The Diverse Roles of Mitochondria in Regulating Cancer Metastasis
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Dissemination
3.1. Stress Selection

3.2. Transdifferentiation
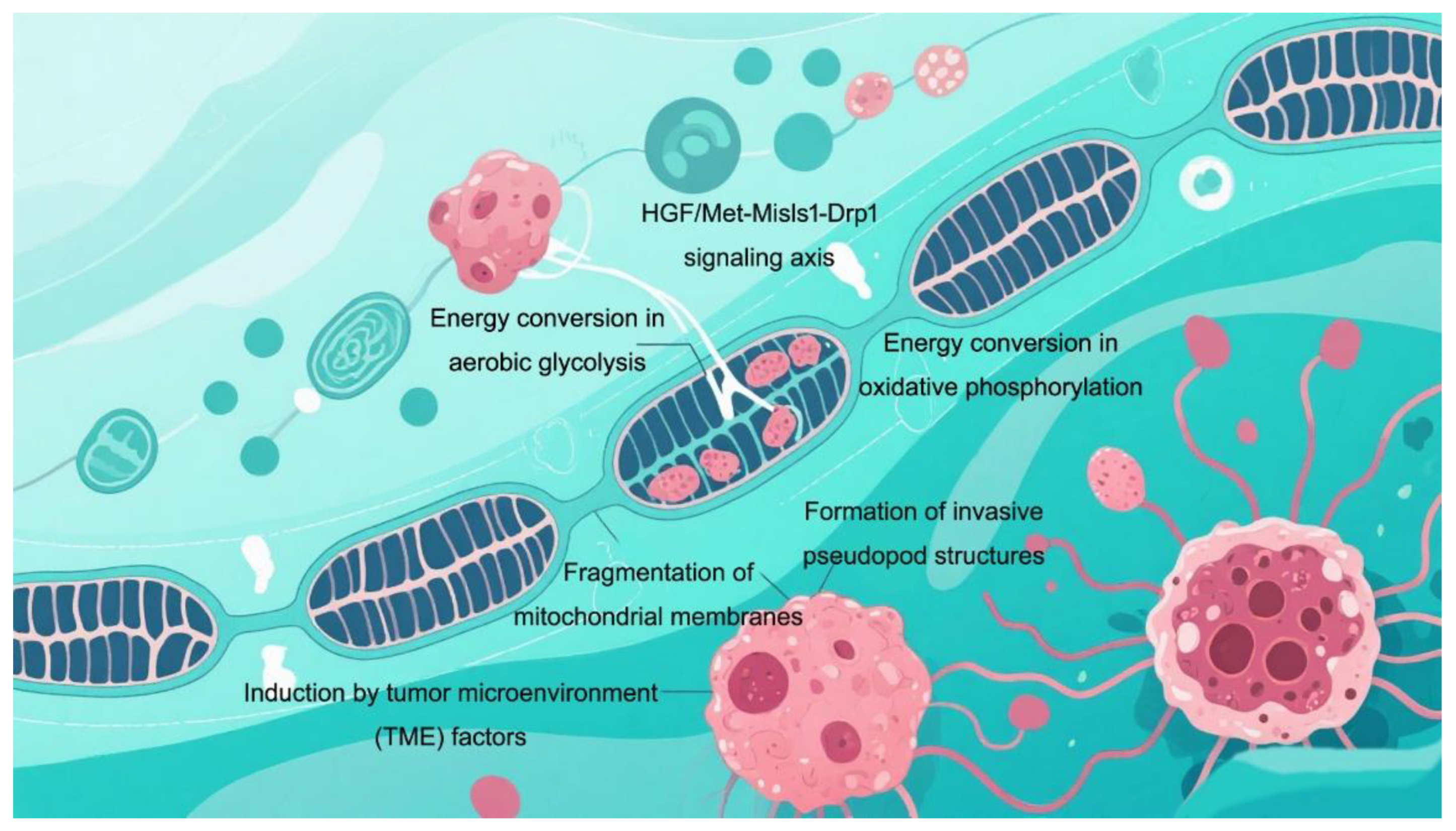
3.3. Migration out of Nest
4. Dormancy
4.1. Dedifferentiation
4.2. Restore Stem Cell Characteristics
5. Colonization
5.1. Differentiation
5.2. Adhesion and Proliferation
6. Regulation of Microenvironment
7. Emerging Perspectives on Therapy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hanahan, D.; Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 2023, 41, 573–580. [Google Scholar] [CrossRef]
- Loukas, I.; Simeoni, F.; Milan, M.; Inglese, P.; Patel, H.; Goldstone, R.; East, P.; Strohbuecker, S.; Mitter, R.; Talsania, B.; et al. Selective advantage of epigenetically disrupted cancer cells via phenotypic inertia. Cancer Cell 2023, 41, 70–87.e14. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Ebright, R.Y.; Haber, D.A.; Maheswaran, S. Translational Regulation of Cancer Metastasis. Cancer Res. 2021, 81, 517–524. [Google Scholar] [CrossRef]
- Fane, M.E.; Chhabra, Y.; Alicea, G.M.; Maranto, D.A.; Douglass, S.M.; Webster, M.R.; Rebecca, V.W.; Marino, G.E.; Almeida, F.; Ecker, B.L.; et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature 2022, 606, 396–405, Erratum in Nature 2025, 638, E31. [Google Scholar] [CrossRef] [PubMed]
- Bertola, N.; Degan, P.; Cappelli, E.; Ravera, S. Mutated FANCA Gene Role in the Modulation of Energy Metabolism and Mitochondrial Dynamics in Head and Neck Squamous Cell Carcinoma. Cells 2022, 11, 2353. [Google Scholar] [CrossRef]
- Li, H.; Guglielmetti, C.; Sei, Y.J.; Zilberter, M.; Le Page, L.M.; Shields, L.; Yang, J.; Nguyen, K.; Tiret, B.; Gao, X.; et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023, 42, 112335. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689, Erratum in Nat. Chem. Biol. 2019, 15, 757. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Torrealba, N.; Tomkova, V.; Jadhav, S.B.; Blazkova, K.; Merta, L.; Lettlova, S.; Adamcová, M.K.; Rosel, D.; Brábek, J.; et al. Targeting Mitochondrial Iron Metabolism Suppresses Tumor Growth and Metastasis by Inducing Mitochondrial Dysfunction and Mitophagy. Cancer Res. 2021, 81, 2289–2303. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Nobre, A.R.; Mondal, C.; Taha, I.; Farias, E.F.; Fertig, E.J.; Naba, A.; Aguirre-Ghiso, J.A.; Bravo-Cordero, J.J. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 2022, 3, 90–107. [Google Scholar] [CrossRef]
