Uncovering the Tumorigenic Blueprint of PFOS and PFOA Through Multi-Organ Transcriptomic Analysis of Biomarkers, Mechanisms, and Therapeutic Targets
Abstract
1. Introduction
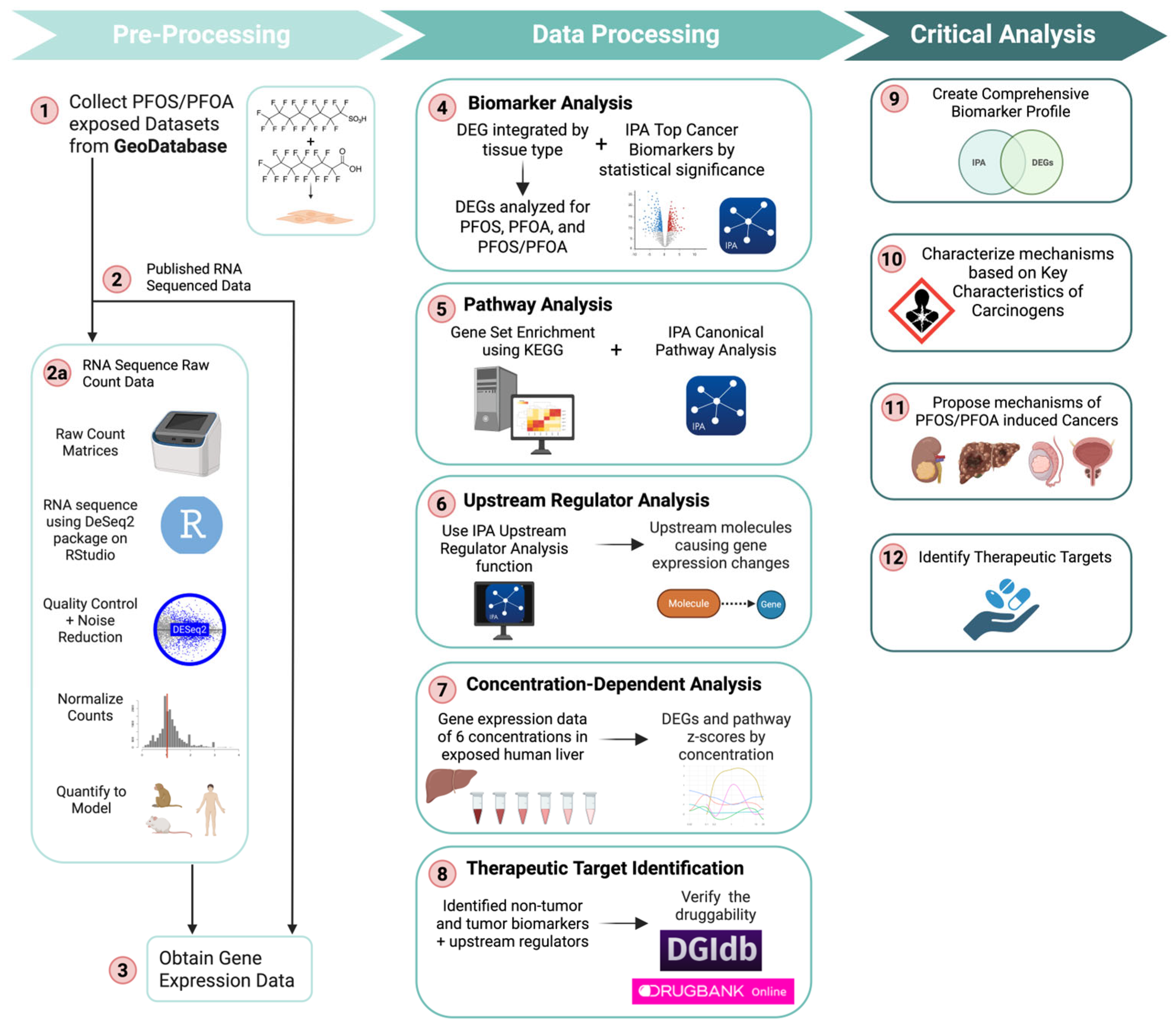
2. Materials and Methods
2.1. Data Acquisition, Processing, and Preparation
2.2. RNA Sequencing with RStudio to Generate Differentially Expressed Genes
2.2.1. Validation of RStudio RNA-Sequencing Workflow
2.2.2. Batch Effect Mitigation and Meta-Analysis Validation
2.3. Tumor Biomarker Discovery
Tumor Biomarker Categorization
2.4. Identification of Significant Pathways
2.5. Identification of Significant Upstream Regulators and Downstream Effects
2.6. Identification of Therapeutic Targets as a Rescue Strategy
3. Results and Discussion
3.1. Correlation Analysis and Batch Effect Assessment
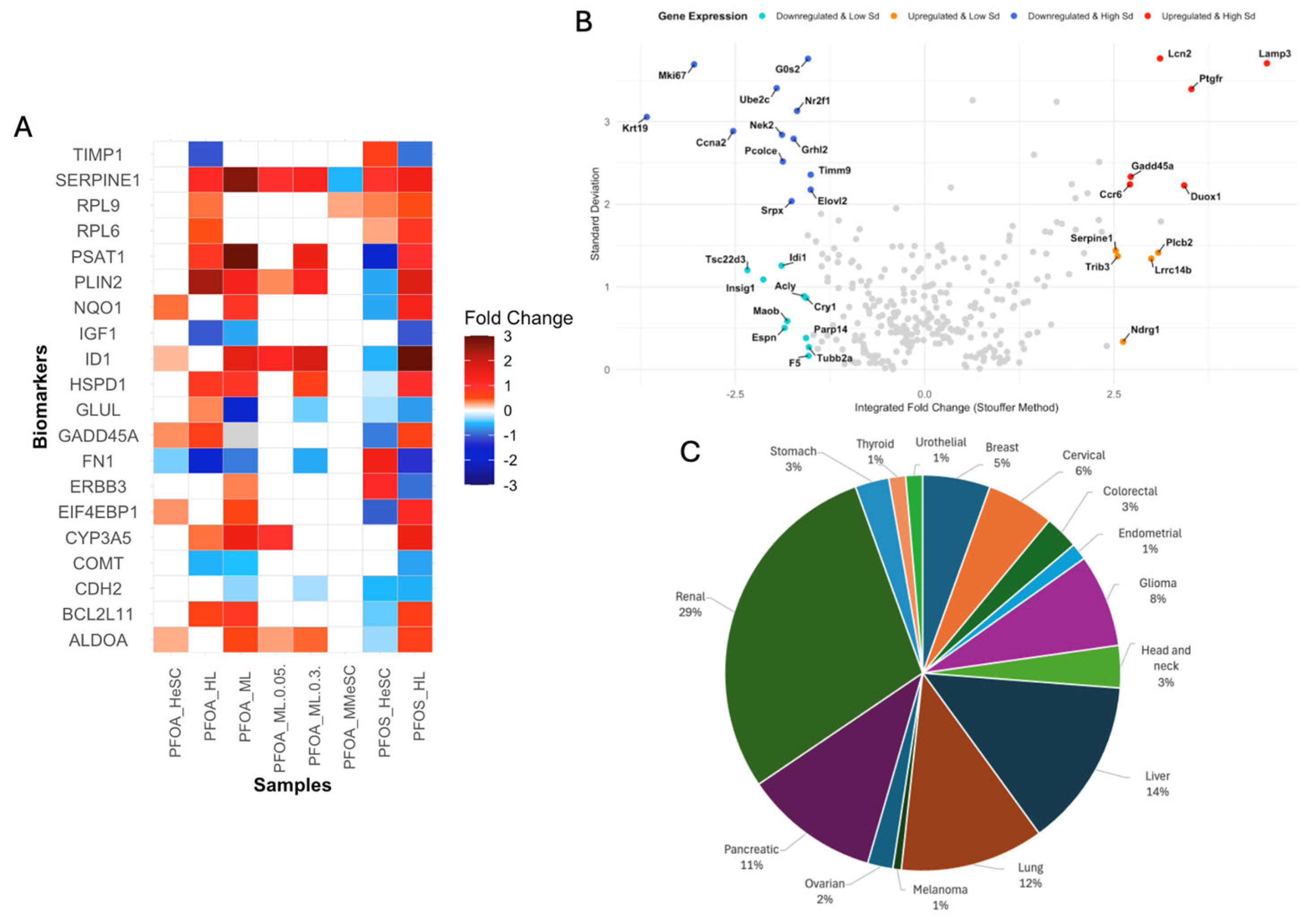
3.2. Identification of PFOS/PFOA-Associated Cancer Biomarkers
3.3. PFOS/PFOA Molecular Markers and Signatures Defined by the Key Characteristics of Carcinogens Framework
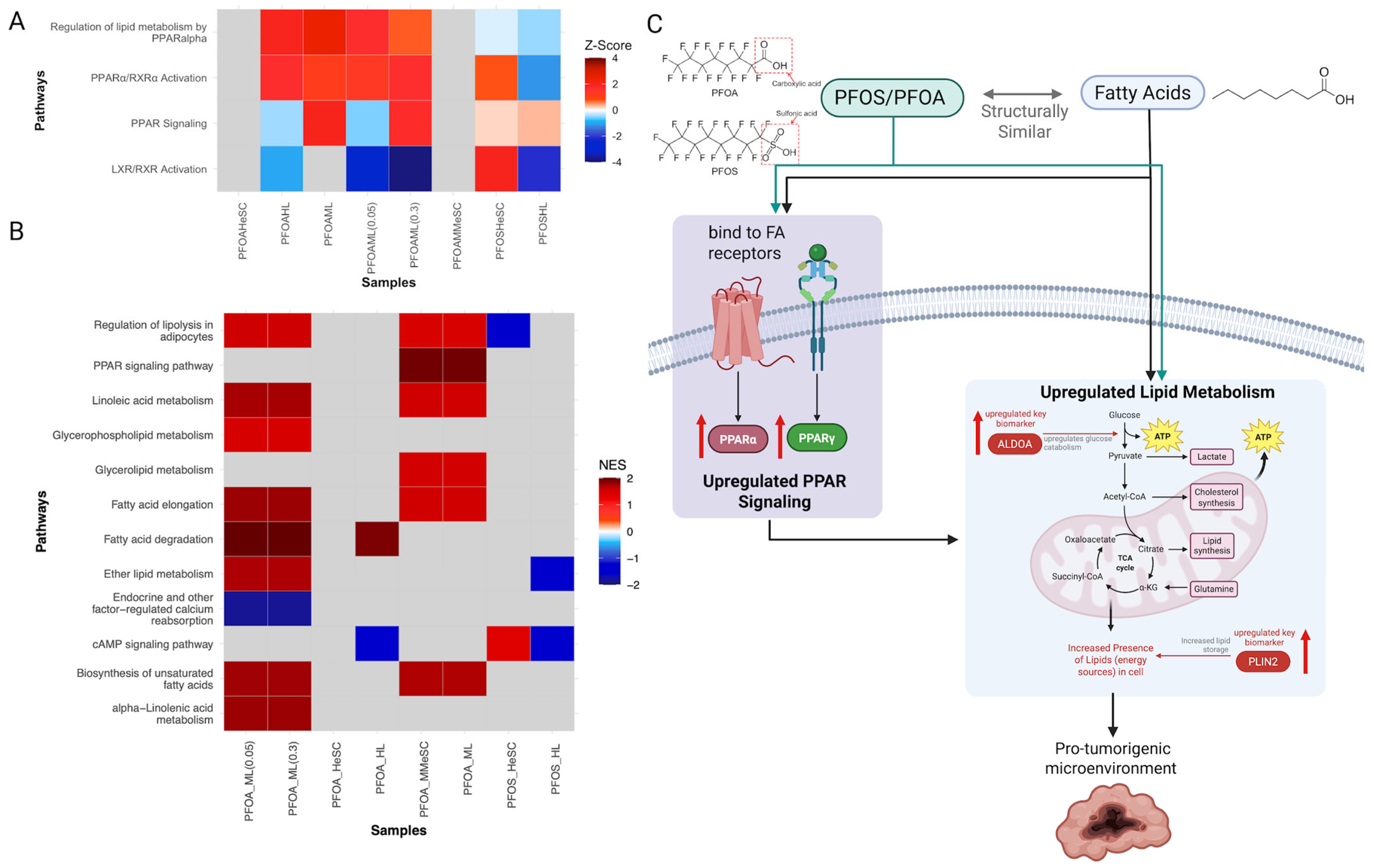
3.3.1. PFOS/PFOA Exposure Modulates Receptor-Mediated Effects
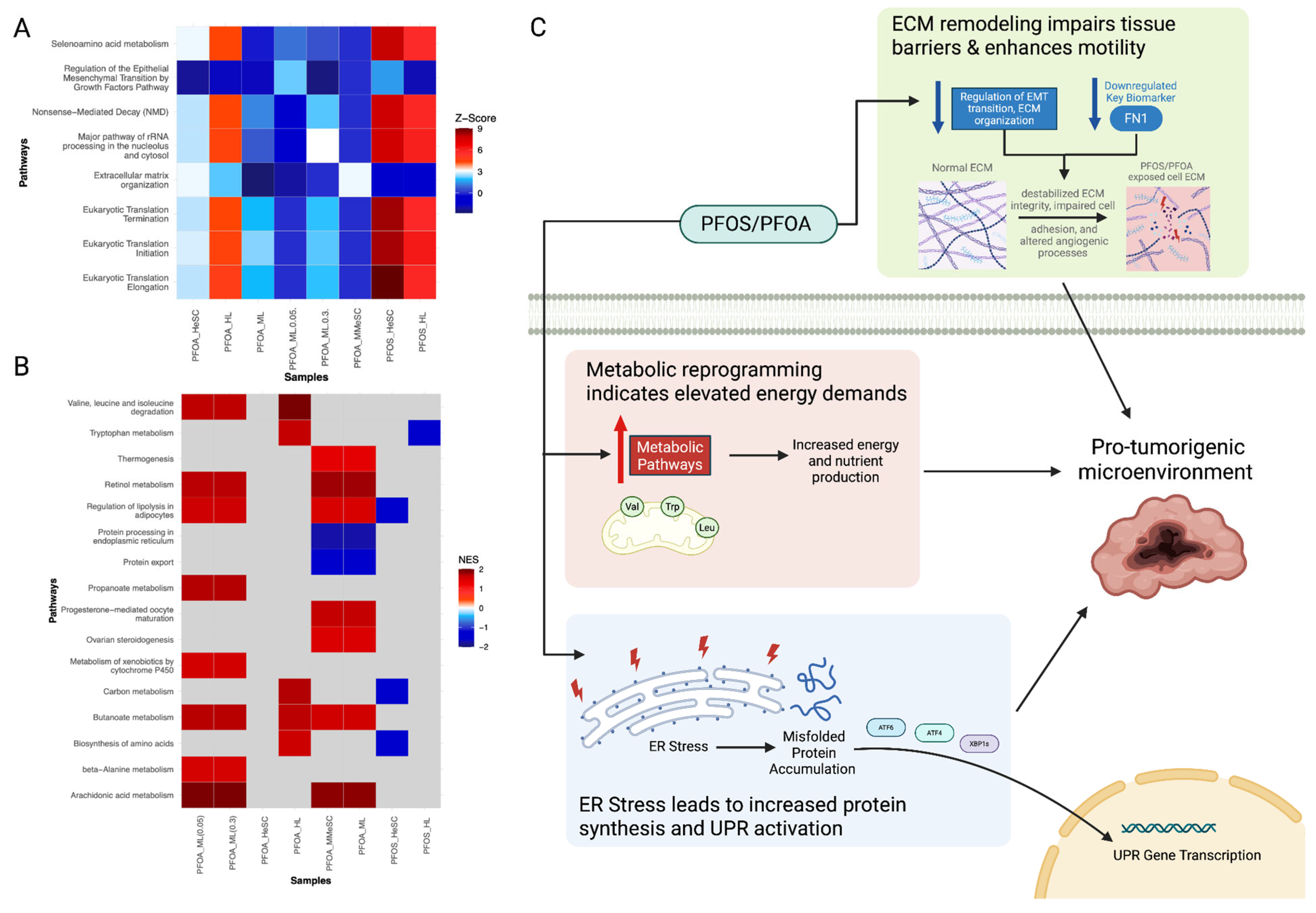
3.3.2. PFOS and PFOA-Induced Alterations in Cell Proliferation, Cell Death, and Nutrient Supply
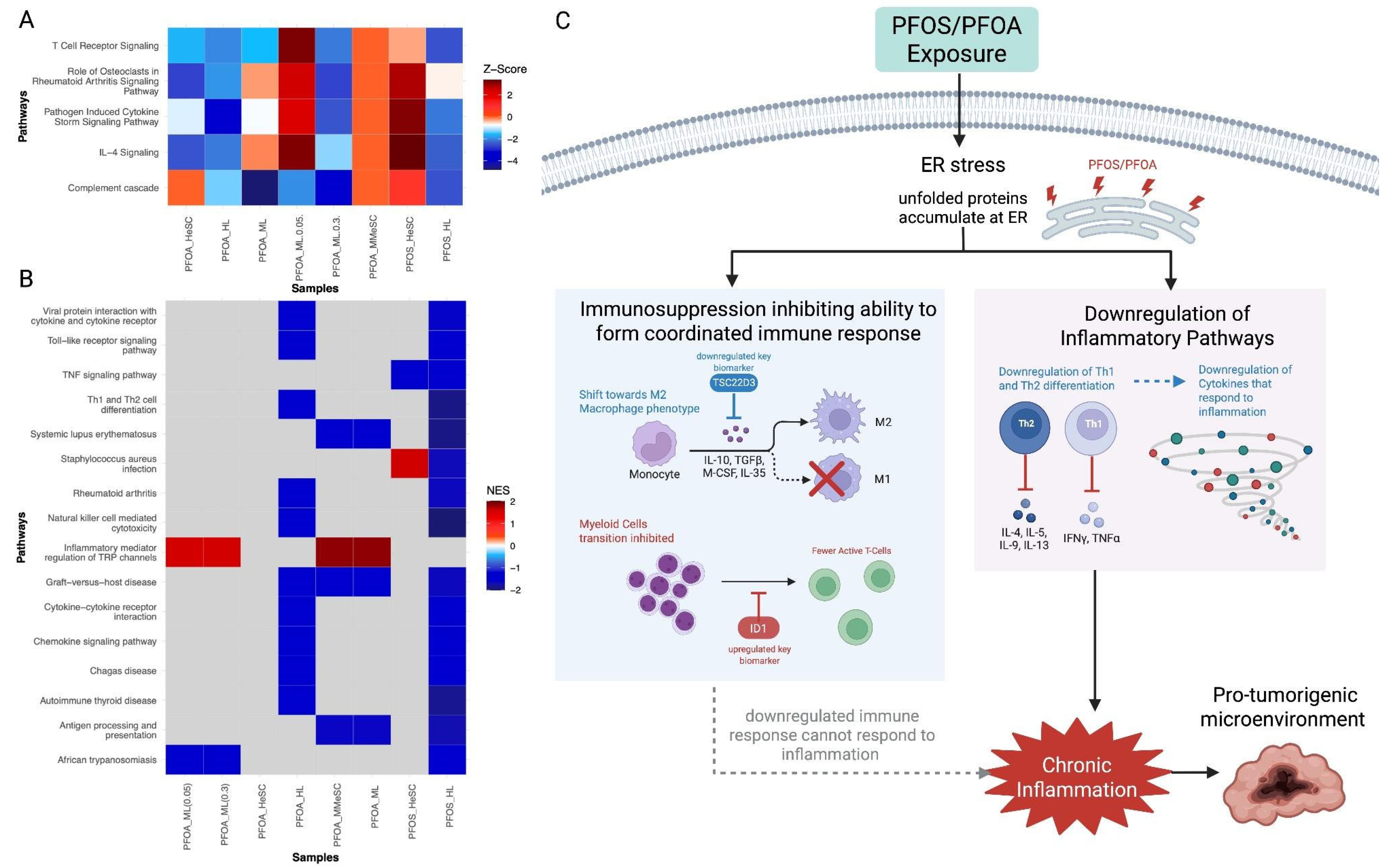
3.3.3. PFOS and PFOA-Induced the Suppression of Immune and Inflammatory Responses
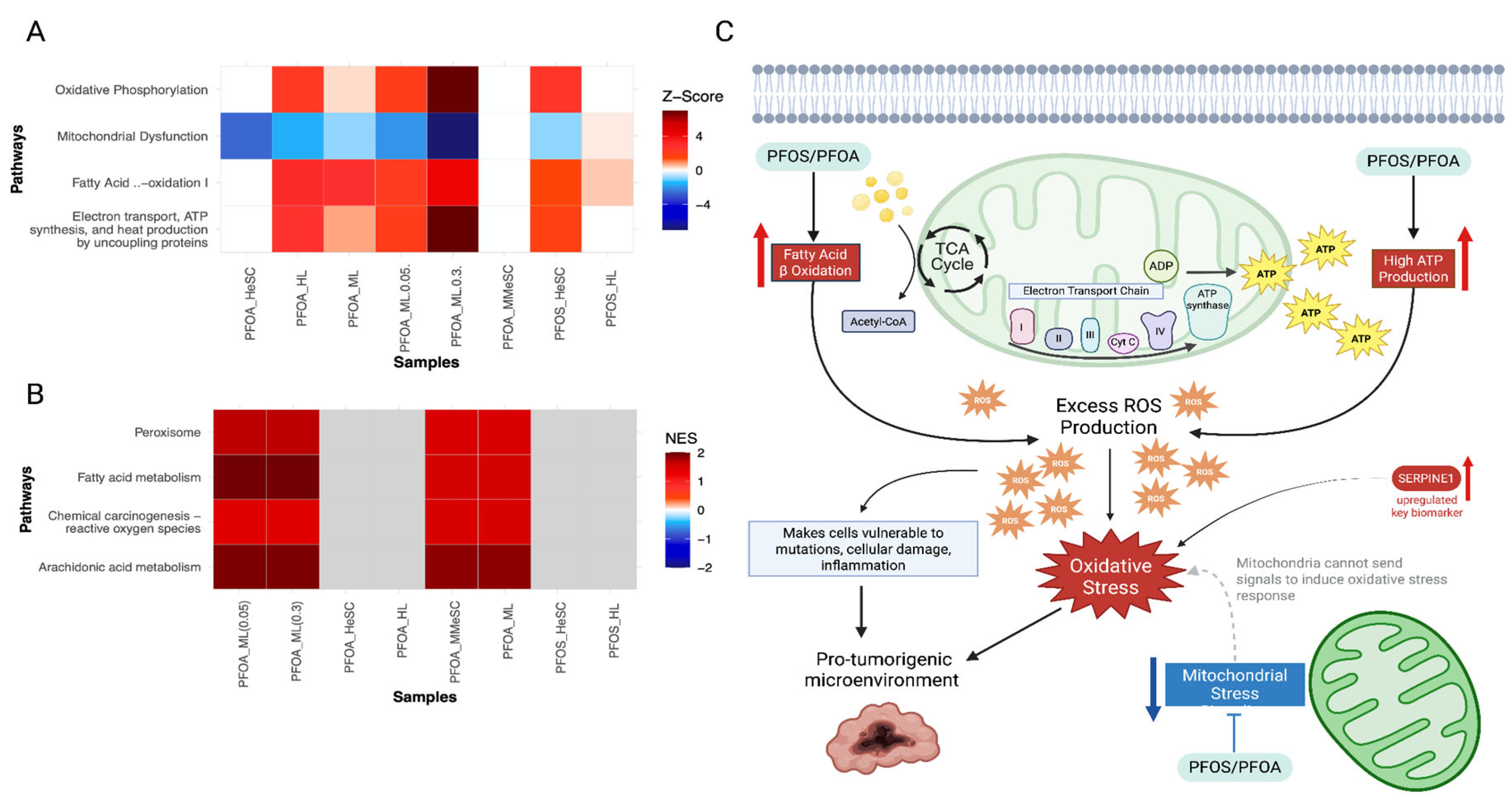
3.3.4. PFOS/PFOA Disrupts Mitochondrial Signaling and Induces Elevated Oxidative Stress
3.3.5. PFOS/PFOA-Induced Epigenetic Reprogramming and Genomic Instability Drive Tumor Promotion
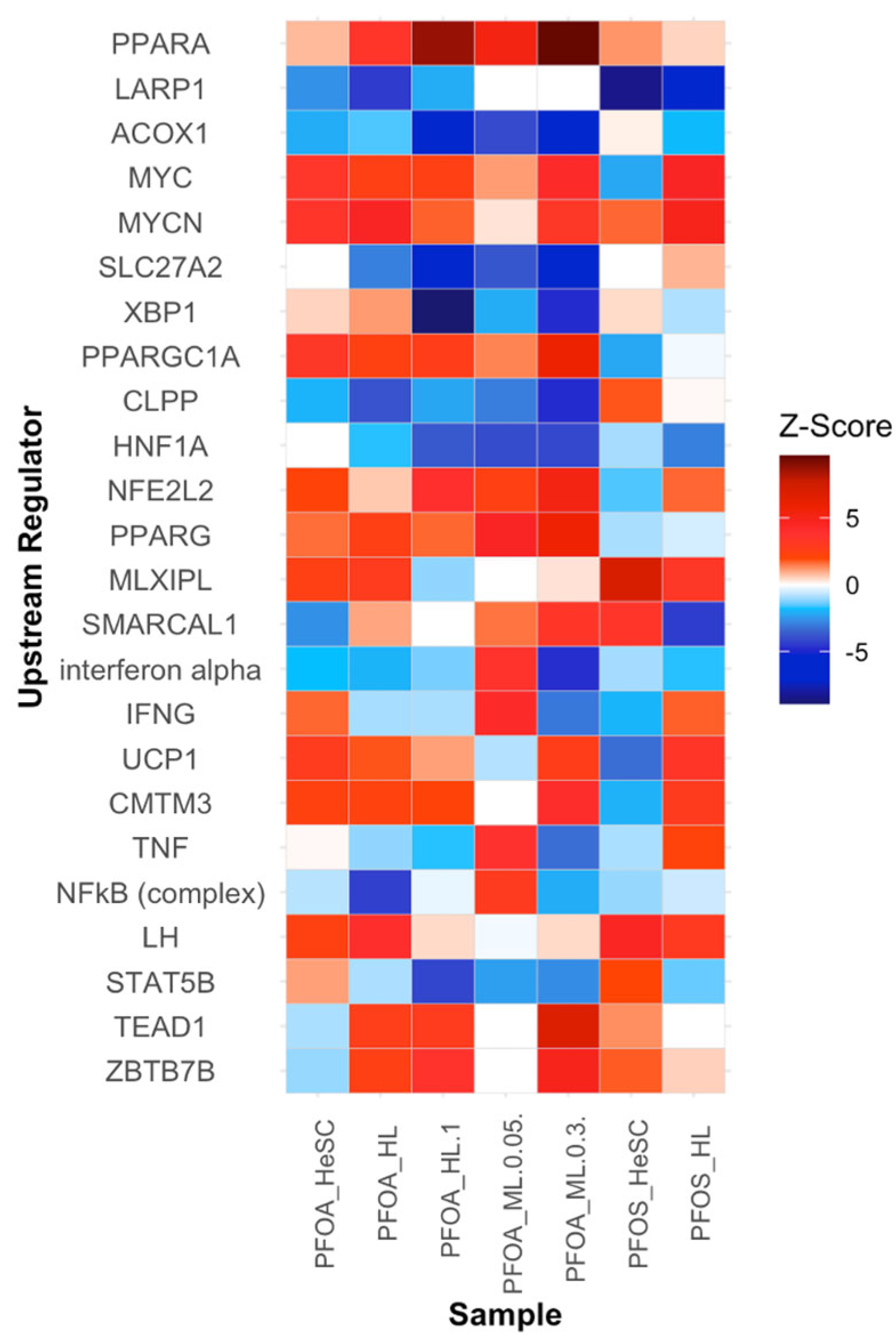
3.3.6. Alterations in the Upstream Regulators PPARα, LARP1, ACOX1, MYC, and MYCN Contribute to Increased Cell Proliferation
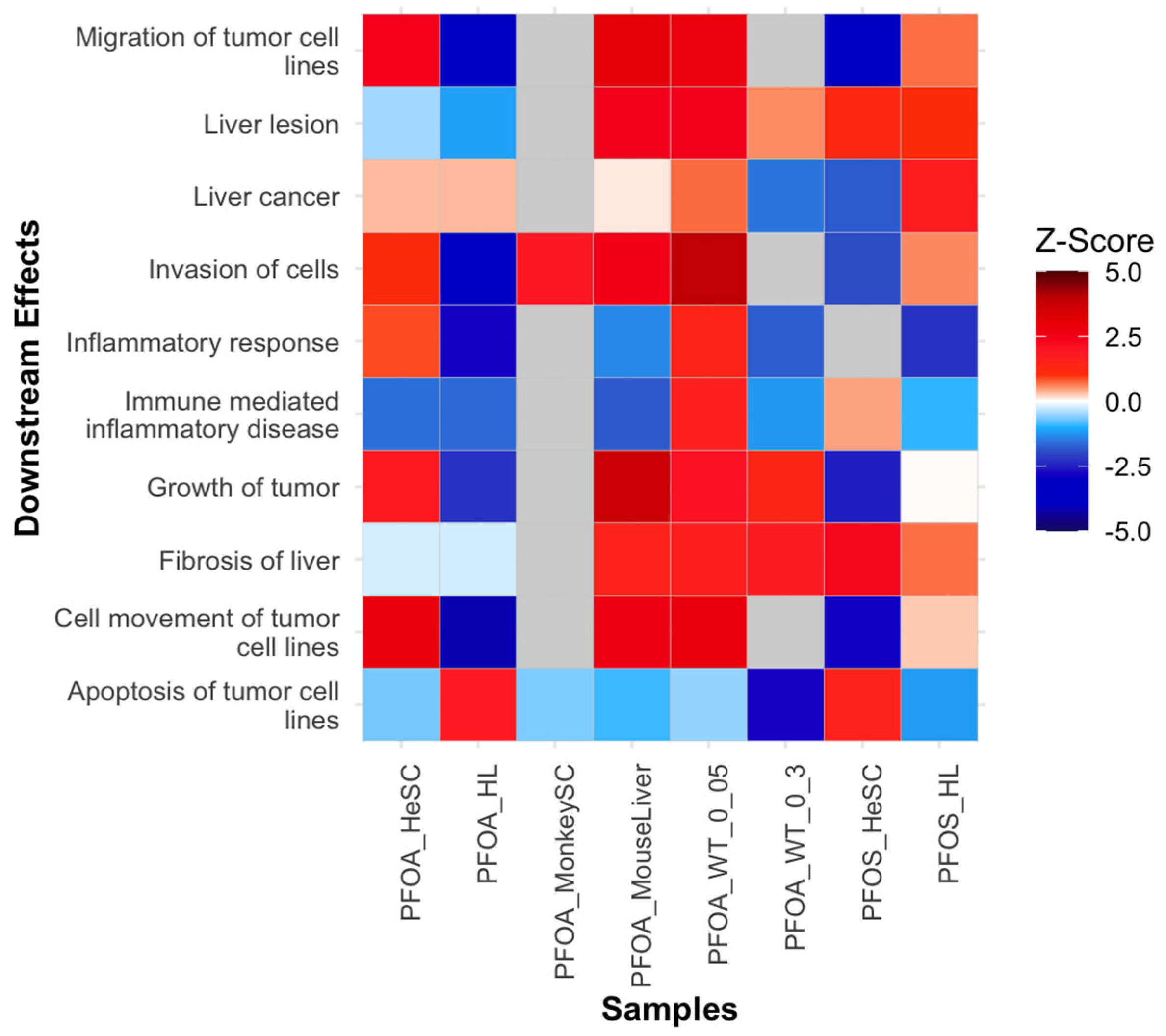
3.3.7. Downstream Diseases and Functions Indicative of Tumor Initiation
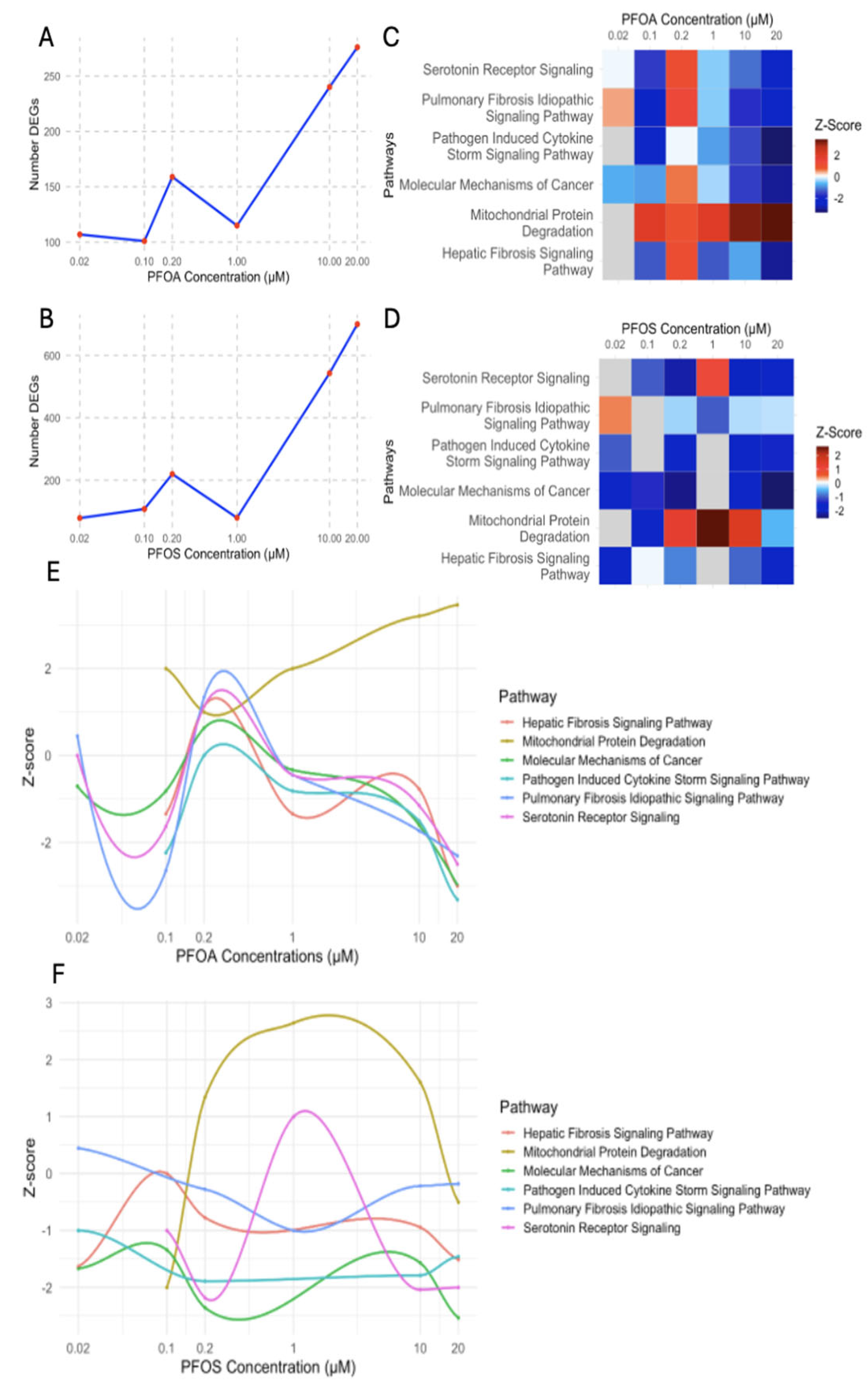
