The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. HEK293T Cultivation and Lentivirus Production
2.3. Direct Reprogramming Technique
2.4. Senescence β-Galactosidase Staining
2.5. Immunofluorescence Staining
2.6. Western Blot
2.7. Statistics
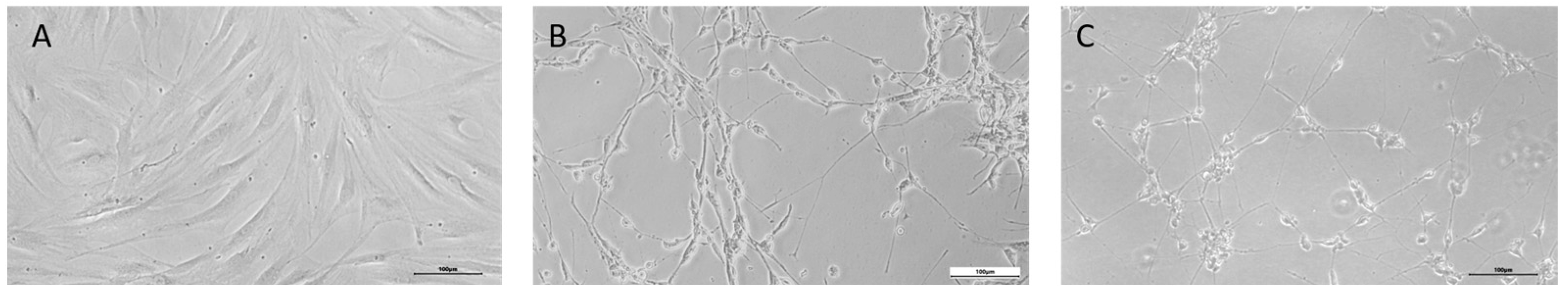
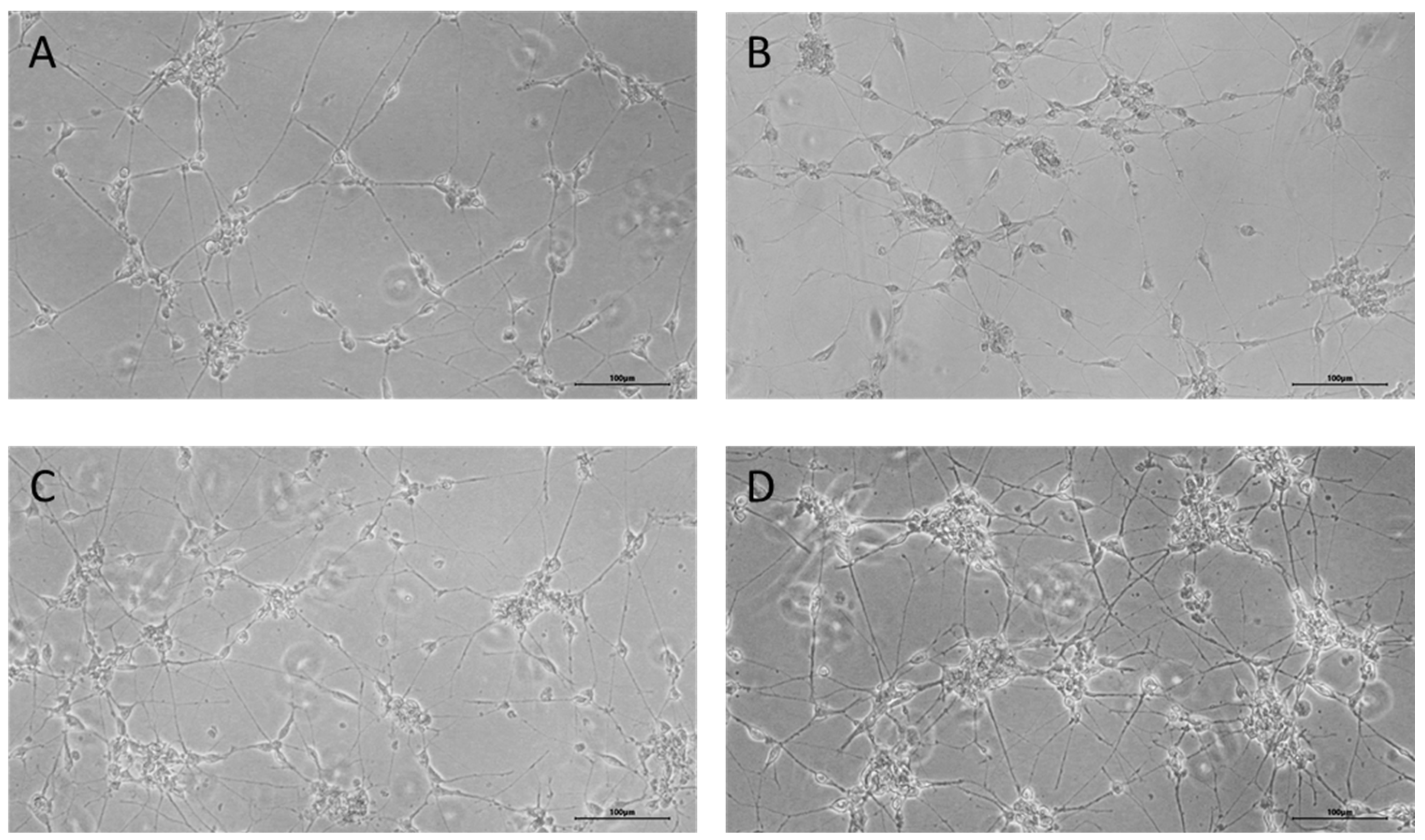
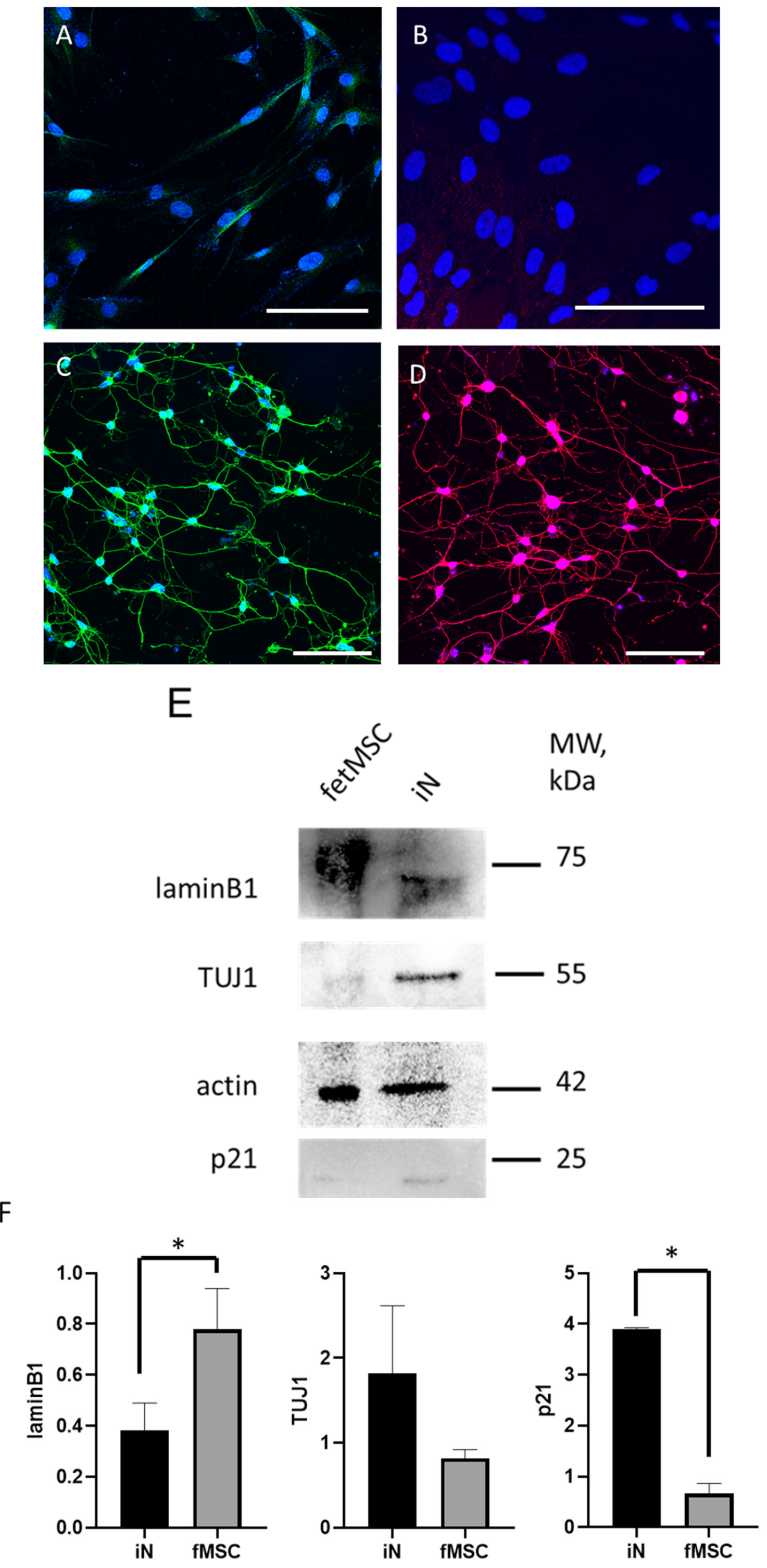
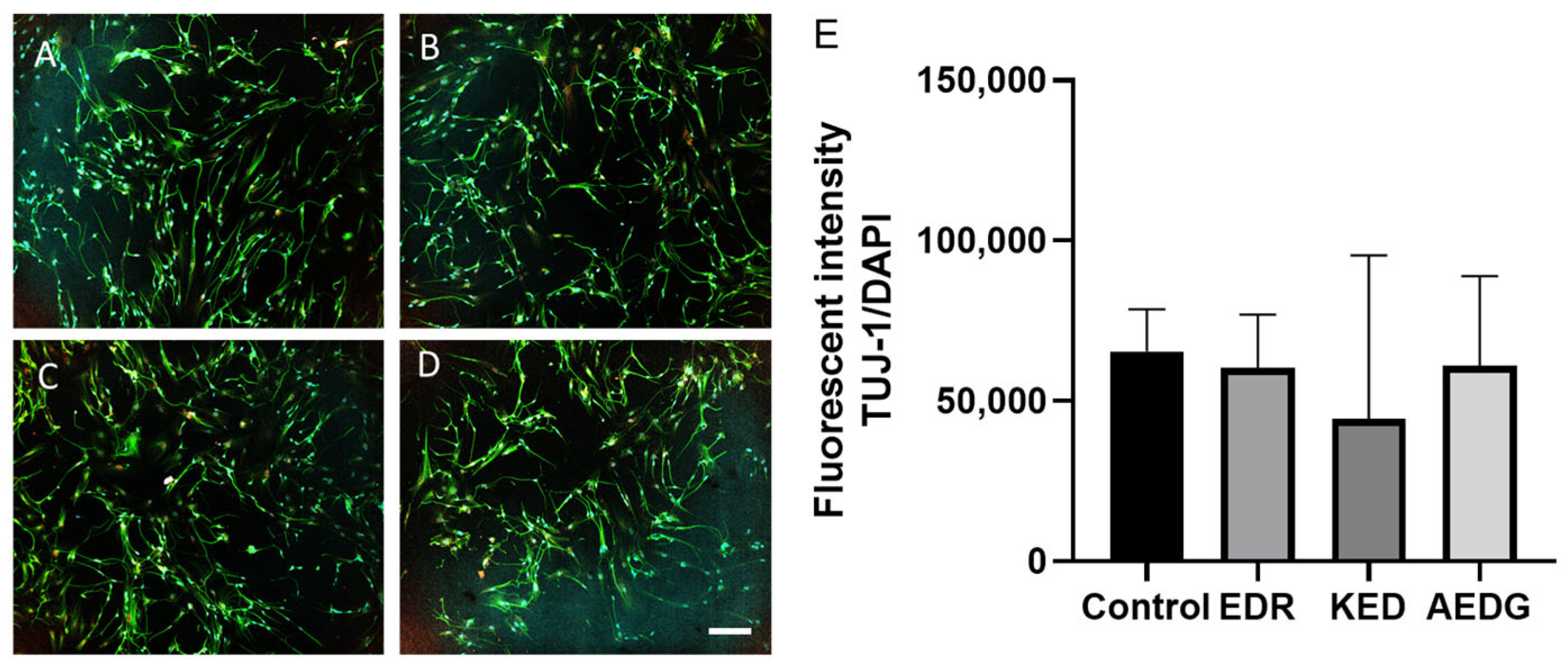
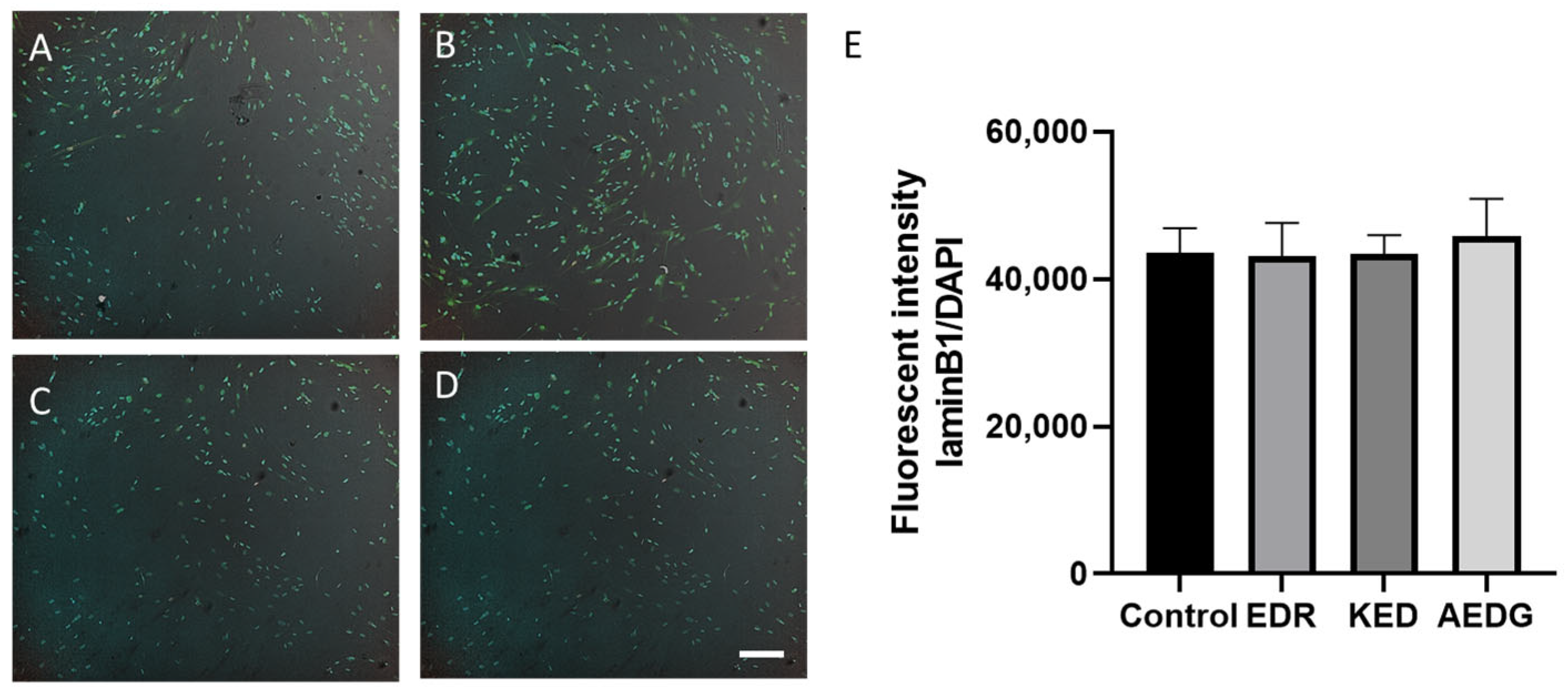
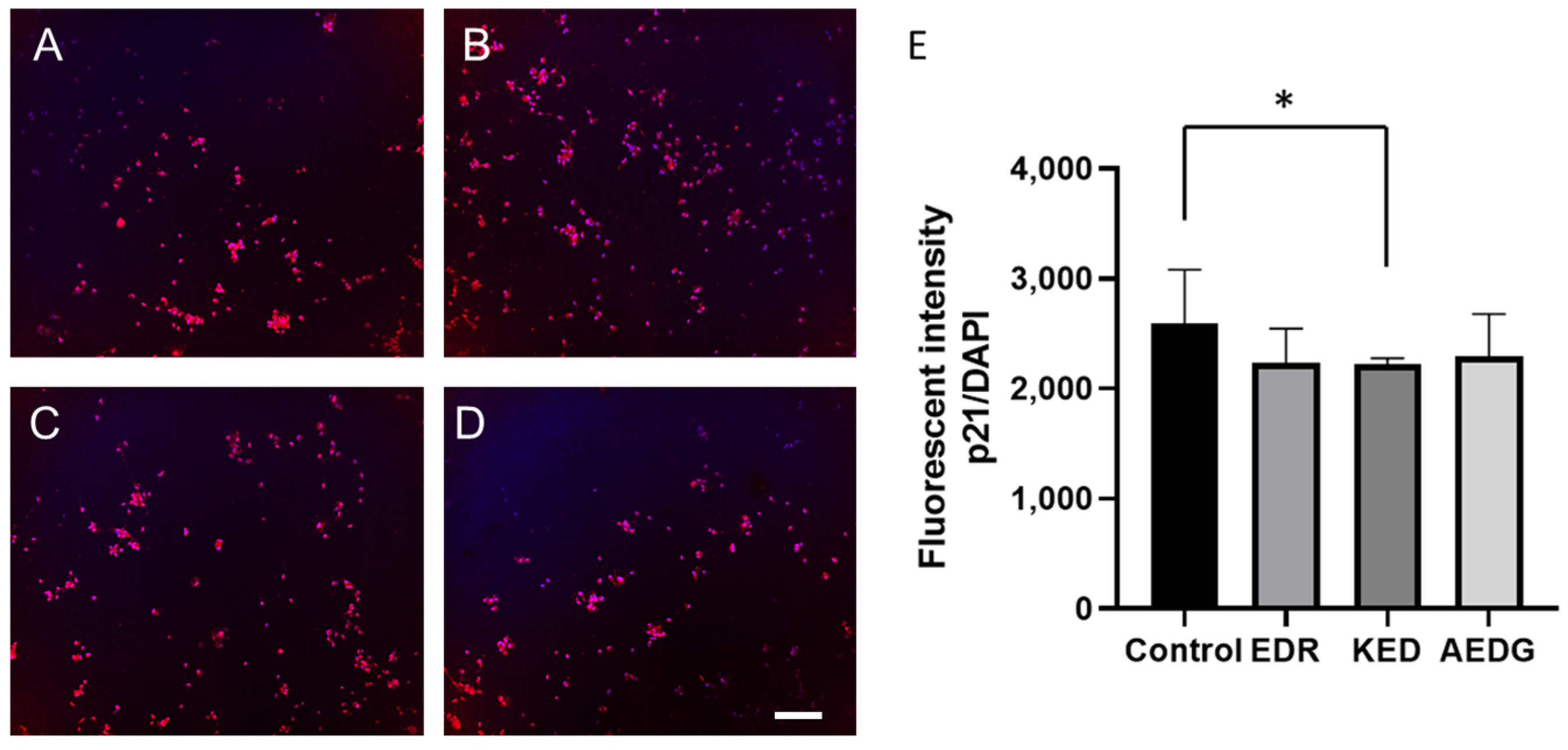
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Sun, S.; Deng, H. Chemical reprogramming for cell fate manipulation: Methods, applications, and perspectives. Cell Stem Cell 2023, 30, 1130–1147. [Google Scholar] [CrossRef]
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jorg, D.J.; Carta, S.; April, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live imaging of neurogenesis in the adult mouse hippocampus. Science 2018, 359, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Bottes, S.; Jaeger, B.N.; Pilz, G.A.; Jorg, D.J.; Cole, J.D.; Kruse, M.; Harris, L.; Korobeynyk, V.I.; Mallona, I.; Helmchen, F.; et al. Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nat. Neurosci. 2021, 24, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.V.; Mira, H. Adult Neural Stem Cells: Born to Last. Front. Cell Dev. Biol. 2019, 7, 96. [Google Scholar] [CrossRef]
- Jessberger, S.; Gage, F.H. Adult neurogenesis: Bridging the gap between mice and humans. Trends Cell Biol. 2014, 24, 558–563. [Google Scholar] [CrossRef]