- Subramanian, S.; Jonas, E.A. Mitochondria: Powerhouses of presynaptic plasticity. J. Physiol. 2021, 599, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Pei, G.; Min, J.; Rajapakshe, K.I.; Branchi, V.; Liu, Y.; Selvanesan, B.C.; Thege, F.; Sadeghian, D.; Zhang, D.; Cho, K.S.; et al. Spatial mapping of transcriptomic plasticity in metastatic pancreatic cancer. Nature 2025, 642, 212–221. [Google Scholar] [CrossRef]
- Zhang, W.; Bado, I.L.; Hu, J.; Wan, Y.W.; Wu, L.; Wang, H.; Gao, Y.; Jeong, H.H.; Xu, Z.; Hao, X.; et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 2021, 184, 2471–2486.e20. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaudhary, A.K.; Woytash, J.; Inigo, J.R.; Gokhale, A.A.; Bshara, W.; Attwood, K.; Wang, J.; Spernyak, J.A.; Rath, E.; et al. A mitochondrial unfolded protein response inhibitor suppresses prostate cancer growth in mice via HSP60. J. Clin. Investig. 2022, 132, e149906. [Google Scholar] [CrossRef]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and ischemia reperfusion injury. Curr. Opin. Organ. Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef]
- Willson, J.A.; Arienti, S.; Sadiku, P.; Reyes, L.; Coelho, P.; Morrison, T.; Rinaldi, G.; Dockrell, D.H.; Whyte, M.K.B.; Walmsley, S.R. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood 2022, 139, 281–286. [Google Scholar] [CrossRef]
- Wu, Z.; Oeck, S.; West, A.P.; Mangalhara, K.C.; Sainz, A.G.; Newman, L.E.; Zhang, X.O.; Wu, L.; Yan, Q.; Bosenberg, M.; et al. Mitochondrial DNA Stress Signalling Protects the Nuclear Genome. Nat. Metab. 2019, 1, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Takehara, M.; Sato, Y.; Kimura, T.; Noda, K.; Miyamoto, H.; Fujino, Y.; Miyoshi, J.; Nakamura, F.; Wada, H.; Bando, Y.; et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci. 2020, 111, 2883–2894. [Google Scholar]
- Zhang, W.; Xu, F.; Yao, J.; Mao, C.; Zhu, M.; Qian, M.; Hu, J.; Zhong, H.; Zhou, J.; Shi, X.; et al. Single-cell metabolic fingerprints discover a cluster of circulating tumor cells with distinct metastatic potential. Nat. Commun. 2023, 14, 2485. [Google Scholar] [CrossRef]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003, Erraum in Nat. Cell Biol. 2014, 16, 1125. [Google Scholar]
- Wang, Y.; Jasper, H.; Toan, S.; Muid, D.; Chang, X.; Zhou, H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021, 45, 102049. [Google Scholar] [CrossRef]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar]
- Steuer, C. 2025. Available online: https://BioRender.com/zqqq2q2 (accessed on 29 August 2025).