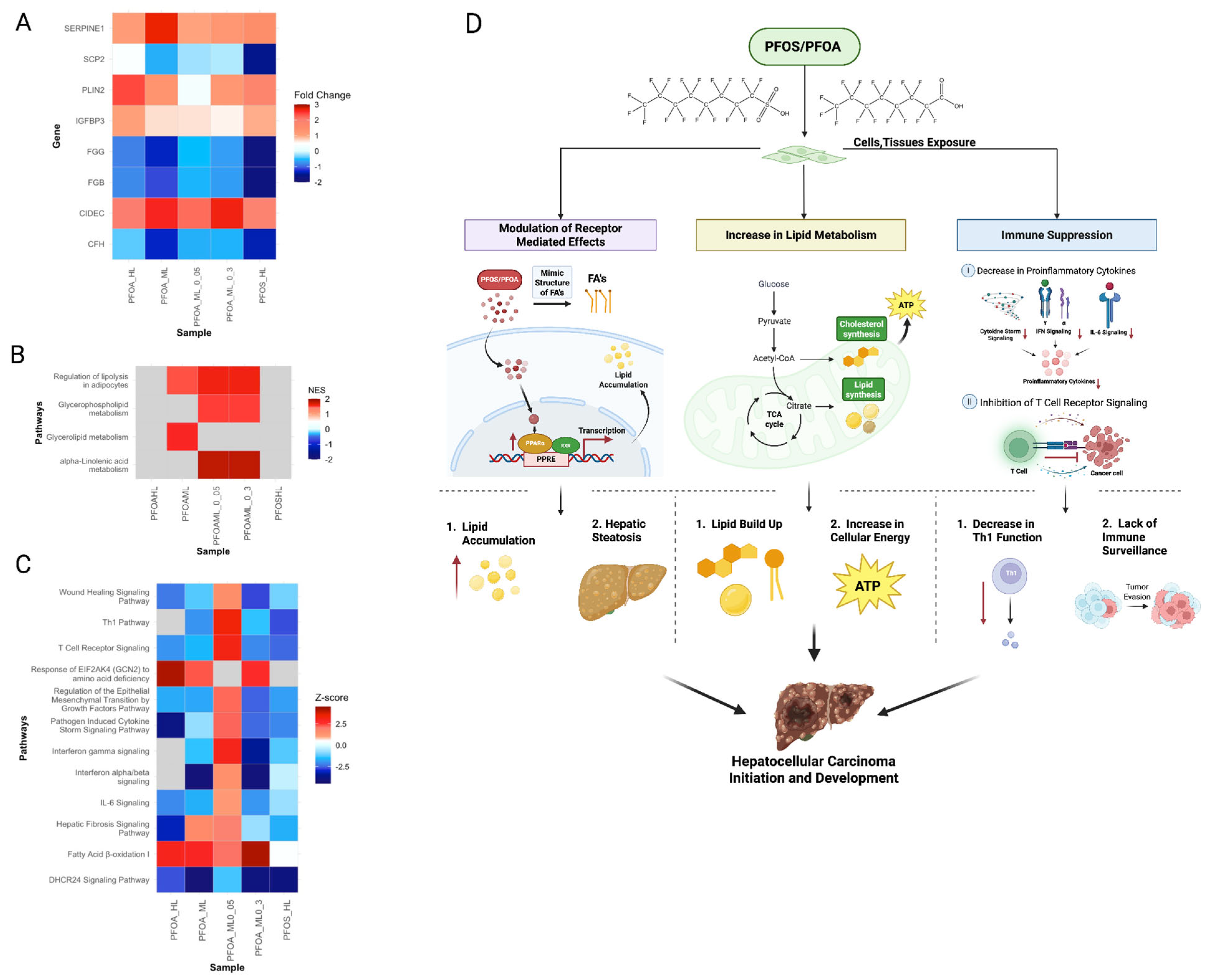
3.4. Evaluation of Concentration-Dependent Responses of PFOS and PFOA Exposure in Human Liver Samples
3.4.1. Differential Regulation of Liver Stress and Fibrosis at Low and High PFOA Concentrations
3.4.2. PFOS-Induced Nonlinear Regulation of Fibrosis, Immune, and Cancer Pathways
3.4.3. PFOS and PFOA Exhibit Distinct and Shared Concentration-Dependent Mechanisms of Liver Toxicity
3.5. PFOS and PFOA Potential Mechanisms Driving Tumorigenesis and Carcinogenesis
3.5.1. PFOS and PFOA Exposure Increases Risk of HCC Initiation and Development
3.5.2. PFOS Promotes Progression of Testicular Cancer
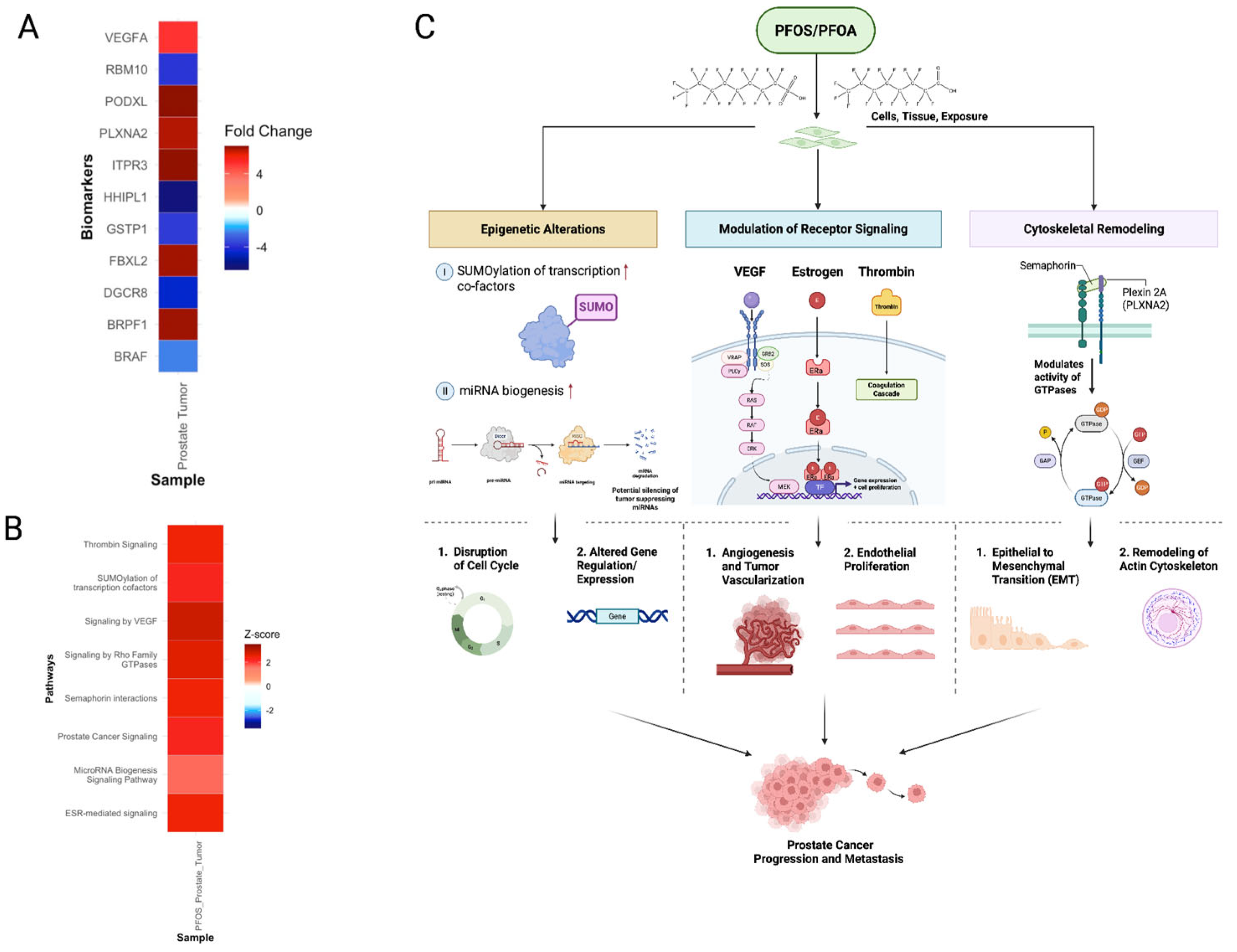
3.5.3. PFOS Drives Progression of Prostate Cancer
3.5.4. PFOS and PFOA-Induced Common Molecular Signatures of ccRCC Development
3.6. Extrapolation of Tumorigenic Signatures from PFOS-Exposed Non-Tumor to Tumor Samples
3.7. Identification of Novel Therapeutic Targets as a Rescue Strategy: Prevention and Treatment Strategy
3.7.1. Preventative Interventions for Cancer Initiation
3.7.2. Treatment Strategies for Prostate and Testicular Tumor Progression
4. Strengths
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of Perfluoroalkyl Substances in Human Tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Chemicals: Perfluoroalkyl and Polyfluoroalkyl (PFAS) Substances|Wisconsin Department of Health Services. Available online: https://www.dhs.wisconsin.gov/chemical/pfas.htm (accessed on 3 February 2025).
- U.S. Department of Health and Human Services. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS); National Institute of Environmental Health Sciences: Durham, NC, USA, 2025. Available online: https://www.niehs.nih.gov/health/topics/agents/pfc (accessed on 3 February 2025).
