Crosstalk Between Metabolic Reprogramming and Epigenetic Modifications in Colorectal Cancer: Mechanisms and Clinical Applications
Abstract
1. Introduction
2. Method
3. Metabolic Reprogramming in CRC
3.1. Glycolytic Reprogramming
3.2. Amino Acid Metabolic Reprogramming
3.3. Lipid Metabolic Reprogramming
3.4. Reprogramming of Other Metabolic Pathways
4. Epigenetic Modifications
4.1. DNA Methylation
4.2. Histone Modification
4.3. Noncoding RNA Dysregulation
4.3.1. miRNA
4.3.2. LncRNAs
4.3.3. CircRNAs
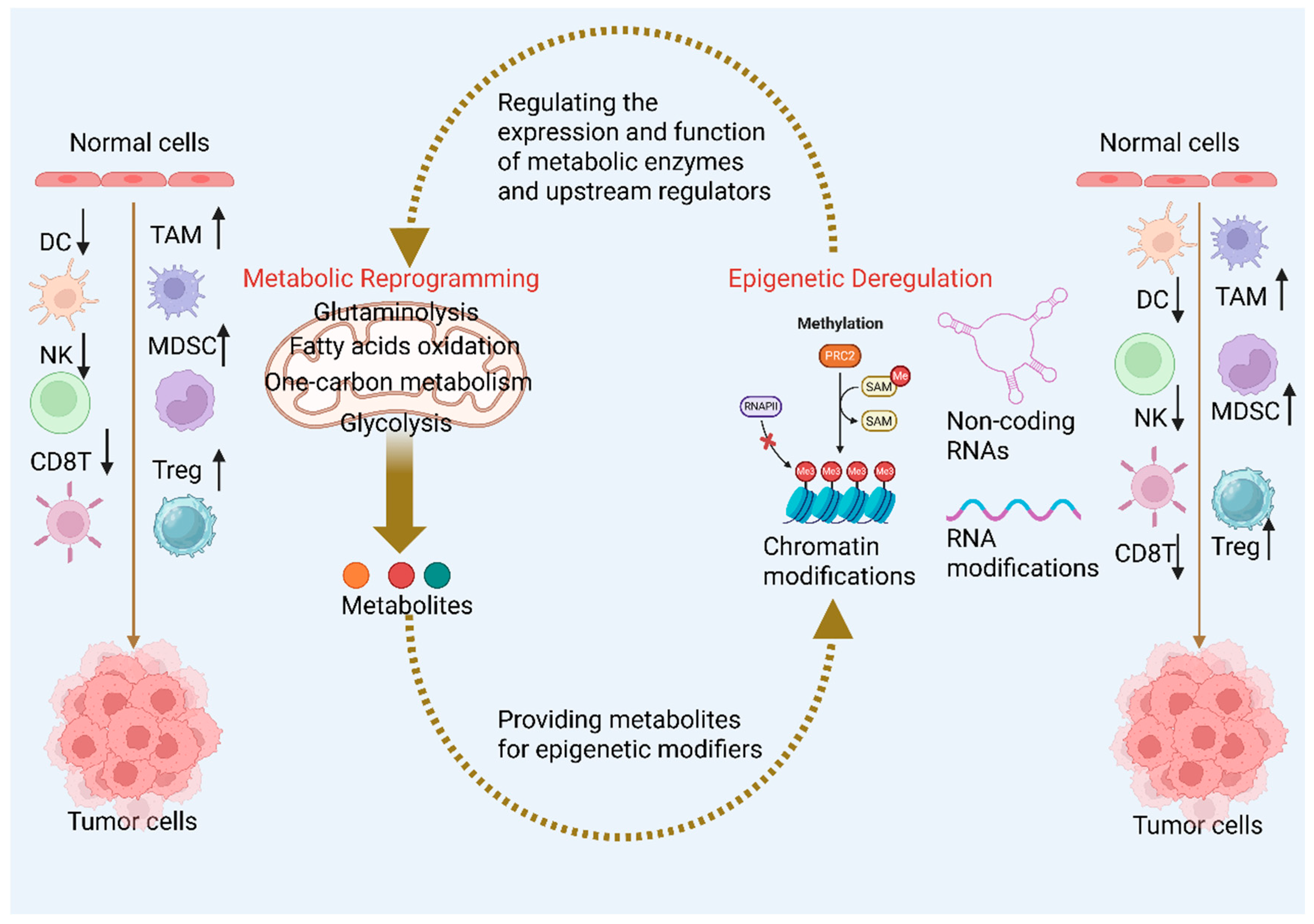
5. Interplay Between Metabolism and Epigenetic Modifications in CRC Development
5.1. Metabolic Regulation of Epigenetics
Metabolic Substrates Influence Epigenetic Enzyme Activity
- (1)
- SAM
- (2)
- Acetyl-CoA
- (3)
- TCA metabolites and demethylases
- (4)
- NAD+/NADH and deacetylases
5.2. Epigenetic Regulation of Metabolism
6. Crosstalk Between Epigenetic Modifications, Metabolic Reprogramming, and the TIME
6.1. T Cell
6.2. MDSCs
6.3. M2 Macrophages
6.4. Other Immune Cells
7. Metabolic Plasticity of Cancer Stem Cells and Therapeutic Resistance
8. Novel Directions in Diagnosis
8.1. Early Detection
8.2. Diagnostic Approaches
8.2.1. Single-Cell and Spatial Omics Analysis
8.2.2. Liquid Biopsy and Non-Invasive Testing
8.2.3. Multi-Omics Integrative Analysis
9. Novel Therapeutic Strategies
9.1. Targeting Metabolic Pathways
9.2. Targeting Epigenetic Therapies
9.3. Therapeutic Targeted Delivery of ncRNAs That Modulate Metabolic Pathways in CRC
10. Limitations and Challenges for Clinical Use of Multi-Omics and Liquid Biopsy
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, C.C.; Zaki, T.A. Changing epidemiology of colorectal cancer—Birth cohort effects and emerging risk factors. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; May, F.P.; Kupfer, S.S.; Murphy, C.C. Birth Cohort Colorectal Cancer (CRC): Implications for Research and Practice. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2024, 22, 455–469.e7. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xia, Z.; Xia, W.; Jiang, P. Metabolic reprogramming, sensing, and cancer therapy. Cell Rep. 2024, 43, 115064. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Song, H.; Li, Y.; Liu, Z.; Ye, Z.; Zhao, J.; Wu, Y.; Tang, J.; Yao, M. Metabolic reprogramming in tumor immune microenvironment: Impact on immune cell function and therapeutic implications. Cancer Lett. 2024, 597, 217076. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Hu, F.; Hu, C.; Sun, Y.; Yang, K.; Yang, S. Metabolic Reprogramming of Immune Cells in the Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 12223. [Google Scholar] [CrossRef]
- Avella Patino, D.M.; Radhakrishnan, V.; Suvilesh, K.N.; Manjunath, Y.; Li, G.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Warren, W.C.; Kaifi, J.T.; Mitchem, J.B. Epigenetic Regulation of Cancer Immune Cells. Semin. Cancer Biol. 2022, 83, 377–383. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Fang, Q.; Shi, S.; Nan, J.; He, J.; Yin, Y.; Li, X.; Li, J.; Hou, L.; et al. Crosstalk between epitranscriptomic and epigenomic modifications and its implication in human diseases. Cell Genom. 2024, 4, 100605. [Google Scholar] [CrossRef]
- Roy, S.; Deka, D.; Kondaveeti, S.B.; Ayyadurai, P.; Siripragada, S.; Philip, N.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. An overview of potential of natural compounds to regulate epigenetic modifications in colorectal cancer: A recent update. Epigenetics 2025, 20, 2491316. [Google Scholar] [CrossRef]
- Guo, L.; Lee, Y.T.; Zhou, Y.; Huang, Y. Targeting epigenetic regulatory machinery to overcome cancer therapy resistance. Semin. Cancer Biol. 2022, 83, 487–502. [Google Scholar] [CrossRef]
- Chen, Z.; Natarajan, R. Epigenetic modifications in metabolic memory: What are the memories, and can we erase them? Am. J. Physiol. Cell Physiol. 2022, 323, C570–C582. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.W.; Liu, H.L.; Su, H.F.; Luo, C.; Liang, H.F.; Zhang, B.X.; Zhang, W. m6A-regulated tumor glycolysis: New advances in epigenetics and metabolism. Mol. Cancer 2023, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel epigenetic therapeutic strategies and targets in cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166552. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2022, 13, 877–919. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, C.Y.; Pai, Y.C.; Lin, B.R.; Lee, T.C.; Liang, P.H.; Yu, L.C. Glucose Metabolites Exert Opposing Roles in Tumor Chemoresistance. Front. Oncol. 2019, 9, 1282. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Zhou, C.; Zeng, Z.; Wang, F.; Hu, T.; He, X.; Wu, X.; Wu, X.; Lan, P. Mutant KRAS triggers functional reprogramming of tumor-associated macrophages in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 144. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, C.; Wang, C.; Deng, J.; Zuo, Z.; Wu, H.; Xiong, J.; Wu, X.; Lu, H.; Hao, Q.; et al. p53-responsive CMBL reprograms glucose metabolism and suppresses cancer development by destabilizing phosphofructokinase PFKP. Cell Rep. 2023, 42, 113426. [Google Scholar] [CrossRef]
- Jing, Z.; Liu, Q.; He, X.; Jia, Z.; Xu, Z.; Yang, B.; Liu, P. NCAPD3 enhances Warburg effect through c-myc and E2F1 and promotes the occurrence and progression of colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 198. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Zhou, M.; He, J.; Li, Y.; Jiang, L.; Ran, J.; Wang, C.; Ju, C.; Du, D.; Xu, X.; Wang, X.; et al. N6-methyladenosine modification of REG1α facilitates colorectal cancer progression via β-catenin/MYC/LDHA axis mediated glycolytic reprogramming. Cell Death Dis. 2023, 14, 557. [Google Scholar] [CrossRef]