- Lackner, L.L.; Ping, H.; Graef, M.; Murley, A.; Nunnari, J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc. Natl. Acad. Sci. USA 2013, 110, E458–E467. [Google Scholar] [CrossRef]
- Kazdal, D.; Harms, A.; Endris, V.; Penzel, R.; Kriegsmann, M.; Eichhorn, F.; Muley, T.; Stenzinger, A.; Pfarr, N.; Weichert, W.; et al. Prevalence of somatic mitochondrial mutations and spatial distribution of mitochondria in non-small cell lung cancer. Br. J. Cancer 2017, 117, 220–226. [Google Scholar] [CrossRef]
- Lewis, S.C.; Uchiyama, L.F.; Nunnari, J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 2016, 353, aaf5549. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kristian, T.; Pivovarova, N.B.; Fiskum, G.; Andrews, S.B. Calcium-induced precipitate formation in brain mitochondria: Composition, calcium capacity, and retention. J. Neurochem. 2007, 102, 1346–1356. [Google Scholar] [CrossRef]
- Baechler, B.L.; Bloemberg, D.; Quadrilatero, J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 2019, 15, 1606–1619. [Google Scholar] [CrossRef]
- Kalkavan, H.; Chen, M.J.; Crawford, J.C.; Quarato, G.; Fitzgerald, P.; Tait, S.W.G.; Goding, C.R.; Green, D.R. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 2022, 185, 3356–3374.e22. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.R. Bax/Bak-dependent, Drp1-independent Targeting of X-linked Inhibitor of Apoptosis Protein (XIAP) into Inner Mitochondrial Compartments Counteracts Smac/DIABLO-dependent Effector Caspase Activation. J. Biol. Chem. 2015, 290, 22005–22018. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, V.; Mikhailova, M.; Pulkrabek, D.J.; Dong, Z.; Venkatachalam, M.A.; Saikumar, P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001, 276, 18361–18374. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Meng, L.; Gao, J.; Lu, M.; Guo, C.; Li, Y.; Rong, Z.; Ye, Y. PHB2 promotes colorectal cancer cell proliferation and tumorigenesis through NDUFS1-mediated oxidative phosphorylation. Cell Death Dis. 2023, 14, 44. [Google Scholar] [CrossRef]
- Sen, K.; Pati, R.; Jha, A.; Mishra, G.P.; Prusty, S.; Chaudhary, S.; Swetalika, S.; Podder, S.; Sen, A.; Swain, M.; et al. NCoR1 controls immune tolerance in conventional dendritic cells by fine-tuning glycolysis and fatty acid oxidation. Redox Biol. 2023, 59, 102575. [Google Scholar]
- Puccini, J.; Wei, J.; Tong, L.; Bar-Sagi, D. Cytoskeletal association of ATP citrate lyase controls the mechanodynamics of macropinocytosis. Proc. Natl. Acad. Sci. USA 2023, 120, e2213272120. [Google Scholar] [PubMed]
- Kidwell, C.U.; Casalini, J.R.; Pradeep, S.; Scherer, S.D.; Greiner, D.; Bayik, D.; Watson, D.C.; Olson, G.S.; Lathia, J.D.; Johnson, J.S.; et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. Elife 2023, 12, e85494. [Google Scholar] [CrossRef]
- Shen, Y.A.; Wang, C.Y.; Hsieh, Y.T.; Chen, Y.J.; Wei, Y.H. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 2015, 14, 86–98. [Google Scholar] [CrossRef]
- Datta, S.R.; Ranger, A.M.; Lin, M.Z.; Sturgill, J.F.; Ma, Y.C.; Cowan, C.W.; Dikkes, P.; Korsmeyer, S.J.; Greenberg, M.E. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 2002, 3, 631–643. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, C.S.; Li, M.; Wang, W.; Wang, Z.; Hawley, S.A.; Ma, T.; Feng, J.W.; Tian, X.; Qi, Q.; et al. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 2019, 29, 460–473. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Zhang, C.S.; Wu, Q.; Yu, X.; Zhou, R.; Meng, F.; Wang, A.; Zhang, F.; Chen, S.; et al. AMPK directly phosphorylates TBK1 to integrate glucose sensing into innate immunity. Mol. Cell 2022, 82, 4519–4536.e7. [Google Scholar] [CrossRef]
- Xie, L.; Yuan, Y.; Xu, S.; Lu, S.; Gu, J.; Wang, Y.; Wang, Y.; Zhang, X.; Chen, S.; Li, J.; et al. Downregulation of hepatic ceruloplasmin ameliorates NAFLD via SCO1-AMPK-LKB1 complex. Cell Rep. 2022, 41, 111498. [Google Scholar] [CrossRef]
- Krebs, A.M.; Mitschke, J.; Lasierra Losada, M.; Schmalhofer, O.; Boerries, M.; Busch, H.; Boettcher, M.; Mougiakakos, D.; Reichardt, W.; Bronsert, P.; et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19, 518–529. [Google Scholar] [CrossRef]
