Comparative Molecular Profiling and Bioactivity Analysis of Algerian Propolis: Antioxidant, Antibacterial Activities, and In Silico NRF2-KEAP1 Pathway Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Propolis Samples
2.3. Dry Propolis Extract
2.4. Determination of the Total Phenolic Content (TPC) and the Total Flavonoid Content (TFC)
2.5. Advanced Analytical Techniques
2.5.1. GC-MS
2.5.2. UHPLC-DAD-ESI/MS
2.6. Antioxidant Activity Evaluation
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging Assays
2.6.2. Ferric Reducing Antioxidant Power (FRAP) and 1,10-Phenanthroline (Phen) Assays
2.7. Antibacterial Activity Assessment
2.8. Computational Analysis
2.8.1. Data Insights
2.8.2. In Silico Molecular Modeling
2.8.3. In Silico ADME-Tox Predictions
3. Results
3.1. TPC and TFC
3.2. Chromatographic Analysis Results
3.2.1. GC-MS Results
3.2.2. UHPLC-DAD-ESI/MS Results
3.3. Antioxidant Activity
3.4. Antibacterial Activity
3.5. Results of Computational Studies
3.5.1. Molecular Modeling
3.5.2. In Silico ADME-Tox Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2:2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ADME-Tox | absorption, distribution, metabolism, excretion, and toxicology |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric reducing antioxidant power |
| GC-MS | gas chromatography–mass spectrometry |
| PCA | principal component analysis |
| PDB | Protein Data Bank |
| PE1 | propolis extract 1 |
| PE2 | propolis extract 2 |
| Phen | 1,10-phenanthroline |
| RMSD | root mean square deviation |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| UHPLC-DAD-ESI/MS | ultra-high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry |
References
- Etxegarai-Legarreta, O.; Sanchez-Famoso, V. The role of beekeeping in the generation of goods and services: The interrelation between environmental, socioeconomic, and sociocultural utilities. Agriculture 2022, 12, 551. [Google Scholar] [CrossRef]
- Fikadu, Z. The contribution of managed honey bees to crop pollination, food security, and economic stability: Case of Ethiopia. Open Agric. J. 2019, 13, 175. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Bobiş, O.; Shah, S.R.A.; Arslan-Acaroz, D.; Segueni, N.; Kurek-Górecka, A.; Olczyk, P.; Nanda, V.; Nayik, G.A. Propolis. In Honey Bees, Beekeeping and Bee Products; CRC Press: Boca Raton, FL, USA, 2024; pp. 147–169. [Google Scholar] [CrossRef]
- Wali, A.F.; Mushtaq, A.H.L.A.M.; Rehman, M.U.; Akbar, S.; Masoodi, M.H. Bee propolis (Bee’s glue): A phytochemistry review. J. Crit. Rev. 2017, 4, 9–13. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; dos Santos Arruda, R.E.; da Cruz Almeida, E.T.; dos Santos Oliveira, J.M.; Basílio-Júnior, I.D.; Celerino de Moraes Porto, I.C.; Sabino, A.R.; Tonholo, J.; Gray, A.; Ebel, R.E.; et al. Comprehensive multivariate correlations between climatic effect, metabolite profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Sci. Rep. 2019, 9, 18293. [Google Scholar] [CrossRef]
- da Cruz, F.B.; Martins, D.H.N.; de Freitas Ferreira, J.; de Oliveira Magalhães, P.; Silveira, D.; Fonseca-Bazzo, Y.M. Antioxidant activity of Apis mellifera bee propolis: A review. J. Nat. Prod. Discov. 2022, 1, 1. [Google Scholar] [CrossRef]