- Lazarov, O.; Marr, R.A. Of mice and men: Neurogenesis. cognition and Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 43. [Google Scholar] [CrossRef]
- Steward, M.M.; Sridhar, A.; Meyer, J.S. Neural regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 163–191. [Google Scholar] [CrossRef]
- Carter, J.L.; Halmai, J.; Fink, K.D. The iNs and Outs of Direct Reprogramming to Induced Neurons. Front. Genome Ed. 2020, 2, 7. [Google Scholar] [CrossRef]
- Huh, C.J.; Zhang, B.; Victor, M.B.; Dahiya, S.; Batista, L.F.; Horvath, S.; Yoo, A.S. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife 2016, 5, e18648. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Dai, P.; Harada, Y.; Takamatsu, T. Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds. J. Clin. Biochem. Nutr. 2015, 56, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, X.; Jing, J.; Ma, Y.; Wang, J.; Liu, D.; Zhu, J.; Du, X.; Xiong, L.; Du, Y.; et al. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell 2015, 17, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Stubblefield, E.A.; Blanchard, B.; Richards, T.L.; Larson, G.A.; He, Y.; Huang, Q.; Tan, A.C.; Zhang, D.; et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012, 22, 321–332. [Google Scholar] [CrossRef]
- Vadodaria, K.C.; Mertens, J.; Paquola, A.; Bardy, C.; Li, X.; Jappelli, R.; Fung, L.; Marchetto, M.C.; Hamm, M.; Gorris, M.; et al. Generation of functional human serotonergic neurons from fibroblasts. Mol. Psychiatry 2016, 21, 49–61. [Google Scholar] [CrossRef]