- Phelps, D.W.; Palekar, A.I.; Conley, H.E.; Ferrero, G.; Driggers, J.H.; Linder, K.E.; Kullman, S.W.; Reif, D.M.; Sheats, M.K.; DeWitt, J.C.; et al. Legacy and Emerging Per- and Polyfluoroalkyl Substances Suppress the Neutrophil Respiratory Burst. J. Immunotoxicol. 2023, 20, 2176953. [Google Scholar] [CrossRef]
- IARC Monographs Evaluate the Carcinogenicity of Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). Available online: https://www.iarc.who.int/news-events/iarc-monographs-evaluate-the-carcinogenicity-of-perfluorooctanoic-acid-pfoa-and-perfluorooctanesulfonic-acid-pfos (accessed on 3 February 2025).
- Beale, D.J.; Sinclair, G.M.; Shah, R.; Paten, A.M.; Kumar, A.; Long, S.M.; Vardy, S.; Jones, O.A.H. A Review of Omics-Based PFAS Exposure Studies Reveals Common Biochemical Response Pathways. Sci. Total Environ. 2022, 845, 157255. [Google Scholar] [CrossRef]
- Messmer, M.F.; Salloway, J.; Shara, N.; Locwin, B.; Harvey, M.W.; Traviss, N. Risk of Cancer in a Community Exposed to Per- and Poly-Fluoroalkyl Substances. Environ. Health Insights 2022, 16, 11786302221076707. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living Near a Chemical Plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Rhee, J.; Chang, V.C.; Cheng, I.; Calafat, A.M.; Botelho, J.C.; Shearer, J.J.; Sampson, J.N.; Setiawan, V.W.; Wilkens, L.R.; Silverman, D.T.; et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma in the Multiethnic Cohort Study. Environ. Int. 2023, 180, 108197. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Winquist, A. PFAS and Cancer, a Scoping Review of the Epidemiologic Evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic Changes by Per- and Polyfluoroalkyl Substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef]
- Beccacece, L.; Costa, F.; Pascali, J.P.; Giorgi, F.M. Cross-Species Transcriptomics Analysis Highlights Conserved Molecular Responses to Per- and Polyfluoroalkyl Substances. Toxics 2023, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers 2022, 14, 2919. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Key Characteristics of Carcinogens. In Tumour Site Concordance and Mechanisms of Carcinogenesis; Baan, R.A., Stewart, B.W., Straif, K., Eds.; IARC Scientific Publications: Lyon, France, 2019; ISBN 978-92-832-2217-0. [Google Scholar]
