IDO Activation Affects BDNF/TrkB Signaling Pathway, Oxidative Stress, and Mitochondrial Enzymatic Activities in Temporal Lobe Epilepsy
Abstract
1. Introduction
2. Materials and Methods
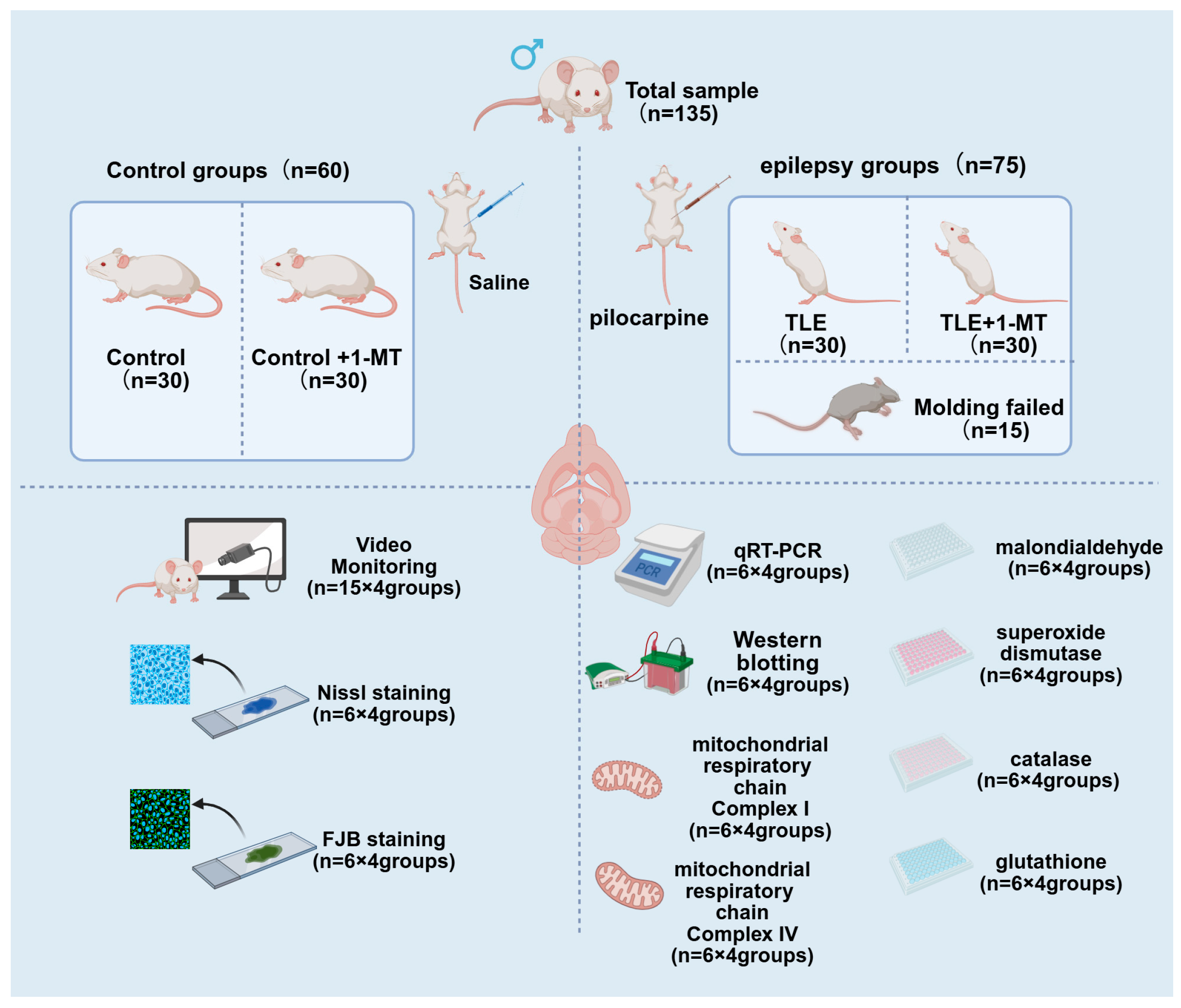
2.1. Experimental Animals
2.2. Tissue Processing
2.3. Video Monitoring
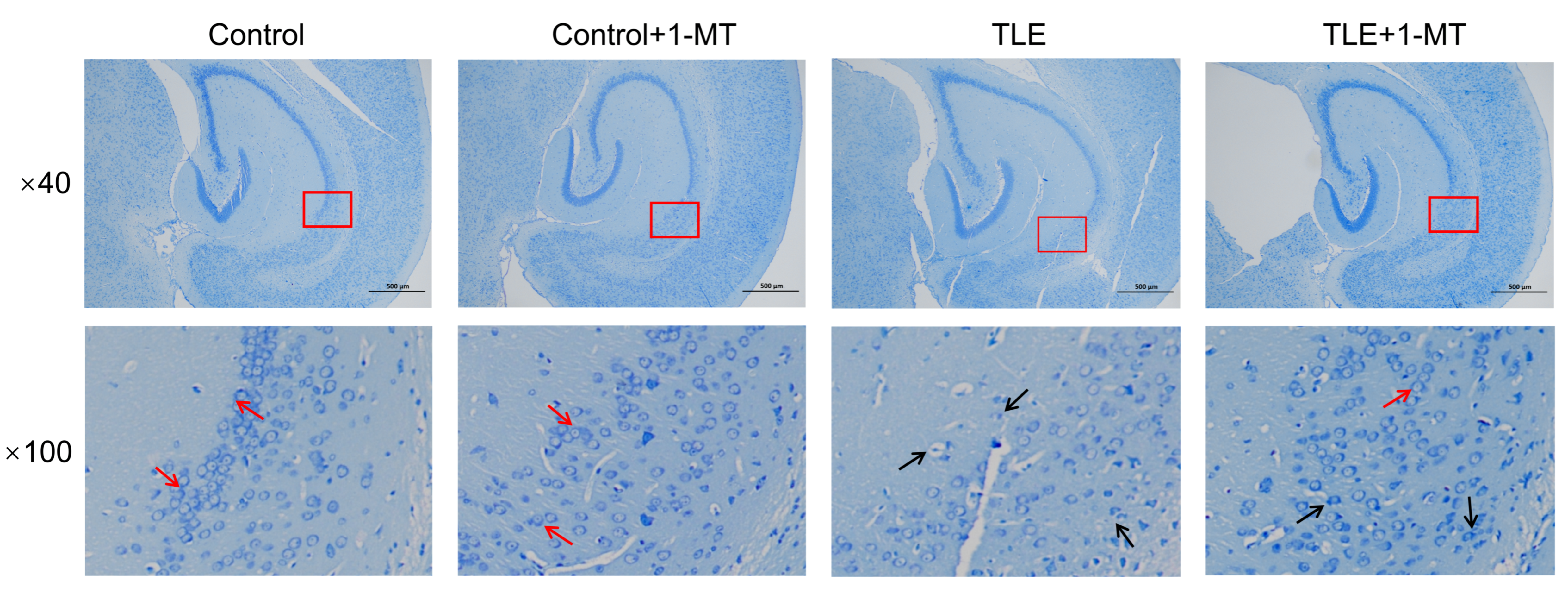
2.4. Nissl Staining
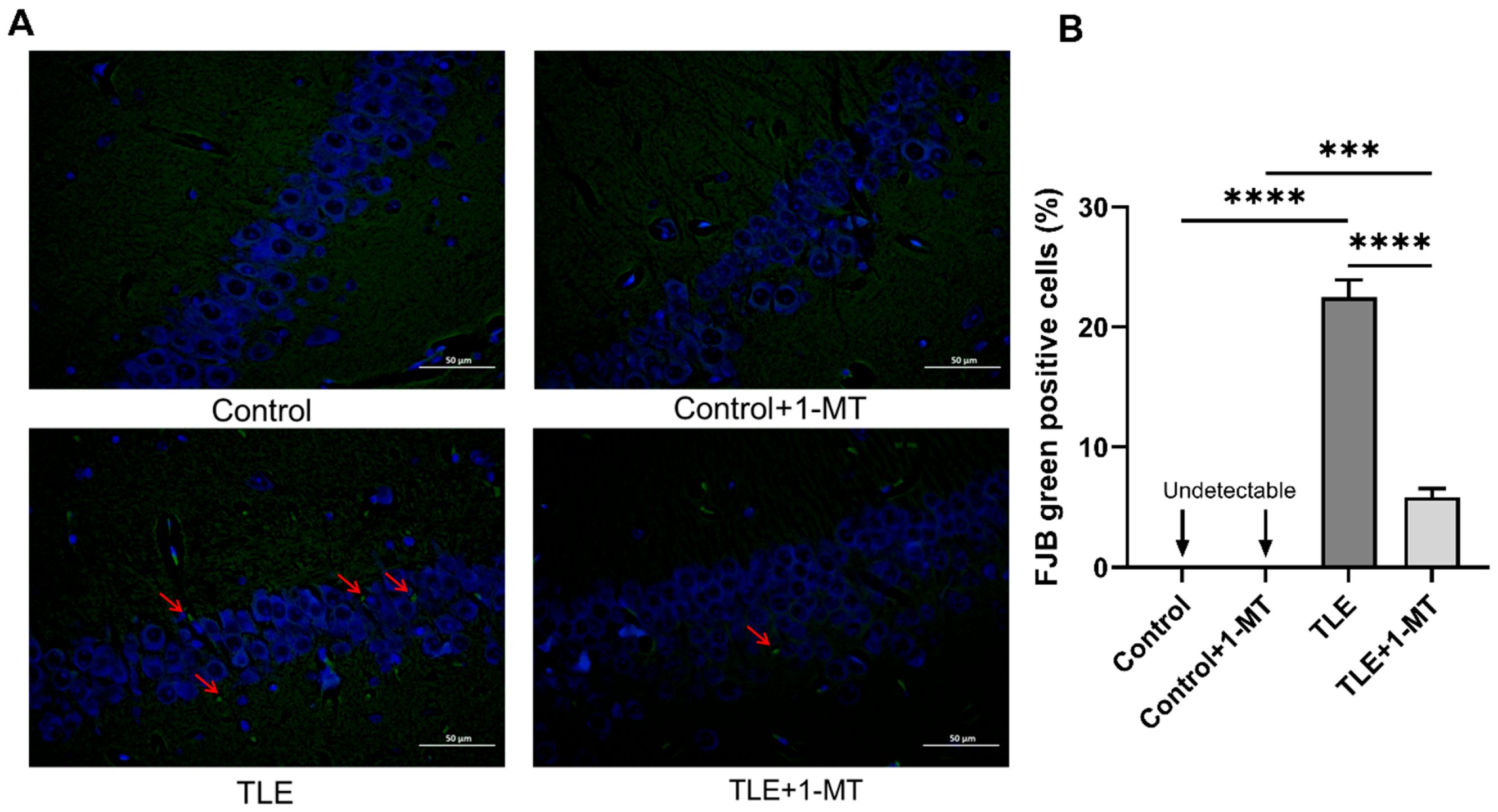
2.5. FJB Staining
2.6. qRT-PCR Analysis
2.7. Western Blot Assay
2.8. Determination of SOD, MDA, CAT, and GSH Levels
2.9. Determination of Mitochondrial Respiratory Chain Complex I and IV Activities
2.10. Statistical Analysis
3. Results
3.1. Effect of IDO Activation on Seizure Behavior in Mice
3.2. IDO Activation Leads to Neuronal Damage
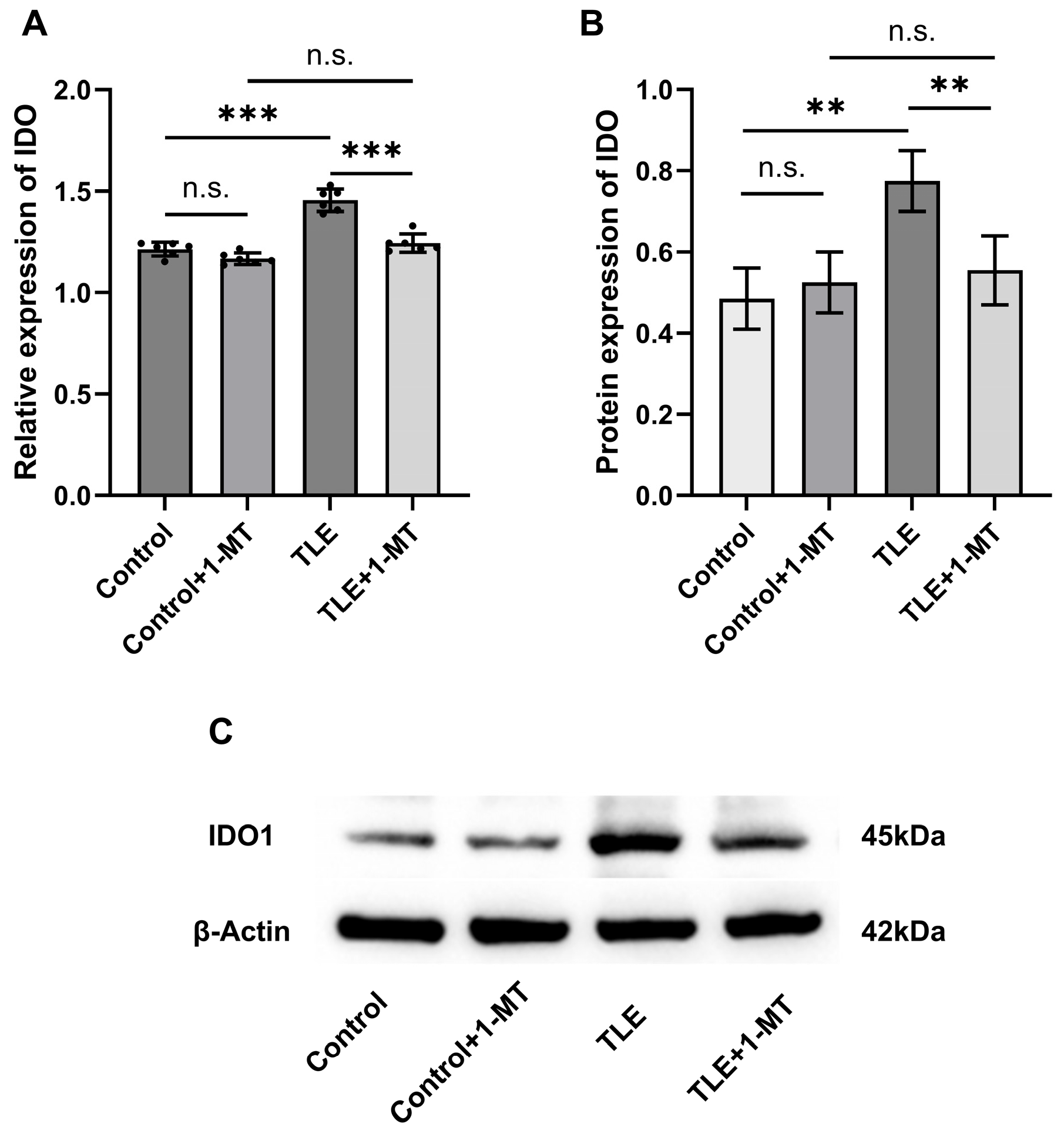
3.3. Expression Changes in IDO in the Hippocampus of TLE Model Mice
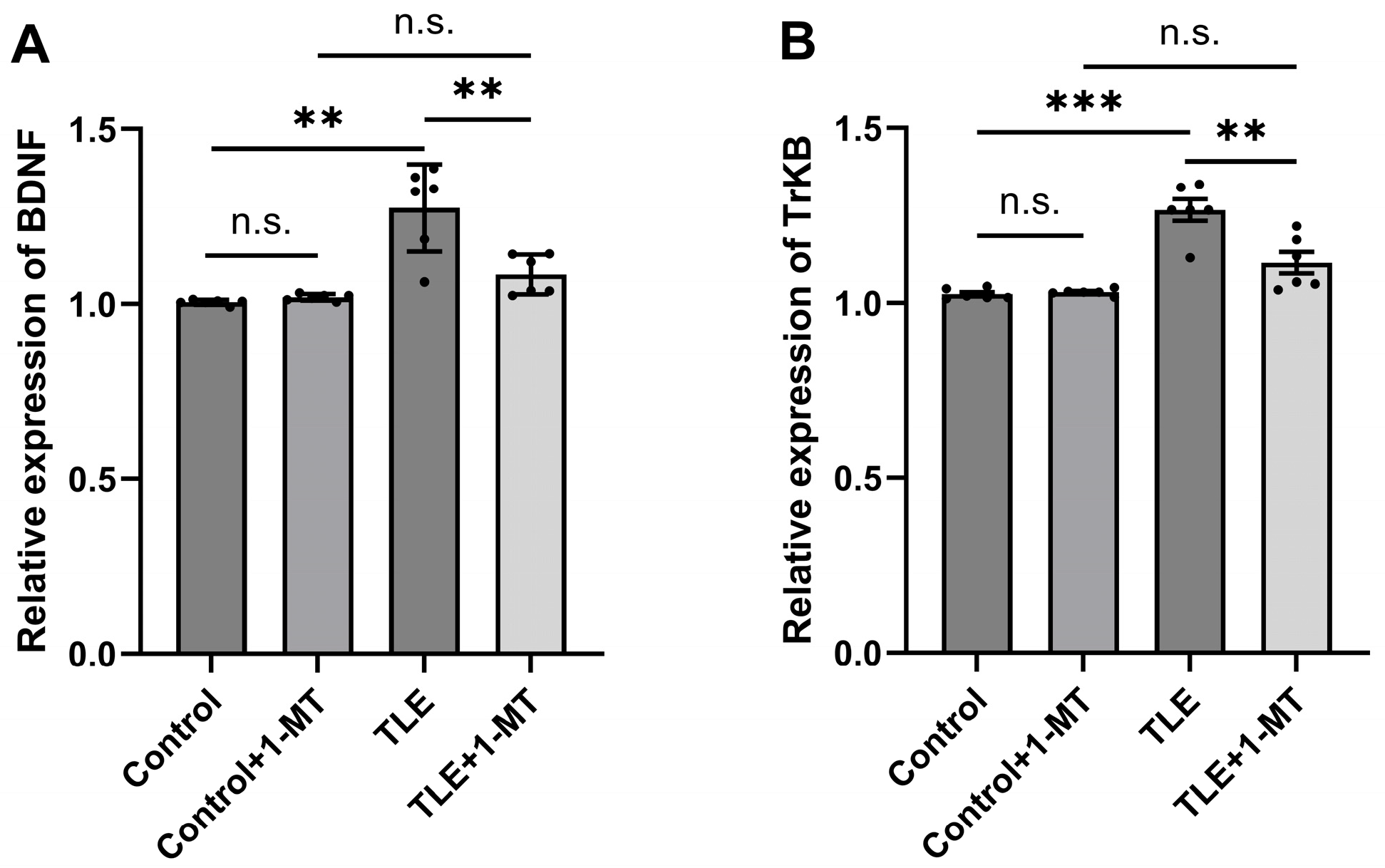
3.4. Overexpression of the BDNF/TrkB Pathway in the Hippocampus of TLE Model Mice Is Associated with IDO Activation
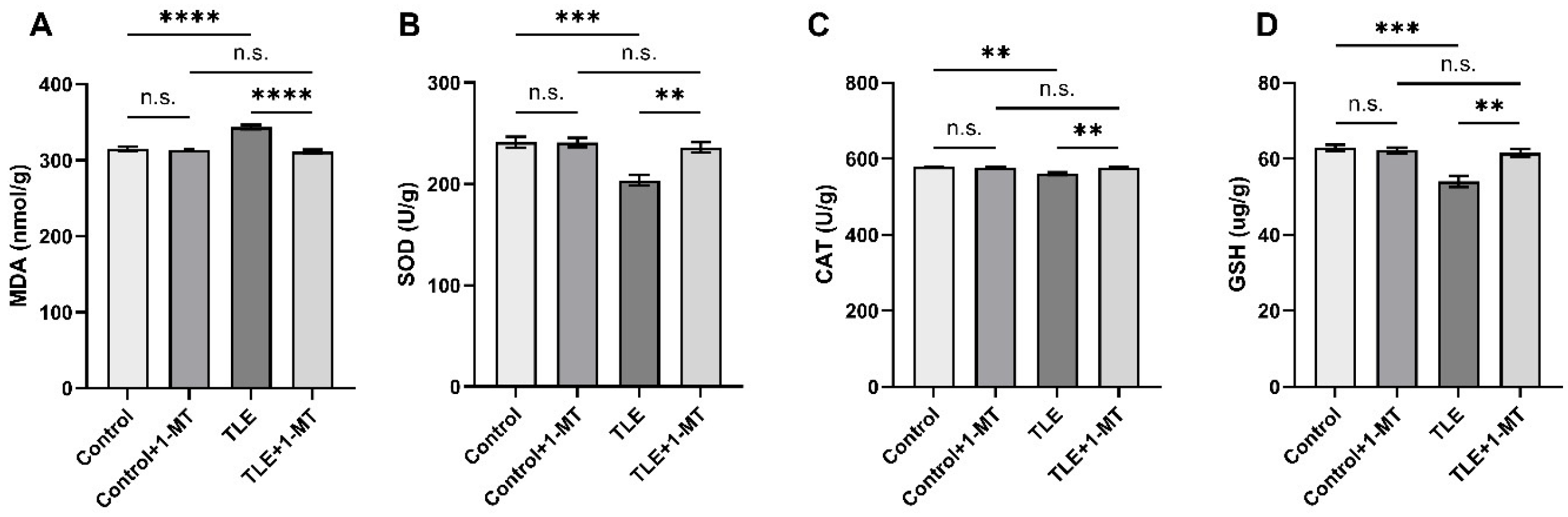
3.5. IDO Activation Induces Oxidative Stress Injury in TLE Model Mice
3.6. Impairment of Mitochondrial Complex Activities Induced by IDO Activation
4. Discussion
4.1. Main Findings
4.2. Comparisons with Previous Studies
4.3. Possible Mechanisms
4.4. Clinical Implications
4.5. Limitations
4.6. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IDO | Indoleamine 2,3-Dioxygenase |
| BDNF | Brain-Derived Neurotrophic Factor |
| TrkB | Tyrosine Kinase B |
| TLE | Temporal Lobe Epilepsy |
| 1-MT | 1-Methyl-DL-Tryptophan |
| ARRIVE SE | Animal Research: Reporting of In Vivo Experiments Status Epilepticus |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| cDNA RIPA NMDA | complementary DNA Radio-Immunoprecipitation Assay N-methyl-D-aspartate |
| FJB | Fluoro-Jade B |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| PVDF | Polyvinylidene Fluoride |
| TBST | Tris-Buffered Saline with Tween 20 |
| HRP | Horseradish Peroxidase |
| ECL | Enhanced Chemiluminescence |
| SPF | Specific Pathogen-Free |
| ANOVA | Analysis of Variance |
| NMDA CA1 | N-Methyl-D-Aspartate Cornu Ammonis 1 |
| KYN TRP 5-HT | Kynurenine Tryptophan 5-Hydroxytryptamine |