- Habekost, M.; Jorgensen, A.L.; Qvist, P.; Denham, M. MicroRNAs and Ascl1 facilitate direct conversion of porcine fibroblasts into induced neurons. Stem Cell Res. 2020, 48, 101984. [Google Scholar] [CrossRef]
- Church, V.A.; Cates, K.; Capano, L.; Aryal, S.; Kim, W.K.; Yoo, A.S. Generation of Human Neurons by microRNA-Mediated Direct Conversion of Dermal Fibroblasts. Methods Mol. Biol. 2021, 2239, 77–100. [Google Scholar] [CrossRef]
- Ambasudhan, R.; Talantova, M.; Coleman, R.; Yuan, X.; Zhu, S.; Lipton, S.A.; Ding, S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 2011, 9, 113–118. [Google Scholar] [CrossRef]
- Yoo, A.S.; Sun, A.X.; Li, L.; Shcheglovitov, A.; Portmann, T.; Li, Y.; Lee-Messer, C.; Dolmetsch, R.E.; Tsien, R.W.; Crabtree, G.R. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011, 476, 228–231. [Google Scholar] [CrossRef]
- Khazaei, M.; Ahuja, C.S.; Nakashima, H.; Nagoshi, N.; Li, L.; Wang, J.; Chio, J.; Badner, A.; Seligman, D.; Ichise, A.; et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci. Transl. Med. 2020, 12, eaau3538. [Google Scholar] [CrossRef]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Klein, S.M.; Aebischer, P.; Svendsen, C.N. GDNF secreting human neural progenitor cells protect dying motor neurons. but not their projection to muscle, in a rat model of familial ALS. PLoS ONE 2007, 2, e689. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, J.; Pineda, J.R.; Irastorza, I.; Uribe-Etxebarria, V.; Garcia-Gallastegui, P.; Encinas, J.M.; Chamero, P.; Unda, F.; Ibarretxe, G. BDNF and NT3 Reprogram Human Ectomesenchymal Dental Pulp Stem Cells to Neurogenic and Gliogenic Neural Crest Progenitors Cultured in Serum-Free Medium. Cell Physiol. Biochem. 2019, 52, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef] [PubMed]
- Hartbauer, M.; Hutter-Paier, B.; Skofitsch, G.; Windisch, M. Antiapoptotic effects of the peptidergic drug cerebrolysin on primary cultures of embryonic chick cortical neurons. J. Neural Transm. 2001, 108, 459–473. [Google Scholar] [CrossRef]
- Caputi, S.; Trubiani, O.; Sinjari, B.; Trofimova, S.; Diomede, F.; Linkova, N.; Diatlova, A.; Khavinson, V. Effect of short peptides on neuronal differentiation of stem cells. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419828613. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: Genome-wide transcriptional analysis. BMC Genom. 2014, 15, 228. [Google Scholar] [CrossRef]
- Shadrina, M.I.; Dolotov, O.V.; Grivennikov, I.A.; Slominsky, P.A.; Andreeva, L.A.; Inozemtseva, L.S.; Limborska, S.A.; Myasoedov, N.F. Rapid induction of neurotrophin mRNAs in rat glial cell cultures by Semax. an adrenocorticotropic hormone analog. Neurosci. Lett. 2001, 308, 115–118. [Google Scholar] [CrossRef]
- Fedoreyeva, L.I.; Kireev, I.I.; Khavinson, V.K.h.; Vanyushin, B.F. Penetration of short fluorescence-labeled peptides into the nucleus in HeLa cells and in vitro specific interaction of the peptides with deoxyribooligonucleotides and DNA. Biochemistry 2011, 76, 1210–1219. [Google Scholar] [CrossRef]