- Nazari-Bonab, H.; Jamilian, P.; Radkhah, N.; Zarezadeh, M.; Ebrahimi-Mameghani, M. The effect of propolis supplementation in improving antioxidant status: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2023, 37, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Boulechfar, S.; Akbulut, Z.; Tepe, H.D.; Zellagui, A.; Aktas, R.G.; Bensouici, C.; Doyuk, F.; Khattabi, L.; Demirel, G.; Lahouel, M. LC–MS/MS analysis, antioxidant and anticancer effects of phenolic-rich extracts from Algerian propolis: A comparative study. J. Food Meas. Charact. 2023, 17, 564–575. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Abu-Seida, A. Potential Benefits of Propolis in Large and Small Animal Practices: A Narrative Review of the Literature. World’s Vet. J. 2023, 13, 441–451. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the propolis chemical composition between 2013 and 2018: A review. Efood 2020, 1, 24–37. [Google Scholar] [CrossRef]
- Yabrir, B.; Touati, M.; Adli, B.; Bezini, E.; Ghafoul, M.; Khalifa, S.; Guit, B. Therapeutic use of spontaneous medicinal flora from an extreme environment (dune cordon) in Djelfa region, Algeria. J. Pharm. Pharm. Res. 2018, 6, 358–373. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 protein–protein interaction inhibitors: Design, pharmacological properties and therapeutic potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Chen, B.; Miao, J.; Ye, H.; Xia, Z.; Huang, W.; Guo, J.; Liang, X.; Yin, Y.; Zheng, Y.; Cao, Y. Purification, identification, and mechanistic investigation of novel selenium-enriched antioxidant peptides from Moringa oleifera seeds. J. Agric. Food Chem. 2023, 71, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Wu, Z.; Xing, C.; Miao, Z. Small molecules inhibiting Keap1–Nrf2 protein–protein interactions: A novel approach to activate Nrf2 function. MedChemComm 2017, 8, 286–294. [Google Scholar] [CrossRef]
- Culletta, G.; Buttari, B.; Arese, M.; Brogi, S.; Almerico, A.M.; Saso, L.; Tutone, M. Natural products as non-covalent and covalent modulators of the KEAP1/NRF2 pathway exerting antioxidant effects. Eur. J. Med. Chem. 2024, 270, 116355. [Google Scholar] [CrossRef]
- Wu, X.; Wei, J.; Yi, Y.; Gong, Q.; Gao, J. Activation of Nrf2 signaling: A key molecular mechanism of protection against cardiovascular diseases by natural products. Front. Pharmacol. 2022, 13, 1057918. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational approaches in preclinical studies on drug discovery and development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Ulker, O.C. Combinative ex vivo studies and in silico models ProTox-II for investigating the toxicity of chemicals used mainly in cosmetic products. Toxicol. Mech. Methods 2022, 32, 542–548. [Google Scholar] [CrossRef]
- Kasote, D.M.; Sharbidre, A.A.; Kalyani, D.C.; Nandre, V.S.; Lee, J.H.J.; Ahmad, A.; Telke, A.A. Propolis: A natural antibiotic to combat multidrug-resistant bacteria. In Non-Traditional Approaches to Combat Antimicrobial Drug Resistance; Wani, M.Y., Ahmad, A., Eds.; Springer Nature: Singapore, 2023; pp. 281–296. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Končić, M.Z.; Kosalec, I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemotype propolis by extraction procedures. LWT–Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Cordeiro, N.; Torres, I.C.; Evtuguin, D. Lipophilic extractives from different morphological parts of banana plant “Dwarf Cavendish”. Ind. Crops Prod. 2006, 23, 201–211. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the antioxidant activity of propolis samples from different geographical regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Akinpelu, D.A.; Alayande, K.A.; Aiyegoro, O.A.; Akinpelu, O.F.; Okoh, A.I. Probable mechanisms of biocidal action of Cocos nucifera husk extract and fractions on bacteria isolates. BMC Complement. Altern. Med. 2015, 15, 116. [Google Scholar] [CrossRef]