| QUIN | Quinolinic Acid |
| GABA 3-HK | Gamma-Aminobutyric Acid 3-hydroxykynurenine |
| CNS IL-6 | Central Nervous System Interleukin-6 |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor Necrosis Factor-α |
| CaMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| CREB mtDNA | cAMP Response Element-Binding Protein mitochondrial DNA |
| NF-κB | Nuclear Factor-Kappa B |
| KM | Kunming (mouse strain) |
| ATP | Adenosine Triphosphate |
| ROS nROS | Reactive Oxygen Species neuronal Nitric Oxide Synthase |
| PBS | Phosphate-Buffered Saline |
References
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Kremen, V.; Sladky, V.; Mivalt, F.; Gregg, N.M.; Brinkmann, B.H.; Balzekas, I.; Marks, V.; Kucewicz, M.; Lundstrom, B.N.; Cui, J.; et al. Modulating limbic circuits in temporal lobe epilepsy: Impacts on seizures, memory, mood and sleep. Brain Commun. 2025, 7, fcaf106. [Google Scholar] [CrossRef]
- Zhvania, M.G.; Sharikadze, I.; Japaridze, N.; Tizabi, Y.; Rzayev, F.; Gasimov, E.; Lobzhanidze, G. Status epilepticus alters hippocampal ultrastructure in kainic acid rat model. Tissue Cell 2025, 94, 102789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, H.; Klein, P.M.; Jensen, F.E. Neonatal seizures alter NMDA glutamate receptor GluN2A and 3A subunit expression and function in hippocampal CA1 neurons. Front. Cell. Neurosci. 2015, 9, 362. [Google Scholar] [CrossRef]
- Ziaei, M.; Arnold, C.; Thompson, K.; Reutens, D.C. Social Cognition in Temporal and Frontal Lobe Epilepsy: Systematic Review, Meta-analysis, and Clinical Recommendations. J. Int. Neuropsychol. Soc. 2023, 29, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Sun, H.; Dong, Z.; Yi, T.; Sander, J.W.; Zhou, D.; Li, J. Prevalence, clinical characteristics, and risk factors for psychosis in people with epilepsy: A multicenter retrospective cohort study. Epilepsia 2025, 66, 2904–2915. [Google Scholar] [CrossRef]