- Padder, R.A.; Bhat, Z.I.; Ahmad, Z.; Singh, N.; Husain, M. DRP1 Promotes BRAFV600E-Driven Tumor Progression and Metabolic Reprogramming in Colorectal Cancer. Front. Oncol. 2020, 10, 592130. [Google Scholar] [CrossRef]
- Guan, Y.; Yao, W.; Yu, H.; Feng, Y.; Zhao, Y.; Zhan, X.; Wang, Y. Chronic stress promotes colorectal cancer progression by enhancing glycolysis through β2-AR/CREB1 signal pathway. Int. J. Biol. Sci. 2023, 19, 2006–2019. [Google Scholar] [CrossRef]
- Barisciano, G.; Colangelo, T.; Rosato, V.; Muccillo, L.; Taddei, M.L.; Ippolito, L.; Chiarugi, P.; Galgani, M.; Bruzzaniti, S.; Matarese, G.; et al. miR-27a is a master regulator of metabolic reprogramming and chemoresistance in colorectal cancer. Br. J. Cancer 2020, 122, 1354–1366, Erratum in Br. J. Cancer 2020, 122, 1576. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, C.; Zheng, Y.; Liu, C.; Wang, X.; Cong, X. Glutamine deficiency promotes recurrence and metastasis in colorectal cancer through enhancing epithelial-mesenchymal transition. J. Transl. Med. 2022, 20, 330. [Google Scholar] [CrossRef]
- Yu, W.; Huang, J.; Dong, Q.; Li, W.; Jiang, L.; Zhang, Q.; Sun, L.; Yuan, S.; He, X. Ag120-Mediated Inhibition of ASCT2-Dependent Glutamine Transport has an Anti-Tumor Effect on Colorectal Cancer Cells. Front. Pharmacol. 2022, 13, 871392. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Pu, Y.; Liu, X.; Liu, Z.; Chen, Y.; Tang, L.; Zhou, J.; Song, Q.; Ji, Q. YTHDF1 regulates GID8-mediated glutamine metabolism to promote colorectal cancer progression in m6A-dependent manner. Cancer Lett. 2024, 601, 217186. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhang, B.; Xu, G.; Wang, L.; Wang, H.; Lin, Z.; Yu, D.; Ren, J.; Zhang, D.; Zhao, L.; et al. CEMIP, a novel adaptor protein of OGT, promotes colorectal cancer metastasis through glutamine metabolic reprogramming via reciprocal regulation of β-catenin. Oncogene 2021, 40, 6443–6455. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, S.; Qiu, Q.; Cui, G.; Zhang, Y.; Yao, M.; Li, X.; Chen, C.; Gu, J.; Wang, T.; et al. Hypoxia-induced cysteine metabolism reprogramming is crucial for the tumorigenesis of colorectal cancer. Redox Biol. 2024, 75, 103286. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, J.; Fang, K.; Jiang, H.; Leng, Z.; Wu, H.; Zhang, Z.; Wang, Z.; Li, Z.; Sun, M.; et al. FOXC1-mediated serine metabolism reprogramming enhances colorectal cancer growth and 5-FU resistance under serine restriction. Cell Commun. Signal. CCS 2025, 23, 13. [Google Scholar] [CrossRef]
- Li, J.; Song, P.; Jiang, T.; Dai, D.; Wang, H.; Sun, J.; Zhu, L.; Xu, W.; Feng, L.; Shin, V.Y.; et al. Heat Shock Factor 1 Epigenetically Stimulates Glutaminase-1-Dependent mTOR Activation to Promote Colorectal Carcinogenesis. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1828–1839. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Aladelokun, O.; Berardi, D.; Shen, X.; Marin, A.; Garcia-Milian, R.; Roper, J.; Khan, S.A.; Johnson, C.H. Asparagine synthetase and G-protein coupled estrogen receptor are critical responders to nutrient supply in KRAS mutant colorectal cancer. Int. J. Cancer 2025, 156, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Yu, F.; Jin, H.; Zhang, P.; Huang, X.; Peng, J.; Xie, X.; Li, X.; Ma, N.; Wei, Y.; et al. eIF3f Mediates SGOC Pathway Reprogramming by Enhancing Deubiquitinating Activity in Colorectal Cancer. Adv. Sci. 2023, 10, e2300759. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Kawada, K.; Iwamoto, M.; Inamoto, S.; Sasazuki, T.; Shirasawa, S.; Hasegawa, S.; Sakai, Y. Metabolic Alterations Caused by KRAS Mutations in Colorectal Cancer Contribute to Cell Adaptation to Glutamine Depletion by Upregulation of Asparagine Synthetase. Neoplasia 2016, 18, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Nenkov, M.; Ma, Y.; Gaßler, N.; Chen, Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2021, 22, 6262. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, X.; Yan, P.; Yang, C.; Li, Y.; Han, L.; Ren, X. Lipid metabolism reprogramming in colorectal cancer. J. Cell. Biochem. 2023, 124, 3–16. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, Y.; Zeng, J.; Chong, S.; Liu, Y.; Bian, Z.; Fan, S.; Chen, X. FUT2 promotes colorectal cancer metastasis by reprogramming fatty acid metabolism via YAP/TAZ signaling and SREBP-1. Commun. Biol. 2024, 7, 1297. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.J.; Zhang, L.; Yang, Q.; Sun, Q.H.; Guo, J.R.; Lei, X.Y.; He, K.Y.; Li, J.Q.; Yang, J.Y.; et al. High mobility group A1 (HMGA1) promotes the tumorigenesis of colorectal cancer by increasing lipid synthesis. Nat. Commun. 2024, 15, 9909. [Google Scholar] [CrossRef]
- Liu, X.; Lu, J.; Ni, X.; He, Y.; Wang, J.; Deng, Z.; Zhang, G.; Shi, T.; Chen, W. FASN promotes lipid metabolism and progression in colorectal cancer via the SP1/PLA2G4B axis. Cell Death Discov. 2025, 11, 122. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.; Zhang, B.; Zhang, Z.; Jia, G.; Liu, S.; Wu, T.; He, X.; Wang, N. SLC25A1 promotes tumor growth and survival by reprogramming energy metabolism in colorectal cancer. Cell Death Dis. 2021, 12, 1108. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Qian, Y.; Wei, B.; Jiang, K.; Sun, Z.; Zhang, F.; Yang, M.; Baldi, S.; Yu, X.; et al. The Lyn/RUVBL1 Complex Promotes Colorectal Cancer Liver Metastasis by Regulating Arachidonic Acid Metabolism Through Chromatin Remodeling. Adv. Sci. 2025, 12, e2406562. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Are, C. PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 3178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Liu, Y.; Li, Q.; Luo, J.; Wang, L.; Chen, Y.; Sang, C.; Zhang, W.; Ge, X.; et al. The lncRNA ZFAS1 regulates lipogenesis in colorectal cancer by binding polyadenylate-binding protein 2 to stabilize SREBP1 mRNA. Mol. Ther. Nucleic Acids 2022, 27, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. 