- Zhang, L.; Louie, A.; Rigutto, G.; Guo, H.; Zhao, Y.; Ahn, S.; Dahlberg, S.; Sholinbeck, M.; Smith, M.T. A Systematic Evidence Map of Chronic Inflammation and Immunosuppression Related to Per- and Polyfluoroalkyl Substance (PFAS) Exposure. Environ. Res. 2023, 220, 115188. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; G, M.M.; G, S.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated Substances (PFAS) Affect Oxidative Stress Biomarkers in Vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Han, D.; Zhao, Y.; Yan, X.; Cui, S. Association between Environmental Chemicals Co-Exposure and Peripheral Blood Immune-Inflammatory Indicators. Front. Public Health 2022, 10, 980987. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Rusyn, I.; Chiu, W.A.; Corpet, D.E.; Van Den Berg, M.; Ross, M.K.; Christiani, D.C.; Beland, F.A.; Smith, M.T. Application of the Key Characteristics of Carcinogens in Cancer Hazard Identification. Carcinogenesis 2018, 39, 614–622. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Li, F.; Wang, Y.; Xu, Y.; Zhang, M.; Zhang, X.; Ying, X.; Zhang, X. Perfluorooctanoic Acid Induces Human Ishikawa Endometrial Cancer Cell Migration and Invasion through Activation of ERK/mTOR Signaling. Oncotarget 2016, 7, 66558–66568. [Google Scholar] [CrossRef]
- Li, X.; Bao, C.; Ma, Z.; Xu, B.; Ying, X.; Liu, X.; Zhang, X. Perfluorooctanoic Acid Stimulates Ovarian Cancer Cell Migration, Invasion via ERK/NF-κB/MMP-2/-9 Pathway. Toxicol. Lett. 2018, 294, 44–50. [Google Scholar] [CrossRef]
- Moysich, K. “Forever Chemicals” and Cancer. Available online: https://www.roswellpark.org/cancertalk/202308/forever-chemicals-cancer (accessed on 3 February 2025).