- Kraskovskaya, N.; Linkova, N.; Sakhenberg, E.; Krieger, D.; Polyakova, V.; Medvedev, D.; Krasichkov, A.; Khotin, M.; Ryzhak, G. Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes. Int. J. Mol. Sci. 2024, 25, 11363. [Google Scholar] [CrossRef]
- Ilina, A.; Khavinson, V.; Linkova, N.; Petukhov, M. Neuroepigenetic Mechanisms of Action of Ultrashort Peptides in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4259. [Google Scholar] [CrossRef]
- Khavinson, V.; Ilina, A.; Kraskovskaya, N.; Linkova, N.; Kolchina, N.; Mironova, E.; Erofeev, A.; Petukhov, M. Neuroprotective Effects of Tripeptides—Epigenetic Regulators in Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 515, Correction in Pharmaceuticals 2025, 18, 111. [Google Scholar] [CrossRef]
- Krylova, T.A.; Koltsova, A.M.; Zenin, V.V.; Musorina, A.S.; Yakovleva, T.K.; Poljanskaya, G.G. Comparative characteristics of new mesenchymal stem cell lines derived from human embryonic stem cells, bone marrow and foreskin. Tsitologiia 2012, 54, 5–16. [Google Scholar] [CrossRef]
- Kraskovskaya, N.; Bolshakova, A.; Khotin, M.; Bezprozvanny, I.; Mikhailova, N. Protocol Optimization for Direct Reprogramming of Primary Human Fibroblast into Induced Striatal Neurons. Int. J. Mol. Sci. 2023, 24, 6799. [Google Scholar] [CrossRef]