2022, 40, 111233. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Goyert, J.W.; Solanki, S.; Kerk, S.A.; Chen, B.; Castillo, C.; Hsu, P.P.; Do, B.T.; Singhal, R.; Dame, M.K.; et al. Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat. Metab. 2021, 3, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chen, Y.; Wang, Y.Q.; Tao, E.W.; Tan, J.; Liu, Q.Q.; Li, C.M.; Tong, X.M.; Gao, Q.Y.; Hong, J.; et al. Sirtuin5 protects colorectal cancer from DNA damage by keeping nucleotide availability. Nat. Commun. 2022, 13, 6121. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Wang, L.; Wang, S.; Tang, Q. Spatial transcriptome and single-cell reveal the role of nucleotide metabolism in colorectal cancer progression and tumor microenvironment. J. Transl. Med. 2024, 22, 702. [Google Scholar] [CrossRef]
- Demetriadou, C.; Raoukka, A.; Charidemou, E.; Mylonas, C.; Michael, C.; Parekh, S.; Koufaris, C.; Skourides, P.; Papageorgis, P.; Tessarz, P.; et al. Histone N-terminal acetyltransferase NAA40 links one-carbon metabolism to chemoresistance. Oncogene 2022, 41, 571–585. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Yu, Y.; Wang, Q.; Yang, C.; Yan, Y.; Wang, F.; Mao, Y. Desulfovibrio desulfuricans and its derived metabolites confer resistance to FOLFOX through METTL3. EBioMedicine 2024, 102, 105041. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.A.; Kim, D.H.; Lee, B.H.; Hwang, D.Y.; Jeong, J.; Lee, H.J.; Matsuo, K.; Tajima, K.; Ahn, Y.O. Dietary intake of folate and alcohol, MTHFR C677T polymorphism, and colorectal cancer risk in Korea. Am. J. Clin. Nutr. 2012, 95, 405–412. [Google Scholar] [CrossRef]
- Wei, W.; Qin, B.; Wen, W.; Zhang, B.; Luo, H.; Wang, Y.; Xu, H.; Xie, X.; Liu, S.; Jiang, X.; et al. FBXW7β loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth. Signal Transduct. Target. Ther. 2023, 8, 187. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Lee, A.V.; Nestler, K.A.; Chiappinelli, K.B. Therapeutic targeting of DNA methylation alterations in cancer. Pharmacol. Ther. 2024, 258, 108640. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ma, S.; Zhang, X.N.; Zhang, Z.Y.; Zhu, H.F.; Ji, Y.H.; Li, J.; Qian, X.L.; Wang, Y.X. Hypermethylation Of ADHFE1 Promotes The Proliferation Of Colorectal Cancer Cell Via Modulating Cell Cycle Progression. OncoTargets Ther. 2019, 12, 8105–8115. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Watkins, D.N.; Jair, K.W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Keith, K.; Calendo, G.; Jelinek, J.; Madzo, J.; Gharaibeh, R.Z.; Ghosh, J.; Sapienza, C.; Jobin, C.; Issa, J.J. Association between gut microbiota and CpG island methylator phenotype in colorectal cancer. Gut Microbes 2024, 16, 2363012. [Google Scholar] [CrossRef]
- Salem, M.E.; Bodor, J.N.; Puccini, A.; Xiu, J.; Goldberg, R.M.; Grothey, A.; Korn, W.M.; Shields, A.F.; Worrilow, W.M.; Kim, E.S.; et al. Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int. J. Cancer 2020, 147, 2948–2956. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Ohashi, T.; Kobayashi, N.; Kanemoto, K.; Nose, M.; Shinozaki, R.; Kataoka, T.; Fujii, H. Aberrant DNA methylation-mediated NF-κB/fatty acid-binding protein 5 (FABP5) feed-forward loop promotes malignancy of colorectal cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159362. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H. Association between MTHFR gene C677T polymorphism and gestational diabetes mellitus in Chinese population: A meta-analysis. Front. Endocrinol. 2023, 14, 1273218. [Google Scholar] [CrossRef]
- Kim, Y.I.; Pogribny, I.P.; Basnakian, A.G.; Miller, J.W.; Selhub, J.; James, S.J.; Mason, J.B. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am. J. Clin. Nutr. 1997, 65, 46–52. [Google Scholar] [CrossRef]
- Kim, Y.I.; Christman, J.K.; Fleet, J.C.; Cravo, M.L.; Salomon, R.N.; Smith, D.; Ordovas, J.; Selhub, J.; Mason, J.B. Moderate folate deficiency does not cause global hypomethylation of hepatic and colonic DNA or c-myc-specific hypomethylation of colonic DNA in rats. Am. J. Clin. Nutr. 1995, 61, 1083–1090. [Google Scholar] [CrossRef]
- He, W.; Li, Q.; Li, X. Acetyl-CoA regulates lipid metabolism and histone acetylation modification in cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188837. [Google Scholar] [CrossRef]
- Yao, W.; Hu, X.; Wang, X. Crossing epigenetic frontiers: The intersection of novel histone modifications and diseases. Signal Transduct. Target. Ther. 2024, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, R.; Wang, B.; Xiong, R.; Cui, L.; Liao, Y.; Ruan, Y.; Fang, L.; Lu, X.; Yu, X.; et al. Inhibition of HDAC2 sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated pyroptosis in colorectal cancer. Clin. Transl. Med. 2024, 14, e1692. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Gui, T.; Zeng, X.; Deng, Y.; Wang, Z.; Wang, Y.; Yang, D.; Li, Q.; Xu, P.; Hu, R.; et al. PRMT1-mediated H4R3me2a recruits SMARCA4 to promote colorectal cancer progression by enhancing EGFR signaling. Genome Med. 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Fan, Y.; Cai, D.; Lei, R.; Wang, Q.; Li, Y.; Shen, L.; Gu, Y.; Zhang, Q.; et al. SNORA28 Promotes Proliferation and Radioresistance in Colorectal Cancer Cells through the STAT3 Pathway by Increasing H3K9 Acetylation in the LIFR Promoter. Adv. Sci. 2024, 11, e2405332. [Google Scholar] [CrossRef]
- Yang, Z.; Su, W.; Zhang, Q.; Niu, L.; Feng, B.; Zhang, Y.; Huang, F.; He, J.; Zhou, Q.; Zhou, X.; et al. Lactylation of HDAC1 Confers Resistance to Ferroptosis in Colorectal Cancer. Adv. Sci. 2025, 12, e2408845. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.; Ren, Y.; Zhang, Y.; Wang, X.; Liu, B. Association analysis of miRNA-related genetic polymorphisms in miR-143/145 and KRAS with colorectal cancer susceptibility and survival. Biosci. Rep. 2021, 41, BSR20204136. [Google Scholar] [CrossRef]