- Bourougaa, L.; Ouassaf, M.; Shtaiwi, A. Discovery of novel potent drugs for influenza by inhibiting the vital function of neuraminidase via fragment-based drug design (FBDD) and molecular dynamics simulation strategies. J. Biomol. Struct. Dyn. 2024, 42, 92494–99308. [Google Scholar] [CrossRef] [PubMed]
- Keighobadi, M.; Emami, S.; Lagzian, M.; Fakhar, M.; Rafiei, A.; Valadan, R. Molecular modeling and structural stability of wild-type and mutant CYP51 from Leishmania major: In vitro and in silico analysis of a laboratory strain. Molecules 2018, 23, 696. [Google Scholar] [CrossRef]
- Bakchi, B.; Krishna, A.D.; Sreecharan, E.; Ganesh, V.B.J.; Niharika, M.; Maharshi, S.; Puttagunta, S.B.; Sigalapalli, D.K.; Bhandare, R.R.; Shaik, A.B. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: A medicinal chemist’s perspective. J. Mol. Struct. 2022, 1259, 132712. [Google Scholar] [CrossRef]
- Diniyah, N.; Alam, M.B.; Javed, A.; Alshammari, F.H.; Choi, H.J.; Lee, S.H. In silico and docking studies on the binding activities of Keap1 of antioxidant compounds in non-oilseed legumes. Arab. J. Chem. 2023, 16, 104414. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A web server for automatic structure matching and RMSD calculations among identical and similar compounds in protein-ligand docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Kouadri, I.; Rebiai, A.; Hemmami, H.; Seghir, B.B.; Zeghoud, S.; Berra, D.; Bouchra, R.M. Impact of geographic variation on the chemical composition and antioxidant activity of Algerian propolis. Appl. Biol. Sahar. Areas 2021, 3, 27–41. Available online: https://www.researchgate.net/publication/357469596 (accessed on 6 June 2024).
- Segueni, N.; Keskin, Ş.; Kadour, B.; Kolaylı, S.; Salah, A. Comparison between phenolic content, antioxidant, and antibacterial activity of Algerian and Turkish propolis. Comb. Chem. High Throughput Screen. 2021, 24, 1679–1687. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.D.A.; Rebouças Silva, J.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Teggar, N.; Bakchiche, B.; Abdel-Aziz, M.E.S.; Bardaweel, S.K.; Ghareeb, M.A. Chemical composition and biological evaluation of Algerian propolis from six different regions. Jordan J. Pharm. Sci. 2023, 16, 184–197. [Google Scholar] [CrossRef]
- Ouahab, A.; Grara, N.; Menaiaia, K.; Fugaldi, A.S.; Bensouici, C. Phytochemical analysis, antioxidant, and acetylcholinesterase inhibitory activity of propolis from northeastern Algeria. Phytothérapie 2023, 21, 119–129. [Google Scholar] [CrossRef]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y.J. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement. Altern. Med. 2016, 16, 65. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis diterpenes as a remarkable bio-source for drug discovery development: A review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef]