- Marjanovic, N.D.; Hofree, M.; Chan, J.E.; Canner, D.; Wu, K.; Trakala, M.; Hartmann, G.G.; Smith, O.C.; Kim, J.Y.; Evans, K.V.; et al. Emergence of a High-Plasticity Cell State during Lung Cancer Evolution. Cancer Cell 2020, 38, 229–246.e13. [Google Scholar] [CrossRef]
- Vaubel, R.A.; Tian, S.; Remonde, D.; Schroeder, M.A.; Mladek, A.C.; Kitange, G.J.; Caron, A.; Kollmeyer, T.M.; Grove, R.; Peng, S.; et al. Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin. Cancer Res. 2020, 26, 1094–1104. [Google Scholar] [CrossRef]
- Foroni, C.; Broggini, M.; Generali, D.; Damia, G. Epithelial-mesenchymal transition and breast cancer: Role, molecular mechanisms and clinical impact. Cancer Treat. Rev. 2012, 38, 689–697. [Google Scholar] [CrossRef]
- Addison, J.B.; Voronkova, M.A.; Fugett, J.H.; Lin, C.C.; Linville, N.C.; Trinh, B.; Livengood, R.H.; Smolkin, M.B.; Schaller, M.D.; Ruppert, J.M.; et al. Functional Hierarchy and Cooperation of EMT Master Transcription Factors in Breast Cancer Metastasis. Mol. Cancer Res. 2021, 19, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Pandey, C.; Sze, S.K.; Thanabalu, T. Hypoxia activated EGFR signaling induces epithelial to mesenchymal transition (EMT). PLoS ONE 2012, 7, e49766. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Zhu, J.; Gu, X.; Yang, Y.; Liu, Y.; Wang, Z.; Zhao, Y.; Wu, H.; Huang, G.; Lu, C. Human Schlafen 5 regulates reversible epithelial and mesenchymal transitions in breast cancer by suppression of ZEB1 transcription. Br. J. Cancer 2020, 123, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ge, X.; Chen, Y.; Hussain, T.; Liang, Z.; Dong, Y.; Wang, Y.; Tang, C.; Zhou, X. Mycobacterium bovis induces mitophagy to suppress host xenophagy for its intracellular survival. Autophagy 2022, 18, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Ordureau, A.; Swarup, S.; Paulo, J.A.; Shen, K.; Sabatini, D.M.; Harper, J.W. RAB7A phosphorylation by TBK1 promotes mitophagy via the PINK-PARKIN pathway. Sci. Adv. 2018, 4, eaav0443. [Google Scholar] [CrossRef]
- Pappas, G.; Wilkinson, M.L.; Gow, A.J. Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy. Nitric Oxide 2023, 131, 8–17. [Google Scholar] [CrossRef]
- Park, C.W.; Hong, S.M.; Kim, E.S.; Kwon, J.H.; Kim, K.T.; Nam, H.G.; Choi, K.Y. BNIP3 is degraded by ULK1-dependent autophagy via MTORC1 and AMPK. Autophagy 2013, 9, 345–360. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate hypoxia promotes skeletal muscle cell growth and hypertrophy in C2C12 cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef]
- Jehanno, C.; Vulin, M.; Richina, V.; Richina, F.; Bentires-Alj, M. Phenotypic plasticity during metastatic colonization. Trends Cell Biol. 2022, 32, 854–867. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, D.; Yang, P.; Guo, R.; Kong, M.; Gao, Y.; Yu, X.; Lu, X.; Fan, X. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA Sequencing. Clin. Transl. Med. 2022, 12, e730. [Google Scholar] [CrossRef] [PubMed]
- Shokouhian, B.; Negahdari, B.; Heydari, Z.; Totonchi, M.; Aboulkheyr Es, H.; Piryaei, A.; Mostafavi, E.; Vosough, M. HNF4α is possibly the missing link between epithelial-mesenchymal transition and Warburg effect during hepatocarcinogenesis. Cancer Sci. 2023, 114, 1337–1352. [Google Scholar] [CrossRef]
- Han, M.; Bushong, E.A.; Segawa, M.; Tiard, A.; Wong, A.; Brady, M.R.; Momcilovic, M.; Wolf, D.M.; Zhang, R.; Petcherski, A.; et al. Spatial mapping of mitochondrial networks and bioenergetics in lung cancer. Nature 2023, 615, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Peng, X.D.; Qian, X.J.; Zhang, K.M.; Huang, X.; Chen, Y.H.; Li, Y.T.; Feng, G.K.; Zhang, H.L.; Xu, X.L.; et al. Fis1 phosphorylation by Met promotes mitochondrial fission and hepatocellular carcinoma metastasis. Signal Transduct. Target. Ther. 2021, 6, 401. [Google Scholar]
- Chen, Z.H.; Cao, S.H.; Ren, Z.Y.; Zhang, T.; Jiang, H.M.; Hu, Z.K.; Dong, L.H. Lactate Dehydrogenase A Crotonylation and Mono-Ubiquitination Maintains Vascular Smooth Muscle Cell Growth and Migration and Promotes Neointima Hyperplasia. J. Am. Heart Assoc. 2025, 14, e036377. [Google Scholar] [CrossRef]