- Chen, W.S.; Leung, C.M.; Pan, H.W.; Hu, L.Y.; Li, S.C.; Ho, M.R.; Tsai, K.W. Silencing of miR-1-1 and miR-133a-2 cluster expression by DNA hypermethylation in colorectal cancer. Oncol. Rep. 2012, 28, 1069–1076. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.M.; Zhang, C.; Li, M.; Gao, B.; Hu, X.H.; Cao, J.; Wang, G.Y. The lncRNA FEZF1-AS1 Promotes the Progression of Colorectal Cancer Through Regulating OTX1 and Targeting miR-30a-5p. Oncol. Res. 2020, 28, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fan, J.B.; Gao, Y.; Zhang, M.; Zhang, L.; Yang, N.; Zhao, X. miR-135b-5p inhibits LPS-induced TNFα production via silencing AMPK phosphatase Ppm1e. Oncotarget 2016, 7, 77978–77986. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Matsuyama, T.; Toiyama, Y.; Takahashi, N.; Ishikawa, T.; Uetake, H.; Yamada, Y.; Kusunoki, M.; Calin, G.; Goel, A. CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21 ‘gene desert’, serve as important prognostic biomarkers in colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, T.; Bao, Y.; Shen, C.; Yu, C.; Zhu, X.; Tian, X.; Guo, F.; Liang, Q.; Liu, Q.; et al. LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat. Commun. 2019, 10, 3499. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.H.; Wu, Q.N.; Jin, Y.; Wang, D.S.; Chen, Y.X.; Liu, J.; Luo, X.J.; Meng, Q.; Pu, H.Y.; et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 2019, 18, 174. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, H.S.; Zhou, C.; Yang, X.; Huang, L.; Jie, H.Q.; Zeng, Z.W.; Zheng, X.B.; Li, W.X.; Liu, Z.Z.; et al. A novel protein encoded by circINSIG1 reprograms cholesterol metabolism by promoting the ubiquitin-dependent degradation of INSIG1 in colorectal cancer. Mol. Cancer 2023, 22, 72. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liu, H.S.; Xiong, L.; Yang, X.; Wang, F.W.; Zeng, Z.W.; He, X.W.; Wu, X.R.; Lan, P. A novel NF-κB regulator encoded by circPLCE1 inhibits colorectal carcinoma progression by promoting RPS3 ubiquitin-dependent degradation. Mol. Cancer 2021, 20, 103. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Cui, K.; Liu, B.; Zhou, M.; Cao, Y.; Zhang, J.; Bian, Z.; Fei, B.; Huang, Z. Hsa_circ_0062682 Promotes Serine Metabolism and Tumor Growth in Colorectal Cancer by Regulating the miR-940/PHGDH Axis. Front. Cell Dev. Biol. 2021, 9, 770006. [Google Scholar] [CrossRef]
- He, J.; Chu, Z.; Lai, W.; Lan, Q.; Zeng, Y.; Lu, D.; Jin, S.; Xu, H.; Su, P.; Yin, D.; et al. Circular RNA circHERC4 as a novel oncogenic driver to promote tumor metastasis via the miR-556-5p/CTBP2/E-cadherin axis in colorectal cancer. J. Hematol. Oncol. 2021, 14, 194. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019, 30, 157–173.E7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Liu, Z.; Lin, C.; Meng, F.; Xu, L.; Zhang, X.; Zhang, C.; Zhang, P.; Gong, S.; et al. CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol. Cancer 2021, 20, 114. [Google Scholar] [CrossRef]
- Wang, Z.; Long, H.; Chang, C.; Zhao, M.; Lu, Q. Crosstalk between metabolism and epigenetic modifications in autoimmune diseases: A comprehensive overview. Cell. Mol. Life Sci. 2018, 75, 3353–3369. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Simile, M.M.; Calvisi, D.F.; Feo, C.F.; Feo, F. S-Adenosylmethionine: From the Discovery of Its Inhibition of Tumorigenesis to Its Use as a Therapeutic Agent. Cells 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Zsigrai, S.; Kalmár, A.; Nagy, Z.B.; Barták, B.K.; Valcz, G.; Szigeti, K.A.; Galamb, O.; Dankó, T.; Sebestyén, A.; Barna, G.; et al. S-Adenosylmethionine Treatment of Colorectal Cancer Cell Lines Alters DNA Methylation, DNA Repair and Tumor Progression-Related Gene Expression. Cells 2020, 9, 1864. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Zhang, J.; Gao, H.; Wang, S.; Li, S.; Wei, P.; Liang, J.; Yu, G.; Wang, X.; et al. Cul4A-DDB1-mediated monoubiquitination of phosphoglycerate dehydrogenase promotes colorectal cancer metastasis via increased S-adenosylmethionine. J. Clin. Investig. 2021, 131, e146187. [Google Scholar] [CrossRef]
- Guertin, D.A.; Wellen, K.E. Acetyl-CoA metabolism in cancer. Nat. Rev. Cancer 2023, 23, 156–172. [Google Scholar] [CrossRef]
- Anderson, G. Tumour Microenvironment: Roles of the Aryl Hydrocarbon Receptor, O-GlcNAcylation, Acetyl-CoA and Melatonergic Pathway in Regulating Dynamic Metabolic Interactions across Cell Types-Tumour Microenvironment and Metabolism. Int. J. Mol. Sci. 2020, 22, 141. [Google Scholar] [CrossRef]
- Budagyan, K.; Cannon, A.C.; Chatoff, A.; Benton, D.; Kurimchak, A.M.; Araiza-Olivera, D.; Gerasimova, A.; Snyder, N.W.; Duncan, J.S.; Uribe-Alvarez, C.; et al. KRAS G12V mutation-selective requirement for ACSS2 in colorectal adenoma formation. Cell Rep. 2025, 44, 115444. [Google Scholar] [CrossRef]
- Eniafe, J.; Jiang, S. The functional roles of TCA cycle metabolites in cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef]
- Pianka, S.T.; Li, T.; Prins, T.J.; Eldred, B.S.C.; Kevan, B.M.; Liang, H.; Zapanta Rinonos, S.; Kornblum, H.I.; Nathanson, D.A.; Pellegrini, M.; et al. D-2-HG Inhibits IDH1mut Glioma Growth via FTO Inhibition and Resultant m6A Hypermethylation. Cancer Res. Commun. 2024, 4, 876–894. [Google Scholar] [CrossRef]
- Hu, S.S.; Han, Y.; Tan, T.Y.; Chen, H.; Gao, J.W.; Wang, L.; Yang, M.H.; Zhao, L.; Wang, Y.Q.; Ding, Y.Q.; et al. SLC25A21 downregulation promotes KRAS-mutant colorectal cancer progression by increasing glutamine anaplerosis. JCI Insight 2023, 8, e167874. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a Relevant Target in Cancer. Cells 2022, 11, 2627. [Google Scholar] [CrossRef]