- Li, W.; Xuemei, G.; Yilin, Z.; Han, W.; Yajun, H.; Yi, H.; Zhongxiang, Z. Anticancer effects of pimaric acid is mediated via endoplasmic reticulum stress, caspase-dependent apoptosis, cell cycle arrest, and inhibition of cell migration in human ovarian cancer cells. Acta Biochim. Pol. 2022, 69, 245–250. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Oriquat, G.A.; Katekhaye, S.; Paradkar, A.; Fearnley, H.; Fearnley, J. Fatty acid analysis, chemical constituents, biological activity and pesticide residues screening in Jordanian propolis. Molecules 2021, 26, 5076. [Google Scholar] [CrossRef]
- Boulechfar, S.; Zellagui, A.; Chawki, B.; Mesbah, L.; Desdous, A. GC-MS based metabolic profile and toxicological evaluation of three Algerian propolis. Nat. Prod. Res. 2023, 39, 1718–1722. [Google Scholar] [CrossRef]
- Kartal, M.; Kaya, S.; Kurucu, S. GC-MS analysis of propolis samples from two different regions of Turkey. Z. Naturforschung C 2002, 57, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.S.; Finger, D.; Caetano, I.K.; Torres, Y.R. Multivariate GC-MS data analysis of the apolar fraction of brown propolis produced in southern Brazil. J. Apic. Res. 2024, 63, 1038–1049. [Google Scholar] [CrossRef]
- Sarıkahya, N.B.; Gören, A.C.; Okkalı, G.S.; Çöven, F.O.; Orman, B.; Kırcı, D.; Yücel, B.; Kışla, D.; Demirci, B.; Altun, M.; et al. Chemical composition and biological activities of propolis samples from different geographical regions of Turkey. Phytochem. Lett. 2021, 44, 129–136. [Google Scholar] [CrossRef]
- Ayad, A.S.; Hébert, M.P.; Doiron, J.A.; Loucif-Ayad, W.; Daas, T.; Smagghe, G.; Alburaki, M.; Barnett, D.A.; Touaibia, M.; Surette, M.E. Algerian propolis from distinct geographical locations: Chemical profiles, antioxidant capacity, cytotoxicity and inhibition of 5-lipoxygenase product biosynthesis. Chem. Biodivers. 2024, 21, e202301758. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Ceylan, F.D.; Eroglu, N.; Kaplan, M.; Olgun, E.O.; Capanoglu, E. Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Food Res. Int. 2019, 122, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The chemical composition of Brazilian green propolis and its protective effects on mouse aortic endothelial cells against inflammatory injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Hernández, I.M.; Fernández, M.C.; Rodríguez-Delgado, I.; De Oca Porto, R.M.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. Chemical characterization and antioxidant potential of Ecuadorian propolis. Phytochemistry 2022, 203, 113415. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Kerebba, N.; Rautenbach, F.; Singh, S.K.; Dua, K.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS phenolic compounds identification and quantification from ethanolic extract of Myrtus communis ‘Variegatha’: In vitro antioxidant and antidiabetic potentials. Arab. J. Chem. 2023, 16, 104447. [Google Scholar] [CrossRef]
- Sorucu, A.; Oruç, H.H. Determination of biologically active phenolic compounds in propolis by LC–MS/MS according to seasons and altitudes. J. Food Meas. Charact. 2019, 13, 2461–2469. [Google Scholar] [CrossRef]
- Anđelković, B.; Vujisić, L.; Vučković, I.; Tešević, V.; Vajs, V.; Gođevac, D. Metabolomics study of Populus type propolis. J. Pharm. Biomed. Anal. 2017, 135, 217–226. [Google Scholar] [CrossRef]
