakibi, A.R.; Tarapore, R.S.; Gardner, S.; Thomas, C.; Cartaxo, R.; Yadav, V.N.; Chi, A.; Kurz, S.; Wen, P.; Arrillaga-Romany, I.; et al. Clinical Efficacy and Predictive Biomarkers of ONC201 in H3K27M-Mutant Diffuse Midline Glioma. Neuro-Oncology 2020, 22, ii45–ii46. [Google Scholar] [CrossRef]
- Bonner, E.R.; Waszak, S.M.; Grotzer, M.A.; Mueller, S.; Nazarian, J. Mechanisms of imipridones in targeting mitochondrial metabolism in cancer cells. Neuro-Oncology 2021, 23, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Borsuk, R.; Zhou, L.; Chang, W.I.; Zhang, Y.; Sharma, A.; Prabhu, V.V.; Tapinos, N.; Lulla, R.R.; El-Deiry, W.S. Potent preclinical sensitivity to imipridone-based combination therapies in oncohistone H3K27M-mutant diffuse intrinsic pontine glioma is associated with induction of the integrated stress response, TRAIL death receptor DR5, reduced ClpX and apoptosis. Am. J. Cancer Res. 2021, 11, 4607–4623. [Google Scholar]
- Nguyen, T.T.T.; Shang, E.; Schiffgens, S.; Torrini, C.; Shu, C.; Akman, H.O.; Prabhu, V.V.; Allen, J.E.; Westhoff, M.A.; Karpel-Massler, G.; et al. Induction of Synthetic Lethality by Activation of Mitochondrial ClpP and Inhibition of HDAC1/2 in Glioblastoma. Clin. Cancer Res. 2022, 28, 1881–1895. [Google Scholar] [CrossRef]
- Przystal, J.M.; Cosentino, C.C.; Yadavilli, S.; Zhang, J.; Laternser, S.; Bonner, E.R.; Prasad, R.; Dawood, A.A.; Lobeto, N.; Chong, W.C.; et al. Imipridones affect tumor bioenergetics and promote cell lineage differentiation in diffuse midline gliomas. Neuro-Oncology 2022, 24, 1438–1451. [Google Scholar] [CrossRef]
- Purow, B. ONC201 and ONC206: Metabolically ClipPing the wings of diffuse midline glioma. Neuro-Oncology 2022, 24, 1452–1453. [Google Scholar] [CrossRef]
