French Maritime Pine Bark Extract Alleviates Lung Injury by Regulating Inflammatory–Oxidative–Apoptotic Pathway and P2X7 Receptor Expression in LPS-Induced Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Materials
2.2. Animals
2.2.1. Experimental Design and Treatments
2.2.2. Sample Collection and Analysis
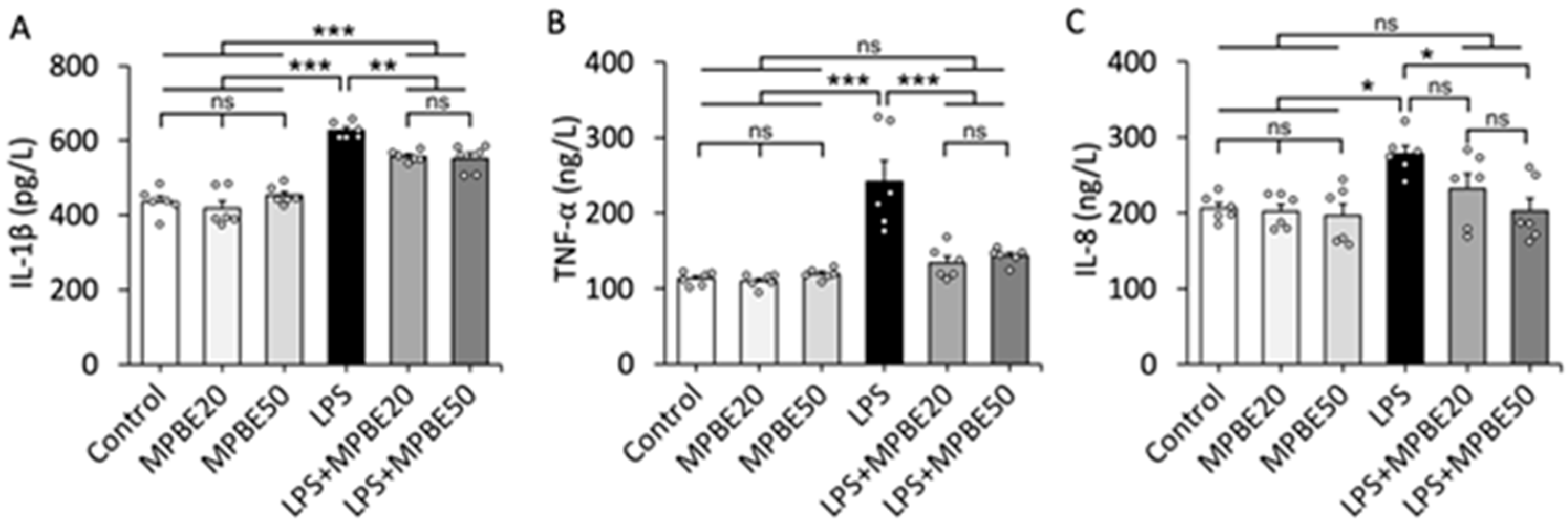
2.2.3. Serum Cytokine Measurements
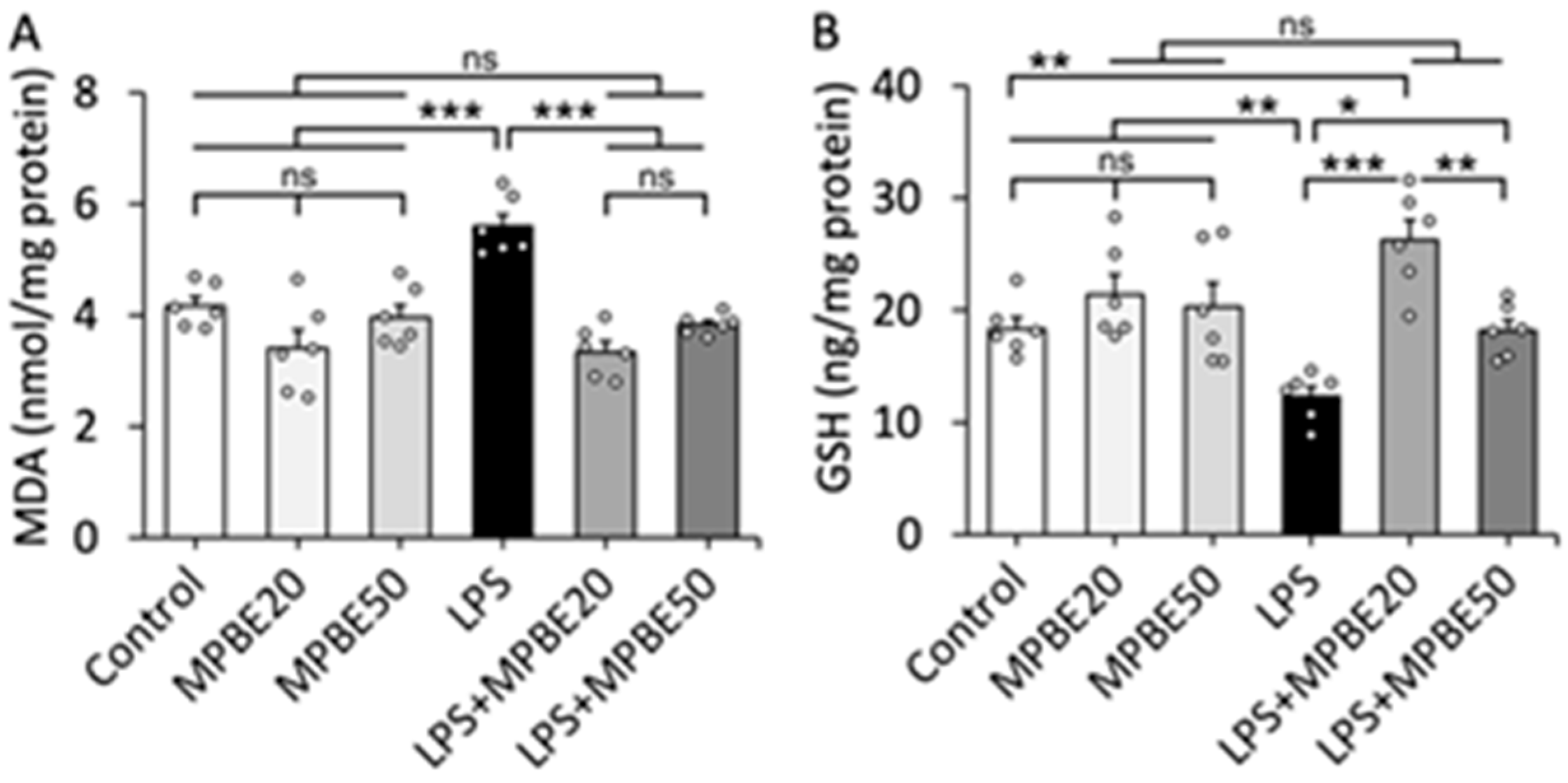
2.2.4. Determination of Oxidative Stress Markers in Lung Tissue
2.2.5. Histopathological Examination
2.2.6. Immunohistochemical Examination
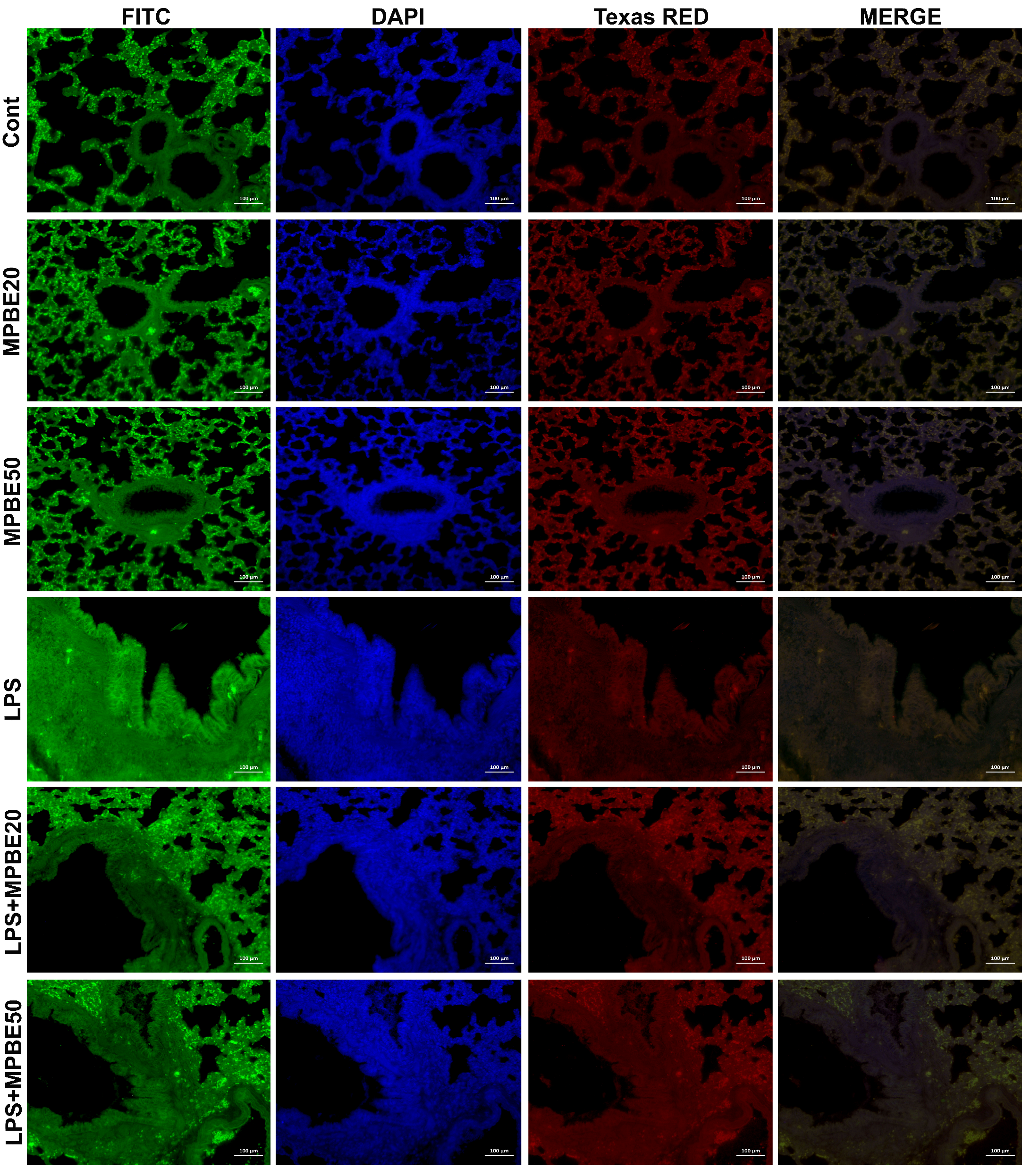
2.2.7. Double Immunofluorescence Examination
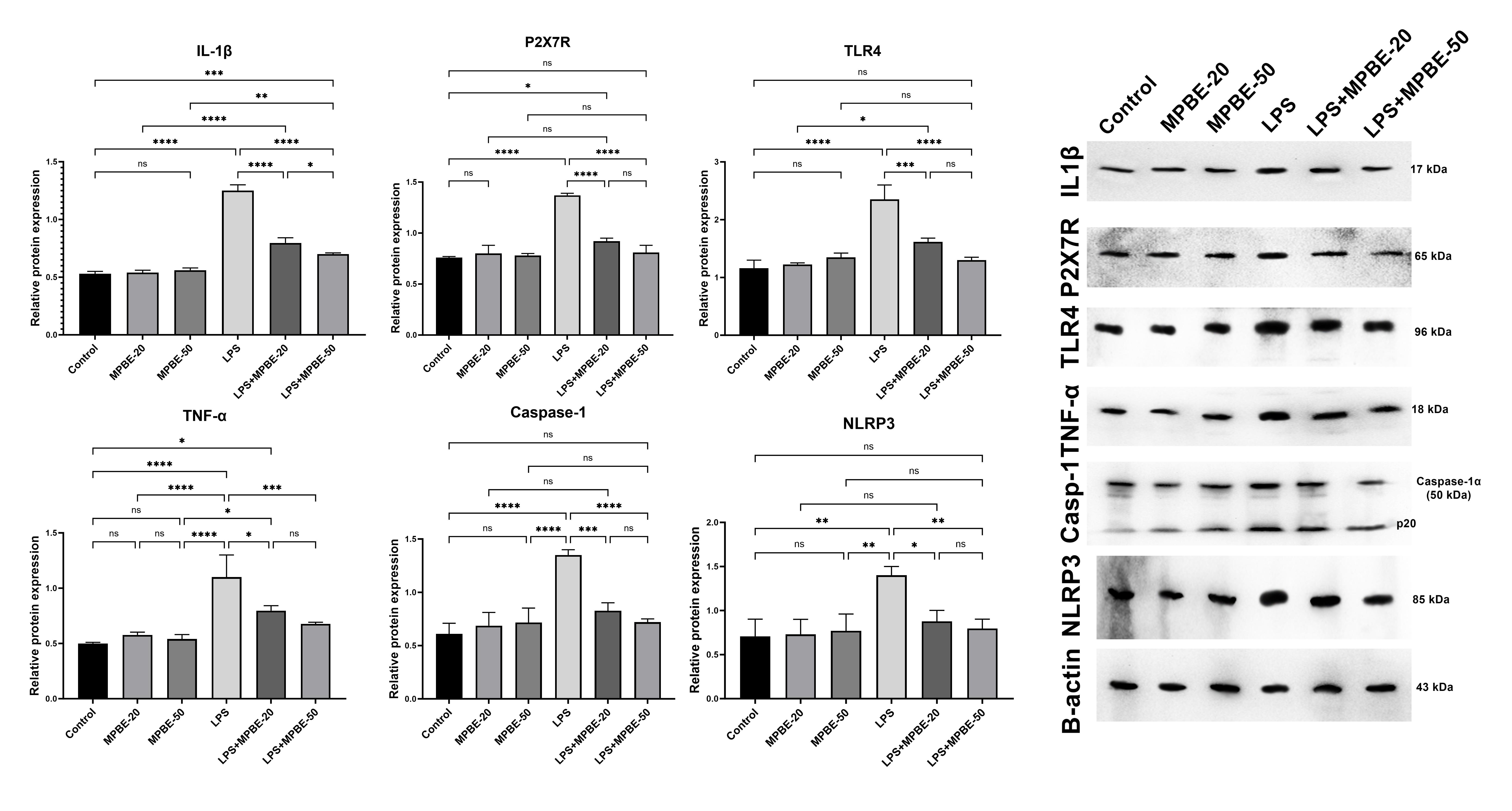
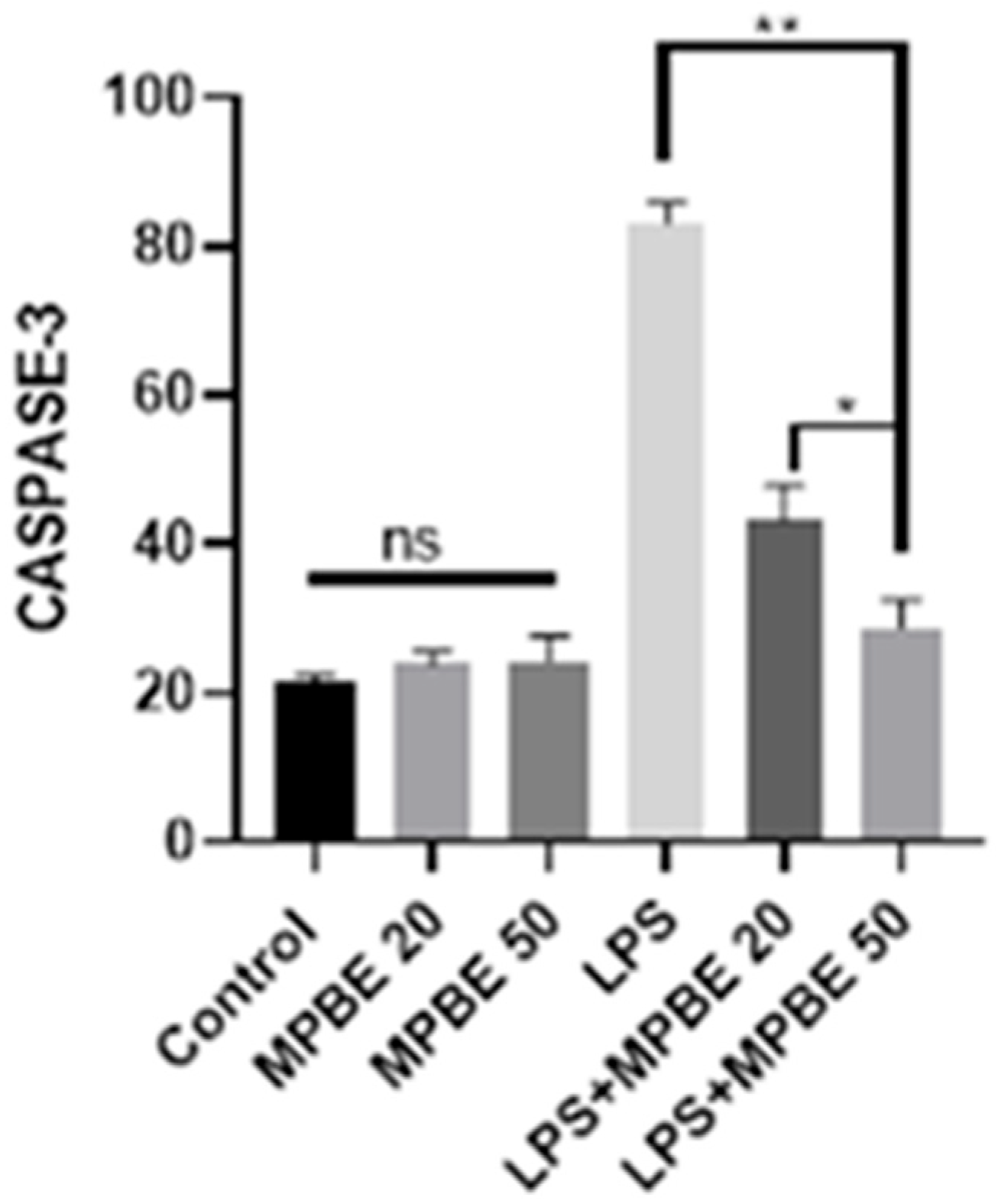
2.2.8. Western Blot Analysis
2.3. Statistical Analysis
3. Results
3.1. LPS Induction Triggers Severe Sepsis by Elevating Cytokine Levels in the Bloodstream
3.2. LPS Induction Causes Severe Oxidative Stress in Lung Tissue That Can Be Prevented by MPBE Administration
3.3. Western Blot Analysis Findings
3.4. Histopathological Findings
3.5. Immunohistochemical Findings
3.6. Immunofluorescence Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| ATP | Adenosine 3′-triphosphate |
| E. coli | Escherichia coli |
| eATP | Extracellular adenosine 3′-triphosphate |
| GSH | Glutathione |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LtxA | Leukotoxin |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MPBE | French maritime pine bark extract |
| NF-κB | Nuclear factor-kappa B |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| PAC | Proanthocyanidins |
| P2X7R | P2X7 receptor |
| TLR | Toll-like receptor |
| TNF-α | Tumor Necrosis Factor-alpha |
| H2AX | Phosphorylated H2A histone family member X |
References