- Dong, W.; Lu, J.; Li, Y.; Zeng, J.; Du, X.; Yu, A.; Zhao, X.; Chi, F.; Xi, Z.; Cao, S. SIRT1: A novel regulator in colorectal cancer. Biomed. Pharmacother. 2024, 178, 117176. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, V.; Campagna, R.; Sartini, D.; Emanuelli, M. Nicotinamide N-Methyltransferase as Promising Tool for Management of Gastrointestinal Neoplasms. Biomolecules 2022, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mazzanti, L.; Pompei, V.; Alia, S.; Vignini, A.; Emanuelli, M. The Multifaceted Role of Endothelial Sirt1 in Vascular Aging: An Update. Cells 2024, 13, 1469. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; Escudé Martinez de Castilla, P.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Z.; Yang, M.; Xiang, J.; Liu, Z. Disrupting CENP-N mediated SEPT9 methylation as a strategy to inhibit aerobic glycolysis and liver metastasis in colorectal cancer. Clin. Exp. Metastasis 2024, 41, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, J.; He, H.; Liu, H. Small molecule NSC1892 targets the CUL4A/4B-DDB1 interactions and causes impairment of CRL4(DCAF4) E3 ligases to inhibit colorectal cancer cell growth. Int. J. Biol. Sci. 2020, 16, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, C.; Yu, L.; Hou, Z.; Liu, H.; Kong, L.; Xu, Y.; He, J.; Lan, J.; Ou, Q.; et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 2024, 20, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zeng, Z. Epigenetic Activation of PTCD3 Promotes CRC Glutamine Metabolism and Metastasis via IGF2BP2-Mediated SLC38A2 m6A Modification. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2025, 39, e70558. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Zhang, W.; Huang, Y.; Wang, X.; Li, Q. Association between Metabolic Reprogramming and Immune Regulation in Digestive Tract Tumors. Oncol. Res. Treat. 2024, 47, 273–286. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Yang, X.; Liu, X.; Guo, Z.; Lin, X.; Li, L.; Huang, Z. Glycerol-3-phosphate acyltransferase 3-mediated lipid droplets accumulation confers chemoresistance of colorectal cancer. MedComm 2024, 5, e486. [Google Scholar] [CrossRef]
- Zi, R.; Zhao, X.; Liu, L.; Wang, Y.; Zhang, R.; Bian, Z.; Jiang, H.; Liu, T.; Sun, Y.; Peng, H.; et al. Metabolic-Immune Suppression Mediated by the SIRT1-CX3CL1 Axis Induces Functional Enhancement of Regulatory T Cells in Colorectal Carcinoma. Adv. Sci. 2025, 12, e2404734. [Google Scholar] [CrossRef]
- Du, Z.; Su, J.; Lin, S.; Chen, T.; Gao, W.; Wang, M.; Li, Y.; Wei, D.; Hu, Z.; Gao, C.; et al. Hydroxyphenylpyruvate Dioxygenase Is a Metabolic Immune Checkpoint for UTX-deficient Colorectal Cancer. Gastroenterology 2023, 164, 1165–1179.e13. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Chen, Y.; Yin, Y.; Chen, Y.; Chen, Q.; Hou, Y.; Shen, S.; Lv, M.; Wang, T. Gut fungi enhances immunosuppressive function of myeloid-derived suppressor cells by activating PKM2-dependent glycolysis to promote colorectal tumorigenesis. Exp. Hematol. Oncol. 2022, 11, 88. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Chen, L.; Wang, X.; Mao, X.; Wu, Z.; Shi, H.; Qi, H.; Chen, L.; Huang, Y.; et al. VSIG4 Promotes Tumour-Associated Macrophage M2 Polarization and Immune Escape in Colorectal Cancer via Fatty Acid Oxidation Pathway. Clin. Transl. Med. 2025, 15, e70340. [Google Scholar] [CrossRef]
- Liu, S.; Gong, H.; Li, P.; Hu, J.; Li, Y.; Xu, R.; Cai, J.; Wang, S.; Cai, J.; Ma, H.; et al. Chemotherapy-induced macrophage CXCL7 expression drives tumor chemoresistance via the STAT1/PHGDH-serine metabolism axis and SAM paracrine feedback to M2 polarization. Cell Death Dis. 2025, 16, 379. [Google Scholar] [CrossRef]
- Miao, H.; Ou, J.; Peng, Y.; Zhang, X.; Chen, Y.; Hao, L.; Xie, G.; Wang, Z.; Pang, X.; Ruan, Z.; et al. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat. Commun. 2016, 7, 11716. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, R.; Xiang, W.; Kang, X.; Tang, B.; Li, C.; Gao, L.; Zhang, X.; Zhang, L.; Dai, R.; et al. The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages. Signal Transduct. Target. Ther. 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Na, R.; Peng, X.; Li, H.; Ouyang, W.; Zhou, W.; You, X.; Li, Y.; Pu, X.; Zhang, K.; et al. Musashi-2 potentiates colorectal cancer immune infiltration by regulating the post-translational modifications of HMGB1 to promote DCs maturation and migration. Cell Commun. Signal. 2024, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, J.; Chen, Q.; Yuan, M.; Zeng, X.; Li, Y.; Zeng, Y.; He, M.; Zhou, Q.; Feng, D.; et al. ELFN1-AS1 promotes GDF15-mediated immune escape of colorectal cancer from NK cells by facilitating GCN5 and SND1 association. Discov. Oncol. 2023, 14, 56. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Yin, L.; Liu, S.; Li, Y.; Liao, W.; Mu, L.; Luo, X.; Qin, J. Histone lactylation enhances GCLC expression and thus promotes chemoresistance of colorectal cancer stem cells through inhibiting ferroptosis. Cell Death Dis. 2025, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, S.; Chen, S.; Chen, J.; Wang, Z.; Wang, Y.; Zheng, H. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021, 12, 449. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakata, A.; Chen, X.; Nishi, K.; Meguro-Horike, M.; Sasaki, S.; Kita, K.; Horike, S.I.; Saitoh, K.; Kato, K.; et al. Cancer stem-like properties and gefitinib resistance are dependent on purine synthetic metabolism mediated by the mitochondrial enzyme MTHFD2. Oncogene 2019, 38, 2464–2481. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, C.; Tang, Z.; Yang, Y.; Tian, Y.; Yao, H.; Zhu, X.; Zhang, Z.; Ji, J.; Zheng, X. Adenylate kinase hCINAP determines self-renewal of colorectal cancer stem cells by facilitating LDHA phosphorylation. Nat. Commun. 2017, 8, 15308. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Hou, Y.; Yang, L.; Wan, X.; Qin, Y.; Liu, Y.; Wang, R.; Zhu, P.; Teng, Y.; et al. A novel lncRNA ROPM-mediated lipid metabolism governs breast cancer stem cell properties. J. Hematol. Oncol. 2021, 14, 178. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.L.; Wu, D.Z.; Yuan, Y.W.; Xin, L. Methionine restriction enhances the chemotherapeutic sensitivity of colorectal cancer stem cells by miR-320d/c-Myc axis. Mol. Cell. Biochem. 2022, 477, 2001–2013. [Google Scholar] [CrossRef]