- Zhao, M.; Yin, N.; Yang, R.; Li, S.; Zhang, S.; Faiola, F. Understanding the Effects of Per- and Polyfluoroalkyl Substances on Early Skin Development: Role of Ciliogenesis Inhibition and Altered Microtubule Dynamics. Sci. Total Environ. 2024, 913, 169702. [Google Scholar] [CrossRef]
- Rowan-Carroll, A.; Reardon, A.; Leingartner, K.; Gagné, R.; Williams, A.; Meier, M.J.; Kuo, B.; Bourdon-Lacombe, J.; Moffat, I.; Carrier, R.; et al. High-Throughput Transcriptomic Analysis of Human Primary Hepatocyte Spheroids Exposed to Per- and Polyfluoroalkyl Substances as a Platform for Relative Potency Characterization. Toxicol. Sci. 2021, 181, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.I.; Shokry, D.; Fazal, Z.; Rennels, B.C.; Freemantle, S.J.; La Frano, M.R.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Singh, R.; et al. Perfluorooctanesulfonic Acid Alters Pro-Cancer Phenotypes and Metabolic and Transcriptional Signatures in Testicular Germ Cell Tumors. Toxics 2024, 12, 232. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.-Y.; Liu, Y.-J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.-Y.; Prins, G.S.; Madak Erdogan, Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Klaunig, J.E. The Effects of Perfluorooctanoate on High Fat Diet Induced Non-Alcoholic Fatty Liver Disease in Mice. Toxicology 2019, 416, 1–14. [Google Scholar] [CrossRef]
- Attema, B.; Janssen, A.W.F.; Rijkers, D.; Van Schothorst, E.M.; Hooiveld, G.J.E.J.; Kersten, S. Exposure to Low-Dose Perfluorooctanoic Acid Promotes Hepatic Steatosis and Disrupts the Hepatic Transcriptome in Mice. Mol. Metab. 2022, 66, 101602. [Google Scholar] [CrossRef]
- Midic, U.; Vincent, K.A.; VandeVoort, C.A.; Latham, K.E. Effects of Long-Term Endocrine Disrupting Compound Exposure on Macaca Mulatta Embryonic Stem Cells. Reprod. Toxicol. 2016, 65, 382–393. [Google Scholar] [CrossRef]
- Pagès, H.; Carlson, M.; Falcon, S.; Li, N. AnnotationDbi: Manipulation of SQLite-Based Annotations in Bioconductor. R package version 1.64.1. 2023. Available online: https://bioconductor.org/packages/AnnotationDbi (accessed on 3 February 2025).
- Zhang, X. Highly effective batch effect correction method for RNA-seq count data. Comput. Struct. Biotechnol. J. 2025, 27, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Upstream Regulator Analysis. Available online: http://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/features/upstream-regulator-analysis/ (accessed on 19 May 2025).
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th anniversary: Update 2025. Nucleic Acids Res. 2025, 53, D1328–D1334. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.; Stevenson, J.; Stahl, K.; Basu, R.; Coffman, A.; Kiwala, S.; McMichael, J.F.; Kuzma, K.; Morrissey, D.; Cotto, K.; et al. DGIdb 5.0: Rebuilding the Drug-Gene Interaction Database for Precision Medicine and Drug Discovery Platforms. Nucleic Acids Res. 2024, 52, D1227–D1235. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.L.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 2 May 2025).
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.; Yuzhalin, A. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de La Forest Divonne, S.; et al. Fibronectin-Guided Migration of Carcinoma Collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Onodera, Y.; Bissell, M.J.; Park, C.C. Breast Cancer Cells in Three-Dimensional Culture Display an Enhanced Radioresponse after Coordinate Targeting of Integrin A5β1 and Fibronectin. Cancer Res. 2010, 70, 5238–5248. [Google Scholar] [CrossRef]
- Shi, K.; Wang, S.; Shen, B.; Yu, F.; Weng, D.; Lin, J. Clinicopathological and Prognostic Values of Fibronectin and Integrin Avβ3 Expression in Primary Osteosarcoma. World J. Surg. Onc. 2019, 17, 23. [Google Scholar] [CrossRef]
- Wang, J.P.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef]
- Yi, W.; Xiao, E.; Ding, R.; Luo, P.; Yang, Y. High Expression of Fibronectin Is Associated with Poor Prognosis, Cell Proliferation and Malignancy via the NF-κB/P53-Apoptosis Signaling Pathway in Colorectal Cancer. Oncol. Rep. 2016, 36, 3145–3153. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Zhu, Y.; Fei, J.; Song, L.; Sun, G.; Guo, L.; Li, X. SERPINE1 Overexpression Promotes Malignant Progression and Poor Prognosis of Gastric Cancer. J. Oncol. 2022, 2022, 2647825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Gao, J.; Ding, M.; Li, H. The Expression of SERPINE1 in Colon Cancer and Its Regulatory Network and Prognostic Value. BMC Gastroenterol. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, Z.; Wang, Q.; Jin, S.; Sun, P.; Wei, Y.; Zhu, G.; Wang, K. Pan-Cancer Analysis of SERPINE1 with a Concentration on Immune Therapeutic and Prognostic in Gastric Cancer. J Cell. Mol. Med. 2024, 28, e18579. [Google Scholar] [CrossRef]
- Jevrić, M.; Matić, I.Z.; Krivokuća, A.; Đorđić Crnogorac, M.; Besu, I.; Damjanović, A.; Branković-Magić, M.; Milovanović, Z.; Gavrilović, D.; Susnjar, S.; et al. Association of uPA and PAI-1 Tumor Levels and 4G/5G Variants of PAI-1 Gene with Disease Outcome in Luminal HER2-Negative Node-Negative Breast Cancer Patients Treated with Adjuvant Endocrine Therapy. BMC Cancer 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, E.; Sawada, K.; Nakamura, K.; Yoshimura, A.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Mabuchi, S.; Makino, H.; Morii, E.; et al. Plasminogen Activator Inhibitor-1 Is an Independent Prognostic Factor of Ovarian Cancer and IMD-4482, a Novel Plasminogen Activator Inhibitor-1 Inhibitor, Inhibits Ovarian Cancer Peritoneal Dissemination. Oncotarget 2017, 8, 89887–89902. [Google Scholar] [CrossRef]
- Becker, M.; Szarvas, T.; Wittschier, M.; Vom Dorp, F.; Tötsch, M.; Schmid, K.W.; Rübben, H.; Ergün, S. Prognostic Impact of Plasminogen Activator Inhibitor Type 1 Expression in Bladder Cancer. Cancer 2010, 116, 4502–4512. [Google Scholar] [CrossRef]
- Zubac, D.P.; Wentzel-Larsen, T.; Seidal, T.; Bostad, L. Type 1 Plasminogen Activator Inhibitor (PAI-1) in Clear Cell Renal Cell Carcinoma (CCRCC) and Its Impact on Angiogenesis, Progression and Patient Survival after Radical Nephrectomy. BMC Urol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Sotiropoulos, G.; Kotopouli, M.; Karampela, I.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Lekka, A.; Papavassiliou, A.; Dalamaga, M. Circulating Plasminogen Activator Inhibitor-1 Activity: A Biomarker for Resectable Non-Small Cell Lung Cancer? J. BU ON. 2019, 24, 943–954. [Google Scholar]
- Liu, W.; Liu, X.; Liu, Y.; Ling, T.; Chen, D.; Otkur, W.; Zhao, H.; Ma, M.; Ma, K.; Dong, B.; et al. PLIN2 Promotes HCC Cells Proliferation by Inhibiting the Degradation of HIF1α. Exp. Cell Res. 2022, 418, 113244. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Feng, K.; Hu, C.; Li, S. High Expression of Aldolase A Is Associated with Tumor Progression and Poor Prognosis in Hepatocellular Carcinoma. J. Gastrointest. Oncol. 2021, 12, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, H.; Liu, Y.; Li, X.; Shi, Q.; Lei, Q.; Hu, W.; Huang, S.; Chen, Z.; He, X. Aldolase A Accelerates Cancer Progression by Modulating mRNA Translation and Protein Biosynthesis via Noncanonical Mechanisms. Adv. Sci. 2023, 10, 2302425. [Google Scholar] [CrossRef] [PubMed]
- Gizak, A.; Wiśniewski, J.; Heron, P.; Mamczur, P.; Sygusch, J.; Rakus, D. Targeting a Moonlighting Function of Aldolase Induces Apoptosis in Cancer Cells. Cell Death Dis. 2019, 10, 712. [Google Scholar] [CrossRef]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a Novel ER Stress-Inducible Gene, Is Induced via ATF4–CHOP Pathway and Is Involved in Cell Death. EMBO J. 2005, 24, 1243–1255. [Google Scholar] [CrossRef]
- Martinez-Campesino, L.; Kocsy, K.; Cañedo, J.; Johnston, J.M.; Moss, C.E.; Johnston, S.A.; Hamby, S.; Goodall, A.H.; Redgrave, J.; Francis, S.E.; et al. Tribbles 3 Deficiency Promotes Atherosclerotic Fibrous Cap Thickening and Macrophage-Mediated Extracellular Matrix Remodelling. Front. Cardiovasc. Med. 2022, 9, 948461. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Zhu, Z.; Chen, S.; Liang, Y.; Shu, L. TSC22D3 as an Immune-Related Prognostic Biomarker for Acute Myeloid Leukemia. iScience 2023, 26, 107451. [Google Scholar] [CrossRef]
- Ayroldi, E.; Cannarile, L.; Delfino, D.V.; Riccardi, C. A Dual Role for Glucocorticoid-Induced Leucine Zipper in Glucocorticoid Function: Tumor Growth Promotion or Suppression? Cell Death Dis. 2018, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bo, Z.; Gong, W.; Guo, Y. Inhibitor of Differentiation 1 (Id1) in Cancer and Cancer Therapy. Int. J. Med. Sci. 2020, 17, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridonos, M.; Matei, I.; Huang, Y.; Do Rosario Andre, M.; Brazier-Mitouart, H.; Waite, J.C.; Chan, A.S.; Kalter, J.; Ramos, I.; Wu, Q.; et al. Id1 Suppresses Anti-Tumour Immune Responses and Promotes Tumour Progression by Impairing Myeloid Cell Maturation. Nat. Commun. 2015, 6, 6840. [Google Scholar] [CrossRef]
- Kidani, Y.; Bensinger, S.J. Liver X Receptor and Peroxisome Proliferator-Activated Receptor as Integrators of Lipid Homeostasis and Immunity. Immunol. Rev. 2012, 249, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-Like Activity of Perfluoroalkyl Acids In Vivo and Interaction with Human and Rainbow Trout Estrogen Receptors In Vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef]