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S. The global burden of sepsis and septic shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, S.; Zhou, S. Advances in sepsis research: Insights into signaling pathways, organ failure, and emerging intervention strategies. Exp. Mol. Pathol. 2025, 142, 104963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, X.; Yao, J.; Yang, C.; Liu, J. Mitophagy and ferroptosis in sepsis-induced ALI/ARDS: Molecular mechanisms, interactions and therapeutic prospects of medicinal plants. J. Inflamm. Res. 2024, 17, 7819–7835. [Google Scholar] [CrossRef]
- Dutta, S.; Dutta, S.; Somanath, P.R.; Narayanan, S.P.; Wang, X.; Zhang, D. Circulating Nucleosomes and Histones in the Development of Lung Injury and Sepsis. Curr. Issues Mol. Biol. 2025, 47, 133. [Google Scholar] [CrossRef]
- Bone, R.C. Gram-negative sepsis: A dilemma of modern medicine. Clin. Microbiol. Rev. 1993, 6, 57–68. [Google Scholar] [CrossRef]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in sepsis-related organ dysfunction: A systematic review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef]
- Reddy, H.; Javvaji, C.K.; Malali, S.; Kumar, S.; Acharya, S.; Toshniwal, S. Navigating the cytokine storm: A comprehensive review of chemokines and cytokines in sepsis. Cureus 2024, 16, e54275. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, J.; Sun, Y.; Wang, X.; Sun, L.; Zhao, F.; Niu, C.; Liu, S. Mechanisms of sepsis-induced acute lung injury and advancements of natural small molecules in its treatment. Pharmaceuticals 2024, 17, 472. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, J.-F.; Illes, P. Purinergic signalling—A perspective from China. Purinergic Signal. 2023, 19, 1–3. [Google Scholar] [CrossRef]
- Savio, L.; De Andrade, M.; Da Silva, C.; Coutinho-Silva, R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016, 12, 59–67. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling: Therapeutic developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Perfettini, J.-L.; Persechini, P.M.; Dautry-Varsat, A.; Ojcius, D.M. Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am. J. Physiol. Cell Physiol. 