- Delaunay, S.; Pascual, G.; Feng, B.; Klann, K.; Behm, M.; Hotz-Wagenblatt, A.; Richter, K.; Zaoui, K.; Herpel, E.; Münch, C.; et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature 2022, 607, 593–603. [Google Scholar] [CrossRef]
- Alshaabi, H.; Shannon, N.; Gravelle, R.; Milczarek, S.; Messier, T.; Cunniff, B. Miro1-mediated mitochondrial positioning supports subcellular redox status. Redox Biol. 2021, 38, 101818. [Google Scholar] [CrossRef]
- Desai, S.P.; Bhatia, S.N.; Toner, M.; Irimia, D. Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys. J. 2013, 104, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Laser-Azogui, A.; Diamant-Levi, T.; Israeli, S.; Roytman, Y.; Tsarfaty, I. Met-induced membrane blebbing leads to amoeboid cell motility and invasion. Oncogene 2014, 33, 1788–1798. [Google Scholar] [CrossRef][Green Version]
- Davis, R.T.; Blake, K.; Ma, D.; Gabra, M.B.I.; Hernandez, G.A.; Phung, A.T.; Yang, Y.; Maurer, D.; Lefebvre, A.E.Y.T.; Alshetaiwi, H.; et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 2020, 22, 310–320. [Google Scholar] [CrossRef]
- Xie, C.; Wang, F.Y.; Sang, Y.; Chen, B.; Huang, J.H.; He, F.J.; Li, H.; Zhu, Y.; Liu, X.; Zhuang, S.M.; et al. Mitochondrial Micropeptide STMP1 Enhances Mitochondrial Fission to Promote Tumor Metastasis. Cancer Res. 2022, 82, 2431–2443. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Santos, F.; Oliveira Santos, M.; Serafim, T.L.; Lopes, A.P.; Coutinho, D.; Carvalho, F.S.; Domingues, R.M.; Domingues, P.; Bernardes de Jesus, B.; et al. Mitochondrial Metabolism Drives Low-density Lipoprotein-induced Breast Cancer Cell Migration. Cancer Res. Commun. 2023, 3, 709–724. [Google Scholar] [CrossRef]
- Jiang, X.; Baig, A.H.; Palazzo, G.; Del Pizzo, R.; Bortecen, T.; Groessl, S.; Zaal, E.A.; Amaya Ramirez, C.C.; Kowar, A.; Aviles-Huerta, D.; et al. P53-dependent hypusination of eIF5A affects mitochondrial translation and senescence immune surveillance. Nat. Commun. 2024, 15, 7458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.J.; Du, Y.; Liu, W.; Huang, X.; Fan, Z.; Lu, J.; Yi, R.; Xiang, X.W.; Xia, X.; et al. DNA nanomachines reveal an adaptive energy mode in confinement-induced amoeboid migration powered by polarized mitochondrial distribution. Proc. Natl. Acad. Sci. USA 2024, 121, e2317492121. [Google Scholar] [CrossRef]
- Caino, M.C.; Seo, J.H.; Aguinaldo, A.; Wait, E.; Bryant, K.G.; Kossenkov, A.V.; Hayden, J.E.; Vaira, V.; Morotti, A.; Ferrero, S.; et al. A neuronal network of mitochondrial dynamics regulates metastasis. Nat. Commun. 2016, 7, 13730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wu, L.; Liu, S.P.; Jiang, M.M.; Hu, B.; Zhou, K.Q.; Guo, W.; Xu, Y.; Zhong, Y.; Zhou, X.R.; et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat. Commun. 2021, 12, 4091. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Li, H.; Mou, H.; Yinwang, E.; Xue, Y.; Wang, S.; Zhang, Y.; Wang, Z.; Chen, T.; et al. Cancer cells reprogram to metastatic state through the acquisition of platelet mitochondria. Cell Rep. 2023, 42, 113147. [Google Scholar] [CrossRef] [PubMed]
- Kiweler, N.; Delbrouck, C.; Pozdeev, V.I.; Neises, L.; Soriano-Baguet, L.; Eiden, K.; Xian, F.; Benzarti, M.; Haase, L.; Koncina, E.; et al. Mitochondria preserve an autarkic one-carbon cycle to confer growth-independent cancer cell migration and metastasis. Nat. Commun. 2022, 13, 2699. [Google Scholar] [CrossRef]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef]
- Yao, R.Q.; Ren, C.; Xia, Z.F.; Yao, Y.M. Organelle-specific autophagy in inflammatory diseases: A potential therapeutic target underlying the quality control of multiple organelles. Autophagy 2021, 17, 385–401. [Google Scholar] [CrossRef]
- Kelly, G.; Kataura, T.; Panek, J.; Ma, G.; Salmonowicz, H.; Davis, A.; Kendall, H.; Brookes, C.; Ayine-Tora, D.M.; Banks, P.; et al. Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule. Dev. Cell 2024, 59, 1924–1939.e7. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.P.; Macleod, K.F. Mitophagy in tumorigenesis and metastasis. Cell Mol. Life Sci. 2021, 78, 3817–3851. [Google Scholar]