- Carter, J.L.; Hege, K.; Kalpage, H.A.; Edwards, H.; Hüttemann, M.; Taub, J.W.; Ge, Y. Targeting mitochondrial respiration for the treatment of acute myeloid leukemia. Biochem. Pharmacol. 2020, 182, 114253. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Yin, Y.; Fan, Y.; Sun, W.; Zhao, X.; Tucker, K.; Staley, A.; Paraghamian, S.; Hawkins, G.; et al. ONC206, an Imipridone Derivative, Induces Cell Death Through Activation of the Integrated Stress Response in Serous Endometrial Cancer In Vitro. Front. Oncol. 2020, 10, 577141. [Google Scholar] [CrossRef]
- Staley, A.; Tucker, K.; Yin, Y.; Zhang, X.; Fan, Y.; Zhang, Y.; Fang, Z.; Sun, W.; Suo, H.; Zhao, X.; et al. Highly potent dopamine receptor D2 antagonist ONC206 demonstrates anti-tumorigenic activity in endometrial cancer. Am. J. Cancer Res. 2021, 11, 5374–5387. [Google Scholar] [CrossRef]
- Tucker, K.; Yin, Y.; Staley, S.A.; Zhao, Z.; Fang, Z.; Fan, Y.; Zhang, X.; Suo, H.; Sun, W.; Prabhu, V.V.; et al. ONC206 has anti-tumorigenic effects in human ovarian cancer cells and in a transgenic mouse model of high-grade serous ovarian cancer. Am. J. Cancer Res. 2022, 12, 521–536. [Google Scholar]
- El-Soussi, S.; Hanna, R.; Semaan, H.; Khater, A.R.; Abdallah, J.; Abou-Kheir, W.; Abou-Antoun, T. A Novel Therapeutic Mechanism of Imipridones ONC201/ONC206 in MYCN-Amplified Neuroblastoma Cells via Differential Expression of Tumorigenic Proteins. Front. Pediatr. 2021, 9, 693145. [Google Scholar] [CrossRef]
- Wu, L.J.; Pinho-Schwermann, M.; Zhou, L.; Zhang, L.; Huntington, K.E.; Malpass, R.; Seyhan, A.A.; Carneiro, B.A.; El-Deiry, W.S. Synergistic combination therapy with ONC201 or ONC206, and enzalutamide or darolutamide in preclinical studies of castration-resistant prostate cancer. Am. J. Cancer Res. 2024, 14, 6012–6036. [Google Scholar] [CrossRef]