- Roy, S.; Moran, J.; Danasekaran, K.; O’Brien, K.; Dakshanamurthy, S. Large-Scale Screening of Per- and Polyfluoroalkyl Substance Binding Interactions and Their Mixtures with Nuclear Receptors. IJMS 2024, 25, 8241. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.P.; Thompson, J.T.; Frame, S.R.; Gillies, P.J. Differential Activation of Nuclear Receptors by Perfluorinated Fatty Acid Analogs and Natural Fatty Acids: A Comparison of Human, Mouse, and Rat Peroxisome Proliferator-Activated Receptor-α, -β, and -γ, Liver X Receptor-β, and Retinoid X Receptor-α. Toxicol. Sci. 2006, 92, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Sun, W.; Liu, R.; Gao, X.; Lin, Z.; Tang, H.; Cui, H.; Zhao, E. Targeting Serine-Glycine-One-Carbon Metabolism as a Vulnerability in Cancers. Biomark. Res. 2023, 11, 48. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Venneti, S. Glutamine Metabolism in Brain Tumors. Cancers 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino Acids in Cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Zheng, J.; Li, X.; Zhang, C.; Zhang, Y. eIF4E Overexpression Is Associated with Poor Prognoses of Ovarian Cancer. Anal. Cell. Pathol. 2020, 2020, 8984526. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Marima, R.; Hull, R.; Syrigos, K.N.; Lolas, G.; Mphahlele, L.; Mbita, Z. Genomics and Molecular Analysis of RPL9 and LIAS in Lung Cancer: Emerging Implications in Carcinogenesis. Inform. Med. Unlocked 2021, 25, 100698. [Google Scholar] [CrossRef]
- Hwang, S.-P.; Denicourt, C. The Impact of Ribosome Biogenesis in Cancer: From Proliferation to Metastasis. NAR Cancer 2024, 6, zcae017. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic Reticulum Stress Signalling—From Basic Mechanisms to Clinical Applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Kamendulis, L.M.; Hocevar, J.M.; Stephens, M.; Sandusky, G.E.; Hocevar, B.A. Exposure to Perfluorooctanoic Acid Leads to Promotion of Pancreatic Cancer. Carcinogenesis 2022, 43, 469–478. [Google Scholar] [CrossRef]
- Shaheen, A. Effect of the Unfolded Protein Response on ER Protein Export: A Potential New Mechanism to Relieve ER Stress. Cell Stress Chaperones 2018, 23, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; Van Obberghen-Schilling, E. Shaping Up the Tumor Microenvironment With Cellular Fibronectin. Front. Oncol. 2020, 10, 641. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of Perfluorinated Compounds: Recent Developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef]
- Bardhan, M.; Kaushik, R. Physiology, Complement Cascade. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mackay, I.R. Tolerance and Autoimmunity. West. J. Med. 2001, 174, 118–123. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic Inflammation and Cancer. Oncology 2002, 16, 217–226, 229; discussion 230–232. [Google Scholar]
- Wen, Y.; Rashid, F.; Fazal, Z.; Singh, R.; Spinella, M.J.; Irudayaraj, J. Nephrotoxicity of Perfluorooctane Sulfonate (PFOS)—Effect on Transcription and Epigenetic Factors. Environ. Epigenetics 2022, 8, dvac010. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Guan, Y.; Liao, D.; Chen, H.; Xiong, H.; Sheng, Y.; Chen, X.; Pang, J. TRIB3 Promotes the Progression of Renal Cell Carcinoma by Upregulating the Lipid Droplet-Associated Protein PLIN2. Cell Death Dis. 2024, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Y.; Guo, X.; Wang, X.; Han, X.; Kanwore, K.; Hong, X.; Zhou, H.; Gao, D. Hypoxia-Induced ROS Aggravate Tumor Progression through HIF-1α-SERPINE1 Signaling in Glioblastoma. J. Zhejiang Univ. Sci. B 2023, 24, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Hattori, N.; Imao, Y.; Nishino, K.; Hattori, N.; Ohgane, J.; Yagi, S.; Tanaka, S.; Shiota, K. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Development, Growth & Differentiation. Genes Cells 2007, 49, 99–107. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takahashi, R.; Kinehara, M.; Yano, K.; Kuramoto, T.; Shimamoto, A.; Tahara, H. Downregulation of Histone H3.3 Induces P53-Dependent Cellular Senescence in Human Diploid Fibroblasts. Genes 2024, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huo, Y.; Zhou, S.; Guo, J.; Ma, X.; Li, T.; Fan, C.; Wang, L. Cancer Cell-Intrinsic XBP1 Drives Immunosuppressive Reprogramming of Intratumoral Myeloid Cells by Promoting Cholesterol Production. Cell Metab. 2022, 34, 2018–2035.e8. [Google Scholar] [CrossRef]
- Ganesh, H.; Moran, J.; Roy, S.; Mathew, J.; Ackah-Blay, J.; Costello, E.; Shan, P.; Dakshanamurthy, S. Impact of Persistent Endocrine-Disrupting Chemicals on Human Nuclear Receptors: Insights from In Silico and Experimental Characterization. IJMS 2025, 26, 2879. [Google Scholar] [CrossRef]
- Hardwick, J.P.; Osei-Hyiaman, D.; Wiland, H.; Abdelmegeed, M.A.; Song, B.-J. PPAR/RXR Regulation of Fatty Acid Metabolism and Fatty Acid Omega-Hydroxylase (CYP4) Isozymes: Implications for Prevention of Lipotoxicity in Fatty Liver Disease. PPAR Res. 2009, 2009, 952734. [Google Scholar] [CrossRef]
- Hong, S.; Freeberg, M.A.; Han, T.; Kamath, A.; Yao, Y.; Fukuda, T.; Suzuki, T.; Kim, J.K.; Inoki, K. LARP1 Functions as a Molecular Switch for mTORC1-Mediated Translation of an Essential Class of mRNAs. elife 2017, 6, e25237. [Google Scholar] [CrossRef]
- Tannoury, M.; Ayoub, M.; Dehgane, L.; Nemazanyy, I.; Dubois, K.; Izabelle, C.; Brousse, A.; Roos-Weil, D.; Maloum, K.; Merle-Béral, H.; et al. ACOX1-Mediated Peroxisomal Fatty Acid Oxidation Contributes to Metabolic Reprogramming and Survival in Chronic Lymphocytic Leukemia. Leukemia 2024, 38, 302–317. [Google Scholar] [CrossRef]
- Mundo, L.; Ambrosio, M.R.; Raimondi, F.; Del Porro, L.; Guazzo, R.; Mancini, V.; Granai, M.; Jim Rocca, B.; Lopez, C.; Bens, S.; et al. Molecular Switch from MYC to MYCN Expression in MYC Protein Negative Burkitt Lymphoma Cases. Blood Cancer J. 2019, 9, 91. [Google Scholar] [CrossRef]
- Kostecki, G.; Chuang, K.; Buxton, A.; Dakshanamurthy, S. Dose-Dependent PFESA-BP2 Exposure Increases Risk of Liver Toxicity and Hepatocellular Carcinoma. CIMB 2025, 47, 98. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Y.; Dong, H.; Wang, L.; Zhang, X.; Han, X.; Sang, X.; Bao, Y.; Peng, M.; Cao, G. From Liver Fibrosis to Hepatocarcinogenesis: Role of Excessive Liver H2O2 and Targeting Nanotherapeutics. Bioact. Mater. 2023, 23, 187–205. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cho, S.Y.; Yun, C.H.; Moon, Y.S.; Lee, T.R.; Kim, S.H. Transcriptional Activation of Cidec by PPARγ2 in Adipocyte. Biochem. Biophys. Res. Commun. 2008, 377, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Feng, B.; Ji, X.; Tian, X.; Zhao, L.; Zhou, J.; Zhang, W.; Li, M.; Fei, Y.; Wu, X. Complement Factor H Attenuates TNF-α-Induced Inflammation by Upregulating EIF3C in Rheumatoid Arthritis. J. Transl. Med. 2023, 21, 846. [Google Scholar] [CrossRef]
- Wang, H.; Meyer, C.A.; Fei, T.; Wang, G.; Zhang, F.; Liu, X.S. A Systematic Approach Identifies FOXA1 as a Key Factor in the Loss of Epithelial Traits during the Epithelial-to-Mesenchymal Transition in Lung Cancer. BMC Genom. 2013, 14, 680. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-Associated Fibroblasts Modulate Growth Factor Signaling and Extracellular Matrix Remodeling to Regulate Tumor Metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy-Morrogh, L.; Martin, P. The Hallmarks of Cancer Are Also the Hallmarks of Wound Healing. Sci. Signal. 2020, 13, eaay8690. [Google Scholar] [CrossRef]
- Lai, P.M.; Gong, X.; Chan, K.M. Roles of Histone H2B, H3 and H4 Variants in Cancer Development and Prognosis. Int. J. Mol. Sci. 2024, 25, 9699. [Google Scholar] [CrossRef]
- Vardabasso, C.; Hasson, D.; Ratnakumar, K.; Chung, C.-Y.; Duarte, L.F.; Bernstein, E. Histone Variants: Emerging Players in Cancer Biology. Cell. Mol. Life Sci. 2014, 71, 379–404. [Google Scholar] [CrossRef]
- Hao, Q.; Zhou, X. The Emerging Role of Long Noncoding RNA RMRP in Cancer Development and Targeted Therapy. Cancer Biol. Med. 2022, 19, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Williams, K.; Yao, J.L.; Dennis, R.A.; Huang, J.; Reeder, J.; Ricke, W.A. Thrombin Expression in Prostate: A Novel Finding. Cancer Investig. 2011, 29, 62–67. [Google Scholar] [CrossRef]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Bonkhoff, H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Martins, J.B.; Cekaite, L.; Sveen, A.; Torres-Ferreira, J.; Graça, I.; Costa-Pinheiro, P.; Eilertsen, I.A.; Antunes, L.; Oliveira, J.; et al. Epigenetic Disruption of miR-130a Promotes Prostate Cancer by Targeting SEC23B and DEPDC1. Cancer Lett. 2017, 385, 150–159. [Google Scholar] [CrossRef]
- Alto, L.T.; Terman, J.R. Semaphorins and Their Signaling Mechanisms. Methods Mol. Biol. 2017, 1493, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.J.; Hui, D.H.F.; Lee, W.W.; Dong, M.; Tombe, T.; Jiao, I.Z.F.; Khosravi, S.; Takeuchi, A.; Peacock, J.W.; Ivanova, L.; et al. Semaphorin 3 C Drives Epithelial-to-Mesenchymal Transition, Invasiveness, and Stem-like Characteristics in Prostate Cells. Sci. Rep. 2017, 7, 11501. [Google Scholar] [CrossRef]
- Le Tran, N.; Wang, Y.; Bilandzic, M.; Stephens, A.; Nie, G. Podocalyxin Promotes the Formation of Compact and Chemoresistant Cancer Spheroids in High Grade Serous Carcinoma. Sci. Rep. 2024, 14, 7539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.; Yue, Y.; Zhang, L.; Liu, T.; Jing, M.; Liang, X.; Ma, M.; Xu, S.; Wang, K.; et al. ITPR3 Facilitates Tumor Growth, Metastasis and Stemness by Inducing the NF-ĸB/CD44 Pathway in Urinary Bladder Carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 65. [Google Scholar] [CrossRef]
- Li, F.; Niu, M.; Qin, K.; Guo, R.; Yi, Y.; Xu, J.; Li, L.; Xie, S.; Fu, M.; Wen, N.; et al. FBXL2 Promotes E47 Protein Instability to Inhibit Breast Cancer Stemness and Paclitaxel Resistance. Oncogene 2023, 42, 339–350. [Google Scholar] [CrossRef]
- Tan, S.K.; Hougen, H.Y.; Merchan, J.R.; Gonzalgo, M.L.; Welford, S.M. Fatty Acid Metabolism Reprogramming in ccRCC: Mechanisms and Potential Targets. Nat. Rev. Urol. 2023, 20, 48–60. [Google Scholar] [CrossRef]