- Hoyer, M.J.; Chitwood, P.J.; Ebmeier, C.C.; Striepen, J.F.; Qi, R.Z.; Old, W.M.; Voeltz, G.K. A Novel Class of ER Membrane Proteins Regulates ER-Associated Endosome Fission. Cell 2018, 175, 254–265.e14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Obara, C.J.; Szczesna, E.; Nixon-Abell, J.; Mahalingan, K.K.; Roll-Mecak, A.; Lippincott-Schwartz, J.; Blackstone, C. ER proteins decipher the tubulin code to regulate organelle distribution. Nature 2022, 601, 132–138, Correction in Nature 2022, 604, E11. https://doi.org/10.1038/s41586-022-04656-7. [Google Scholar] [CrossRef]
- Kakadia, J.; Biggar, K.; Jain, B.; Chen, A.W.; Nygard, K.; Li, C.; Nathanielsz, P.W.; Jansson, T.; Gupta, M.B. Mechanisms linking hypoxia to phosphorylation of insulin-like growth factor binding protein-1 in baboon fetuses with intrauterine growth restriction and in cell culture. FASEB J. 2021, 35, e21788. [Google Scholar] [CrossRef]
- Horak, P.; Crawford, A.R.; Vadysirisack, D.D.; Nash, Z.M.; DeYoung, M.P.; Sgroi, D.; Ellisen, L.W. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 4675–4680. [Google Scholar] [CrossRef]
- Miao, Z.F.; Adkins-Threats, M.; Burclaff, J.R.; Osaki, L.H.; Sun, J.X.; Kefalov, Y.; He, Z.; Wang, Z.N.; Mills, J.C. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell 2020, 26, 910–925.e6. [Google Scholar] [CrossRef]
- Miao, Z.F.; Lewis, M.A.; Cho, C.J.; Adkins-Threats, M.; Park, D.; Brown, J.W.; Sun, J.X.; Burclaff, J.R.; Kennedy, S.; Lu, J.; et al. A Dedicated Evolutionarily Conserved Molecular Network Licenses Differentiated Cells to Return to the Cell Cycle. Dev. Cell 2020, 55, 178–194.e7. [Google Scholar] [CrossRef]
- Mayer, D.; Stadler, M.B.; Rittirsch, M.; Hess, D.; Lukonin, I.; Winzi, M.; Smith, A.; Buchholz, F.; Betschinger, J. Zfp281 orchestrates interconversion of pluripotent states by engaging Ehmt1 and Zic2. EMBO J. 2020, 39, e102591. [Google Scholar] [CrossRef]
- Xiang, L.; Mou, J.; Shao, B.; Wei, Y.; Liang, H.; Takano, N.; Semenza, G.L.; Xie, G. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 2019, 10, 40. [Google Scholar] [CrossRef]
- Nakagawa, M.; Takizawa, N.; Narita, M.; Ichisaka, T.; Yamanaka, S. Promotion of direct reprogramming by transformation-deficient Myc. Proc. Natl. Acad. Sci. USA 2010, 107, 14152–14157. [Google Scholar] [CrossRef]
- Ying, Z.; Xiang, G.; Zheng, L.; Tang, H.; Duan, L.; Lin, X.; Zhao, Q.; Chen, K.; Wu, Y.; Xing, G.; et al. Short-Term Mitochondrial Permeability Transition Pore Opening Modulates Histone Lysine Methylation at the Early Phase of Somatic Cell Reprogramming. Cell Metab. 2018, 28, 935–945.e5, Erratum in Cell Metab. 2019, 29, 502. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Beppu, A.K.; Zhao, J.; Yao, C.; Carraro, G.; Israely, E.; Coelho, A.L.; Drake, K.; Hogaboam, C.M.; Parks, W.C.; Kolls, J.K.; et al. Epithelial plasticity and innate immune activation promote lung tissue remodeling following respiratory viral infection. Nat. Commun. 2023, 14, 5814. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Recondo, G.; Che, J.; Jänne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef]
- Corr, B.R.; Erickson, B.K.; Barber, E.L.; Fisher, C.M.; Slomovitz, B. Advances in the management of endometrial cancer. BMJ 2025, 388, e080978. [Google Scholar] [CrossRef]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Conti, S.; Venturini, V.; Cañellas-Socias, A.; Cortina, C.; Abenza, J.F.; Stephan-Otto Attolini, C.; Middendorp Guerra, E.; Xu, C.K.; Li, J.H.; Rossetti, L.; et al. Membrane to cortex attachment determines different mechanical phenotypes in LGR5+ and LGR5- colorectal cancer cells. Nat. Commun. 2024, 15, 3363. [Google Scholar] [CrossRef]
- Tang, F.; Tie, Y.; Lan, T.X.; Yang, J.Y.; Hong, W.Q.; Chen, S.Y.; Shi, H.H.; Li, L.Q.; Zeng, H.; Min, L.; et al. Surgical Treatment of Osteosarcoma Induced Distant Pre-Metastatic Niche in Lung to Facilitate the Colonization of Circulating Tumor Cells. Adv. Sci. 2023, 10, e2207518. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Tábara, L.C.; Tilokani, L.; Paupe, V.; Anand, H.; Pogson, J.H.; Zunino, R.; McBride, H.M.; Prudent, J. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science 2020, 367, 1366–1371. [Google Scholar] [CrossRef]