- Neto, M.R.; Tintino, S.R.; da Silva, A.R.P.; do Socorro Costa, M.; Boligon, A.A.; Matias, E.F.; Balbino, V.d.Q.; Menezes, I.R.; Coutinho, H.D.M. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef]
- Hadjab, W.; Zellagui, A.; Mokrani, M.; Öztürk, M.; Ceylan, Ö.; Gherraf, N.; Bensouici, C. Pharmacological potential effects of Algerian propolis against oxidative stress, multidrug-resistant pathogens biofilm and quorum-sensing. Turk. J. Pharm. Sci. 2024, 21, 71. [Google Scholar] [CrossRef]
- Bedaida, I.K.; Masry, S.H.D.; Mamache, B.; Shehata, M.G.; Benammar, L.; Ayachi, A. Ethanolic extract of Algerian propolis induced cell damage in Staphylococcus aureus: A promising alternative as a natural bio-preservative in food products. Acta Aliment. 2020, 49, 505–514. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Hadi, I.; Mamdooh, I.; Al-Fahham, A. The chemical compositions and antimicrobial activity of propolis: A review article. Int. J. Health Med. Res. 2024, 3, 535–539. [Google Scholar] [CrossRef]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis extract and its bioactive compounds—From traditional to modern extraction technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef] [PubMed]
- Alzain, A.A.; Mukhtar, R.M.; Abdelmoniem, N.; Shoaib, T.H.; Osman, W.; Alsulaimany, M.; Aljohani, A.K.B.; Almadani, S.A.; Alsaadi, B.H.; Althubyani, M.M.; et al. Modulation of NRF2/KEAP1-mediated oxidative stress for cancer treatment by natural products using pharmacophore-based screening, molecular docking, and molecular dynamics studies. Molecules 2023, 28, 6003. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Wilkinson, B. Drug discovery beyond the “rule-of-five”. Curr. Opin. Biotechnol. 2007, 18, 478–488. [Google Scholar] [CrossRef]
| Peak | Rt (min) | Profiled Volatile | Quantification (µg Compound/g Extract) | p-Value | |
|---|---|---|---|---|---|
| PE1 | PE2 | ||||
| 1 | 6.74 | Verbenyl ethyl ether | 29.99 ± 0.01 | - | - |
| 5 | 8.22 | α-Pinene | 12.81 ± 0.03 | - | - |
| 7 | 8.44 | α-Terpineol | 55.75 ± 0.01 | - | - |
| 8 | 8.72 | α-Terpinyl acetate | 14.65 ± 0.01 | - | - |
| 11 | 10.38 | δ-Cadinene | 12.35 ± 0.01 | - | - |
| 12 | 10.74 | β-Terpineol | 35.01 ± 0.05 | - | - |
| 13 | 10.80 | Cubebol | 14.64 ± 0.01 | - | - |
| 14 | 11.51 | Valerianol (4bH,5a-Eremophil-1(10)-ene) | 28.88 ± 0.01 | - | - |
| 30 | 15.98 | (13R)-8,13-Epoxylabd-14-ene | 17.78 ± 0.04 | - | - |
| 35 | 17.34 | Pimaric acid | 1631.19 ± 0.07 | 1505.00 ± 0.65 | 0.756 |
| 39 | 20.36 | Isopimaric acid | 404.72 ± 0.01 | 298.50 ± 0.16 | 0.319 |
| 45 | 21.00 | Callitrisic acid | 98.39 ± 0.02 | - | - |
| 42 | 20.71 | 4-epi-Abietic acid | 22.13 ± 0.07 | - | - |
| 43 | 20.97 | Dehydroabietic acid | - | 95.27 ± 0.03 | - |
| 47 | 21.93 | Methyl 7-β-hydroxydehydroabietate | 116.48 ± 0.09 | 41.96 ± 0.01 | 0.001 |
| 48 | 22.38 | Farnesol | - | 9.08 ± 0.02 | - |
| 55 | 36.56 | α-Amyrin | - | 24.87 ± 0.01 | - |
| 56 | 38.15 | Methyl commate C | - | 12.82 ± 0.04 | - |
| 57 | 38.85 | Lupeol | - | 84.97 ± 0.04 | - |
| Total terpenoids | 2494.77 μg/g | 2072.47 μg/g | 0.383 | ||
| 4 | 8.04 | Dihydrocinnamic acid | 26.68 ± 0.01 | - | - |
| 10 | 9.93 | Malic acid | - | 148.60 ± 0.06 | - |
| 20 | 13.38 | Citric acid | - | 118.51 ± 0.01 | - |
| 22 | 13.85 | Quininic acid | - | 74.02 ± 0.03 | - |
| 26 | 15.01 | 10,12-Docosadiynedioic acid | 344.62 ± 0.11 | - | - |