2001, 280, C81–C89. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Silva, R.; Persechini, P.M.; Bisaggio, R.D.C.; Perfettini, J.-L.; Neto, A.C.T.D.S.; Kanellopoulos, J.M.; Motta-Ly, I.; Dautry-Varsat, A.; Ojcius, D.M. P2Z/P2X7receptor-dependent apoptosis of dendritic cells. Am. J. Physiol. Cell Physiol. 1999, 276, C1139–C1147. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered cytokine production in mice lacking P2X7Receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Ozkanlar, S.; Ulas, N.; Kaynar, O.; Satici, E. P2X7 receptor antagonist A-438079 alleviates oxidative stress of lung in LPS-induced septic rats. Purinergic Signal. 2023, 19, 699–707. [Google Scholar] [CrossRef]
- Danielski, L.G.; Giustina, A.D.; Bonfante, S.; Barichello, T.; Petronilho, F. The NLRP3 inflammasome and its role in sepsis development. Inflammation 2020, 43, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef]
- Enseleit, F.; Sudano, I.; Periat, D.; Winnik, S.; Wolfrum, M.; Flammer, A.J.; Fröhlich, G.M.; Kaiser, P.; Hirt, A.; Haile, S.R. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: A double-blind, randomized, placebo-controlled, cross-over study. Eur. Heart J. 2012, 33, 1589–1597. [Google Scholar] [CrossRef]
- Devaraj, S.; Vega-López, S.; Kaul, N.; Schönlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef]
- Ryan, J.; Croft, K.; Mori, T.; Wesnes, K.; Spong, J.; Downey, L.; Kure, C.; Lloyd, J.; Stough, C. An examination of the effects of the antioxidant Pycnogenol® on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J. Psychopharmacol. 2008, 22, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Canali, R.; Comitato, R.; Schonlau, F.; Virgili, F. The anti-inflammatory pharmacology of Pycnogenol® in humans involves COX-2 and 5-LOX mRNA expression in leukocytes. Int. Immunopharmacol. 2009, 9, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Chovanová, Z.; Muchová, J.; Sumegová, K.; Liptáková, A.; Ďuračková, Z.; Högger, P. Inhibition of NF-κB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J. Inflamm. 2006, 3, 1. [Google Scholar] [CrossRef]