- Cappelletto, P.; Accolla, C.; Preti, M.; Pisano, T.; Barba, C.; Guerrini, R. Psychiatric disorders in children and adolescents with temporal lobe epilepsy: A narrative review. Epilepsia Open 2025, 10, 74–84. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Z.; Yang, Y.; Zhang, S.; Zheng, R.; Li, X.; Qiu, X.; Zheng, Y.; Wang, S.; Liu, W.; et al. Prediction of Pharmacoresistance in Drug-Naïve Temporal Lobe Epilepsy Using Ictal EEGs Based on Convolutional Neural Network. Neurosci. Bull. 2025, 41, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Kyriatzis, G.; Bernard, A.; Bôle, A.; Khrestchatisky, M.; Ferhat, L. In the Rat Hippocampus, Pilocarpine-Induced Status Epilepticus Is Associated with Reactive Glia and Concomitant Increased Expression of CD31, PDGFRβ, and Collagen IV in Endothelial Cells and Pericytes of the Blood-Brain Barrier. Int. J. Mol. Sci. 2024, 25, 1693. [Google Scholar] [CrossRef]
- Zawadzka, M.; Pietruszka, M.; Krygier, M.; Mazurkiewicz-Bełdzińska, M. Role of neuroinflammation factors as potential biomarkers of epilepsy: A narrative review. Neurol. Neurochir. Pol. 2025, 59, 210–220. [Google Scholar] [CrossRef]
- Yang, H.; Yin, F.; Gan, S.; Pan, Z.; Xiao, T.; Kessi, M.; Yang, Z.; Zhang, V.W.; Wu, L. The Study of Genetic Susceptibility and Mitochondrial Dysfunction in Mesial Temporal Lobe Epilepsy. Mol. Neurobiol. 2020, 57, 3920–3930. [Google Scholar] [CrossRef]
- Yao, C.; Xie, D.; Zhang, Y.; Shen, Y.; Sun, P.; Ma, Z.; Li, J.; Tao, J.; Fang, M. Tryptophan metabolism and ischemic stroke: An intricate balance. Neural Regen. Res. 2026, 21, 466–477. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1–20. [Google Scholar] [CrossRef]
- Tan, Q.; Deng, S.; Xiong, L. Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. Int. J. Mol. Sci. 2025, 26, 968. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J. Neurochem. 2025, 169, e70075. [Google Scholar] [CrossRef] [PubMed]
- Sezen, S.; Karadayi, M.; Yesilyurt, F.; Burul, F.; Gulsahin, Y.; Ozkaraca, M.; Okkay, U.; Gulluce, M. Acyclovir provides protection against 6-OHDA-induced neurotoxicity in SH-SY5Y cells through the kynurenine pathway. Neurotoxicology 2025, 106, 1–9. [Google Scholar] [CrossRef]