- Iwata, R.; Casimir, P.; Vanderhaeghen, P. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science 2020, 369, 858–862. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Fan, H.; Lu, Y.; Qin, H.; Zhou, Y.; Gu, Y.; Zhou, J.; Wang, X.; Fan, D. High Ran level is correlated with poor prognosis in patients with colorectal cancer. Int. J. Clin. Oncol. 2013, 18, 856–863. [Google Scholar] [CrossRef]
- Mondal, C.; Di Martino, J.S.; Bravo-Cordero, J.J. Actin dynamics during tumor cell dissemination. Int. Rev. Cell Mol. Biol. 2021, 360, 65–98. [Google Scholar]
- Lin, S.; Huang, C.; Gunda, V.; Sun, J.; Chellappan, S.P.; Li, Z.; Izumi, V.; Fang, B.; Koomen, J.; Singh, P.K.; et al. Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments. Cell Rep. 2019, 28, 2824–2836.e8. [Google Scholar] [CrossRef]
- Rangaraju, V.; Lauterbach, M.; Schuman, E.M. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 2019, 176, 73–84.e15. [Google Scholar] [CrossRef] [PubMed]
- Virga, D.M.; Hamilton, S.; Osei, B.; Morgan, A.; Kneis, P.; Zamponi, E.; Park, N.J.; Hewitt, V.L.; Zhang, D.; Gonzalez, K.C.; et al. Activity-dependent compartmentalization of dendritic mitochondria morphology through local regulation of fusion-fission balance in neurons in vivo. Nat. Commun. 2024, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Fluegen, G.; Avivar-Valderas, A.; Wang, Y.; Padgen, M.R.; Williams, J.K.; Nobre, A.R.; Calvo, V.; Cheung, J.F.; Bravo-Cordero, J.J.; Entenberg, D.; et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 2017, 19, 120–132. [Google Scholar] [CrossRef]
- Castaño, Z.; San Juan, B.P.; Spiegel, A.; Pant, A.; DeCristo, M.J.; Laszewski, T.; Ubellacker, J.M.; Janssen, S.R.; Dongre, A.; Reinhardt, F.; et al. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat. Cell Biol. 2018, 20, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Mineo, M.; Ricklefs, F.; Rooj, A.K.; Lyons, S.M.; Ivanov, P.; Ansari, K.I.; Nakano, I.; Chiocca, E.A.; Godlewski, J.; Bronisz, A. The Long Non-coding RNA HIF1A-AS2 Facilitates the Maintenance of Mesenchymal Glioblastoma Stem-like Cells in Hypoxic Niches. Cell Rep. 2016, 15, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647.e7. [Google Scholar] [CrossRef]
- Waschke, J.; Drenckhahn, D.; Adamson, R.H.; Curry, F.E. Role of adhesion and contraction in Rac 1-regulated endothelial barrier function in vivo and in vitro. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H704–H711. [Google Scholar] [CrossRef]
- Herkenne, S.; Ek, O.; Zamberlan, M.; Pellattiero, A.; Chergova, M.; Chivite, I.; Novotná, E.; Rigoni, G.; Fonseca, T.B.; Samardzic, D.; et al. Developmental and Tumor Angiogenesis Requires the Mitochondria-Shaping Protein Opa1. Cell Metab. 2020, 31, 987–1003.e8. [Google Scholar] [CrossRef]
- Wright, G.L.; Maroulakou, I.G.; Eldridge, J.; Liby, T.L.; Sridharan, V.; Tsichlis, P.N.; Muise-Helmericks, R.C. VEGF stimulation of mitochondrial biogenesis: Requirement of AKT3 kinase. FASEB J. 2008, 22, 3264–3275. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, C.; Zhang, F.; Kesarwani, M.; Tu, Z.; Cai, X.; Davis, A.K.; Xu, L.; Hochstetler, C.L.; Chen, X.; et al. Adaptive responses to mTOR gene targeting in hematopoietic stem cells reveal a proliferative mechanism evasive to mTOR inhibition. Proc. Natl. Acad. Sci. USA 2021, 118, e2020102118. [Google Scholar] [CrossRef]
- Parida, P.K.; Marquez-Palencia, M.; Ghosh, S.; Khandelwal, N.; Kim, K.; Nair, V.; Liu, X.Z.; Vu, H.S.; Zacharias, L.G.; Gonzalez-Ericsson, P.I.; et al. Limiting mitochondrial plasticity by targeting DRP1 induces metabolic reprogramming and reduces breast cancer brain metastases. Nat. Cancer 2023, 4, 893–907. [Google Scholar] [CrossRef]
