Targeting the cGAS-STING Pathway to Modulate Immune Inflammation in Diabetes and Cardiovascular Complications: Mechanisms and Therapeutic Insights
Abstract
1. Introduction
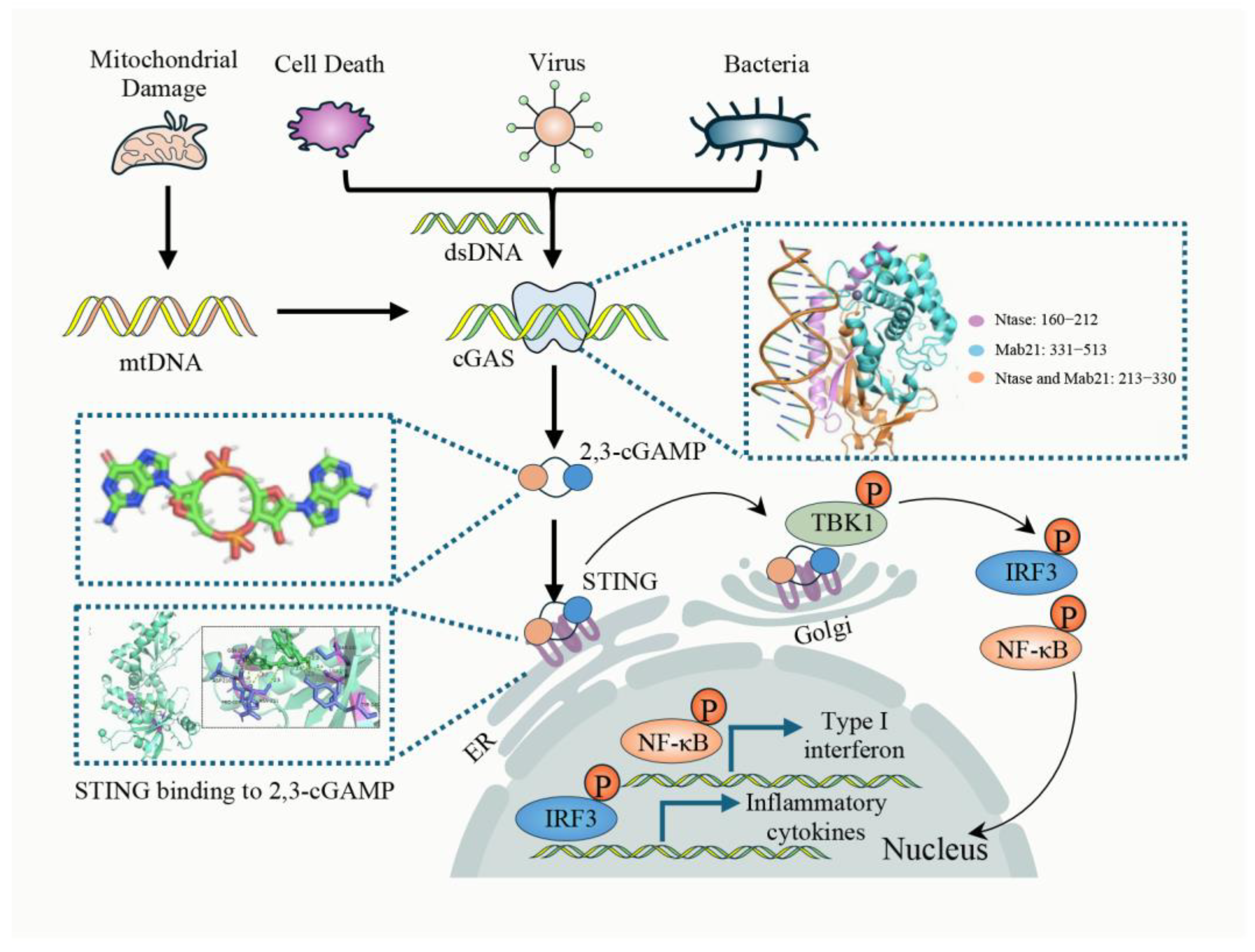
2. cGAS-STING Pathway
2.1. Canonical cGAS-STING Pathway
2.2. Non-Canonical cGAS-STING Pathway
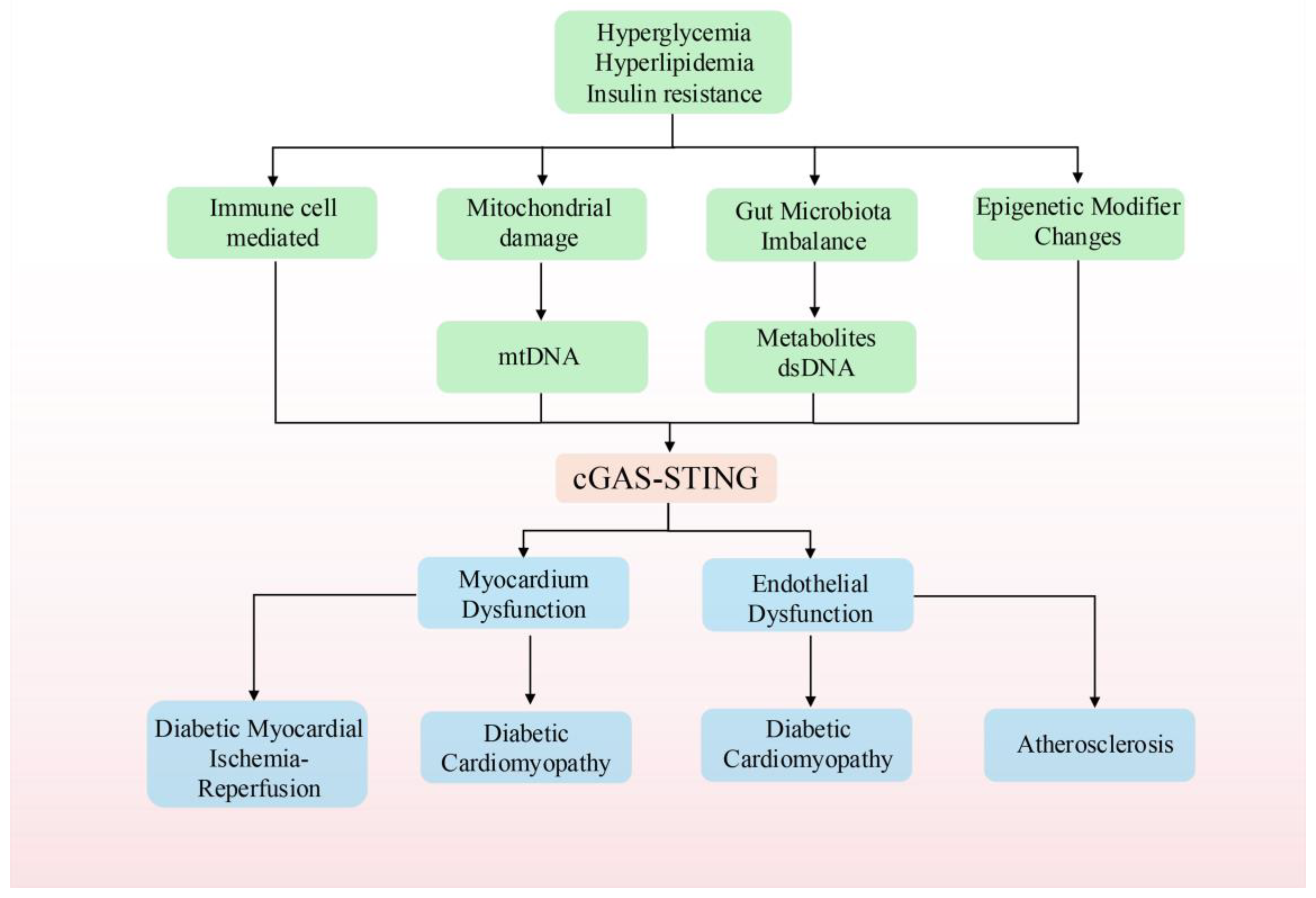
3. The cGAS-STING Pathway and T2DM
3.1. The cGAS-STING Pathway and Its Involvement in Inflammatory Responses Mediated by Immune Cells in Diabetes
3.2. The cGAS-STING Pathway and Gut Microbiota Dysbiosis in T2DM
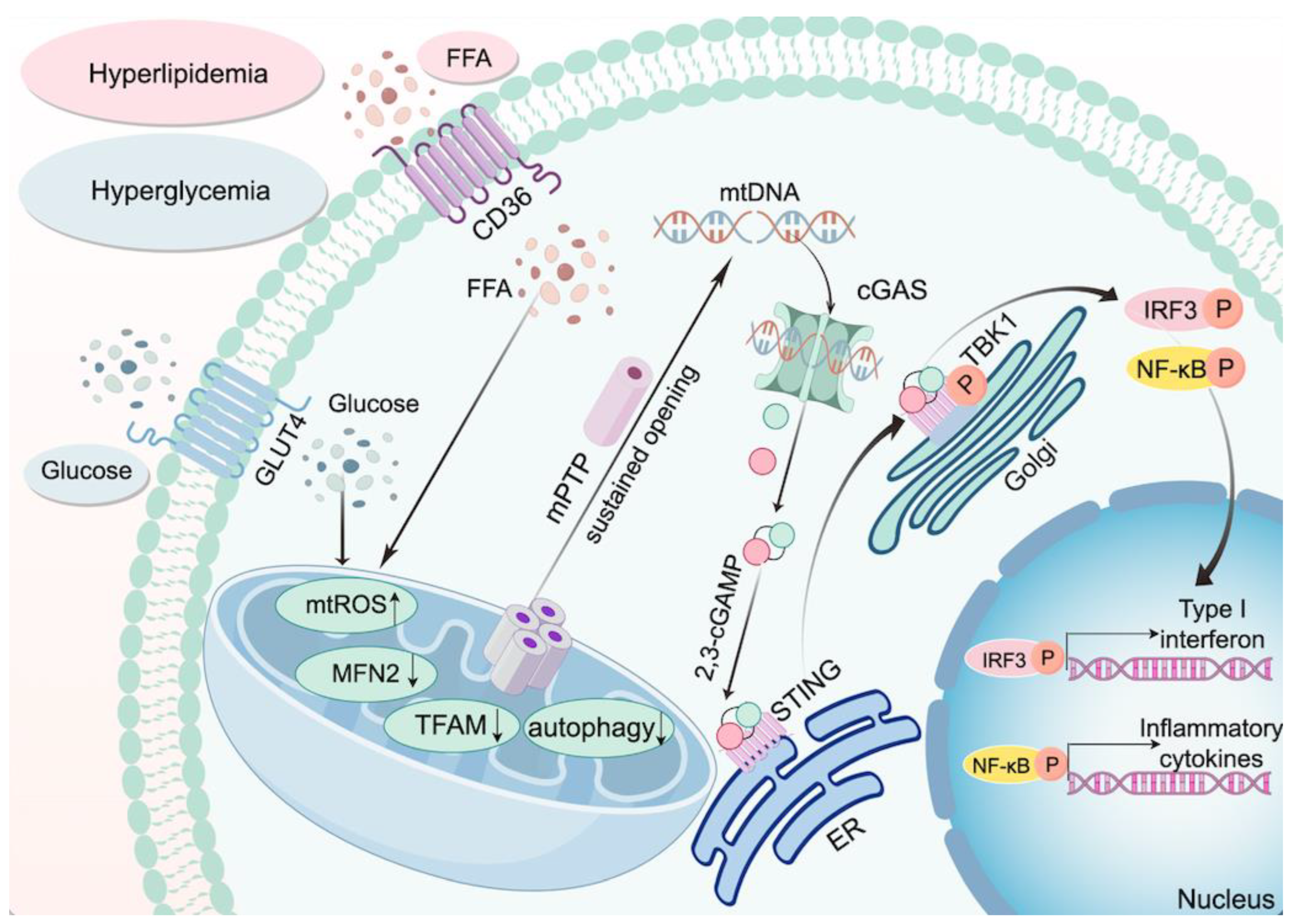
3.3. The cGAS-STING Pathway and Mitochondrial Damage in Diabetes
3.4. cGAS-STING and Epigenetic Modifier Changes in Diabetes
3.5. cGAS-STING and Cell Death in Diabetes
3.6. cGAS-STING and Other Factors in T2DM
4. cGAS-STING and Cardiovascular Complications in Diabetes
4.1. cGAS-STING and Diabetic Cardiomyopathy
4.2. cGAS-STING and Diabetic Myocardial Ischemia/Reperfusion Injury
4.3. cGAS-STING and Diabetic Atherosclerosis
5. Therapeutic Potential of cGAS-STING in T2DM and Its Cardiovascular Complications
5.1. cGAS Inhibitors
5.2. STING Inhibitors
5.3. TBK1 Inhibitors
5.4. cGAS/STING Degraders
5.5. Herbal Medicines and Monomers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Eckel, R.H.; Bornfeldt, K.E.; Goldberg, I.J. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021, 33, 1519–1545. [Google Scholar] [CrossRef]
- Xu, M.; Liu, P.P.; Li, H. Innate Immune Signaling and Its Role in Metabolic and Cardiovascular Diseases. Physiol. Rev. 2019, 99, 893–948. [Google Scholar] [CrossRef]
- An, C.; Li, Z.; Chen, Y.; Huang, S.; Yang, F.; Hu, Y.; Xu, T.; Zhang, C.; Ge, S. The cGAS-STING pathway in cardiovascular diseases: From basic research to clinical perspectives. Cell Biosci. 2024, 14, 58. [Google Scholar] [CrossRef]
- He, X.; Wedn, A.; Wang, J.; Gu, Y.; Liu, H.; Zhang, J.; Lin, Z.; Zhou, R.; Pang, X.; Cui, Y. IUPHAR ECR review: The cGAS-STING pathway: Novel functions beyond innate immune and emerging therapeutic opportunities. Pharmacol. Res. 2024, 201, 107063. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the cGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef]
- Yu, L.; Liu, P. Cytosolic DNA sensing by cGAS: Regulation, function, and human diseases. Signal Transduct. Target. Ther. 2021, 6, 170. [Google Scholar] [CrossRef]
- Michalski, S.; de Oliveira Mann, C.C.; Stafford, C.A.; Witte, G.; Bartho, J.; Lammens, K.; Hornung, V.; Hopfner, K.-P. Structural basis for sequestration and autoinhibition of cGAS by chromatin. Nature 2020, 587, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Ralph, E.C.; Shanker, S.; Wang, H.; Byrnes, L.J.; Horst, R.; Wong, J.; Brault, A.; Dumlao, D.; Smith, J.F. The catalytic mechanism of cyclic GMP-AMP synthase (cGAS) and implications for innate immunity and inhibition. Protein Sci. 2017, 26, 2367–2380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Whiteley, A.T.; de Oliveira Mann, C.C.; Morehouse, B.R.; Nowak, R.P.; Fischer, E.S.; Gray, N.S.; Mekalanos, J.J.; Kranzusch, P.J. Structure of the human cGAS–DNA complex reveals enhanced control of immune surveillance. Cell 2018, 174, 300–311.e11. [Google Scholar] [CrossRef]
- Dowling, Q.M.; Volkman, H.E.; Gray, E.E.; Ovchinnikov, S.; Cambier, S.; Bera, A.K.; Sankaran, B.; Johnson, M.R.; Bick, M.J.; Kang, A. Computational design of constitutively active cGAS. Nat. Struct. Mol. Biol. 2023, 30, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G. Cyclic [G (2′, 5′) pA (3′, 5′) p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Yi, G.; Watts, T.; Kao, C.C.; Li, P. Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat. Struct. Mol. Biol. 2012, 19, 722–724. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Ablasser, A. Regulation of the cGAS-STING Pathway. Annu. Rev. Immunol. 2025, 43, 667–692. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Lu, D.; Wang, X.; Shang, G. cGAS-STING: Mechanisms and therapeutic opportunities. Sci. China Life Sci. 2025, 68, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.-C.; Chen, Z.J. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 2017, 171, 1110–1124.e18. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chao, H.; Chen, L.; Craig, P.A.; Crichlow, G.V.; Dalenberg, K.; Duarte, J.M.; et al. RCSB Protein Data Bank (RCSB.org): Delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Res. 2023, 51, D488–D508. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Zhou, Z.; Han, L.; Karapetyan, K.; Dracheva, S.; Shoemaker, B.A.; et al. PubChem’s BioAssay Database. Nucleic Acids Res. 2012, 40, D400–D412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Zhu, Y.; Zhang, Q.; Guan, H.; Liu, S.; Chen, S.; Mei, C.; Chen, C.; Liao, Z.; et al. A non-canonical cGAS–STING–PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat. Cell Biol. 2022, 24, 766–782. [Google Scholar] [CrossRef]
- Bao, T.; Liu, J.; Leng, J.; Cai, L. The cGAS–STING pathway: More than fighting against viruses and cancer. Cell Biosci. 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Cui, C.; Qing, L.; Wang, L.; He, T.; Yan, F.; Liu, F.; Shen, Y.; Hou, X.; Chen, L. Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism 2018, 81, 13–24. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Dahik, V.D.; Frisdal, E.; Le Goff, W. Rewiring of Lipid Metabolism in Adipose Tissue Macrophages in Obesity: Impact on Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5505. [Google Scholar] [CrossRef]