- Qiao, C.-M.; Ma, X.-Y.; Tan, L.-L.; Xia, Y.-M.; Li, T.; Wu, J.; Cui, C.; Zhao, W.-J.; Shen, Y.-Q. Indoleamine 2, 3-dioxygenase 1 inhibition mediates the therapeutic effects in Parkinson’s disease mice by modulating inflammation and neurogenesis in a gut microbiota dependent manner. Exp. Neurol. 2025, 385, 115142. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rodrigues, L.; Fonseca-Gomes, J.; Paulo, S.L.; Viais, R.; Ribeiro, F.F.; Miranda-Lourenço, C.; Mouro, F.M.; Belo, R.F.; Ferreira, C.B.; Tanqueiro, S.R.; et al. Cleavage of the TrkB-FL receptor during epileptogenesis: Insights from a kainic acid-induced model of epilepsy and human samples. Pharmacol. Res. 2025, 215, 107707. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Jia, M.; Kong, X.; Lyu, W.; Feng, H.; Sun, X.; Li, J.; Yang, J. Human umbilical cord-derived mesenchymal stem cells ameliorate perioperative neurocognitive disorder by inhibiting inflammatory responses and activating BDNF/TrkB/CREB signaling pathway in aged mice. Stem Cell Res Ther. 2023, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Kajihara, R. The Role of Brain-Derived Neurotrophic Factor as an Essential Mediator in Neuronal Functions and the Therapeutic Potential of Its Mimetics for Neuroprotection in Neurologic and Psychiatric Disorders. Molecules 2025, 30, 848. [Google Scholar] [CrossRef]
- Perveen, N.; Alqahtani, F.; Ashraf, W.; Fawad Rasool, M.; Muhammad Muneeb Anjum, S.; Kaukab, I.; Ahmad, T.; Alqarni, S.A.; Imran, I. Perampanel increases seizure threshold in pentylenetetrazole-kindled mice and improves behavioral dysfunctions by modifying mRNA expression levels of BDNF/TrkB and inflammatory markers. Saudi Pharm. J. 2024, 32, 101930. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Cho, K.-O.; Lybrand, Z.R.; Ito, N.; Brulet, R.; Tafacory, F.; Zhang, L.; Good, L.; Ure, K.; Kernie, S.G.; Birnbaum, S.G.; et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015, 6, 6606. [Google Scholar] [CrossRef] [PubMed]
- Batulin, D.; Lagzi, F.; Vezzani, A.; Jedlicka, P.; Triesch, J. A mathematical model of neuroimmune interactions in epileptogenesis for discovering treatment strategies. iScience 2022, 25, 104343. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Xie, W.; Cai, L.; Yu, Y.; Gao, L.; Xiao, L.; He, Q.; Ren, Z.; Liu, Y. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J. Neuroinflammation 2014, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Deng, N.; Hu, J.; Hong, Y.; Ding, Y.; Xiong, Y.; Wu, Z.; Xie, W. Indoleamine-2,3-Dioxygenase 1 Deficiency Suppresses Seizures in Epilepsy. Front. Cell Neurosci. 2021, 15, 638854. [Google Scholar] [CrossRef]