- Cui, F.; Chen, Y.; Wu, X.; Zhao, W. Mesenchymal stem cell-derived exosomes carrying miR-486-5p inhibit glycolysis and cell stemness in colorectal cancer by targeting NEK2. BMC Cancer 2024, 24, 1356. [Google Scholar] [CrossRef]
- Stintzing, S.; Wirapati, P.; Lenz, H.J.; Neureiter, D.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef]
- Mo, S.; Ye, L.; Wang, D.; Han, L.; Zhou, S.; Wang, H.; Dai, W.; Wang, Y.; Luo, W.; Wang, R.; et al. Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation. JAMA Oncol. 2023, 9, 770–778. [Google Scholar] [CrossRef]
- Xie, Z.; Niu, L.; Zheng, G.; Du, K.; Dai, S.; Li, R.; Dan, H.; Duan, L.; Wu, H.; Ren, G.; et al. Single-cell analysis unveils activation of mast cells in colorectal cancer microenvironment. Cell Biosci. 2023, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, X.; Li, Q.; Qin, J.; Ye, L.; Zhang, X.; Huang, X.; Wen, X.; Wang, Z.; He, W.; et al. Single-cell and spatial-resolved profiling reveals cancer-associated fibroblast heterogeneity in colorectal cancer metabolic subtypes. J. Transl. Med. 2025, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Li, Q.Q.; Zeng, Y. Clinical application of liquid biopsy in colorectal cancer: Detection, prediction, and treatment monitoring. Mol. Cancer 2024, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A.; Weng, Y.; Schoen, R.E.; Dominitz, J.A.; Desai, M.; Lieberman, D. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology 2024, 167, 378–391. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Du, M.; Gu, D.; Xin, J.; Peters, U.; Song, M.; Cai, G.; Li, S.; Ben, S.; Meng, Y.; Chu, H.; et al. Integrated multi-omics approach to distinct molecular characterization and classification of early-onset colorectal cancer. Cell Rep. Med. 2023, 4, 100974. [Google Scholar] [CrossRef]
- Kamel, F.; Eltarhoni, K.; Nisar, P.; Soloviev, M. Colorectal Cancer Diagnosis: The Obstacles We Face in Determining a Non-Invasive Test and Current Advances in Biomarker Detection. Cancers 2022, 14, 1889. [Google Scholar] [CrossRef]
- Laussel, C.; Léon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 2020, 182, 114213. [Google Scholar] [CrossRef]
- Mu, M.; Zhang, Q.; Zhao, C.; Li, X.; Chen, Z.; Sun, X.; Yu, J. 3-Bromopyruvate overcomes cetuximab resistance in human colorectal cancer cells by inducing autophagy-dependent ferroptosis. Cancer Gene Ther. 2023, 30, 1414–1425. [Google Scholar] [CrossRef]
- Miyamoto, R.; Takigawa, H.; Yuge, R.; Shimizu, D.; Ariyoshi, M.; Otani, R.; Tsuboi, A.; Tanaka, H.; Yamashita, K.; Hiyama, Y.; et al. Analysis of anti-tumor effect and mechanism of GLS1 inhibitor CB-839 in colorectal cancer using a stroma-abundant tumor model. Exp. Mol. Pathol. 2024, 137, 104896. [Google Scholar] [CrossRef] [PubMed]
- Fries, B.D.; Hummon, A.B. FAS Inhibited Proteomics and Phosphoproteomics Profiling of Colorectal Cancer Spheroids Shows Activation of Ferroptotic Death Mechanism. J. Proteome Res. 2024, 23, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Wu, D.S.; Shen, Y.A. Fatty acid synthase inhibitor cerulenin hinders liver cancer stem cell properties through FASN/APP axis as novel therapeutic strategies. J. Lipid Res. 2024, 65, 100660. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Chamoto, K.; Saeki, S.; Hatae, R.; Ikematsu, Y.; Sakai, K.; Ando, N.; Sonomura, K.; Kojima, S.; Taketsuna, M.; et al. Combination bezafibrate and nivolumab treatment of patients with advanced non-small cell lung cancer. Sci. Transl. Med. 2022, 14, eabq0021. [Google Scholar] [CrossRef]
- Laranjeira, A.B.A.; Nguyen, D.; Pelosof, L.C.; Doroshow, J.H.; Yang, S.X. Upregulation of TET2 and Resistance to DNA Methyltransferase (DNMT) Inhibitors in DNMT1-Deleted Cancer Cells. Diseases 2024, 12, 163. [Google Scholar] [CrossRef]
- Huang, K.C.; Ke, T.W.; Lai, C.Y.; Hong, W.Z.; Chang, H.Y.; Lee, C.Y.; Wu, C.H.; Chiang, S.F.; Liang, J.A.; Chen, J.Y.; et al. Inhibition of DNMTs increases neoantigen-reactive T-cell toxicity against microsatellite-stable colorectal cancer in combination with radiotherapy. Biomed. Pharmacother. 2024, 177, 116958. [Google Scholar] [CrossRef]
- Wei, T.T.; Lin, Y.T.; Tang, S.P.; Luo, C.K.; Tsai, C.T.; Shun, C.T.; Chen, C.C. Metabolic targeting of HIF-1α potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene 2020, 39, 414–427. [Google Scholar] [CrossRef]
- Humphreys, K.J.; Cobiac, L.; Le Leu, R.K.; Van der Hoek, M.B.; Michael, M.Z. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol. Carcinog. 2013, 52, 459–474. [Google Scholar] [CrossRef]
- Song, Y.; Ren, S.; Wu, S.; Liu, W.; Hu, C.; Feng, S.; Chen, X.; Tu, R.; Gao, F. Glucocorticoid promotes metastasis of colorectal cancer via co-regulation of glucocorticoid receptor and TET2. Int. J. Cancer 2025, 156, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Murphy, A.G.; Zahurak, M.; Gianino, N.; Parkinson, R.; Walker, R.; Lopez-Vidal, T.Y.; Zheng, L.; Rosner, G.; Ahuja, N.; et al. A study of using epigenetic modulators to enhance response to pembrolizumab (MK-3475) in microsatellite stable advanced colorectal cancer. Clin. Epigenetics 2023, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, J.; Guo, Z.; Gong, Y.; Zhang, T.; Huang, J.; Cheng, R.; Yu, X.; Li, Y.; Chen, L.; et al. EZH2 Inhibitors Suppress Colorectal Cancer by Regulating Macrophage Polarization in the Tumor Microenvironment. Front. Immunol. 2022, 13, 857808. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cao, Q.; Chen, C.; Du, X.; Jin, B.; Pan, J. Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells. BMC Cancer 2015, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Govan, D.; Penny, C. Epigenetic regulation in colorectal cancer: The susceptibility of microRNAs 145, 143 and 133b to DNA demethylation and histone deacetylase inhibitors. PLoS ONE 2023, 18, e0289800. [Google Scholar] [CrossRef]