- Varga, K.Z.; Gyurina, K.; Radványi, Á.; Pál, T.; Sasi-Szabó, L.; Yu, H.; Felszeghy, E.; Szabó, T.; Röszer, T. Stimulator of Interferon Genes (STING) Triggers Adipocyte Autophagy. Cells 2023, 12, 2345. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, Z.; Chen, D.; Zhu, L.; Yang, Y. A role of STING signaling in obesity-induced lung inflammation. Int. J. Obes. 2023, 47, 325–334. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Q.; Yang, R.; Fu, L.; Jiang, H.; Zhu, Q.; Tai, S. Aberrant STING activation promotes macrophage senescence by suppressing autophagy in vascular aging from diabetes. iScience 2025, 28, 111594. [Google Scholar] [CrossRef]
- Memon, B.; Abdelalim, E.M. Stem cell therapy for diabetes: Beta cells versus pancreatic progenitors. Cells 2020, 9, 283. [Google Scholar] [CrossRef]
- Yin, Y.; Hao, H.; Cheng, Y.; Zang, L.; Liu, J.; Gao, J.; Xue, J.; Xie, Z.; Zhang, Q.; Han, W.; et al. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; She, S.; Li, W.; Zhu, J.; Li, X.; Yang, F.; Dai, K. Inhibition of cGAS-STING pathway by stress granules after activation of M2 macrophages by human mesenchymal stem cells against drug induced liver injury. Mol. Immunol. 2024, 165, 42–54. [Google Scholar] [CrossRef]
- Sarikonda, G.; Pettus, J.; Phatak, S.; Sachithanantham, S.; Miller, J.F.; Wesley, J.D.; Cadag, E.; Chae, J.; Ganesan, L.; Mallios, R.; et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J. Autoimmun. 2014, 50, 77–82. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Z.; Li, X.; Liang, Y.; Pei, S.; Hao, S.; Zhu, Q.; Yu, T.; Pei, Y.; Yuan, J.; et al. Cytoplasmic DNA sensing by KU complex in aged CD4(+) T cell potentiates T cell activation and aging-related autoimmune inflammation. Immunity 2021, 54, 632–647.e9. [Google Scholar] [CrossRef]
- Larkin, B.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Vannier, E.; Poltorak, A. Cutting edge: Activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 2017, 199, 397–402. [Google Scholar] [CrossRef]
- Cerboni, S.; Jeremiah, N.; Gentili, M.; Gehrmann, U.; Conrad, C.; Stolzenberg, M.-C.; Picard, C.; Neven, B.; Fischer, A.; Amigorena, S. Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J. Exp. Med. 2017, 214, 1769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, X.; Hu, J.; Wang, L.; Luo, H.; Zhang, J.; Zhang, Y.; Li, G.; Ji, Y.; Zhang, J.; et al. DsbA-L deficiency in T cells promotes diet-induced thermogenesis through suppressing IFN-γ production. Nat. Commun. 2021, 12, 326. [Google Scholar] [CrossRef]
- Bai, J.; Cervantes, C.; Liu, J.; He, S.; Zhou, H.; Zhang, B.; Cai, H.; Yin, D.; Hu, D.; Li, Z.; et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 12196–12201. [Google Scholar] [CrossRef]
- Gao, H.; Luo, Z.; Ji, Y.; Tang, K.; Jin, Z.; Ly, C.; Sears, D.D.; Mahata, S.; Ying, W. Accumulation of microbial DNAs promotes to islet inflammation and β cell abnormalities in obesity in mice. Nat. Commun. 2022, 13, 565. [Google Scholar] [CrossRef]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.; Damgaard, M.T.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef]
- Massier, L.; Chakaroun, R.; Tabei, S.; Crane, A.; Didt, K.D.; Fallmann, J.; Von Bergen, M.; Haange, S.-B.; Heyne, H.; Stumvoll, M. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 2020, 69, 1796–1806. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Wu, H.; Wu, Q.; Duan, M.; Deng, W.; Tang, Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019, 24, 101215. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, I.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Tian, X.; Zeng, Y.; Tu, Q.; Jiao, Y.; Yao, S.; Chen, Y.; Sun, L.; Xia, Q.; Luo, Y.; Yuan, L.; et al. Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-kappaB/p65 pathway. Int. Immunopharmacol. 2023, 124 Pt B, 111010. [Google Scholar] [CrossRef]
- Hasan, A.U.; Rahman, A.; Kobori, H. Interactions between Host PPARs and Gut Microbiota in Health and Disease. Int. J. Mol. Sci. 2019, 20, 387. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, R.; Ma, X.; Liang, W.; Hong, Y.; Li, H.; Zhou, K.; Du, Y.; Takahashi, Y.; Zhang, X. Regulation of monocyte activation by PPARα through interaction with the cGAS-STING pathway. Diabetes 2023, 72, 958–972. [Google Scholar] [CrossRef]
- Xu, P.; Hong, F.; Wang, J.; Wang, J.; Zhao, X.; Wang, S.; Xue, T.; Xu, J.; Zheng, X.; Zhai, Y. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 2690–2701. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics 2022, 12, 1220. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Teng, Y.; Mu, J.; Xu, Q.; Xu, F.; Sriwastva, M.K.; Zhang, L.; Park, J.W.; Zhang, X.; Yan, J.; et al. Outer Membrane Vesicles Released from Garlic Exosome-like Nanoparticles (GaELNs) Train Gut Bacteria that Reverses Type 2 Diabetes via the Gut-Brain Axis. Small 2024, 20, e2308680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Cui, H.; Fan, C.; Xue, Y.; Liu, H.; Li, H.; Li, J.; Li, H.; Sun, Y. Inhibition of fatty acid uptake by TGR5 prevents diabetic cardiomyopathy. Nat. Metab. 2024, 6, 1161–1177. [Google Scholar] [CrossRef]
- van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017, 5, 224–233. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Zhao, N.; Peng, Y.; Shen, D.; Chen, Y.; Zhang, X.; Tang, C.E.; Chai, J. STING-mediated IL-6 Inhibits OATP1B1 Expression via the TCF4 Signaling Pathway in Cholestasis. J. Clin. Transl. Hepatol. 2024, 12, 701–712. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [PubMed]