- Purcell, C.; Srinivasan, P.R.; Pinho-Schwermann, M.; MacDonald, W.J.; Ding, E.; El-Deiry, W.S. Neuroendocrine prostate cancer drivers SOX2 and BRN2 confer differential responses to imipridones ONC201, ONC206, and ONC212 in prostate cancer cell lines. Am. J. Transl. Res. 2024, 16, 7972–7982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huntington, K.E.; Seyhan, A.A.; Tapinos, N.; Lulla, R.R.; Monje, M.; Wong, E.T.; Chen, C.C.; El-Deiry, W.S. Reduced EZH1/2 expression in imipridone-treated cells correlates with synergy following combinations with EZH1/2 or HDAC inhibitors in diffuse glioma and other tumors. Am. J. Cancer Res. 2025, 15, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Kaur, K.; Gharamanians, N. Supercharged NK Cells as a Promising Therapeutic Strategy to Target and Eliminate Aggressive DIPG Tumors in Pediatric Patients. Crit. Rev. Immunol. 2025, 45, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tapinos, N.; Lulla, R.; El-Deiry, W.S. Dopamine pre-treatment impairs the anti-cancer effect of integrated stress response- and TRAIL pathway-inducing ONC201, ONC206 and ONC212 imipridones in pancreatic, colorectal cancer but not DMG cells. Am. J. Cancer Res. 2024, 14, 2453–2464. [Google Scholar] [CrossRef]
- Nii, T.; Prabhu, V.V.; Ruvolo, V.; Madhukar, N.; Zhao, R.; Mu, H.; Heese, L.; Nishida, Y.; Kojima, K.; Garnett, M.J.; et al. Imipridone ONC212 activates orphan G protein-coupled receptor GPR132 and integrated stress response in acute myeloid leukemia. Leukemia 2019, 33, 2805–2816. [Google Scholar] [CrossRef]
- Basu, V.; Shabnam; Murghai, Y.; Ali, M.; Sahu, S.; Verma, B.K.; Seervi, M. ONC212, Alone or in Synergistic Conjunction with Navitoclax (ABT-263), Promotes Cancer Cell Apoptosis via Unconventional Mitochondrial-Independent Caspase-3 Activation. Cell Commun. Signal. 2024, 22, 441. [Google Scholar] [CrossRef]
- Szász, Z.; Takács, A.; Kalabay, M.; Bárány, P.; Czuczi, T.; Csámpai, A.; Lajkó, E.; Kőhidai, L. Comparative Study of the Anti-Tumour Effects of the Imipridone, ONC201 and Its Fluorinated Analogues on Pancreatic Cancer Cell Line. Sci. Rep. 2025, 15, 15925. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Zhang, J.; Zhang, S.; El-Deiry, W.S. Abstract 3331: Preclinical Combination of ONC206 with Radiotherapy and Temozolomide in a GBM Mouse Orthotopic Model. Cancer Res. 2024, 84, 3331. [Google Scholar] [CrossRef]