- Huang, X.; Xu, X.; Ke, H.; Pan, X.; Ai, J.; Xie, R.; Lan, G.; Hu, Y.; Wu, Y. microRNA-16-5p suppresses cell proliferation and angiogenesis in colorectal cancer by negatively regulating forkhead box K1 to block the PI3K/Akt/mTOR pathway. Eur. J. Histochem. 2022, 66, 3333. [Google Scholar] [CrossRef]
- Aziret, M.; Güney Eskiler, G.; Çakar, G.; Özkan, A.D.; Ercan, M.; Bilir, C.; Polat, E.; Koçer, H.B.; Yıldırım, E.K.; Duman, M. Effect of the MiR-99b and MiR-135b on peritoneal carcinomatosis and liver metastasis in colorectal cancer. Clinics 2023, 78, 100271. [Google Scholar] [CrossRef]
- Philip, P.A.; Buyse, M.E.; Alistar, A.T.; Rocha Lima, C.M.; Luther, S.; Pardee, T.S.; Van Cutsem, E. A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. Future Oncol. 2019, 15, 3189–3196. [Google Scholar] [CrossRef]
- Pujalte-Martin, M.; Belaïd, A.; Bost, S.; Kahi, M.; Peraldi, P.; Rouleau, M.; Mazure, N.M.; Bost, F. Targeting cancer and immune cell metabolism with the complex I inhibitors metformin and IACS-010759. Mol. Oncol. 2024, 18, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Koyama, T.; Matsubara, N.; Naito, Y.; Kondo, S.; Harano, K.; Yonemori, K.; Yoh, K.; Gu, Y.; Mita, T.; et al. PD-1 inhibition with retifanlimab and/or arginase inhibition with INCB001158 in Japanese patients with solid tumors: A phase I study. Cancer Med. 2024, 13, e6980. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Saebfar, H.; Mahabady, M.K.; Orouei, S.; Hushmandi, K.; Entezari, M.; Hashemi, M.; Aref, A.R.; Hamblin, M.R.; Ang, H.L.; et al. Overcoming doxorubicin resistance in cancer: siRNA-loaded nanoarchitectures for cancer gene therapy. Life Sci. 2022, 298, 120463. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Ga, Y.J.; Kim, S.H.; Cho, Y.H.; Kim, J.W.; Kim, C.; Yeh, J.Y. Small interfering RNA (siRNA)-based therapeutic applications against viruses: Principles, potential, and challenges. J. Biomed. Sci. 2023, 30, 88. [Google Scholar] [CrossRef]
- Sendi, H.; Yazdimamaghani, M.; Hu, M.; Sultanpuram, N.; Wang, J.; Moody, A.S.; McCabe, E.; Zhang, J.; Graboski, A.; Li, L.; et al. Nanoparticle Delivery of miR-122 Inhibits Colorectal Cancer Liver Metastasis. Cancer Res. 2022, 82, 105–113. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, J.E.; Kim, E.; Lee, H.; Lee, H.; Shin, E.A.; Lee, J.W. Liver-originated small extracellular vesicles with TM4SF5 target brown adipose tissue for homeostatic glucose clearance. J. Extracell. Vesicles 2022, 11, e12262. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Schlegel, M.K.; Matsuda, S.; Brown, C.R.; Harp, J.M.; Barry, J.D.; Berman, D.; Castoreno, A.; Schofield, S.; Szeto, J.; Manoharan, M.; et al. Overcoming GNA/RNA base-pairing limitations using isonucleotides improves the pharmacodynamic activity of ESC+ GalNAc-siRNAs. Nucleic Acids Res. 2021, 49, 10851–10867. [Google Scholar] [CrossRef]


| Regulation | ncRNA | Key Mechanism | Impact on CRC | Ref. |
|---|---|---|---|---|
| ↑ | miR-21 | Suppresses autophagy via PTEN/AKT/TFEB | ↑ invasion, ↓ 5-FU sensitivity | [68] |
| ↑ | miR-452-5p | Represses PKN2/DUSP6; activates MAPK-ERK | ↑ proliferation, chemoresistance | [68] |
| ↑ | miR-496 | Inhibits RASSF6; activates Wnt signaling | ↑ EMT, motility | [69] |
| ↑ | miR-298 | Targets PTEN; activates AKT/ERK and mTOR | ↑ metabolic activity, growth | [69] |
| ↑ | miR-645 | Targets EFNA5 | ↑ migration, metastasis | [70] |
| ↓ | miR-130a-3p | Targets WNT1; blocks Wnt | ↓ growth (cells and xenograft) | [71] |
| ↓ | miR-144-5p | Represses RNF187 | ↓ migration, invasion | [71] |
| ↓ | miR-144-3p | Targets BCL6; dampens β-catenin | ↓ proliferation, cell cycle | [72] |
| ↓ | miR-215-5p | Binds CTNNBIP1→restrains Wnt | ↓ clonogenicity, liver metastasis | [72] |
| ↓ | miR-148b | p53-induced; targets p55PIK | ↓ proliferation, tumor growth | [72] |
| ↓ | miR-16 | Represses surviving (BIRC5) | ↑ apoptosis, ↓ growth | [73] |
| ↓ | miR-654-3p | Suppresses SRC | ↓ proliferation and invasion; ↑ apoptosis | [73] |
| ↑ | lncRNA CTBP1-AS2 | Sponges miR-93-5p; TGF-β1/Smad2/3 | ↑ proliferation and invasion; ↓ apoptosis | [74] |
| ↑ | lncRNA COL4A2-AS1 | Sponges miR-20b-5p; ↑HIF1A | ↑ proliferation and aerobic glycolysis | [74] |
| ↑ | lncRNA NEAT1 | Sponges miR-34a; ↑ SIRT1 (Wnt) | ↑ growth and invasion | [74] |
| ↑ | lncRNA RoR | Sponges miR-6833-3p; ↑ SMC4 | ↑ proliferation; ↓ apoptosis | [74] |
| ↑ | lncRNA SNHG8 | Sponges miR-588; ↑ ATG7 | ↑ proliferation and autophagy | [74] |
| ↑ | lncRNA CASC21 | Sponges miR-7-5p; ↑ YAP1 | ↑ migration and EMT; ↓ apoptosis | [75] |
| ↑ | lncRNA MCF2L-AS1 | Sponges miR-874-3p; ↑ CCNE1 | ↑ proliferation and EMT; ↓ apoptosis | [75] |
| ↑ | lncRNA RNCR3 | Sponges miR-1301-3p; ↑ AKT1 | ↑ proliferation and invasion; ↓ apoptosis | [75] |
| ↓ | lncRNA MIR503HG | Sponges miR-107; ↑ PAR4 | ↓ migration and invasion | [76] |
| ↓ | lncRNA DPP10-AS1 | Sponges miR-127-3p; ↓ ADCY1 | ↓ stemness and invasion; ↑ apoptosis | [76] |
| ↓ | lncRNA MCM3AP-AS1 | Sponges miR-19a-3p; ↑ FOXF2 | ↓ proliferation and migration | [76] |
| Study/Approach | Key Biomarkers/Targets | Sample Types | Use Case/Setting | Key Performance Metrics |
|---|---|---|---|---|