| 31 | 16.10 | 3,4-Dimethoxycinnamic acid | - | 39.01 ± 0.01 | - |
| 33 | 16.79 | Ferulic acid | - | 55.10 ± 0.01 | - |
| 40 | 20.53 | Caffeic acid | 151.81 ± 0.03 | 297.22 ± 0.03 | 0.004 |
| Total carboxylic acids | 523.11 μg/g | 732.44 μg/g | 0.004 | ||
| 6 | 8.29 | Butanedioic acid | 55.75 ± 0.03 | 105.95 ± 0.05 | 0.205 |
| 19 | 13.50 | Myristoleic acid | 273.55 ± 0.07 | - | - |
| 32 | 16.37 | Palmitic Acid | - | 408.71 ± 0.14 | - |
| 36 | 18.596 | Linoleic acid | - | 436.92 ± 0.10 | - |
| 37 | 18.68 | Oleic acid | - | 994.56 ± 0.15 | - |
| 41 | 20.61 | Oleamide | NQ | - | - |
| Total fatty acids | 329.30 μg/g | 1946.14 μg/g | 0.205 | ||
| 17 | 12.85 | D-Tagatose | - | 62.95 ± 0.02 | - |
| 18 | 13.35 | D-Fructose | 90.64 ± 0.73 | 394.83 ± 0.15 | 0.032 |
| 21 | 13.63 | D-Talose | - | 43.39 ± 0.01 | - |
| 23 | 14.22 | D-Psicose | 50.26 ± 0.09 | 128.86 ± 0.04 | 0.031 |
| 24 | 14.28 | D-Mannose | 73.44 ± 0.02 | 102.69 ± 0.06 | |
| 25 | 14.83 | D-Galactose | 43.75 ± 0.01 | 49.04 ± 0.01 | 0.505 |
| 28 | 15.38 | D-Glucose | 93.70 ± 0.02 | 147.76 ± 0.05 | 0.769 |
| 52 | 24.36 | Sucrose | - | 96.33 ± 0.02 | - |
| 53 | 24.88 | Methyl galactoside | - | 101.45 ± 0.02 | - |
| 54 | 26.52 | Mannobiose | - | 54.98 ± 0.03 | - |
| Total sugars | 351.79 μg/g | 1095.64 μg/g | 0.045 | ||
| 38 | 20.24 | Allocholic acid | 38.99 ± 0.04 | - | - |
| 44 | 20.81 | 17-Methylandrosta-1,4-dien-3-one | 26.59 ± 0.01 | - | - |
| 51 | 23.82 | Androsta-3,5-diene-3,17-dione | 43.16 ± 0.05 | 34.27 ± 0.07 | 0.151 |
| Total steroids | 108.74 μg/g | 34.27 μg/g | 0.151 | ||
| 3 | 7.81 | Glycerol | 15.26 ± 0.04 | 52.58 ± 0.03 | 0.140 |
| 27 | 15.09 | scyllo-Inositol | 7.43 ± 0.01 | - | - |
| 34 | 16.88 | myo-Inositol | - | 107.60 ± 0.04 | - |
| Total alcohols | 22.69 μg/g | 160.18 μg/g | 0.140 | ||
| 2 | 7.32 | Epimethendiol | 127.19 ± 0.08 | - | - |
| 9 | 9.61 | 1-Methyl 2-cyclohexene-1-methanol | 5.47 ± 0.02 | - | - |
| 15 | 11.53 | Gluconolactone | - | NQ | - |
| 16 | 12.54 | 2-Furanacetaldehyde | - | NQ | - |
| 29 | 15.66 | Galactonic acid | - | 86.65 ± 0.02 | - |
| 49 | 23.42 | Retroretinol | 15.78 ± 0.01 | - | - |
| 50 | 23.53 | Naringenin | NQ | NQ | - |
| Other compounds | |||||
| Profiled Molecule | Rt (min) | λ (nm) | [M-H]− | Quantification (µg/g Extract) | p-Value | ||
|---|---|---|---|---|---|---|---|
| PE1 | PE2 | ||||||
| 1 | Unknown | 1.66 | 208, 258, 282, 319 | 377 | - | ||
| 2 | Caffeic acid | 2.38 | 208, 324 | 179 | 572.08 ± 0.04 | - | 0.003 |
| 2.40 | 208, 290, 323 | - | 1666.71 ± 0.44 | ||||
| 3 | p-Coumaric acid | 3.44 | 208, 312 | 163 | - | 511.68 ± 0.27 | - |
| 4 | Ferulic acid derivative | 4.09 | 208,323 | 397 | 277.47 ± 0.17 | - | |
| 7.45 | 208, 324 | 137.57 ± 0.07 | - | 0.023 | |||
| 7.54 | 208, 322 | 456.98 ± 0.20 | |||||
| 5 | Gallic acid 4-O-glucoside | 17.37 | 208, 311 | 331 (377) | - | NQ | - |
| 6 | Caffeic acid isoprenyl ester I | 21.44 | 209, 269, 322 | 247 | 3518.39 ± 0.14 | - | <0.0001 |
| 21.54 | 208, 268, 320 | - | 12,127.35 ± 0.02 | ||||
| 7 | Caffeic acid isoprenyl ester II | 22.27 | 207, 299, 325 | 247 | 2572.2 ± 1.63 | - | 0.349 |
| 22.37 | - | 6820.26 ± 2.77 | |||||
| 8 | Caffeic acid isoprenyl ester III | 22.85 | 208, 290, 330 | 247 | 2743.55 ± 0.22 | - | 0.964 |
| 22.93 | - | 8932.12 ± 1.39 | |||||
| 9 | Caffeic acid phenylethyl ester | 25.39 | 209, 326 | 283 | 449.38 ± 0.46 | - | 0.157 |