- Mikhael, S.; Fayyad, R.; Harfouch, L.A.; Prabhu, V.V.; Bahmad, H.F.; Abou-Kheir, W.; Daoud, G. Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models. Curr. Issues Mol. Biol. 2025, 47, 451. [Google Scholar] [CrossRef]
- Goodell, J.C.; Zimmerman, S.M.; Peer, C.J.; Prabhu, V.; Yin, T.; Richardson, W.J.; Azinfar, A.; Dunn, J.A.; Mullin, M.; Theeler, B.J.; et al. Quantitation of the next-generation imipridone ONC206 in human plasma by a simple and sensitive UPLC-MS/MS assay for clinical pharmacokinetic application. J. Pharm. Biomed. Anal. 2022, 213, 114685. [Google Scholar] [CrossRef]
- Czuczi, T.; Murányi, J.; Bárány, P.; Móra, I.; Borbély, A.; Csala, M.; Csámpai, A. Synthesis and Antiproliferative Activity of Novel Imipridone-Ferrocene Hybrids with Triazole and Alkyne Linkers. Pharmaceuticals 2022, 15, 468. [Google Scholar] [CrossRef]
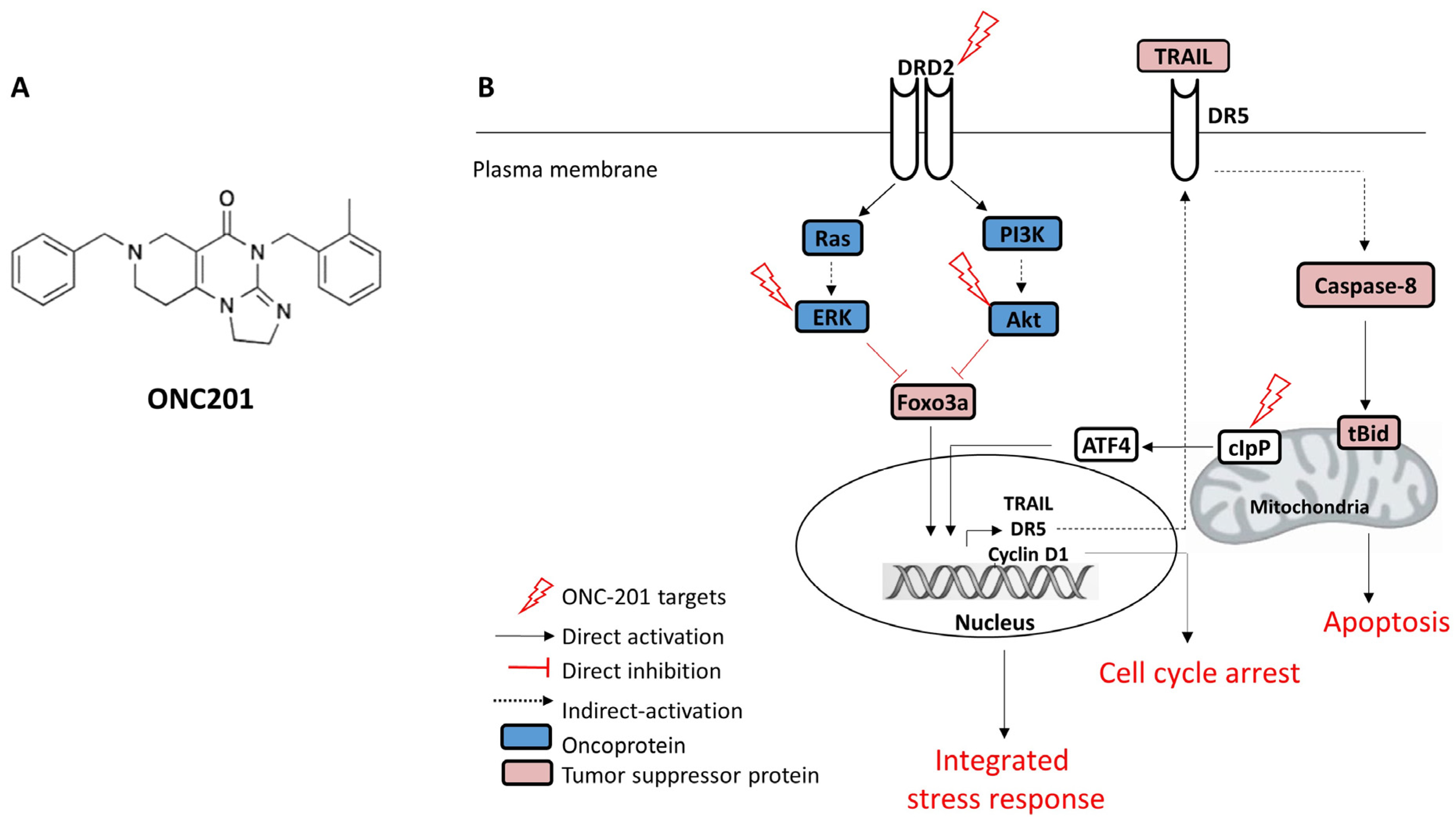
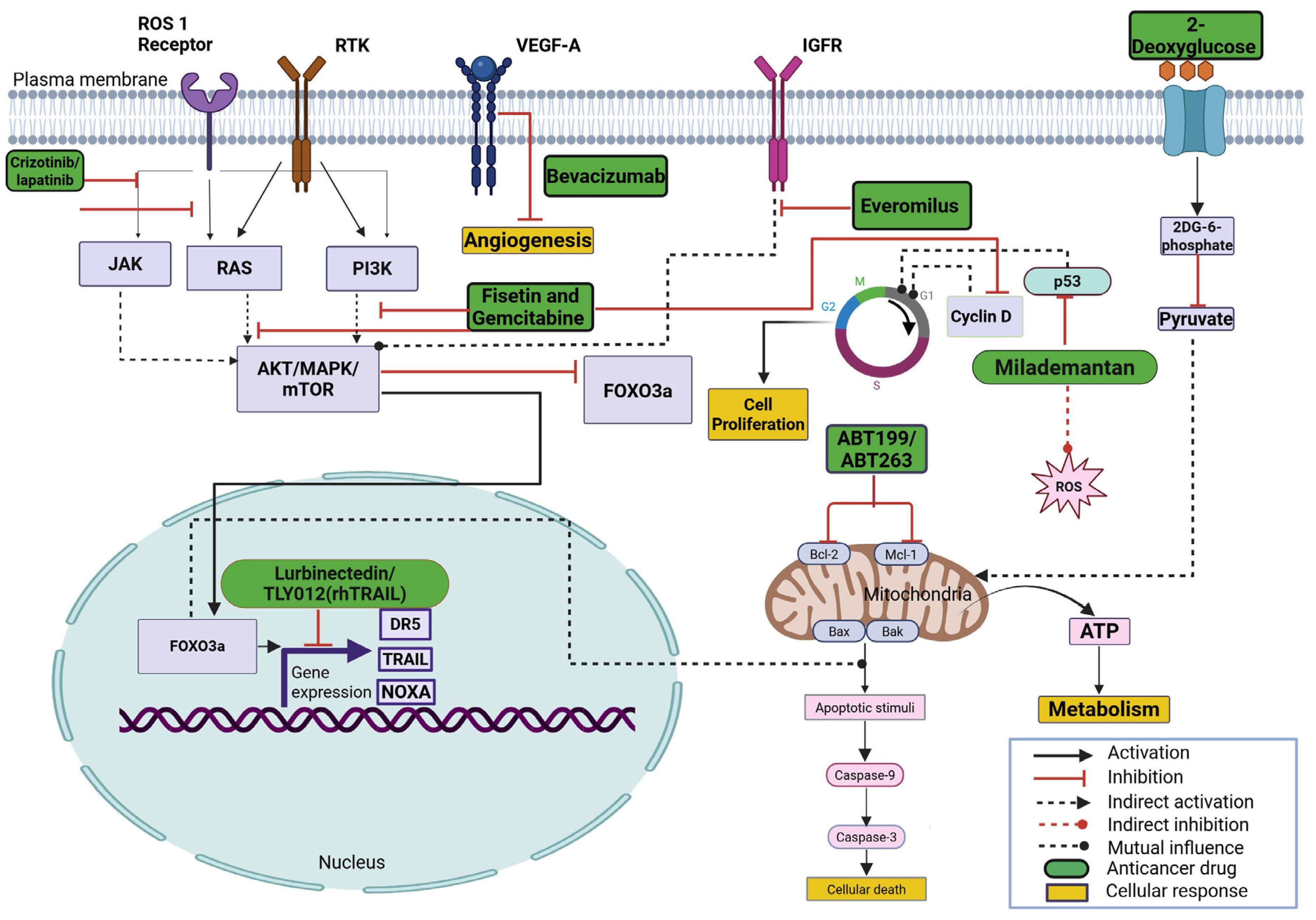
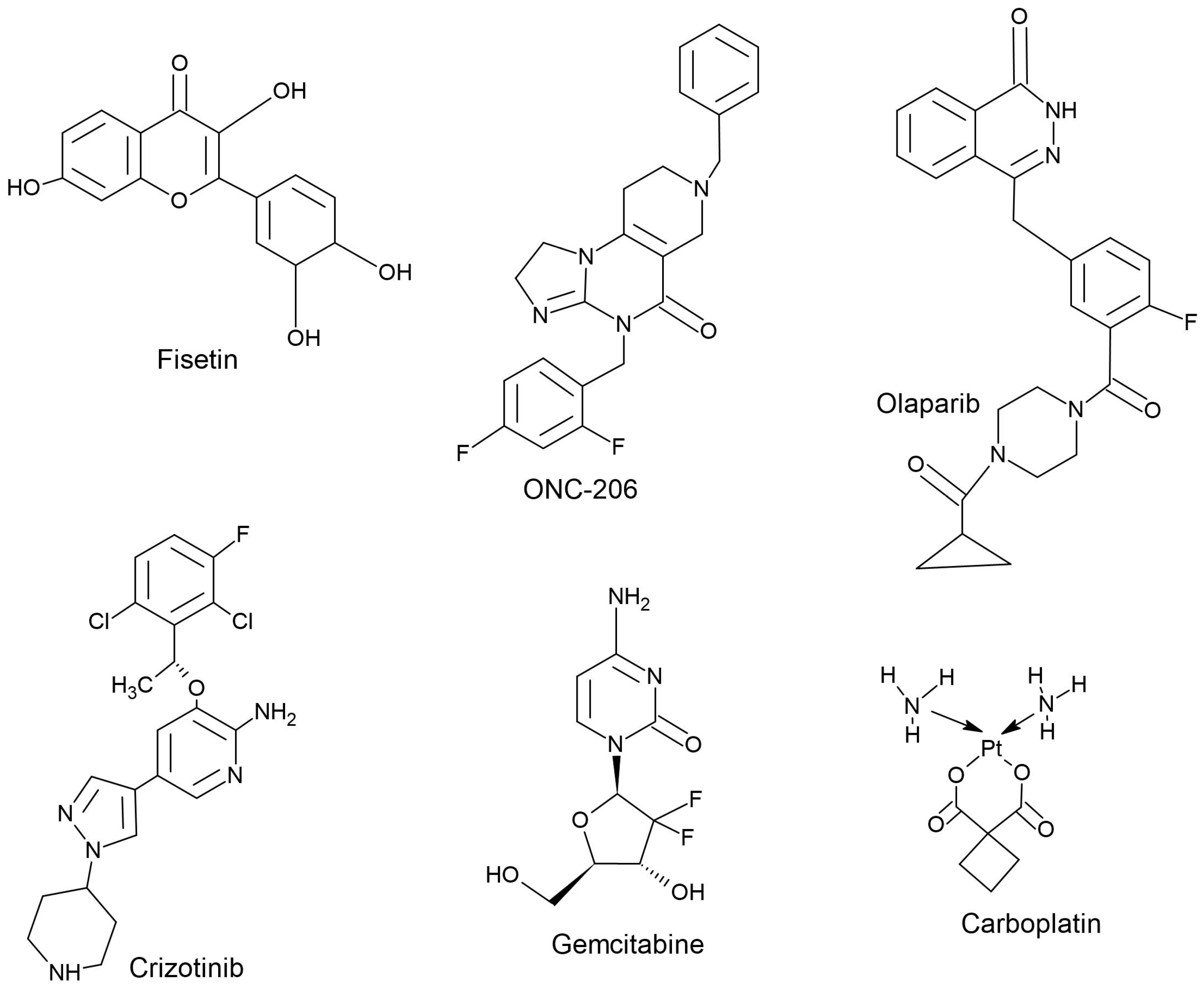
| Tumor Type | Drug Used in Combination with ONC201 | Cell Lines/Xenograft Models Used | References |
|---|---|---|---|
| Colorectal cancer | VEGF inhibitors like bevacizumab (Avastin) with ONC201 | Mice harboring human xenografts of CRC | [128] |
| CRC xenograft and patient-derived xenograft (PDX) | [1] | ||
| Fisetin (an active compound in T. vernicifllum) with ONC201 | HCT116 human colon cancer cell line | [40] | |
| mTOR inhibitors with ONC201 | HT-29, HCT116, and DLD-1 CRC cell lines, chemoresistant CRC xenograft model | [42] | |
| Pancreatic cancer | Lurbinectedin/irinotecan with ONC201/ONC212 | PANC-1, BxPC-3, and HPAF-II cell lines | [55] |
| TLY012 (TRAIL mimetic) | AsPC-1, BxPC3, Capan-1, and PANC-1 cell lines, patient-derived xenograft (PDX) | [52,129] | |