- Shlush, L.I.; Itzkovitz, S.; Cohen, A.; Rutenberg, A.; Berkovitz, R.; Yehezkel, S.; Shahar, H.; Selig, S.; Skorecki, K. Quantitative digital in situ senescence-associated beta-galactosidase assay. BMC Cell Biol. 2011, 12, 16. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism. Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef]
- Kim, Y.; Sharov, A.A.; McDole, K.; Cheng, M.; Hao, H.; Fan, C.M.; Gaiano, N.; Ko, M.S.; Zheng, Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 2011, 334, 1706–1710. [Google Scholar] [CrossRef]
- Giacomini, C.; Mahajani, S.; Ruffilli, R.; Marotta, R.; Gasparini, L. Lamin B1 protein is required for dendrite development in primary mouse cortical neurons. Mol. Biol. Cell 2016, 27, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Laberge, R.M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Takamori, Y.; Tamura, Y.; Kataoka, Y.; Cui, Y.; Seo, S.; Kanazawa, T.; Kurokawa, K.; Yamada, H. Differential expression of nuclear lamin. the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur. J. Neurosci. 2007, 25, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Wong, C.S. Effects of p21 on adult hippocampal neuronal development after irradiation. Cell Death Discov. 2018, 4, 79, Correction in Cell Death Discov. 2018, 5, 116. [Google Scholar] [CrossRef]
- Marques-Torrejon, M.A.; Porlan, E.; Banito, A.; Gomez-Ibarlucea, E.; Lopez-Contreras, A.J.; Fernandez-Capetillo, O.; Vidal, A.; Gil, J.; Torres, J.; Farinas, I. Cyclin-dependent kinase inhibitor p21 controls adult neural stem cell expansion by regulating Sox2 gene expression. Cell Stem Cell 2013, 12, 88–100. [Google Scholar] [CrossRef]