- Bodur, C.; Kazyken, D.; Huang, K.; Tooley, A.S.; Cho, K.W.; Barnes, T.M.; Lumeng, C.N.; Myers, M.G.; Fingar, D.C. TBK1-mTOR Signaling Attenuates Obesity-Linked Hyperglycemia and Insulin Resistance. Diabetes 2022, 71, 2297–2312. [Google Scholar] [CrossRef]
- Krako Jakovljevic, N.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Stanarcic Gajovic, J.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, T.; Li, J.; Wang, Y.; Shi, H.; Yu, Y.; Ji, Q.; Shen, X.; Sun, T.; Shi, H.; et al. IL-37 ameliorates myocardial fibrosis by regulating mtDNA-enriched vesicle release in diabetic cardiomyopathy mice. J. Transl. Med. 2024, 22, 494. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.C.; Arany, Z. Genetic models of PGC-1 and glucose metabolism and homeostasis. Rev. Endocr. Metab. Disord. 2014, 15, 21–29. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; Jiang, Y.; Wei, L.; Chen, Y.; Zhang, J.; Gao, P.; Zhu, S.; Fang, C.; Du, Y.; et al. The role of chemerin in the regulation of cGAS-STING pathway in gestational diabetes mellitus placenta. FASEB J. 2023, 37, e22806. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Cai, M.-X.; Wang, Z.-L.; Zhuang, M.; Tan, C.-Y.; Xie, T.-H.; Yao, Y.; Wei, T.-T. TGR5 supresses cGAS/STING pathway by inhibiting GRP75-mediated endoplasmic reticulum-mitochondrial coupling in diabetic retinopathy. Cell Death Dis. 2023, 14, 583. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Tian, H.; Deng, X.; Li, W.; Li, W.; Xia, Z. Decreased MFN2 activates the cGAS-STING pathway in diabetic myocardial ischaemia-reperfusion by triggering the release of mitochondrial DNA. Cell Commun. Signal 2023, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Gong, D.J.; Zhang, J.J.; Liu, X.H.; Wang, L. Diabetes aggravates renal ischemia-reperfusion injury by repressing mitochondrial function and PINK1/Parkin-mediated mitophagy. Am. J. Physiol. Renal Physiol. 2019, 317, F852–F864. [Google Scholar] [CrossRef]
- Ye, B.; Pei, Y.; Li, H.; Jiang, Y.; Jin, W.; Gao, Y.; Liu, W.; Guan, X.; Qiao, Y.; Gao, X.; et al. PINK1 Deficiency Facilitates Palmitic Acid-Induced Inflammation by Disrupting Mitochondrial Function to Activate mtDNA-cGAS-STING Signaling. Cell Biochem Funct 2025, 43, e70092. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Komar, A.; Vulf, M.; Quang, H.V.; Shunkin, E.; Volkova, L.; Gazatova, N.; Zatolokin, P.; Litvinova, L. IL-6 Reduces Mitochondrial Replication, and IL-6 Receptors Reduce Chronic Inflammation in NAFLD and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Meng, L.; Gong, M.; Li, J.; Shi, W.; Qiu, J.; Yang, Y.; Zhao, J.; Suo, Y.; et al. Pioglitazone Inhibits Diabetes-Induced Atrial Mitochondrial Oxidative Stress and Improves Mitochondrial Biogenesis, Dynamics, and Function Through the PPAR-γ/PGC-1α Signaling Pathway. Front. Pharmacol. 2021, 12, 658362. [Google Scholar] [CrossRef]
- Yuan, L.; Mao, Y.; Luo, W.; Wu, W.; Xu, H.; Wang, X.L.; Shen, Y.H. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J. Biol. Chem. 2017, 292, 15002–15015. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Geng, K.; Law, B.Y.; Wang, P.; Pu, Y.L.; Chen, Q.; Xu, H.W.; Tan, X.Z.; Jiang, Z.Z.; Xu, Y. Lipotoxicity-induced mtDNA release promotes diabetic cardiomyopathy by activating the cGAS-STING pathway in obesity-related diabetes. Cell Biol. Toxicol. 2023, 39, 277–299. [Google Scholar] [CrossRef]
- Lin, C.; Guo, Y.; Xia, Y.; Li, C.; Xu, X.; Qi, T.; Zhang, F.; Fan, M.; Hu, G.; Zhao, H.; et al. FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress. J. Mol. Cell. Cardiol. 2021, 160, 27–41. [Google Scholar] [CrossRef]
- Lu, L.; Shao, Y.; Xiong, X.; Ma, J.; Zhai, M.; Lu, G.; Jiang, L.; Jin, P.; Tang, J.; Yang, J.; et al. Irisin improves diabetic cardiomyopathy-induced cardiac remodeling by regulating GSDMD-mediated pyroptosis through MITOL/STING signaling. Biomed. Pharmacother. 2024, 171, 116007. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, L.; Escobar Marcillo, D.I.; Simonelli, V.; Iorio, E.; Rinaldi, T.; Sbraccia, P.; Fortini, P.; Guglielmi, V. The Crosstalk between Gut Microbiota and White Adipose Tissue Mitochondria in Obesity. Nutrients 2023, 15, 1723. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin. Int. J. Mol. Sci. 2024, 25, 1137. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, C.; Li, T.; Gao, W.; Ren, Y.; Wang, J.; Zhang, Y. Leptin attenuates diabetic cardiomyopathy-induced cardiac remodeling via regulating cGAS/STING signaling and Opa1-mediated mitochondrial fusion. Cell Signal 2025, 132, 111805. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Xu, A.; Lam, K.S.; Wetzel, M.D.; Xiang, R.; Zhang, J.; Xin, X.; Dong, L.Q.; Liu, F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc. Natl. Acad. Sci. USA 2008, 105, 18302–18307. [Google Scholar] [CrossRef]
- Zhao, P.; Wong, K.I.; Sun, X.; Reilly, S.M.; Uhm, M.; Liao, Z.; Skorobogatko, Y.; Saltiel, A.R. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell 2018, 172, 731–743.e12. [Google Scholar] [CrossRef]
- Gong, Y.; Li, G.; Tao, J.; Wu, N.N.; Kandadi, M.R.; Bi, Y.; Wang, S.; Pei, Z.; Ren, J. Double knockout of Akt2 and AMPK accentuates high fat diet-induced cardiac anomalies through a cGAS-STING-mediated mechanism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165855, Erratum in Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166457. [Google Scholar] [CrossRef]
- White, C.D.; Erdemir, H.H.; Sacks, D.B. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal 2012, 24, 826–834. [Google Scholar] [CrossRef]