| 25.50 | 208, 295, 326 | - | 2928.35 ± 1.62 | ||||
| 10 | p-Coumaric acid isoprenyl ester | 30.21 | 208, 314 | 231 | - | 728.37 ± 0.30 | - |
| 11 | Caffeic acid cinnamyl ester | 30.68 | 207, 294, 326 | 295 | - | 842.25 ± 0.39 | - |
| 12 | p-Coumaric acid hexose | 35.60 | 209, 290, 328 | 325 | - | NQ | - |
| 13 | Caffeic acid derivative | 36.85 | 209, 263, 318 | 315 (361) | 314.24 ± 0.14 | - | - |
| 14 | Ferulic acid 4-O-glucoside | 39.21 | 208, 293, 335 | 355 | - | NQ | - |
| 15 | p-Methoxycinnamic acid cinnamyl ester | 41.55 | 206, 281 | 293 (339) | - | 282.75 ± 0.35 | - |
| 16 | Benzofuran derivative | 45.83 | 229, 278 | 301 | 1273.47 ± 0.23 | - | 0.064 |
| 45.86 | 231, 276 | - | 361.13 ± 0.07 | ||||
| 17 | Ferulic acid derivative | 47.35 | 252, 326 | 517 | - | 584.66 ± 0.12 | - |
| Total phenolic acids | 11,858.35 µg/g | 35,448.18 µg/g | 0.550 | ||||
| 18 | Querceti-3-O-rhamnoside | 4.15 | 208, 297, 343 | 447 | - | NQ | - |
| 19 | Pinobanksin-5-methyl ether | 10.16 | 208, 287 | 285 | - | 1257.76 ± 1.28 | - |
| 20 | Quercetin-3-methyl-ether | 10.74 | 207, 265, 355 | 315 | - | 1398.68 ± 0.61 | 0.604 |
| 36.17 | 207, 267, 353 | 315 (361) | 1263.72 ± 0.63 | - | |||
| 21 | Pinobanksin | 12.31 | 208, 292, 335 | 271 | 3140.4 ± 0.37 | - | 0.006 |
| 12.41 | - | 7102.82 ± 0.82 | |||||
| 22 | Kaempferol | 12.80 | 207, 265, 368 | 285 | 1773.39 ± 1.53 | - | 0.598 |
| 12.89 | 206, 266, 367 | - | 646.02 ± 0.05 | ||||
| 23 | Kaempferide | 14.27 | 207, 266, 351 | 299 | 292 ± 0.11 | 339.23 ± 0.24 | 0.132 |
| 24 | Quercetin-dimethyl ether | 19.41 | 207, 261, 356 | 329 | - | 470.40 ± 0.37 | - |
| 25 | Apigenin | 23.43 | 207, 266, 358 | 269 | 1894.54 ± 0.58 | - | 0.036 |
| 23.53 | 202, 265, 358 | - | 4158.55 ± 1.62 | ||||
| 26 | Pinobanksin-3-O-acetate | 24.84 | 208, 293 | 313 | 4530.01 ± 1.47 | - | 0.038 |
| 24.93 | - | 9730.09 ± 3.59 | |||||
| 27 | Naringenin hexoside | 26.13 | 208, 331 | 433 | NQ | - | - |
| 26.24 | 203, 300, 331 | - | NQ | - | |||
| 28 | Chrysin | 29.35 | 208, 316 | 253 | - | 1151.80 ± 0.92 | - |
| 29 | Pinobanksin-3-O-propionate | 31.78 | 209, 292, 328 | 327 | 470.08 ± 0.09 | 1121.68 ± 0.13 | 0.026 |
| 30 | Pinobanksin-3-O-pentanoate | 35.21 | 288. 329 | 355 | NQ | - | - |
| 35.26 | 208, 268, 329 | - | NQ | - | |||
| 31 | Hesperetin | 35.56 | 210, 289, 368 | 317 (363) | NQ | - | - |
| 32 | Isorhamnetin | 35.85 | 210, 270, 372 | 315 | 907.19 ± 0.31 | 2767.44 ± 0.28 | 0.040 |
| 33 | Quercetin | 47.81 | 255, 367 | 301 (347) | 129.67 ± 0.09 | - | - |
| Total flavonoids | 14,401.00 µg/g | 30,144.47 µg/g | 0.009 | ||||
| 34 | 1,3-O-Caffeoyl-dihydrocaffeoylglycerol | 35.88 | 208, 260, 294, 324 | 417 | - | NQ | - |
| Other phenolic compounds | |||||||
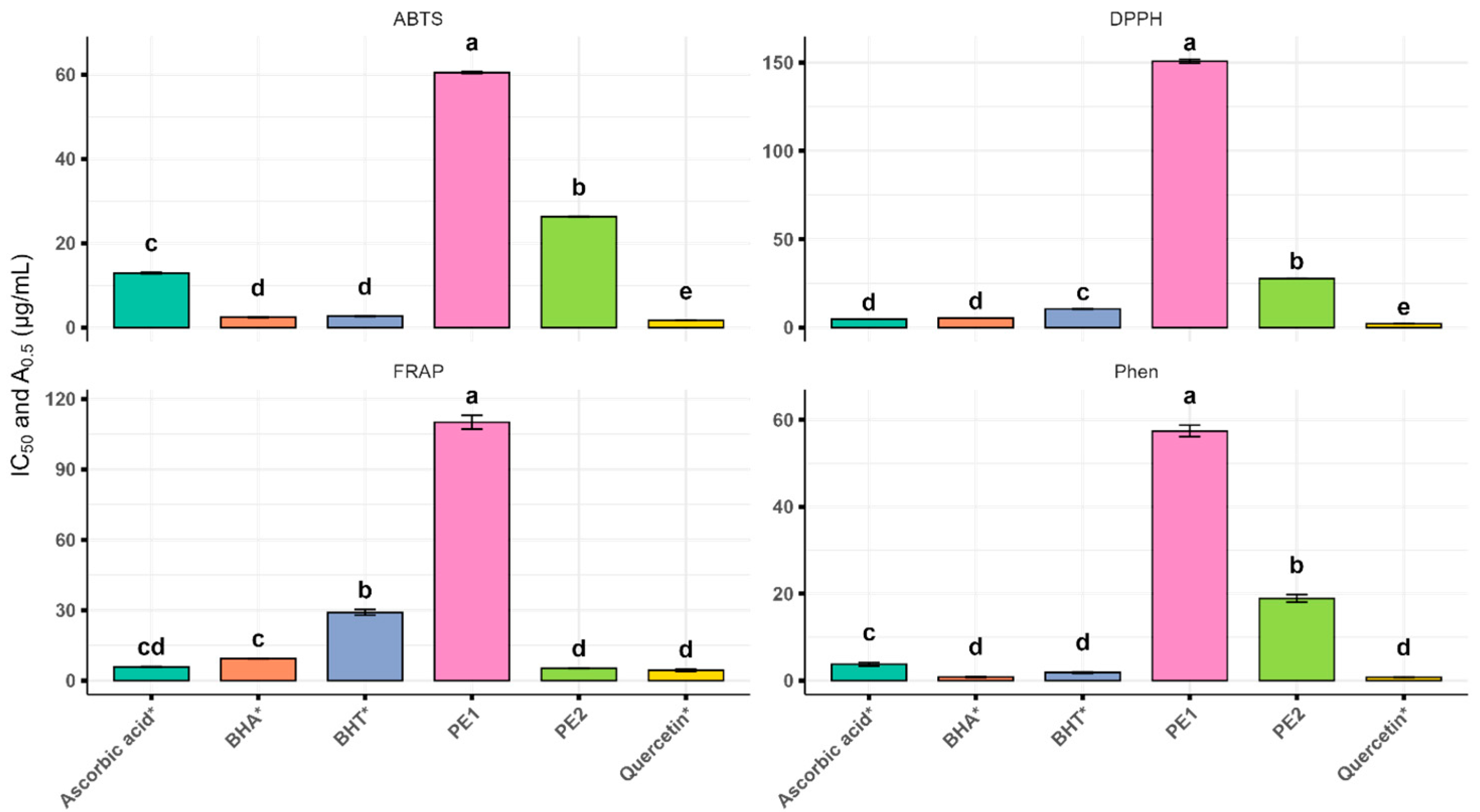
| IC50 (μg/mL) | A0.5 (μg/mL) | |||
|---|---|---|---|---|
| Sample | DPPH | ABTS | FRAP | Phen |
| PE1 | 150.68 ± 1.44 e | 60.49 ± 0.22 e | 110.09 ± 2.97 d | 57.44 ± 1.35 d |
| PE2 | 27.74 ± 0.19 d | 26.30 ± 0.12 d | 5.16 ± 0.12 a | 18.90 ± 1.13 c |
| Ascorbic acid * | 4.70 ± 0.01 b | 12.92 ± 0.18 c | 5.47 ± 0.20 a | 3.74 ± 0.17 b |
| BHT * | 10.49 ± 0.07 c | 2.68 ± 0.08 b | 28.96 ± 1.87 c | 1.84 ± 0.15 a,b |
| BHA * | 5.41 ± 0.01 b | 2.42 ± 0.11 b | 9.33 ± 0.06 b | 0.79 ± 0.09 a |
| Quercetin * | 2.12 ± 0.04 a | 1.52 ± 0.02 a | 4.38 ± 0.09 a | 0.75 ± 0.01 a |
| Sample | Strain | MIC (µg/mL) | MCB (µg/mL) |