- Gandin, V.; Nyström, C.; Rundlöf, A.K.; Jönsson-Videsäter, K.; Schönlau, F.; Hörkkö, J.; Björnstedt, M.; Fernandes, A.P. Effects of the antioxidant Pycnogenol® on cellular redox systems in U1285 human lung carcinoma cells. FEBS J. 2009, 276, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Torras, M.A.C.; Faura, C.A.; Schönlau, F.; Rohdewald, P. Antimicrobial activity of Pycnogenol. Phytother. Res. 2005, 19, 647–648. [Google Scholar] [CrossRef]
- Shin, N.-R.; Ryu, H.-W.; Ko, J.-W.; Park, J.-W.; Kwon, O.-K.; Oh, S.-R.; Kim, J.-C.; Shin, I.-S.; Ahn, K.-S. A standardized bark extract of Pinus pinaster Aiton (Pycnogenol®) attenuated chronic obstructive pulmonary disease via Erk-sp1 signaling pathway. J. Ethnopharmacol. 2016, 194, 412–420. [Google Scholar] [CrossRef]
- Wilson, D.; Evans, M.; Guthrie, N.; Sharma, P.; Baisley, J.; Schonlau, F.; Burki, C. A randomized, double-blind, placebo-controlled exploratory study to evaluate the potential of pycnogenol® for improving allergic rhinitis symptoms. Phytother. Res. 2010, 24, 1115–1119. [Google Scholar] [CrossRef]
- Taner, G.; Aydın, S.; Bacanlı, M.; Sarıgöl, Z.; Şahin, T.; Başaran, A.A.; Başaran, N. Modulating effects of pycnogenol® on oxidative stress and DNA damage induced by sepsis in rats. Phytother. Res. 2014, 28, 1692–1700. [Google Scholar] [CrossRef]
- Yang, Y.S.; Ahn, T.H.; Lee, J.C.; Moon, C.J.; Kim, S.H.; Jun, W.; Park, S.C.; Kim, H.C.; Kim, J.C. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 2008, 46, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Bukumirović, N.; Paut Kusturica, M.; Milić, N.; Čabarkapa, V.; Borišev, I.; Čapo, I.; Miljković, D.; Stilinović, N.; Mikov, M. Hepatoprotective and antioxidant potential of Pycnogenol® in acetaminophen-induced hepatotoxicity in rats. Phytother. Res. 2019, 33, 631–639. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Lu, D.; Qi, N.; Liu, Q. The defensive action of LYRM03 on LPS-induced acute lung injury by NF-κB/TLR4/NLRP3 signals. J. Investig. Surg. 2021, 34, 284–296. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, W.; Dong, Y.; Luo, X.; Miao, H.; Maimaijuma, T.; Xu, X.; Jiang, H.; Huang, Z.; Qi, L. Early identification and diagnosis, pathophysiology, and treatment of sepsis-related acute lung injury: A narrative review. J. Thorac. Dis. 2024, 16, 5457. [Google Scholar] [CrossRef]
- Kardaş, S.; Çınaroğlu, O.S.; Bora, E.S.; Erbaş, O. Gallic Acid Protects from Sepsis-Induced Acute Lung Injury. Curr. Issues Mol. Biol. 2023, 46, 1–10. [Google Scholar] [CrossRef]
- Yimam, M.; Horm, T.; O’Neal, A.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q.; Lin, M.; Gauthier, A.; Wu, J.; et al. A Standardized Botanical Composition Mitigated Acute Inflammatory Lung Injury and Reduced Mortality through Extracellular HMGB1 Reduction. Molecules 2023, 28, 6560. [Google Scholar] [CrossRef]
- Cao, C.; Yin, C.; Shou, S.; Wang, J.; Yu, L.; Li, X.; Chai, Y. Ulinastatin protects against LPS-induced acute lung injury by attenuating TLR4/NF-κB pathway activation and reducing inflammatory mediators. Shock 2018, 50, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Su, B.-H.; Cheng, W.-H.; Zou, C.-T.; Yeh, E.T.; Yang, F.-M. CYLD links the TRAF6/sNASP axis to TLR4 signaling in sepsis-induced acute lung injury. Cell. Mol. Life Sci. 2025, 82, 124. [Google Scholar] [CrossRef] [PubMed]
- Can, I.; Guraslan, A.; Baser, O.F.; Yildiz, G.N.; Toplaoglu, I.; Aksak Karamese, S.; Karamese, M. The Protective Effects of a Single Dose Myricetin Application on CLP-Induced Rat Sepsis Model by Analyzing Some Immune Mechanisms. Immunopharmacol. Immunotoxicol. 2025, 47, 305–316. [Google Scholar] [CrossRef]
- Martínez-García, J.J.; Martínez-Banaclocha, H.; Angosto-Bazarra, D.; de Torre-Minguela, C.; Baroja-Mazo, A.; Alarcón-Vila, C.; Martínez-Alarcón, L.; Amores-Iniesta, J.; Martín-Sánchez, F.; Ercole, G.A. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat. Commun. 2019, 10, 2711. [Google Scholar] [CrossRef]