- An, C.; Sun, F.; Liu, C.; Huang, S.; Xu, T.; Zhang, C.; Ge, S. IQGAP1 promotes mitochondrial damage and activation of the mtDNA sensor cGAS-STING pathway to induce endothelial cell pyroptosis leading to atherosclerosis. Int. Immunopharmacol. 2023, 123, 110795. [Google Scholar] [CrossRef]
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Cancer Res. 2022, 41, 100. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, H.; Huang, X.; Weng, J.; Peng, F.; Wu, S. METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA. Cell Death Dis. 2022, 13, 38. [Google Scholar] [CrossRef]
- Qi, K.; Zhong, J. LncRNA HOTAIR improves diabetic cardiomyopathy by increasing viability of cardiomyocytes through activation of the PI3K/Akt pathway. Exp. Ther. Med. 2018, 16, 4817–4823. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yao, B.; Xu, W.; Chen, J.; Zhou, X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci. Rep. 2016, 6, 36340. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Xie, R.; Zhang, X.; Nie, X.; Shi, X.; Zhan, J.; Yin, Z.; Zhao, Y.; Dai, B. LncRNA ZNF593-AS alleviates contractile dysfunction in dilated cardiomyopathy. Circ. Res. 2021, 128, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Fan, J.; Wen, J.; Jin, K.; Zhan, J.; Yuan, S.; Tang, Y.; Nie, X.; Wen, Z.; Li, H. LncRNA ZNF593-AS alleviates diabetic cardiomyopathy via suppressing IRF3 signaling pathway. Mol. Ther.-Nucleic Acids 2023, 32, 689–703. [Google Scholar] [CrossRef]
- Xu, T.; Chu, Q.; Cui, J. Rhabdovirus-Inducible MicroRNA-210 Modulates Antiviral Innate Immune Response via Targeting STING/MITA in Fish. J. Immunol. 2018, 201, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Zheng, D.; Luo, Y.; Mou, T.; Chen, Q.; Huang, Z.; Wu, Z. MicroRNA-24-3p alleviates hepatic ischemia and reperfusion injury in mice through the repression of STING signaling. Biochem. Biophys. Res. Commun. 2020, 522, 47–52. [Google Scholar] [CrossRef]
- Yu, Q.; Chu, L.; Li, Y.; Wang, Q.; Zhu, J.; Wang, C.; Cui, S. miR-23a/b suppress cGAS-mediated innate and autoimmunity. Cell. Mol. Immunol. 2021, 18, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Ramirez, M.D.J.E.; Goncharov, E.; Ivliev, D.; Shumadalova, A.; Ilyasova, T.; Wang, C. MiRNAs and lncRNAs in the regulation of innate immune signaling. Non-Coding RNA Res. 2023, 8, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Chowdhury, I.H.; Garg, N.J. Mitochondrial regulation of macrophage response against pathogens. Front. Immunol. 2021, 11, 622602. [Google Scholar] [CrossRef]
- Ali, H.S.; Kamel, M.M.; Agwa, S.H.A.; Hakeem, M.S.A.; Meteini, M.S.E.; Matboli, M. Analysis of mRNA-miRNA-lncRNA differential expression in prediabetes/type 2 diabetes mellitus patients as potential players in insulin resistance. Front. Endocrinol. 2023, 14, 1131171. [Google Scholar] [CrossRef]
- Murthy, A.M.; Robinson, N.; Kumar, S. Crosstalk between cGAS–STING signaling and cell death. Cell Death Differ. 2020, 27, 2989–3003. [Google Scholar] [CrossRef]
- Tornovsky-Babeay, S.; Dadon, D.; Ziv, O.; Tzipilevich, E.; Kadosh, T.; Schyr-Ben Haroush, R.; Hija, A.; Stolovich-Rain, M.; Furth-Lavi, J.; Granot, Z.; et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. Cell Metab. 2014, 19, 109–121. [Google Scholar] [CrossRef]
- Hall, E.; Jönsson, J.; Ofori, J.K.; Volkov, P.; Perfilyev, A.; Dekker Nitert, M.; Eliasson, L.; Ling, C.; Bacos, K. Glucolipotoxicity Alters Insulin Secretion via Epigenetic Changes in Human Islets. Diabetes 2019, 68, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tong, J.; Yang, L.; Wei, L.; Stolz, D.B.; Yu, J.; Zhang, J.; Zhang, L. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc. Natl. Acad. Sci. USA 2018, 115, 3930–3935. [Google Scholar] [CrossRef]
- Ni, B.; Yang, Z.; Zhou, T.; Zhou, H.; Zhou, Y.; Lin, S.; Xu, H.; Lin, X.; Yi, W.; He, C. Therapeutic intervention in neuroinflammation for neovascular ocular diseases through targeting the cGAS-STING-necroptosis pathway. J. Neuroinflamm. 2024, 21, 164. [Google Scholar] [CrossRef]
- Xiang, Q.; Geng, Z.-X.; Yi, X.; Wei, X.; Zhu, X.-H.; Jiang, D.-S. PANoptosis: A novel target for cardiovascular diseases. Trends Pharmacol. Sci. 2024, 45, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Müller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Mei, Y.; Yan, M.; Liang, H. Gasdermin D-Mediated Pyroptosis in Diabetic Cardiomyopathy: Molecular Mechanisms and Pharmacological Implications. Molecules 2023, 28, 7813. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, J.; Ouyang, X.; Zhong, J.; Huang, Y.; Huang, Z.; Zheng, B.; Peng, L.; Tang, X.; et al. Lipotoxicity Induces Cardiomyocyte Ferroptosis via Activating the STING Pathway. Antioxid. Redox Signal 2024, 42, 184–198. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Zhu, C.; Li, J.; Sun, W.; Li, D.; Wang, Y.; Shen, X.-C. Signaling Mechanism of Cuproptosis Activating cGAS-STING Immune Pathway. JACS Au 2024, 4, 3988–3999. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Liu, H.; Shi, M.; Wang, J.; Wang, Y. The molecular mechanisms of defective copper metabolism in diabetic cardiomyopathy. Oxidative Med. Cell. Longev. 2022, 2022, 5418376. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hu, Y.; Lv, D.; Xie, L.; Yang, S.; Zuo, D.; Xue, Y.; He, A. Recurrent hypoglycemia impaired vascular function in advanced T2DM rats by inducing pyroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 7812407. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Qu, D.; Wang, L.; Wong, C.M.; Lau, C.W.; Huang, Y.; Wang, Y.F.; Huang, H.; Xia, Y.; et al. Serum exosomes mediate delivery of arginase 1 as a novel mechanism for endothelial dysfunction in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, E6927–E6936. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, L.; Zou, X.; Wang, J.; Zhong, J.; Zhong, T. Extracellular vesicles in type 2 diabetes mellitus: Key roles in pathogenesis, complications, and therapy. J. Extracell. Vesicles 2019, 8, 1625677. [Google Scholar] [CrossRef]