|---|---|---|---|
| PE1 | S. aureus ATCC 25923 | 37.5 a | 37.5 a |
| E. coli ATCC 25922 | 300 b | 300 b | |
| PE2 | S. aureus ATCC 25923 | 18.75 c | 18.75 c |
| E. coli ATCC 25922 | 133 d | 133 d |
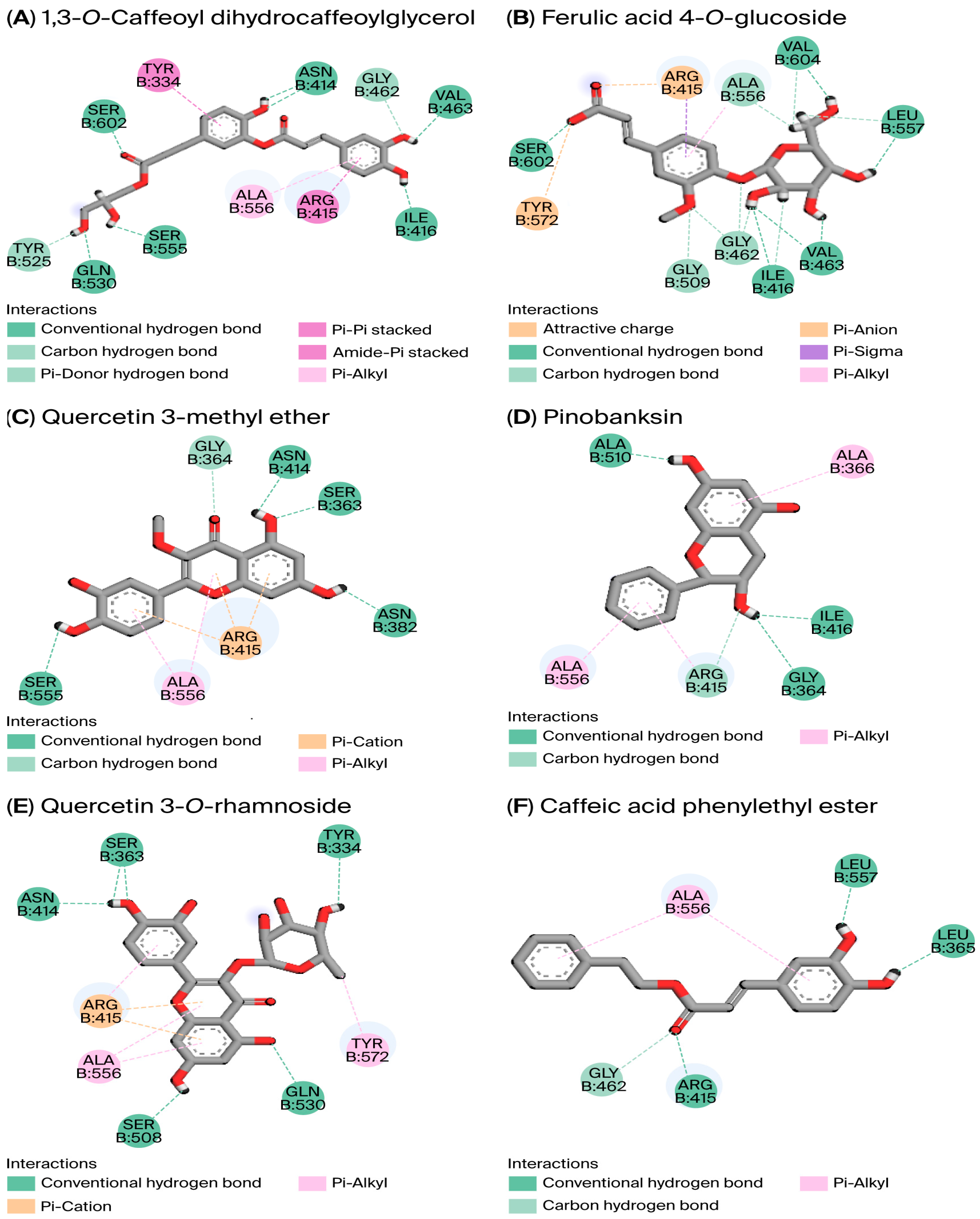
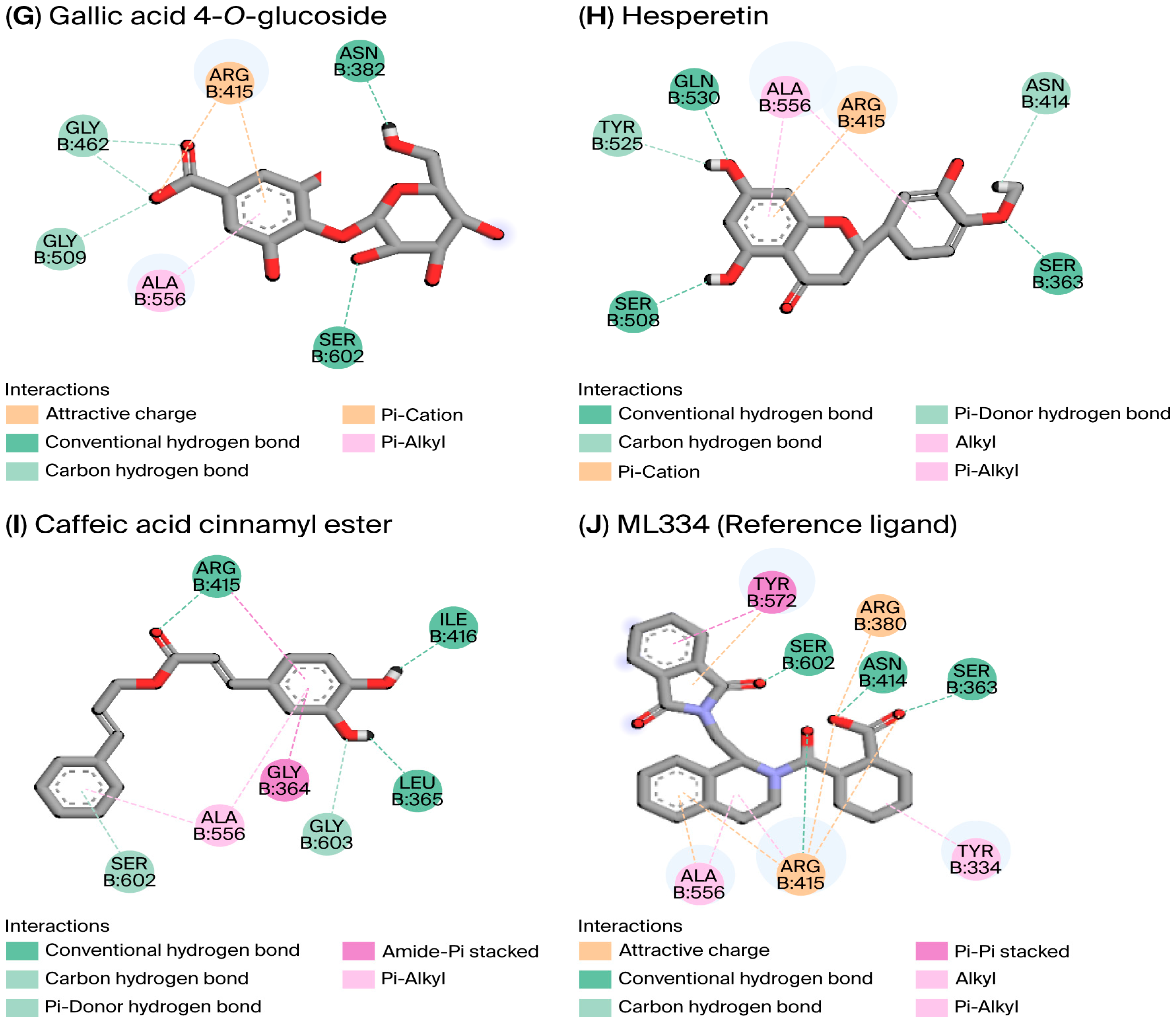
| Complex (4L7B–Compound) | Binding Affinity (kcal/mol) | Hydrogen Bonds | Distance (Å) | Hydrophobic Interactions | Distance (Å) |
|---|---|---|---|---|---|
| 1,3-O-Caffeoyl-dihydrocaffeoylglycerol | −11.020 | Asn414, Gln530, Ser602, Ser555, Asn414, Val463, Ile416, Gly462, Tyr525 | [1.81–2.99] | Tyr334, Arg415, Ile416, Ala556, | [4.63–5.15] |
| Ferulic acid 4-O-glucoside | −10.021 | Ser602, Ile416, Val463, Leu557, Val604, Gly462, Gly509, Ala556 | [1.61–2.81] | Arg415, Ala556 | [2.83–3.45] |
| Quercetin 3-methyl ether | −8.991 | Ser363, Asn414, Asn382, Ser555, Gly364, | [1.72–2.79] | Arg415, Ala556 | [5.21–5.42] |
| Pinobanksin | −8.379 | Gly364, Ile416, Ala510, Arg415 | [1.64–2.99] | Ala336, Arg415, Ala556 | [3.62–5.47] |
| Quercetin 3-O-rhamnoside | −8.335 | Ser363, Gln530, Ser508, Asn414, Tyr334 | [1.91–2.82] | Tyr572, Ala556, Arg415 | [4.34–4.89] |
| Caffeic acid phenylethyl ester | −7.180 | Arg415, Leu557, Leu365, Gly462 | [1.83–2.78] | Ala556 | [4.09] |
| Gallic acid 4-O-glucoside | −6.971 | Ser602, Asn382, Arg415, Gly462, Gly509 | [1.83–3.10] | Arg415, Ala556 | [3.59–4.77] |
| Hesperetin | −6.990 | Ser363, Gln530, Ser508, Asn414, Tyr525 | [1.95–2.68] | Ala556 | [4.87] |
| Caffeic acid cinnamyl ester | −7.009 | Arg415, Leu365, Ile416, Gly603, Ser602 | [1.88–2.97] | Gly364, Leu365, Arg415, Ile416, Ala556, | [4.83–5.03] |
| ML334 (reference ligand) | −6.807 | Ser363, Asn414, Arg415, Ser602, Arg380 | [1.71–3.04] | Arg415, Ala556, Tyr334 | [3.48–4.85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reguig, A.; Messai, A.; Bedaida, I.K.; Pinto, D.C.G.A.; Bensouici, C.; Ouamane, A.T.; Silva, A.M.S.; Roy, J.-P. Comparative Molecular Profiling and Bioactivity Analysis of Algerian Propolis: Antioxidant, Antibacterial Activities, and In Silico NRF2-KEAP1 Pathway Modulation. Curr. Issues Mol. Biol. 2025, 47, 761. https://doi.org/10.3390/cimb47090761
Reguig A, Messai A, Bedaida IK, Pinto DCGA, Bensouici C, Ouamane AT, Silva AMS, Roy J-P. Comparative Molecular Profiling and Bioactivity Analysis of Algerian Propolis: Antioxidant, Antibacterial Activities, and In Silico NRF2-KEAP1 Pathway Modulation. Current Issues in Molecular Biology. 2025; 47(9):761. https://doi.org/10.3390/cimb47090761
Chicago/Turabian StyleReguig, Amel, Ahmed Messai, Ibtissam Kahina Bedaida, Diana C. G. A. Pinto, Chawki Bensouici, Abdelmoneim Tarek Ouamane, Artur M. S. Silva, and Jean-Philippe Roy. 2025. "Comparative Molecular Profiling and Bioactivity Analysis of Algerian Propolis: Antioxidant, Antibacterial Activities, and In Silico NRF2-KEAP1 Pathway Modulation" Current Issues in Molecular Biology 47, no. 9: 761. https://doi.org/10.3390/cimb47090761
APA StyleReguig, A., Messai, A., Bedaida, I. K., Pinto, D. C. G. A., Bensouici, C., Ouamane, A. T., Silva, A. M. S., & Roy, J.-P. (2025). Comparative Molecular Profiling and Bioactivity Analysis of Algerian Propolis: Antioxidant, Antibacterial Activities, and In Silico NRF2-KEAP1 Pathway Modulation. Current Issues in Molecular Biology, 47(9), 761. https://doi.org/10.3390/cimb47090761