- Torrico, S.; Hotter, G.; Muñoz, Á.; Calle, P.; García, M.; Poch, E.; Játiva, S. PBMC therapy reduces cell death and tissue fibrosis after acute kidney injury by modulating the pattern of monocyte/macrophage survival in tissue. Biomed. Pharmacother. 2024, 178, 117186. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, S.; Li, Z.; Zeng, J.; Zeng, H. Hypercapnia promotes NLRP3 inflammasome activation in microglia by activating P2X7R after lipopolysaccharide-induced activation of the TLR4/NF-κB signaling pathway. Cytokine 2025, 185, 156806. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Fang, S.-H.; Wang, S.-C.; Cheng, W.-C.; Liu, P.-L.; Su, C.-C.; Chen, C.-S.; Huang, M.-Y.; Hua, K.-F.; Shen, K.-H. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef]
- Krishnadasan, B.; Naidu, B.V.; Byrne, K.; Fraga, C.; Verrier, E.D.; Mulligan, M.S. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2003, 125, 261–272. [Google Scholar] [CrossRef]
- Ulas, N.; Ozkanlar, Y.; Ozkanlar, S.; Timurkan, M.O.; Aydin, H. Clinical and inflammatory response to antiviral treatments in dogs with parvoviral enteritis. J. Vet. Sci. 2024, 25, e11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Su, Y.; Ding, J.; He, J.; Lai, L.; Song, Y. Lobetyolin protects mice against LPS-induced sepsis by downregulating the production of inflammatory cytokines in macrophage. Front. Pharmacol. 2024, 15, 1405163. [Google Scholar] [CrossRef] [PubMed]
- Ulaş, N.; Üstündağ, H.; Özkanlar, S.; Erbaş, E.; Kara, A.; Özkanlar, Y. D-carvone attenuates LPS-induced acute lung injury via TLR4/NF-κB and Nrf2/HO-1 signaling pathways in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 9, 12215–12225. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Xu, Z.; Deng, X. Pycnogenol, a compound isolated from the bark of pinus maritime mill, attenuates ventilator-induced lung injury through inhibiting NF-κB-mediated inflammatory response. Int. J. Clin. Exp. Med. 2015, 8, 1824–1833. [Google Scholar]