- Cai, L.; Chen, W.; Liu, T.; Wei, L.; Xie, W. Chaihu Shugan Decoction improves temporal lobe epilepsy associated depressive-like behavior which was resisted to fluoxetine by normalizing periphery tryptophan metabolites. China J. Tradit. Chin. Med. Pharm. 2018, 33, 1554–1557. [Google Scholar]
- Sodhi, R.K.; Bansal, Y.; Singh, R.; Saroj, P.; Bhandari, R.; Kumar, B.; Kuhad, A. IDO-1 inhibition protects against neuroinflammation, oxidative stress and mitochondrial dysfunction in 6-OHDA induced murine model of Parkinson’s disease. Neurotoxicology 2021, 84, 184–197. [Google Scholar] [CrossRef]
- Walker, A.K.; Wing, E.E.; Banks, W.A.; Dantzer, R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry 2019, 24, 1523–1532. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Németh, H.; Toldi, J.; Vécsei, L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci. Lett. 2020, 714, 134576. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, H.; Yang, G.; Yang, Y.; Li, W.; Song, M.; Shao, M.; Su, X.; Lv, L. Associations between expression of indoleamine 2, 3-dioxygenase enzyme and inflammatory cytokines in patients with first-episode drug-naive Schizophrenia. Transl. Psychiatry 2021, 11, 595. [Google Scholar] [CrossRef]
- Wichers, M.C.; Maes, M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [CrossRef]
- Reyes-Ocampo, J.; Ramírez-Ortega, D.; Cervantes, G.I.V.; Pineda, B.; Balderas, P.M.d.O.; González-Esquivel, D.; Sánchez-Chapul, L.; Lugo-Huitrón, R.; Silva-Adaya, D.; Ríos, C.; et al. Mitochondrial dysfunction related to cell damage induced by 3-hydroxykynurenine and 3-hydroxyanthranilic acid: Non-dependent-effect of early reactive oxygen species production. Neurotoxicology 2015, 50, 81–91. [Google Scholar] [CrossRef]
- Kubicova, L.; Hadacek, F.; Chobot, V. Quinolinic acid: Neurotoxin or oxidative stress modulator? Int. J. Mol. Sci. 2013, 14, 21328–21338. [Google Scholar] [CrossRef]
- Pukoli, D.; Vécsei, L. Kynurenines and Mitochondrial Disturbances in Multiple Sclerosis. Int. J. Mol. Sci. 2025, 26, 5098. [Google Scholar] [CrossRef]