- Luo, Z.; Ji, Y.; Gao, H.; Gomes Dos Reis, F.C.; Bandyopadhyay, G.; Jin, Z.; Ly, C.; Chang, Y.-J.; Zhang, D.; Kumar, D.; et al. CRIg+ Macrophages Prevent Gut Microbial DNA–Containing Extracellular Vesicle–Induced Tissue Inflammation and Insulin Resistance. Gastroenterology 2021, 160, 863–874. [Google Scholar] [CrossRef]
- Grace, A.; Chan, E.; Giallauria, F.; Graham, P.L.; Smart, N.A. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 37. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, Z.; Zhao, X.; Zhang, B. Aerobic exercise mitigates high-fat diet-induced cardiac dysfunction, pyroptosis, and inflammation by inhibiting STING-NLRP3 signaling pathway. Mol. Cell. Biochem. 2024, 479, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B.; Ding, Y.; Liu, Y.; Wang, Z.C.; Wu, Y.J.; Niu, K.M.; Li, K.X.; Zhang, J.R.; Sun, H.J. Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J. Adv. Res. 2023, 51, 161–179. [Google Scholar] [CrossRef]
- Zhang, T.; Yi, Q.; Huang, W.; Feng, J.; Liu, H. New insights into the roles of Irisin in diabetic cardiomyopathy and vascular diseases. Biomed. Pharmacother. 2024, 175, 116631. [Google Scholar] [CrossRef]
- Li, H.; Irwin, M.G.; Xia, Z. Hyperglycemia-induced Protein Kinase Cβ2 Activation Causes Diastolic Cardiac Dysfunction by Disrupting Brg1-mediated Suppression of RIP3 in Diabetic Rats. FASEB J. 2017, 31, 673.3. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, X.; Li, J.; Yuan, X.; Li, Y.; Zhang, X.; Kang, Z.; Ouyang, Z.; Zeng, J.; Hou, N. BRG1 Deficiency Promotes Cardiomyocyte Inflammation and Apoptosis by Activating the cGAS-STING Signaling in Diabetic Cardiomyopathy. Inflammation 2024, 48, 299–315. [Google Scholar] [CrossRef]
- Li, W.; Qin, R.; Tang, Z.; Wang, C.; Xu, H.; Li, W.; Leng, Y.; Wang, Y.; Xia, Z. Inhibition of inflammation and apoptosis through the cyclic GMP-AMP synthase-stimulator of interferon genes pathway by stress granules after ALKBH5 demethylase activation during diabetic myocardial ischaemia-reperfusion injury. Diabetes Obes. Metab. 2024, 26, 3940–3957. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef]
- An, Y.; Geng, K.; Wang, H.-Y.; Wan, S.-R.; Ma, X.-M.; Long, Y.; Xu, Y.; Jiang, Z.-Z. Hyperglycemia-induced STING signaling activation leads to aortic endothelial injury in diabetes. Cell Commun. Signal. 2023, 21, 365. [Google Scholar] [CrossRef]
- Mao, Y.; Luo, W.; Zhang, L.; Wu, W.; Yuan, L.; Xu, H.; Song, J.; Fujiwara, K.; Abe, J.-i.; LeMaire, S.A. STING–IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 920–929, Erratum in Arterioscler. Thromb. Vasc. Biol. 2018, 38, e60. [Google Scholar] [CrossRef]
- Hedman, A.C.; Li, Z.; Gorisse, L.; Parvathaneni, S.; Morgan, C.J.; Sacks, D.B. IQGAP1 binds AMPK and is required for maximum AMPK activation. J. Biol. Chem. 2021, 296, 100075. [Google Scholar] [CrossRef]
- Hertzog, J.; Rehwinkel, J. Regulation and inhibition of the DNA sensor cGAS. EMBO Rep. 2020, 21, e51345. [Google Scholar] [CrossRef]
- Dai, J.; Huang, Y.J.; He, X.; Zhao, M.; Wang, X.; Liu, Z.S.; Xue, W.; Cai, H.; Zhan, X.Y.; Huang, S.Y.; et al. Acetylation Blocks cGAS Activity and Inhibits Self-DNA-Induced Autoimmunity. Cell 2019, 176, 1447–1460.e14. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Woodward, J.J.; Sasaki, T.; Minie, M.; Elkon, K.B. Cutting edge: Antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase-DNA interaction. J. Immunol. 2015, 194, 4089–4093. [Google Scholar] [CrossRef]
- Steinhagen, F.; Zillinger, T.; Peukert, K.; Fox, M.; Thudium, M.; Barchet, W.; Putensen, C.; Klinman, D.; Latz, E.; Bode, C. Suppressive oligodeoxynucleotides containing TTAGGG motifs inhibit cGAS activation in human monocytes. Eur. J. Immunol. 2018, 48, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sooreshjani, M.A.; Mikek, C.; Opoku-Temeng, C.; Sintim, H.O. Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-β levels. Future Med. Chem. 2018, 10, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zou, Y.; Dietrich, H.; Wick, G.; Xu, Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation 1999, 100, 861–868. [Google Scholar] [CrossRef]
- Vincent, J.; Adura, C.; Gao, P.; Luz, A.; Lama, L.; Asano, Y.; Okamoto, R.; Imaeda, T.; Aida, J.; Rothamel, K.; et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat. Commun. 2017, 8, 750, Correction in Nat. Commun. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Lama, L.; Adura, C.; Xie, W.; Tomita, D.; Kamei, T.; Kuryavyi, V.; Gogakos, T.; Steinberg, J.I.; Miller, M.; Ramos-Espiritu, L.; et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat. Commun. 2019, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiong, H.; Zhu, W.; Liu, Y.; Du, Y. Small molecule inhibition of cyclic GMP-AMP synthase ameliorates sepsis-induced cardiac dysfunction in mice. Life Sci. 2020, 260, 118315. [Google Scholar] [CrossRef]
- Hall, J.; Brault, A.; Vincent, F.; Weng, S.; Wang, H.; Dumlao, D.; Aulabaugh, A.; Aivazian, D.; Castro, D.; Chen, M.; et al. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS ONE 2017, 12, e0184843. [Google Scholar] [CrossRef]