- Montini, L.; Sole, P.D.; Pennisi, M.A.; Rossi, C.; Scatena, R.; Pascale, G.D.; Bello, G.; Cutuli, S.L.; Antonelli, M. Prognostic value of the reactive oxygen species in severe sepsis and septic shock patients: A pilot study. Minerva Anestesiol. 2016, 82, 1306–1313. [Google Scholar]
- Quoilin, C.; Mouithys-Mickalad, A.; Lécart, S.; Fontaine-Aupart, M.-P.; Hoebeke, M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim. Biophys. Acta 2014, 1837, 1790–1800. [Google Scholar] [CrossRef]
- Ou, Y.; An, R.; Wang, H.; Chen, L.; Shen, Y.; Cai, W.; Zhu, W. Oxidative stress-related circulating miRNA-27a is a potential biomarker for diagnosis and prognosis in patients with sepsis. BMC Immunol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dai, X.; Li, X.; Jiang, H. Antioxidant and anti-inflammatory effects of Rhamnazin on lipopolysaccharide-induced acute lung injury and inflammation in rats. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 201–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parveen, K.; Khan, M.R.; Mujeeb, M.; Siddiqui, W.A. Protective effects of Pycnogenol on hyperglycemia-induced oxidative damage in the liver of type 2 diabetic rats. Chem. Biol. Interact. 2010, 186, 219–227. [Google Scholar] [CrossRef]
- Bockstiegel, J.; Engelhardt, J.; Weindl, G. P2X7 receptor activation leads to NLRP3-independent IL-1β release by human macrophages. Cell Commun. Signal. 2023, 21, 335. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Howell, A.; Grenier, D. Cranberry Proanthocyanidins Neutralize the Effects of Aggregatibacter actinomycetemcomitans Leukotoxin. Toxins 2019, 11, 662. [Google Scholar] [CrossRef]
- Kim, G.O.; Park, D.H.; Bae, J.S. Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 7930. [Google Scholar] [CrossRef]