- Cao, Q.; Ruan, H.; Wang, K.; Song, Z.; Bao, L.; Xu, T.; Xiao, H.; Wang, C.; Cheng, G.; Tong, J.; et al. Overexpression of PLIN2 Is a Prognostic Marker and Attenuates Tumor Progression in Clear Cell Renal Cell Carcinoma. Int. J. Oncol. 2018, 53, 137–147. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Xu, Z.; Li, L.; Hu, H.; Li, Y.; Yu, S.; Wang, M.; Gao, L. Identification and Validation of SERPINE1 as a Prognostic and Immunological Biomarker in Pan-Cancer and in ccRCC. Front. Pharmacol 2023, 14, 1213891. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; An, T.; Wan, Z.; Huang, Z.; Chong, T. SERPINE1 and Its Co-Expressed Genes Are Associated with the Progression of Clear Cell Renal Cell Carcinoma. BMC Urol. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Kim, Y.; Kim, M.-J.; Hyun, S.H.; Kim, H.; Chung, S.W.; Joe, Y.; Chung, H.T.; Shin, D.-M.; Back, S.H. Phosphorylation of eIF2α Suppresses the Impairment of GSH/NADPH Homeostasis and Mitigates the Activation of Cell Death Pathways, Including Ferroptosis, during ER Stress. Mol. Cells 2025, 48, 100210. [Google Scholar] [CrossRef]
- Li, X.; Zhou, D.; Cai, Y.; Yu, X.; Zheng, X.; Chen, B.; Li, W.; Zeng, H.; Hassan, M.; Zhao, Y.; et al. Endoplasmic Reticulum Stress Inhibits AR Expression via the PERK/eIF2α/ATF4 Pathway in Luminal Androgen Receptor Triple-Negative Breast Cancer and Prostate Cancer. NPJ Breast Cancer 2022, 8, 2. [Google Scholar] [CrossRef]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Men’s Health 2020, 38, 484–494. [Google Scholar] [CrossRef]
- Issa, N.T.; Stathias, V.; Schürer, S.; Dakshanamurthy, S. Machine and Deep Learning Approaches for Cancer Drug Repurposing. Semin. Cancer Biol. 2021, 68, 132–142. [Google Scholar] [CrossRef]
- Issa, N.T.; Peters, O.J.; Byers, S.W.; Dakshanamurthy, S. RepurposeVS: A Drug Repurposing-Focused Computational Method for Accurate Drug-Target Signature Predictions. Comb. Chem. High Throughput Screen. 2015, 18, 784–794. [Google Scholar] [CrossRef]
- Issa, N.T.; Kruger, J.; Wathieu, H.; Raja, R.; Byers, S.W.; Dakshanamurthy, S. DrugGenEx-Net: A Novel Computational Platform for Systems Pharmacology and Gene Expression-Based Drug Repurposing. BMC Bioinform. 2016, 17, 202. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dakshanamurthy, S.; Gaur, A.; Chen, Y.-S.; Fang, H.-B.; Abdussamad, M.; Zhou, H.; Zapas, J.; Calvert, V.; Petricoin, E.F.; et al. The Repurposed Anthelmintic Mebendazole in Combination with Trametinib Suppresses Refractory NRASQ61K Melanoma. Oncotarget 2017, 8, 12576–12595. [Google Scholar] [CrossRef]
- Dakshanamurthy, S.; Issa, N.T.; Assefnia, S.; Seshasayee, A.; Peters, O.J.; Madhavan, S.; Uren, A.; Brown, M.L.; Byers, S.W. Predicting New Indications for Approved Drugs Using a Proteochemometric Method. J. Med. Chem. 2012, 55, 6832–6848. [Google Scholar] [CrossRef] [PubMed]
- Ballav, S.; Lokhande, K.B.; Yadav, R.S.; Ghosh, P.; Swamy, K.V.; Basu, S. Exploring Binding Mode Assessment of Novel Kaempferol, Resveratrol, and Quercetin Derivatives with PPAR-α as Potent Drug Candidates against Cancer. Mol. Divers. 2023, 27, 2867–2885. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA Reduce LPS-Induced Inflammation Responses in HK-2 Cells: Evidence for a PPAR-Gamma-Dependent Mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Fu, J.; Wu, Z.; Liu, J.; Wu, T. Vitamin C: A Stem Cell Promoter in Cancer Metastasis and Immunotherapy. Biomed. Pharmacother. 2020, 131, 110588. [Google Scholar] [CrossRef]
- Li, W.-N.; Zhang, S.-J.; Feng, J.-Q.; Jin, W.-L. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers 2022, 14, 2608. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Assefnia, S.; Dakshanamurthy, S.; Guidry Auvil, J.M.; Hampel, C.; Anastasiadis, P.Z.; Kallakury, B.; Uren, A.; Foley, D.W.; Brown, M.L.; Shapiro, L.; et al. Cadherin-11 in Poor Prognosis Malignancies and Rheumatoid Arthritis: Common Target, Common Therapies. Oncotarget 2014, 5, 1458–1474. [Google Scholar] [CrossRef]
- Al-Najjar, B.O.; Helal, M.; Saqallah, F.G.; Bandy, B. Isozyme-Specific Inhibition of GSTP1-1: A Crucial Element in Cancer-Targeting Drugs. RSC Med. Chem. 2025, 16, 1516–1531. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Liu, Y.; Wang, X.; Ren, M. Multidimensional Pan-Cancer Analysis of HSPA5 and Its Validation in the Prognostic Value of Bladder Cancer. Heliyon 2024, 10, e27184. [Google Scholar] [CrossRef]
- Peran, I.; Dakshanamurthy, S.; McCoy, M.D.; Mavropoulos, A.; Allo, B.; Sebastian, A.; Hum, N.R.; Sprague, S.C.; Martin, K.A.; Pishvaian, M.J.; et al. Cadherin 11 Promotes Immunosuppression and Extracellular Matrix Deposition to Support Growth of Pancreatic Tumors and Resistance to Gemcitabine in Mice. Gastroenterology 2021, 160, 1359–1372.e13. [Google Scholar] [CrossRef]
- Vidal, I.; Zheng, Q.; Hicks, J.L.; Chen, J.; Platz, E.A.; Trock, B.J.; Kulac, I.; Baena-Del Valle, J.A.; Sfanos, K.S.; Ernst, S.; et al. GSTP1 Positive Prostatic Adenocarcinomas Are More Common in Black than White Men in the United States. PLoS ONE 2021, 16, e0241934. [Google Scholar] [CrossRef]
- Bevacizumab—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab (accessed on 24 June 2025).
- Rathinavelu, A.; Pattabiraman, N.; Sridhar, J.; Dakshanamurthy, S. Vascular Endothelial Receptor Specific Inhibitors. U.S. Patent 7,939,557, 10 May 2011. [Google Scholar]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging Nanopharmaceuticals and Nanonutraceuticals in Cancer Management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Raghow, S.; Hooshdaran, M.Z.; Katiyar, S.; Steiner, M.S. Toremifene Prevents Prostate Cancer in the Transgenic Adenocarcinoma of Mouse Prostate Model. Cancer Res. 2002, 62, 1370–1376. [Google Scholar] [PubMed]
- Mediavilla-Varela, M.; Boateng, K.; Noyes, D.; Antonia, S.J. The Anti-Fibrotic Agent Pirfenidone Synergizes with Cisplatin in Killing Tumor Cells and Cancer-Associated Fibroblasts. BMC Cancer 2016, 16, 176. [Google Scholar] [CrossRef]
- Maas, N.L.; Diehl, J.A. Molecular Pathways: The PERKs and Pitfalls of Targeting the Unfolded Protein Response in Cancer. Clin. Cancer Res. 2015, 21, 675–679. [Google Scholar] [CrossRef]




| GEO ID | Samples | PFOS and PFOA + Control Samples (Total 529) | PFAS Type | Organism | Cell Line/ Tissue Type | PFOS/PFOA Concentration | Reference |
|---|---|---|---|---|---|---|---|
| GSE236956 | 42 | 18 | PFOS, PFOA | Homo sapiens | Embryonic stem cells hESC H1 line from the National Stem Cell Bank ℅ the WiCell Research Institute Differentiated into non-neuroectoderm (NNE) cells | 10 μM | Zhao et al., 2024 [25] |
| GSE144775 | 607 | 406 | PFOS, PFOA | Homo sapiens | Human liver spheroids 3D InSight Human Liver Microtissues from InSphero. Spheroids from this manufacturer are a co-culture model pooled from 10 different human liver donors. | 0.02, 0.1, 0.2, 1, 2, 10, 20, 50, 100 µM | Rowan-Carroll et al., 2021 [26] |
| GSE262137 | 26 | 26 | PFOS | Homo sapiens | Testicular germ cell tumor cells Human embryonal carcinoma cell lines 2102EP and NT2/D1 from ATCC Xenografted into immunocompromised athymic nude mice | 5 µM | Boyd et al., 2024 [27] |
| GSE185183 | 23 | 23 | PFOS | Mus musculus | Prostate cancer xenograft Congenic RWPE-1 (non-tumorigenic) and RWPE-kRAS (tumorigenic w/K-ras oncogene transfection) from ATCC. Xenografted 2 × 106 RWPE-kRAS cells into athymic nude male mice after HFD treatment. | 10 mg/kg/d Cells were treated with PFOS at 10 µM, 1 µM, 0.1 µM, 0.01 µM, 0.001 µM, 0.0001 µM, with or without 0.001 µM DHT, before xenograft. | Imir et al., 2021 [28] |
| GSE119441 | 16 | 16 | PFOA | Mus musculus | Mouse liver cells Mice genotype: C57BL/6 | 1 mg/kg/ bw/day | Li et al., 2019 [29] |
| GSE212294 | 32 | 24 | PFOA | Mus musculus | Mouse liver cells in C57BL/6 wildtype and PPARα knockout mice | 0.05 mg/kg bw/day 0.3 mg/kg bw/day | Attema et al., 2022 [30] |
| GSE86939 | 29 | 10 | PFOA | Macaca mulatta | Embryonic stem cells Rhesus embryonic stem cells (ESCs) Line: Oregon Rhesus Macaque Embryonic Stem (ORMES)-6, 42XX from the Oregon National Primate Research Center | 0.1 µM | Midic et al., 2016 [31] |
| RStudio DeSeq2 Analysis | Experimental Value | Difference Between R Sequencing and Published Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Name | log2FoldChange | p-Value | FDR | log2FoldChange | p-Value | FDR | log2FoldChange | p-Value | FDR |
| CEP131 | −0.171 | 0.239 | 0.486 | −0.171 | 0.243 | 0.553 | 0 | −0.004 | −0.067 |
| EGFR | 0.857 | 2.60 × 10−18 | 1.49 × 10−16 | 0.857 | 2.11 × 10−18 | 1.36 × 10−16 | 0 | 4.90 × 10−19 | 1.30 × 10−17 |
| A1BG | −0.017 | 0.956 | 0.985 | −0.018 | 0.954 | 0.993 | 0.001 | 0.002 | −0.008 |
| SOX7 | −0.55 | 0.169 | 0.391 | −0.548 | 0.175 | 0.452 | −0.002 | −0.006 | −0.061 |
| GPC5 | 1.141 | 0.016 | 0.067 | 1.142 | 0.018 | 0.086 | −0.001 | −0.002 | −0.019 |
| Key Characteristics | PFOS | PFOA | Pathways Evidence Across PFOS/PFOA-Exposed Datasets | Biomarkers |
|---|---|---|---|---|
| 1. Is electrophilic or can be metabolically activated | No Data | No Data | No Data | No Data |
| 2. Is genotoxic | No Data | No Data | No Data | No Data |
| 3. Alters DNA repair or causes genomic instability | Upregulated | Upregulated | Disruption of pathways crucial for maintaining DNA integrity, proper cell cycle progression, and accurate protein synthesis promote DNA damage and genomic instability | XBP1, GADD45a |
| 4. Induced epigenetic alterations | Upregulated | Upregulated | Disruption of pathways that impair DNA methylation, chromatin remodeling, and histone modifications, silencing tumor suppressor genes and promoting epigenetic modifications | FOXO3 |
| 5. Induces oxidative stress | Upregulated | Upregulated | Upregulation of genes involved in oxidative phosphorylation, fatty B-oxidation, and mitochondrial dysfunction that are associated with ROS production, implicating PFOS and PFOA exposure with inducing oxidative stress | CYP3A5, ALDOA, SERPINE1, NQO1, HSPD1, PLIN2, ALDH3A2 |
| 6. Induces chronic inflammation | Downregulated | Downregulated | Downregulation of inflammatory pathways that resolve inflammation, indicating PFOS and PFOA exposure can lead to a state of chronic inflammation | FN1, ID1, HSPD1, TRIB3, SESN2 |
| 7. Is immunosuppressive | Downregulated | Downregulated | Suppression of immune surveillance and elimination of potentially malignant cell pathways, PFOS and PFOA may act as carcinogen by impairing immune function | ID1, HSPD1, TSC22D3 |