- Ticli, G.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Revisiting the Function of p21(CDKN1A) in DNA Repair: The Influence of Protein Interactions and Stability. Int. J. Mol. Sci. 2022, 23, 7058. [Google Scholar] [CrossRef]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Saffrey, M.J.; Cameron, K.; et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods Mol. Biol. 2007, 371, 21–31. [Google Scholar] [CrossRef]
- Chernova, T.; Nicotera, P.; Smith, A.G. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Mol. Pharmacol. 2006, 69, 697–705. [Google Scholar] [CrossRef]
- Geng, Y.Q.; Guan, J.T.; Xu, X.H.; Fu, Y.C. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem. Biophys. Res. Commun. 2010, 396, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Ingram, J.A.; Norton, H.J.; Hanley, E.N., Jr. Senescence in cells of the aging and degenerating intervertebral disc: Immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine 2007, 32, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.K.; Lin’kova, N.S.; Umnov, R.S. Peptide KED: Molecular-Genetic Aspects of Neurogenesis Regulation in Alzheimer’s Disease. Bull. Exp. Biol. Med. 2021, 171, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.; Diomede, F.; Mironova, E.; Linkova, N.; Trofimova, S.; Trubiani, O.; Caputi, S.; Sinjari, B. AEDG Peptide (Epitalon) Stimulates Gene Expression and Protein Synthesis during Neurogenesis: Possible Epigenetic Mechanism. Molecules 2020, 25, 609. [Google Scholar] [CrossRef]