- Kovács, Z.; Kékesi, K.A.; Dobolyi, Á.; Lakatos, R.; Juhász, G. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience 2015, 300, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, S.; Sancho-Balsells, A.; Longueville, S.; Hervé, D.; Gruart, A.; Delgado-García, J.M.; Alberch, J.; Giralt, A. Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe epilepsy. Cell Death Dis. 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.-D.; Ding, J.; Han, J.; Zhang, D.-D.; Liu, J.-M.; Feng, L.-L.; Xiao, R. The effect of soybean isoflavone on the dysregulation of NMDA receptor signaling pathway induced by β-amyloid peptides 1-42 in rats. Cell Mol. Neurobiol. 2015, 35, 555–562. [Google Scholar] [CrossRef]
- Lv, D.; Xiao, B.; Liu, H.; Wang, L.; Li, Y.; Zhang, Y.H.; Jin, Q. Enhanced NMDA receptor pathway and glutamate transmission in the hippocampal dentate gyrus mediate the spatial learning and memory impairment of obese rats. Pflugers Arch. 2024, 476, 821–831. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, H.; Li, K.; Wang, L.; Wu, Y.; Dong, X.; Wang, X.; Chen, Y.; Xu, Y. Trans-cinnamaldehyde improves neuroinflammation-mediated NMDA receptor dysfunction and memory deficits through blocking NF-κB pathway in presenilin1/2 conditional double knockout mice. Brain Behav. Immun. 2019, 82, 45–62. [Google Scholar] [CrossRef]
- Zou, J.; Crews, F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell. Mol. Neurobiol. 2006, 26, 385–405. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Wang, C.; Sun, Y.; Gong, B.; Li, D.; Wu, Y.; Liu, Y.; Wei, J. Antidepressant Activity of Agarwood Essential Oil: A Mechanistic Study on Inflammatory and Neuroprotective Signaling Pathways. Pharmaceuticals 2025, 18, 255. [Google Scholar] [CrossRef]
- Rowley, S.; Patel, M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic. Biol. Med. 2013, 62, 121–131. [Google Scholar] [CrossRef]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert. Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Vanhauwaert, R.; Bharat, V.; Wang, X. Surveillance and transportation of mitochondria in neurons. Curr. Opin. Neurobiol. 2019, 57, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef]