- Lai, K.; Song, C.; Gao, M.; Deng, Y.; Lu, Z.; Li, N.; Geng, Q. Uridine Alleviates Sepsis-Induced Acute Lung Injury by Inhibiting Ferroptosis of Macrophage. Int. J. Mol. Sci. 2023, 24, 5093. [Google Scholar] [CrossRef] [PubMed]





| Primary Antibody | Manufacturer | Dilution |
|---|---|---|
| Caspase-1 | Affinity Biotechnology, AF548, Jiangsu, China | 1/1000 |
| IL-1β | Affinity Biotechnology, AF5103, Jiangsu, China | 1/1000 |
| P2X7R | Santa Cruz, Sc-514962 | 1/1000 |
| TLR4 | Santa Cruz, Sc-293072 | 1/1000 |
| NLRP3/Cryopyrin | Santa Cruz, Sc-134306 | 1/1000 |
| TNF-α | Santa Cruz, Sc-52746 | 1/1000 |
| Beta actin | Santa Cruz, Sc-130065 | 1/1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, N.; Ozkanlar, S.; Yildirim, S.; Aydin, O.; Ozkanlar, Y. French Maritime Pine Bark Extract Alleviates Lung Injury by Regulating Inflammatory–Oxidative–Apoptotic Pathway and P2X7 Receptor Expression in LPS-Induced Sepsis. Curr. Issues Mol. Biol. 2025, 47, 770. https://doi.org/10.3390/cimb47090770
Ulas N, Ozkanlar S, Yildirim S, Aydin O, Ozkanlar Y. French Maritime Pine Bark Extract Alleviates Lung Injury by Regulating Inflammatory–Oxidative–Apoptotic Pathway and P2X7 Receptor Expression in LPS-Induced Sepsis. Current Issues in Molecular Biology. 2025; 47(9):770. https://doi.org/10.3390/cimb47090770
Chicago/Turabian StyleUlas, Nergis, Seckin Ozkanlar, Serkan Yildirim, Omer Aydin, and Yunusemre Ozkanlar. 2025. "French Maritime Pine Bark Extract Alleviates Lung Injury by Regulating Inflammatory–Oxidative–Apoptotic Pathway and P2X7 Receptor Expression in LPS-Induced Sepsis" Current Issues in Molecular Biology 47, no. 9: 770. https://doi.org/10.3390/cimb47090770
APA StyleUlas, N., Ozkanlar, S., Yildirim, S., Aydin, O., & Ozkanlar, Y. (2025). French Maritime Pine Bark Extract Alleviates Lung Injury by Regulating Inflammatory–Oxidative–Apoptotic Pathway and P2X7 Receptor Expression in LPS-Induced Sepsis. Current Issues in Molecular Biology, 47(9), 770. https://doi.org/10.3390/cimb47090770