- Tan, J.; Wu, B.; Chen, T.; Fan, C.; Zhao, J.; Xiong, C.; Feng, C.; Xiao, R.; Ding, C.; Tang, W.; et al. Synthesis and Pharmacological Evaluation of Tetrahydro-γ-carboline Derivatives as Potent Anti-inflammatory Agents Targeting Cyclic GMP-AMP Synthase. J. Med. Chem. 2021, 64, 7667–7690. [Google Scholar] [CrossRef]
- Mullard, A. Biotechs step on cGAS for autoimmune diseases. Nat. Rev. Drug Discov. 2023, 22, 939–941. [Google Scholar] [CrossRef]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, Z.; Wang, Z.; Li, F.; Mei, J.; Huang, L.; Lou, X.; Zhao, S.; Song, L.; Chen, W.; et al. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018, 25, 3405–3421.e7. [Google Scholar] [CrossRef]
- Hong, Z.; Mei, J.; Li, C.; Bai, G.; Maimaiti, M.; Hu, H.; Yu, W.; Sun, L.; Zhang, L.; Cheng, D.; et al. STING inhibitors target the cyclic dinucleotide binding pocket. Proc. Natl. Acad. Sci. USA 2021, 118, e2105465118. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, H.; Lv, J.; Song, W.; Xing, C.; Hui, C.; Zhang, D.; Zhang, C.; Zhao, L.; Li, Y.; et al. Gelsevirine is a novel STING-specific inhibitor and mitigates STING-related inflammation in sepsis. Front. Immunol. 2023, 14, 1190707. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Buchan, G.J.; Rühl, M.; Mukai, K.; Salvatore, S.R.; Ogawa, E.; Andersen, S.D.; Iversen, M.B.; Thielke, A.L.; Gunderstofte, C.; et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E7768–E7775. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Mehrkens, D.; Klinke, A.; Lange, M.; Remane, L.; Friedrichs, K.; Braumann, S.; Geißen, S.; Simsekyilmaz, S.; Nettersheim, F.S.; et al. Nitro-fatty acids suppress ischemic ventricular arrhythmias by preserving calcium homeostasis. Sci. Rep. 2020, 10, 15319. [Google Scholar] [CrossRef]
- Oduro, P.K.; Zheng, X.; Wei, J.; Yang, Y.; Wang, Y.; Zhang, H.; Liu, E.; Gao, X.; Du, M.; Wang, Q. The cGAS–STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm. Sin. B 2022, 12, 50–75. [Google Scholar] [CrossRef] [PubMed]
- Siu, T.; Altman, M.D.; Baltus, G.A.; Childers, M.; Ellis, J.M.; Gunaydin, H.; Hatch, H.; Ho, T.; Jewell, J.; Lacey, B.M. Discovery of a novel cGAMP competitive ligand of the inactive form of STING. ACS Med. Chem. Lett. 2018, 10, 92–97. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, M.; Wu, X.; Zhang, H.; Su, H.; Dang, Y.; Ma, M.; Wang, F.; Xu, J.; Chen, L.; et al. CDK inhibitor Palbociclib targets STING to alleviate autoinflammation. EMBO Rep. 2022, 23, e53932. [Google Scholar] [CrossRef]
- Ozasa, K.; Temizoz, B.; Kusakabe, T.; Kobari, S.; Momota, M.; Coban, C.; Ito, S.; Kobiyama, K.; Kuroda, E.; Ishii, K.J. Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor. Front. Immunol. 2019, 10, 2212. [Google Scholar] [CrossRef]
- Reilly, S.M.; Chiang, S.-H.; Decker, S.J.; Chang, L.; Uhm, M.; Larsen, M.J.; Rubin, J.R.; Mowers, J.; White, N.M.; Hochberg, I. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat. Med. 2013, 19, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Beyett, T.S.; Gan, X.; Reilly, S.M.; Chang, L.; Gomez, A.V.; Saltiel, A.R.; Showalter, H.D.; Tesmer, J.J.G. Carboxylic Acid Derivatives of Amlexanox Display Enhanced Potency toward TBK1 and IKKε and Reveal Mechanisms for Selective Inhibition. Mol. Pharmacol. 2018, 94, 1210–1219. [Google Scholar] [CrossRef]
- Adzika, G.K.; Hou, H.; Adekunle, A.O.; Rizvi, R.; Adzraku, S.Y.; Li, K.; Deng, Q.-M.; Mprah, R.; Ndzie Noah, M.L.; Adu-Amankwaah, J. Amlexanox and forskolin prevents isoproterenol-induced cardiomyopathy by subduing cardiomyocyte hypertrophy and maladaptive inflammatory responses. Front. Cell Dev. Biol. 2021, 9, 719351. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Song, S.; Tang, H.; Smaill, J.B.; Wang, A.; Xie, H.; Lu, X. TANK-binding kinase 1 (TBK1): An emerging therapeutic target for drug discovery. Drug Discov. Today 2021, 26, 2445–2455. [Google Scholar] [CrossRef]
- Clark, K.; Plater, L.; Peggie, M.; Cohen, P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: A distinct upstream kinase mediates Ser-172 phosphorylation and activation. J. Biol. Chem. 2009, 284, 14136–14146. [Google Scholar] [CrossRef]
- Shu, C.; Sankaran, B.; Chaton, C.T.; Herr, A.B.; Mishra, A.; Peng, J.; Li, P. Structural insights into the functions of TBK1 in innate antimicrobial immunity. Structure 2013, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Godl, K.; Gruss, O.J.; Eickhoff, J.; Wissing, J.; Blencke, S.; Weber, M.; Degen, H.; Brehmer, D.; Orfi, L.; Horváth, Z.; et al. Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res. 2005, 65, 6919–6926. [Google Scholar] [CrossRef]
- Thomson, D.W.; Poeckel, D.; Zinn, N.; Rau, C.; Strohmer, K.; Wagner, A.J.; Graves, A.P.; Perrin, J.; Bantscheff, M.; Duempelfeld, B.; et al. Discovery of GSK8612, a Highly Selective and Potent TBK1 Inhibitor. ACS Med. Chem. Lett. 2019, 10, 780–785. [Google Scholar] [CrossRef]
- Wu, K.; Xu, Y.; Liu, P.; Chen, K.; Zhao, Y. STING inhibitors and degraders: Potential therapeutic agents in inflammatory diseases. Eur. J. Med. Chem. 2025, 291, 117632. [Google Scholar] [CrossRef]
- Lui, W.Y.; Bharti, A.; Wong, N.M.; Jangra, S.; Botelho, M.G.; Yuen, K.S.; Jin, D.Y. Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1. PLoS Pathog. 2023, 19, e1011186. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, Y.; Liu, C.; Guey, B.; Li, L.; Melenec, P.; Ricci, J.; Ablasser, A. The CRL5-SPSB3 ubiquitin ligase targets nuclear cGAS for degradation. Nature 2024, 627, 873–879. [Google Scholar] [CrossRef]