| 8. Modulates receptor-mediated effects | Upregulated | Upregulated | Upregulation of lipid metabolism and metabolic reprogramming pathways suggests PFOS and PFOA can act as ligands for carcinogenic pathways | ALDOA, PLIN2, IGF1, LDLR |
| 9. Causes immortalization | No Data | No Data | No Data | No Data |
| 10. Alters cell proliferation, cell death, or nutrient supply | Upregulated | Upregulated | Upregulation of pathways involved in protein synthesis, metabolic reprogramming, and ECM organization indicates disruption in cell proliferation, death, and nutrient supply | BCL2L11, PSAT1, SERPINE1, CDH2, EIF4EBP1, ERBB3, GLUL, COMT, IGF1, RPL9, RPS6, TRIB3, LAMP3, HSD17B11, ATF4, P4HA2, NUCB2, SDC1, ACTA1 |
| Gene | Oncogene or Tumor Suppressor Gene | Basic Functionality | Therapeutic Uses Based on Drug–Gene Interactions | Drugs that Interact with Gene Based on DGIdb | Drugs with Gene as a Target Based on DrugBank |
|---|---|---|---|---|---|
| SERPINE1 | N/A | Plasminogen-activator inhibitor, primary inhibitor of proteins that breaks down blood clots | Used in thrombotic and antithrombotic therapy as well as an antineoplastic agents | Epirubicin, Urokinase, Arsenic Trioxide, Cetrorelix | Drotrecogin Alfa, Urokinase, Troglitazone, Alteplase, Anistreplase, Reteplase, Copper |
| ALDOA | Oncogene | Key enzyme in glycolysis pathway | Therapeutic use in metabolic disorders | Propionic Acid, Acetate | Dihydroxyacetone Phosphate, Zinc, Artenimol, Zinc Acetate, Zinc Chloride, Zinc Sulfate |
| FOXO3 | Oncogene | Transcription factor involved in cell cycle regulation, apoptosis, and oxidative stress response | Targeted in cancer therapy and longevity research | Resveratrol, Syringaresinol | None identified |
| IGFBP3 | N/A | Carrier protein for insulin-like growth factor signaling | Therapeutic use in palliative chemotherapy for gastric cancer | Fluorouracil, Celecoxib | Mecasermin, Alitretinoin |
| FN1 | N/A | Responsible for cell adhesion and migration due to role in ECM | Antineoplastic therapeutic use in chemotherapy | Dacarbazine, Ocriplasmin | Ocriplasmin, Lanoteplase, Zinc, Zinc Acetate, Zinc Chloride, Zinc Sulfate |
| NQO1 | Tumor Suppressor Gene | Antioxidant enzyme regulating redox balance, detoxification, and tumor suppression | Antioxidant defense, chemotherapy activation, tumor suppression, and proteasome regulation | Itraconazole, Dicumarol, Benzene | alpha-Tocopherol succinate, Cannabidiol, Carboplatin, Cisplatin, D-alpha-Tocopheryl acetate, Vitamin E, Flavin adenine dinucleotide, Menadione, Oxaliplatin, Phenytoin |
| CDH2 | Tumor Suppressor Gene | Encodes N-cadherin, involved in cell–cell adhesion and EMT | Targeted in cancer therapy, particularly metastasis inhibition | Methadone, hydrochloride Adh1 | None identified |
| ERBB3 | Oncogene | Member of the EGFR family, involved in cell growth and differentiation Targeted in cancer therapy for inhibition of signaling pathways | Targeted in cancer therapy for inhibition of signaling pathways | AV-203 | Patritumab, LJM716, Duligotuzumab |
| PLIN2 | N/A | Promotes formation in lipid droplets resulting in lipid accumulation | Therapeutic use in NAFLD and metabolic disorders | RTI-122 | No current drugs using gene as a target |
| PFOS/PFOA Mechanism | Target | Compound | Source | Reasoning | Therapeutic Benefit |
|---|---|---|---|---|---|
| PPARα upregulation + lipid metabolism dysregulation | PPARα | Kaempferol [137] | flavonoid found in plant-based foods, including green tea, broccoli, kale, and berries | Kaempferol stably binds to PPARα restoring lipid homeostasis and exhibit anticancer properties | Reduces PFOS/PFOA-induced lipid dysregulation and potentially mitigates tumorigenic effects |
| Lipid accumulation and inflammation | PPARs | n-3 PUFAs: (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) [138] | Found in fish oil, flaxseeds, and walnuts | N-3 PUFAs activate PPARs, restoring PPAR signaling to mitigate lipid accumulation and inflammation | Counteracts PFOS/PFOA-induced metabolic dysregulation and inflammation |
| ECM remodeling and hypoxia | ECM proteins and HIF-1 | Vitamin C [139,140] | Found in citrus fruits, bell peppers, and leafy greens | Vitamin C promotes ECM repair and inhibits HIF-1 activation | May prevent fibrosis and hypoxia-driven tumor progression, driven by dysregulation of FN1 and PLIN2 genes, induced by PFOS/PFOA exposure |
| Oxidative and ER stress | Antioxidant response genes | Glutathione [141] | Endogenously synthesized; also found in spinach, avocados, and asparagus | Glutathione increases antioxidant capacity and reduces ER stress | Protects against oxidative damage and cellular stress responses triggered by PFOS/PFOA |
| Gene | Basic Functionality | Therapeutic Uses Based on Drug–Gene Interactions | Drugs That Interact with Gene Based on DGIdb | Drugs with Gene as a Target Based on DrugBank | Prostate/Testicular |
|---|---|---|---|---|---|
| HSPA5 | RNA binding function, regulates calcium, and responds to ER stress | Inhibition of HSPA5 assists in managing ER stress and protein abundance | Mianserin | No interactions found | Prostate |
| CDH11 | Regulates cell adhesion-known tumor suppressor gene | Antibodies can target overexpressed CDH11 to inhibit tumor progression | No interactions found | Celecoxib | Prostate |
| GSTP1 | Detoxifies foreign substances, preserves DNA and prevents damage to DNA | Under hypoxic conditions where GSTP1 is overexpressed, inhibitors can reduce resistance to therapy and metastasis | Cytarabine Docetaxel Anhydrous Daunoribicin Liposomal | Canfosfamide 2-(N-morpholino)ethanesulfonic acid Cibacron Blue Clomipramine Clozapine Curcumin | Prostate |
| FGF10 | Growth factor involved in EMT and tissue repair | FGFR signaling inhibitors are used in tumors to inhibit EMT and tumor progression | No interactions found | Erdafitinib, Futibatinib, Infigratinib, Lenvatinib, Nintedanib, Palifermin, Pemigatinib, Regorafenib | Testicular |
| HMGB1 | Involved in replication, transcription, chromatin remodeling, V(D)J recombination, DNA repair and genome stability | Antagonists can inhibit HMGB1 by interfering with the cytoplasmic function. Inhibition of HMGB1 decreases inflammation | Itraconazole Prednisolone | Chloroquine Ethyl pyruvate | Prostate and Testicular |
| Mechanism | Target | Reasoning | Cancer Type Targeted | Drug/Compound | Drug Status | Mechanism of Action |
|---|---|---|---|---|---|---|
| Overactive estrogen receptor signaling | ERα | PFOS promotes Erα-mediated transcription, which supports hormone-driven tumor progression | Prostate | Toremifene (nano-targeted delivery) [149] | Chemopreventive (off-label); FDA approved for metastatic breast cancer [150] | Selective estrogen receptor modulator (SERM); antagonizes ERα and downregulates estrogen responsive genes |
| VEGF promoted angiogenesis | VEGF-A | PFOS upregulates VEGF signaling, which enhances tumor vascularization and angiogenic signaling | Prostate | Bevacizumab | FDA-approved for several cancers | Humanized monoclonal antibody targeting VEGF-A; inhibits angiogenesis |
| VEGF promoted angiogenesis | VEGFR1/2 | VEGFR1/2 activation promotes angiogenesis and metastasis in tumors; inhibition can suppress PFOS-induced vascular remodeling | Prostate | Isoindole-based inhibitors (VGA1102, VGA1155) | Experimental, patented small molecules | Small molecule inhibitors of VEGFR1 and VEGFR2; block VEGF binding and signaling |
| Fibrosis associated progression | TGF-β and collagen synthesis | PFOS alters ECM and fibrosis-related pathway which promotes pro-tumorigenic microenvironment | Testicular | Pirfenidone [151] | FDA-approved for idiopathic pulmonary fibrosis | Anti-fibrotic; inhibits TGF-β signaling and collagen production |
| ER stress | PERK (EIF2AK3) | PFOS exposure induces ER stress which initiates the UPR via PERK. This promotes cell survival and contributes to tumor maintenance under ER stress | Testicular and Prostate | GSK2606414 or GSK2656157 [152] | Experimental | PERK inhibitors: bind to PERK kinase domain to prevent EIF2α phosphorylation |
| EIF2 signaling | EIF2α (EIF2S1) | PFOS upregulated EIF2 phosphorylation in non-tumor and tumor samples. This suppresses translation while enhancing stress response | Testicular and Prostate | ISRIB (Integrated Stress Response Inhibitor) | Experimental | Allosteric activator of eIF2B that restores protein translation despite phosphorylated EIF2α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathur, K.; Khaliq, A.; Park, S.; Chu, N.; Burra, V.M.; Kanukolanu, N.; Costello, E.; Dakshanamurthy, S. Uncovering the Tumorigenic Blueprint of PFOS and PFOA Through Multi-Organ Transcriptomic Analysis of Biomarkers, Mechanisms, and Therapeutic Targets. Curr. Issues Mol. Biol. 2025, 47, 763. https://doi.org/10.3390/cimb47090763
Mathur K, Khaliq A, Park S, Chu N, Burra VM, Kanukolanu N, Costello E, Dakshanamurthy S. Uncovering the Tumorigenic Blueprint of PFOS and PFOA Through Multi-Organ Transcriptomic Analysis of Biomarkers, Mechanisms, and Therapeutic Targets. Current Issues in Molecular Biology. 2025; 47(9):763. https://doi.org/10.3390/cimb47090763
Chicago/Turabian StyleMathur, Krisha, Aleezah Khaliq, Stephanie Park, Nathan Chu, Vaishnavi M. Burra, Norah Kanukolanu, Ellen Costello, and Sivanesan Dakshanamurthy. 2025. "Uncovering the Tumorigenic Blueprint of PFOS and PFOA Through Multi-Organ Transcriptomic Analysis of Biomarkers, Mechanisms, and Therapeutic Targets" Current Issues in Molecular Biology 47, no. 9: 763. https://doi.org/10.3390/cimb47090763
APA StyleMathur, K., Khaliq, A., Park, S., Chu, N., Burra, V. M., Kanukolanu, N., Costello, E., & Dakshanamurthy, S. (2025). Uncovering the Tumorigenic Blueprint of PFOS and PFOA Through Multi-Organ Transcriptomic Analysis of Biomarkers, Mechanisms, and Therapeutic Targets. Current Issues in Molecular Biology, 47(9), 763. https://doi.org/10.3390/cimb47090763