| The IGF-1R inhibitor (insulin-like growth factor) AG1024 with ONC201/ONC212 | AsPC-1, BxPC3, Capan-1, Capan-2, CFPAC-1, PANC-1, HPAF-II | [51] | |
| Crizotinib or lapatinib with ONC201/ONC212 | PANC-1, BxPC3, Capan-2, and HPAF-II cell lines and xenograft model | [51] | |
| Lipid–gemcitabine with ONC201 | MIA-PaCa-2, syngeneic Kras-mutated pancreatic cancer xenograft mouse model | [54] | |
| Glioblastoma (GBM) | BRD4 antagonist with ONC201/ONC206/ONC212 | NCH421k, NCH644, NCH690, SF188 < LN229, U87, T98G, and GBM14 GBM cell lines, patient-derived xenograft models of GBM | [130] |
| Bcl-2 inhibitors | Patient-derived glioblastoma cells, SF188 (pediatric), T98G (adult), and MGPP-3 (murine, transgenically derived) glioblastoma cells | [18] | |
| Temozolomide and radiotherapy with ONC201/ONC206 | SNB19, T98G, U138, U251 glioblastoma cell lines, the SF8628 diffuse intrinsic pontine glioma (DIPG) cell line, orthotopic model of GBM in mice | [131] | |
| Lung cancer | Lurbinectedin with ONC201 | H1048, H1105, H1882, and H1417 SCLC cell lines | [132] |
| Etoposide and carboplatin with ONC201 | H1048 and H1105 SCLC cell lines | [72] | |
| Paclitaxel or docetaxel with ONC201 | H460 human non-small-cell lung cancer xenografts in athymic nude mice | [3] | |
| Endometrial cancer | TRAIL with ONC201 | AN3CA, HEC1A, and KLE endometrial cancer cell lines | [85] |
| The MDM2 inhibitor milademetan with ONC201 | - | [88] | |
| Ovarian cancer | The ATR kinase inhibitor ceralasertib with ONC201 | OVCAR3, KURAMOCHI, TOV21G high-grade serous ovarian cancer cell lines | [97] |
| The PARP inhibitor olaparib or rucaparib with ONC201/ONC212 | HCC1937, PEO1, KURAMOCHI, 22RV1, LNCAP (BRCA-deficient cell lines of breast, ovarian, and prostate cancer) | [111] | |
| Breast cancer (Triple-negative and ER+) | Everolimus with ONC201 | MCF7, T47D ER+ breast cancer cell lines, cell lines derived from patients sensitive or resistant to everolimus | [110] |