- Jourdain, A.A.; Begg, B.E.; Mick, E.; Shah, H.; Calvo, S.E.; Skinner, O.S.; Sharma, R.; Blue, S.M.; Yeo, G.W.; Burge, C.B.; et al. Loss of LUC7L2 and U1 snRNP subunits shifts energy metabolism from glycolysis to OXPHOS. Mol. Cell 2021, 81, 1905–1919.e12. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, Y.K.; Kim, H.; Lee, J.; Oh, M.J.; Kim, S.B.; Kim, M.; Kim, K.H.; Yoon, H.J.; Lee, M.S.; et al. Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun. 2022, 42, 716–749. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, S.; Klimcakova, E.; Johnson, R.M.; Tabariès, S.; Annis, M.G.; McGuirk, S.; Northey, J.J.; Chénard, V.; Sriram, U.; Papadopoli, D.J.; et al. PGC-1α Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017, 26, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lim, J.H.; Lee, Y.; Granter, S.R.; Thomas, A.; Vazquez, F.; Widlund, H.R.; Puigserver, P. A PGC1α-mediated transcriptional axis suppresses melanoma metastasis. Nature 2016, 537, 422–426. [Google Scholar] [CrossRef]
- Valcarcel-Jimenez, L.; Macchia, A.; Crosas-Molist, E.; Schaub-Clerigué, A.; Camacho, L.; Martín-Martín, N.; Cicogna, P.; Viera-Bardón, C.; Fernández-Ruiz, S.; Rodriguez-Hernandez, I.; et al. PGC1α Suppresses Prostate Cancer Cell Invasion through ERRα Transcriptional Control. Cancer Res. 2019, 79, 6153–6165. [Google Scholar] [CrossRef]
- Fang, Y.; Liang, S.; Gao, J.; Wang, Z.; Li, C.; Wang, R.; Yu, W. Extracellular matrix stiffness mediates radiosensitivity in a 3D nasopharyngeal carcinoma model. Cancer Cell Int. 2022, 22, 364. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; El Khalki, L.; Wang, W.; Szpendyk, J.; Sossey-Alaoui, K. Kindlin-2 regulates the oncogenic activities of integrins and TGF-β in triple-negative breast cancer progression and metastasis. Oncogene 2024, 43, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Bado, I.L.; Zhang, W.; Hu, J.; Xu, Z.; Wang, H.; Sarkar, P.; Li, L.; Wan, Y.W.; Liu, J.; Wu, W.; et al. The bone microenvironment increases phenotypic plasticity of ER+ breast cancer cells. Dev. Cell 2021, 56, 1100–1117.e9. [Google Scholar] [CrossRef]
- Tang, H.L.; Yuen, K.L.; Tang, H.M.; Fung, M.C. Reversibility of apoptosis in cancer cells. Br. J. Cancer 2009, 100, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.N.; Khosravi, M.; Rahman, H.S.; Adili, A.; Kamali, N.; Soloshenkov, P.P.; Thangavelu, L.; Saeedi, H.; Shomali, N.; Tamjidifar, R.; et al. Anastasis: Cell recovery mechanisms and potential role in cancer. Cell Commun. Signal 2022, 20, 81, Erratum in Cell Commun. Signal 2022, 20, 91. [Google Scholar]
- Moorman, A.; Benitez, E.K.; Cambulli, F.; Jiang, Q.; Mahmoud, A.; Lumish, M.; Hartner, S.; Balkaran, S.; Bermeo, J.; Asawa, S.; et al. Progressive plasticity during colorectal cancer metastasis. Nature 2025, 637, 947–954, Correction in Nature 2025, 637, E28. [Google Scholar] [CrossRef]
- Baidwan, S.; Chekuri, A.; Hynds, D.L.; Kowluru, A. Glucotoxicity promotes aberrant activation and mislocalization of Ras-related C3 botulinum toxin substrate 1 [Rac1] and metabolic dysfunction in pancreatic islet β-cells: Reversal of such metabolic defects by metformin. Apoptosis 2017, 22, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, B.; Long, L.; Luo, P.; Xiang, W.; Li, X.; Wang, H.; Jiang, Q.; Tan, X.; Luo, S.; et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat. Commun. 2021, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Guo, D.; Lin, L.; Zhao, H.; Xu, W.; Luo, S.; Jiang, X.; Li, S.; He, X.; Zhu, R.; et al. Glycolytic enzyme PFKL governs lipolysis by promoting lipid droplet-mitochondria tethering to enhance β-oxidation and tumor cell proliferation. Nat. Metab. 2024, 6, 1092–1107. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Yang, B. The Diverse Roles of Mitochondria in Regulating Cancer Metastasis. Curr. Issues Mol. Biol. 2025, 47, 760. https://doi.org/10.3390/cimb47090760
Tang S, Yang B. The Diverse Roles of Mitochondria in Regulating Cancer Metastasis. Current Issues in Molecular Biology. 2025; 47(9):760. https://doi.org/10.3390/cimb47090760
Chicago/Turabian StyleTang, Shiyu, and Biao Yang. 2025. "The Diverse Roles of Mitochondria in Regulating Cancer Metastasis" Current Issues in Molecular Biology 47, no. 9: 760. https://doi.org/10.3390/cimb47090760
APA StyleTang, S., & Yang, B. (2025). The Diverse Roles of Mitochondria in Regulating Cancer Metastasis. Current Issues in Molecular Biology, 47(9), 760. https://doi.org/10.3390/cimb47090760