- He, P.; Wen, C.; Zhang, X.; Yin, H. Discovery of a Novel CRBN-Recruiting cGAS PROTAC Degrader for the Treatment of Ulcerative Colitis. J. Med. Chem. 2025, 68, 5551–5572. [Google Scholar] [CrossRef]
- Su, M.; Zheng, J.; Gan, L.; Zhao, Y.; Fu, Y.; Chen, Q. Second messenger 2’3’-cyclic GMP-AMP (2’3’-cGAMP): Synthesis, transmission, and degradation. Biochem. Pharmacol. 2022, 198, 114934. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Wang, M.; Sintim, H.O. ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1. Molecules 2019, 24, 4192. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Liu, P.; Wei, X.; Zhang, Z.; Fan, S.; Zhang, L.; Han, F.; Song, Y.; Chu, L.; et al. SMPDL3A is a cGAMP-degrading enzyme induced by LXR-mediated lipid metabolism to restrict cGAS-STING DNA sensing. Immunity 2023, 56, 2492–2507.e10. [Google Scholar] [CrossRef]
- Yang, L.L.; Xiao, W.C.; Li, H.; Hao, Z.Y.; Liu, G.Z.; Zhang, D.H.; Wu, L.M.; Wang, Z.; Zhang, Y.Q.; Huang, Z.; et al. E3 ubiquitin ligase RNF5 attenuates pathological cardiac hypertrophy through STING. Cell Death Dis. 2022, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lian, Q.; Yang, B.; Yan, S.; Zhou, H.; He, L.; Lin, G.; Lian, Z.; Jiang, Z.; Sun, B. TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING. PLoS Pathog. 2015, 11, e1005012. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, L.; Tong, Y.; Liu, Y.; Mou, J.; Wang, X.; Wang, X.; Gong, Y.; Zhao, Y.; Liu, Y.; et al. TRIM29 negatively controls antiviral immune response through targeting STING for degradation. Cell Discov. 2018, 4, 13, Correction in Cell Discov. 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Gentili, M.; Liu, B.; Papanastasiou, M.; Dele-Oni, D.; Schwartz, M.A.; Carlson, R.J.; Al’Khafaji, A.M.; Krug, K.; Brown, A.; Doench, J.G.; et al. ESCRT-dependent STING degradation inhibits steady-state and cGAMP-induced signalling. Nat. Commun. 2023, 14, 611. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, Y.; Wu, Y.; Sun, Y.; Zhao, L.; Xue, Z.; Sun, M.; Wei, X.; He, Z.; Wu, S.A.; et al. SEL1L-HRD1 endoplasmic reticulum-associated degradation controls STING-mediated innate immunity by limiting the size of the activable STING pool. Nat. Cell Biol. 2023, 25, 726–739. [Google Scholar] [CrossRef]
- Zhu, Z.; Johnson, R.L.; Zhang, Z.; Herring, L.E.; Jiang, G.; Damania, B.; James, L.I.; Liu, P. Development of VHL-recruiting STING PROTACs that suppress innate immunity. Cell Mol. Life Sci. 2023, 80, 149. [Google Scholar] [CrossRef] [PubMed]
- Crew, A.P.; Raina, K.; Dong, H.; Qian, Y.; Wang, J.; Vigil, D.; Serebrenik, Y.V.; Hamman, B.D.; Morgan, A.; Ferraro, C.; et al. Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J. Med. Chem. 2018, 61, 583–598. [Google Scholar] [CrossRef]
- Guo, J.; Tang, H.; Zhao, W.; Li, Y.; Song, S.; Feng, F.; Huang, S.; Wang, X.; Zhou, Y.; Pei, J.; et al. Discovery of TBK1Molecular Glue Degraders as a Potential Strategy for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). J. Med. Chem. 2025, 68, 12862–12880. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Dey, S.K.; Kundu, S. Herbs and their bioactive ingredients in cardio-protection: Underlying molecular mechanisms and evidences from clinical studies. Phytomedicine 2021, 92, 153753, Erratum in Phytomedicine 2022, 98, 153828. [Google Scholar] [CrossRef]
- Fan, L.; Tang, K.; Li, J.; Tan, Y.; Liu, X.; Bai, Z.; Tao, A.; Tan, N. Mailuoning oral liquid ameliorates vasculitis in thromboangiitis obliterans rats via inactivating cGAS-STING-IRF3 and TLR4-MAPKs/NF-κB signaling pathways. J. Ethnopharmacol. 2025, 337, 118707. [Google Scholar] [CrossRef]
- Su, Y.; Yin, X.; Huang, X.; Guo, Q.; Ma, M.; Guo, L. Astragaloside IV ameliorates sepsis-induced myocardial dysfunction by regulating NOX4/JNK/BAX pathway. Life Sci. 2022, 310, 121123. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, J.; Han, M.; Zhao, M.; Li, K.; Lu, T.; Guo, Q.; Jiang, Q. Tanshinone IIA inhibits osteoclastogenesis in rheumatoid arthritis via LDHC-regulated ROS generation. Chin. Med. 2023, 18, 54. [Google Scholar] [CrossRef]
- Zhai, P.; Chen, Q.; Wang, X.; Ouyang, X.; Yang, M.; Dong, Y.; Li, J.; Li, Y.; Luo, S.; Liu, Y. The combination of Tanshinone IIA and Astragaloside IV attenuates myocardial ischemia–reperfusion injury by inhibiting the STING pathway. Chin. Med. 2024, 19, 34. [Google Scholar] [CrossRef]
- Su, L.; Cao, P.; Wang, H. Tetrandrine mediates renal function and redox homeostasis in a streptozotocin-induced diabetic nephropathy rat model through Nrf2/HO-1 reactivation. Ann. Transl. Med. 2020, 8, 990. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Luo, Y.; Cui, Y.; Xu, M.; Luo, W.; Wu, G.; Liang, G. Tetrandrine alleviates atherosclerosis via inhibition of STING-TBK1 pathway and inflammation in macrophages. Int. Immunopharmacol. 2023, 119, 110139. [Google Scholar] [CrossRef]
- Li, C.-Y.; Yang, P.; Jiang, Y.-L.; Lin, Z.; Pu, Y.-W.; Xie, L.-Q.; Sun, L.; Lu, D. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomed. Pharmacother. 2020, 125, 109913. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Cui, J.; Wang, P.; Yang, Q.; Chen, Y.; Zhang, T. Ginsenoside Rb1 mitigates acute catecholamine surge-induced myocardial injuries in part by suppressing STING-mediated macrophage activation. Biomed. Pharmacother. 2024, 175, 116794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-inflammatory and antioxidant properties of β-sitosterol in copper sulfate-induced inflammation in zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Z.-Y.; Wen, H.; Lu, C.-H.; Qin, Y.; Xie, Y.-M.; Chen, Q.; Lv, J.-H.; Huang, F.; Zeng, Z.-Y. β-sitosterol alleviates pulmonary arterial hypertension by altering smooth muscle cell phenotype and DNA damage/cGAS/STING signaling. Phytomedicine 2024, 135, 156030. [Google Scholar] [CrossRef]