- Craveiro, M.; Cretenet, G.; Mongellaz, C.; Matias, M.I.; Caron, O.; de Lima, M.C.P.; Zimmermann, V.S.; Solary, E.; Dardalhon, V.; Dulic, V.; et al. Resveratrol stimulates the metabolic reprogramming of human CD4(+) T cells to enhance effector function. Sci. Signal. 2017, 10, eaal3024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakhenberg, E.; Linkova, N.; Kraskovskaya, N.; Krieger, D.; Polyakova, V.; Medvedev, D.; Krasichkov, A.; Khotin, M.; Ryzhak, G. The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation. Curr. Issues Mol. Biol. 2025, 47, 739. https://doi.org/10.3390/cimb47090739
Sakhenberg E, Linkova N, Kraskovskaya N, Krieger D, Polyakova V, Medvedev D, Krasichkov A, Khotin M, Ryzhak G. The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation. Current Issues in Molecular Biology. 2025; 47(9):739. https://doi.org/10.3390/cimb47090739
Chicago/Turabian StyleSakhenberg, Elena, Natalia Linkova, Nina Kraskovskaya, Daria Krieger, Victoria Polyakova, Dmitrii Medvedev, Alexander Krasichkov, Mikhail Khotin, and Galina Ryzhak. 2025. "The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation" Current Issues in Molecular Biology 47, no. 9: 739. https://doi.org/10.3390/cimb47090739
APA StyleSakhenberg, E., Linkova, N., Kraskovskaya, N., Krieger, D., Polyakova, V., Medvedev, D., Krasichkov, A., Khotin, M., & Ryzhak, G. (2025). The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation. Current Issues in Molecular Biology, 47(9), 739. https://doi.org/10.3390/cimb47090739