| The MEK inhibitor trametinib with ONC201 | BT-20, HCC38, HCC70, HCC1187, HCC1395, HCC1806, HCC1937, MDA-MB-157, MDA-MB-231, MDA-MB-453, and MDA-MB-468 TNBC cell lines | [112] | |
| Prostate cancer | Darolutamide or enzalutamide with ONC201 | 22RV1 and LNCaP cell lines, mouse xenograft models with luciferase expressing the 22RV1 and LNCaP cell lines | [118] |
| The ATR kinase inhibitor ceralasertib with ONC201 | - | [120] | |
| Gastric adenocarcinoma | Recombinant TRAIL (rhTRAIL) with ONC201 | AGS, SNU-1, SNU-5, and SNU-16 cell lines AGS and SNU-1 cells in an organoid model and a mouse subcutaneous xenograft model | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenoy, B.; Mandani, M.; Chintamaneni, M.; Manohar, S.M. Combination of the First-in-Class Imipridone ONC201 and Standard Anticancer Therapies as a Rational Approach for Therapeutic Benefit. Curr. Issues Mol. Biol. 2025, 47, 775. https://doi.org/10.3390/cimb47090775
Shenoy B, Mandani M, Chintamaneni M, Manohar SM. Combination of the First-in-Class Imipridone ONC201 and Standard Anticancer Therapies as a Rational Approach for Therapeutic Benefit. Current Issues in Molecular Biology. 2025; 47(9):775. https://doi.org/10.3390/cimb47090775
Chicago/Turabian StyleShenoy, Brahmi, Miloni Mandani, Meena Chintamaneni, and Sonal M. Manohar. 2025. "Combination of the First-in-Class Imipridone ONC201 and Standard Anticancer Therapies as a Rational Approach for Therapeutic Benefit" Current Issues in Molecular Biology 47, no. 9: 775. https://doi.org/10.3390/cimb47090775
APA StyleShenoy, B., Mandani, M., Chintamaneni, M., & Manohar, S. M. (2025). Combination of the First-in-Class Imipridone ONC201 and Standard Anticancer Therapies as a Rational Approach for Therapeutic Benefit. Current Issues in Molecular Biology, 47(9), 775. https://doi.org/10.3390/cimb47090775