- Fischer, J.C.; Bscheider, M.; Eisenkolb, G.; Lin, C.C.; Wintges, A.; Otten, V.; Lindemans, C.A.; Heidegger, S.; Rudelius, M.; Monette, S.; et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 2017, 9, eaag2513. [Google Scholar] [CrossRef] [PubMed]
| Target | Medicines Vocabularies | Molecular Mechanism | Ref |
|---|---|---|---|
| cGAS | Aspirin | Enhancing the acetylation of cGAS at lysine residues 384, 394, and 414 effectively inhibits the activity of cGAS. | [140] |
| cGAS | A151, Suramin, HCQ, QC, X6 | Competitively binds to cGAS, preventing the interaction between dsDNA and cGAS. | [141,142] |
| cGAS | RU. 521, G150, G108, PF-06928215, VENT-03 (compound 25) | Binding to key residues in the catalytic site of cGAS to reduce the binding of cGAS to ATP/GTP. | [145,146,148] |
| STING | Astin C, SN-011 Gelsevirine | Targeting the CDN binding pocket of STING to block CDN binding. | [152,153,154] |
| STING | C-176, C-178, C-170, C-171, H-151, CXA-10 | Targeting Cys91 on STING to block activation-induced palmitoylation. | [136,155,157] |
| STING | Tetradroisoquinolone acetic acid (compound 18) | Binding to the cGAMP binding site, thereby displacing the cGAMP binding site on STING. | [158] |
| STING | The cyclin-dependent protein kinase (CDK) inhibitor 29 (palbociclib) | Directly binds to STING and targets the Y167 residue to block its dimerization and translocation. | [159] |
| TBK1 | Amlexanox | Inhibiting the phosphorylation of STING at Ser366 induced by TBK1 to block the full activation of STING. | [160] |
| TBK1 | BX795, MRT67307, GSK8612, SU6668 | Competitively occupying its ATP-binding pocket, thereby preventing ATP access and abrogating kinase activity | [165,166,167,168] |
| Target | Medicines Vocabularies | Molecular Mechanism | Ref |
|---|---|---|---|
| cGAS | Cullin-RING Ligase 5 | Targets nuclear cGAS for ubiquitination and degradation through the CRL5 E3 ligase complex. | [171] |
| cGAS | TH35(PROTAC) | Recruits CRBN E3 ligase via PROTAC to induce ubiquitination and proteasomal degradation of cGAS. | [172] |
| 2′3′-cGAMP | ENPP1 | Degrades 2′3′-cGAMP through phosphodiesterase activity, thereby blocking downstream STING activation. | [174] |
| 2′3′-cGAMP | SMPDL3A | Hydrolyzes 2′3′-cGAMP under regulation of lipid metabolic signals to inhibit innate immune activation. | [175] |
| STING | RNF5/TRIM30a/TRIM29 | Catalyzes K48-linked ubiquitination of STING, targeting it for degradation via the proteasome pathway. | [176,177,178] |
| STING | ESCRT | Promotes K63-linked ubiquitination at Lys288, triggering ESCRT-mediated microautophagy of STING. | [179] |
| STING | HRD1 | Regulates homeostasis of nascent STING through ubiquitin-mediated degradation in the endoplasmic reticulum. | [180] |
| STING | UNC9036 | Activates and phosphorylates STING, then recruits VHL E3 ligase to induce its proteasomal degradation. | [181] |
| TBK1 | 3i (PROTAC) | Links a TBK1-targeting ligand with a VHL ligand to induce ubiquitination and potent proteasomal degradation. | [182] |
| TBK1 | degrader 30 (molecular glue) | Recruits RNF126 E3 ligase via molecular glue strategy to promote TBK1 degradation. | [183] |
| Drug Name | Animal Type | Animal Disease Model | Drug Effect | Biomarker Changes | Ref |
|---|---|---|---|---|---|
| MLNO | Wistar Rats | TAO | Inflammation ↓ Coagulation ↓ | IL-1β, IL-6, TNF-α, CCL2, PAI-1, TF, ICAM-1, VCAM-1 ↓ | [185] |
| As-IV and Ta-IIA | C57BL/6 J mice | MI/RI | Inflammation ↓ Oxidative stress ↓ Myocardial Function ↑ | GSH; SOD ↑ CK, CKMB, LDH, MDA, IL-6, IL-1β, TNF-α ↓ | [188] |
| TET | ApoE−/− mice | AS | Inflammation ↓ atherosclerotic plaque ↓ | Ccl2, TNF-α, IL-6 ↓ | [190] |
| Ginsenoside Rb1 | C57BL/6J mice | SCM | Inflammation ↓ Myocardial Function ↑ | cTnI, IL-6, IL-1β, CCL2 ↓ | [192] |
| SITO | SD rats | PH | Pulmonary Artery Pressure ↓ Myocardial Function ↑ | BAX ↑ PCNA, Bcl-2, γ-H2AX ↓ | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, G.; Zhang, X.; Jiao, J.; Du, W.; Yan, M. Targeting the cGAS-STING Pathway to Modulate Immune Inflammation in Diabetes and Cardiovascular Complications: Mechanisms and Therapeutic Insights. Curr. Issues Mol. Biol. 2025, 47, 750. https://doi.org/10.3390/cimb47090750
Cai G, Zhang X, Jiao J, Du W, Yan M. Targeting the cGAS-STING Pathway to Modulate Immune Inflammation in Diabetes and Cardiovascular Complications: Mechanisms and Therapeutic Insights. Current Issues in Molecular Biology. 2025; 47(9):750. https://doi.org/10.3390/cimb47090750
Chicago/Turabian StyleCai, Guida, Xi Zhang, Jiexi Jiao, Weijie Du, and Meiling Yan. 2025. "Targeting the cGAS-STING Pathway to Modulate Immune Inflammation in Diabetes and Cardiovascular Complications: Mechanisms and Therapeutic Insights" Current Issues in Molecular Biology 47, no. 9: 750. https://doi.org/10.3390/cimb47090750
APA StyleCai, G., Zhang, X., Jiao, J., Du, W., & Yan, M. (2025). Targeting the cGAS-STING Pathway to Modulate Immune Inflammation in Diabetes and Cardiovascular Complications: Mechanisms and Therapeutic Insights. Current Issues in Molecular Biology, 47(9), 750. https://doi.org/10.3390/cimb47090750