- De Marchi, F.; Venkatesan, S.; Saraceno, M.; Mazzini, L.; Grossini, E. Acetyl-L-carnitine and Amyotrophic Lateral Sclerosis: Current Evidence and Potential use. CNS Neurol. Disord. Drug Targets 2024, 23, 588–601. [Google Scholar] [CrossRef]
- Ramsay, R.R. Electron carriers and energy conservation in mitochondrial respiration. ChemTexts 2019, 5, 9. [Google Scholar] [CrossRef]
- Kageyama, Y.; Okura, S.; Sukigara, A.; Matsunaga, A.; Maekubo, K.; Oue, T.; Ishihara, K.; Deguchi, Y.; Inoue, K. The Association Among Bipolar Disorder, Mitochondrial Dysfunction, and Reactive Oxygen Species. Biomolecules 2025, 15, 383. [Google Scholar] [CrossRef]
- Nesari, A.; Mardani, E.; Goudarzi, M.; Sabbagh, S.; Nooshabadi, M.R.; Bakhtiari, N.; Malayeri, A.R. The antioxidant and anticonvulsant effects of ellagic acid in kainic acid-induced temporal lobe epilepsy in mice. Tissue Cell 2025, 95, 102889. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Lv, X.; Wu, G.; Zhou, X.; Tian, H.; Qv, X.; Sun, H.; He, Y.; Zhang, Y.; et al. Herkinorin ameliorates neuronal damage in a pentylenetetrazol-induced epilepsy rat model through altering microglial and astrocytic activation by inhibiting PARP1 and NF-κB. Int. Immunopharmacol. 2025, 155, 114588. [Google Scholar] [CrossRef]
- Carreño-González, A.J.; Liberato, J.L.; Celani, M.V.B.; Lopes, N.P.; Lopes, J.L.C.; Gobbo-Neto, L.; Fontana, A.C.K.; Dos Santos, W.F. Neuroprotective effects of chlorogenic acid against oxidative stress in rats subjected to lithium-pilocarpine-induced status epilepticus. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 6989–6999. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.S.; Neves, D.; Tomé, D.; Costa, F.J.; Bruno, I.C.; Trigo, D.; Silva, R.M.; Almeida, R.D. Neuroprotection by Mitochondrial NAD Against Glutamate-Induced Excitotoxicity. Cells 2025, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, Q.; Ji, J.; Lu, W.; Liu, Z.; Tian, H.; Guo, L.; Wang, Y. Mitochondrial transplantation ameliorates hippocampal damage following status epilepticus. Anim. Model. Exp. Med. 2023, 6, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Pai, M.-S.; Yeh, K.-C.; Hung, C.-F.; Wang, S.