| Multi-omics consensus molecular subtyping (CMS1, CMS3) | Integrated genomic, epigenetic, transcriptomic, proteomic features | Tumor tissue | Molecular subtyping, prognosis, treatment selection | CMS3 (p = 0.002) |
| Plasma cfDNA methylation panel (six-marker example) | BCAN, BCAT1, IKZF1, SEPTIN9_1, SEPTIN9_2, VAV3 | Plasma | Noninvasive detection and recurrence risk stratification | Sensitivity: 78.0%; Specificity: 90.2%; |
| Single-cell RNA sequencing (scRNA-seq) of CRC and adjacent tissues | Cell type–specific metabolic and epigenetic state signatures | Tumor and adjacent tissue | Personalized diagnostic “fingerprints,” prognostic modeling | Model C-index/AUC (if available): 0.86 |
| ctDNA methylation markers and multigene panels | SDC2, TFPI2, NDRG4, SEPTIN9 (and related panels) | Plasma | Noninvasive screening and adjuvant monitoring | Sensitivity: 88%; Specificity: 93.4% |
| Single-cell sequencing of circulating tumor cells (CTCs) | Whole-genome and metabolic/epigenetic phenotypes at single-cell level | Peripheral blood | Metastatic potential prediction and molecular classification | PFS (p < 0.001) |
| Exosomal nucleic acids and metabolites | Exosomal miRNAs such as miR-21; metabolic enzyme mRNAs/metabolites | Plasma/serum | Diagnostic aid and progression assessment | Sensitivity: 91.1% Specificity: 95.5% |
| Multi-omics liquid biopsy with AI integration | ctDNA mutations, methylation, fragmentation plus circulating proteins/metabolites | Plasma (optionally combined with stool/clinical data) | Noninvasive early detection and MRD monitoring | Sensitivity: 92%; Specificity: 89.5% |
| Multi-omics integrative diagnostic framework | Mutations, methylation, RNA expression, proteins/metabolites, microbiome | Tissue and liquid biopsies | Precision diagnosis and early detection | Concordance with pathology/external validation metrics: R = 0.82; AUC = 0.86 |
| NCT Number | Study Title | Conditions | Interventions | Phases |
|---|---|---|---|---|
| NCT06944548 | Evaluation of the Effect of Adapted Physical Activity on the Modification of Lipid Metabolism During Chemotherapy for Metastatic COLorectal Cancer | Metastatic Colorectal Cancer (CRC)|Volunteer Subjects | BIOLOGICAL: Lipidomic analyses|OTHER: Adapted physical activity program | PHASE2 |
| NCT03831698 | Omega 3 Fatty Acids in Colorectal Cancer (CRC) Prevention in Patients With Lynch Syndrome (COLYNE) | Colorectal Cancer|Lynch Syndrome | DRUG: Omega-3 fatty acid ethyl esters (2 g) | PHASE2 |
| NCT06886022 | Diagnosis, Treatment, and Prevention of Colorectal Cancer Amid Abnormal Lipid Metabolism | Colorectal Cancer | PROCEDURE: Colorectal cancer procedures | NA |
| NCT01591590 | Correlating the Tumoral Metabolic Progression Index to Patient’s Outcome in Advanced Colorectal Cancer | Colorectal Cancer | OTHER: FDG PET-CT|OTHER: Diffusion MRI|OTHER: Blood samples (plasma preparation and CTC) | NA |
| NCT02903914 | Arginase Inhibitor INCB001158 as a Single Agent and in Combination With Immune Checkpoint Therapy in Patients With Advanced/Metastatic Solid Tumors | Metastatic Cancer|Solid Tumors|Colorectal Cancer (CRC)|Gastric Cancer|Head and Neck Cancer|Lung Cancer|Renal Cell Carcinoma (RCC)|Bladder Cancer|UC (Urothelial Cancer)|Mesothelioma | DRUG: INCB001158|DRUG: Pembrolizumab | PHASE1 |
| NCT03550885 | Diet Modulation of Bacterial Sulfur and Bile Acid Metabolism and Colon Cancer Risk | Colorectal Cancer | OTHER: High taurine and saturated fat diet|OTHER: Low in taurine and saturated fat diet | NA |
| NCT01426490 | The Effects of Vitamin B-6 Status on Homocysteine, Oxidative Stress, One-carbon Metabolism and Methylation: Cross-section, Case-control, Intervention and Follow-up Studies in Colorectal Cancer | Colorectal Cancer | DIETARY_SUPPLEMENT: Vitamin C|DIETARY_SUPPLEMENT: Vitamin B6|DIETARY_SUPPLEMENT: Folic acid|DIETARY_SUPPLEMENT: Vitamin B6 plus folic acid | PHASE2| |
| NCT02699047 | Fish Oil Supplementation in Gastrointestinal Cancer | Gastrointestinal Cancer|Colorectal Cancer|Stomach Cancer | DIETARY_SUPPLEMENT: Encapsuled fish oil|DIETARY_SUPPLEMENT: Encapsulated Olive oil | NA |
| NCT06710314 | A Metabolomics-based Study to Explore the Mechanism of Remission of Metabolic Syndrome Radical Resection of Colorectal Cancer | Metabolomics | DIAGNOSTIC_TEST: Colorectal cancer patients with hypertension|DIAGNOSTIC_TEST: Colorectal cancer patients with diabetes|DIAGNOSTIC_TEST: Colorectal cancer patients with fatty liver | NA |
| NCT05494658 | Impact of Preoperative Oral Branched-chain Amino Acids on Reducing Postoperative Insulin Resistance. | Insulin Resistance|Colorectal Cancer | DIETARY_SUPPLEMENT: BCAA|DIETARY_SUPPLEMENT: water | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-H.; Zhang, J.-X.; Jin, H.-S.; Huang, J. Crosstalk Between Metabolic Reprogramming and Epigenetic Modifications in Colorectal Cancer: Mechanisms and Clinical Applications. Curr. Issues Mol. Biol. 2025, 47, 751. https://doi.org/10.3390/cimb47090751
Sun Y-H, Zhang J-X, Jin H-S, Huang J. Crosstalk Between Metabolic Reprogramming and Epigenetic Modifications in Colorectal Cancer: Mechanisms and Clinical Applications. Current Issues in Molecular Biology. 2025; 47(9):751. https://doi.org/10.3390/cimb47090751
Chicago/Turabian StyleSun, Yu-Hui, Jing-Xian Zhang, Han-Shu Jin, and Jin Huang. 2025. "Crosstalk Between Metabolic Reprogramming and Epigenetic Modifications in Colorectal Cancer: Mechanisms and Clinical Applications" Current Issues in Molecular Biology 47, no. 9: 751. https://doi.org/10.3390/cimb47090751
APA StyleSun, Y.-H., Zhang, J.-X., Jin, H.-S., & Huang, J. (2025). Crosstalk Between Metabolic Reprogramming and Epigenetic Modifications in Colorectal Cancer: Mechanisms and Clinical Applications. Current Issues in Molecular Biology, 47(9), 751. https://doi.org/10.3390/cimb47090751