-J. Hydrogen inhalation exerts anti-seizure effects by preventing oxidative stress and inflammation in the hippocampus in a rat model of kainic acid-induced seizures. Neurochem. Int. 2025, 183, 105925. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-M.; Huang, Y.; Wan, P.-P.; Lu, Y.-H.; Zhou, N.; Li, J.-J.; Yu, C.-Y.; Chou, J.-J.; Zhang, L.; Zhang, C.; et al. Ursolic Acid Protects Neurons in Temporal Lobe Epilepsy and Cognitive Impairment by Repressing Inflammation and Oxidation. Front. Pharmacol. 2022, 13, 877898. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| IDO | Forward: GTGTGTGAATGGTCTGGTCTCTGTG Reverse: ACATTTGAGGGCTCTTCCGACTTG |
| BDNF | Forward: TGACAGTATTAGCGAGTGGG Reverse: GCAGCCTTCCTTGGTGTGTGTA |
| TrkB | Forward: CGCTTCAGTGGTTCTACA Reverse: CCTTCCCATACTCGTTCTT |
| β-Actin | Forward: GTGCTATGTTGCTCTAGACTTCG Reverse: ATGCCACAGGATTCCATACC |
| Group | Seizure Frequency (Times/Day) | Duration (s) |
|---|---|---|
| Control Group | ND | ND |
| Control + 1-MT Group | ND | ND |
| TLE Group | 5 ± 0.38 | 58.68 ± 2.78 |
| TLE + 1-MT Group | 3.63 ± 0.38 * | 39.13 ± 1.88 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wei, L.; Fu, J.; Kong, Z.; Cai, L. IDO Activation Affects BDNF/TrkB Signaling Pathway, Oxidative Stress, and Mitochondrial Enzymatic Activities in Temporal Lobe Epilepsy. Curr. Issues Mol. Biol. 2025, 47, 764. https://doi.org/10.3390/cimb47090764
Xu J, Wei L, Fu J, Kong Z, Cai L. IDO Activation Affects BDNF/TrkB Signaling Pathway, Oxidative Stress, and Mitochondrial Enzymatic Activities in Temporal Lobe Epilepsy. Current Issues in Molecular Biology. 2025; 47(9):764. https://doi.org/10.3390/cimb47090764
Chicago/Turabian StyleXu, Jingwen, Liping Wei, Junling Fu, Ziting Kong, and Lun Cai. 2025. "IDO Activation Affects BDNF/TrkB Signaling Pathway, Oxidative Stress, and Mitochondrial Enzymatic Activities in Temporal Lobe Epilepsy" Current Issues in Molecular Biology 47, no. 9: 764. https://doi.org/10.3390/cimb47090764
APA StyleXu, J., Wei, L., Fu, J., Kong, Z., & Cai, L. (2025). IDO Activation Affects BDNF/TrkB Signaling Pathway, Oxidative Stress, and Mitochondrial Enzymatic Activities in Temporal Lobe Epilepsy. Current Issues in Molecular Biology, 47(9), 764. https://doi.org/10.3390/cimb47090764






