Mechanisms of the Effects of Polyphenols on Diabetic Nephropathy
Abstract
1. Introduction
2. Chemistry of Polyphenols
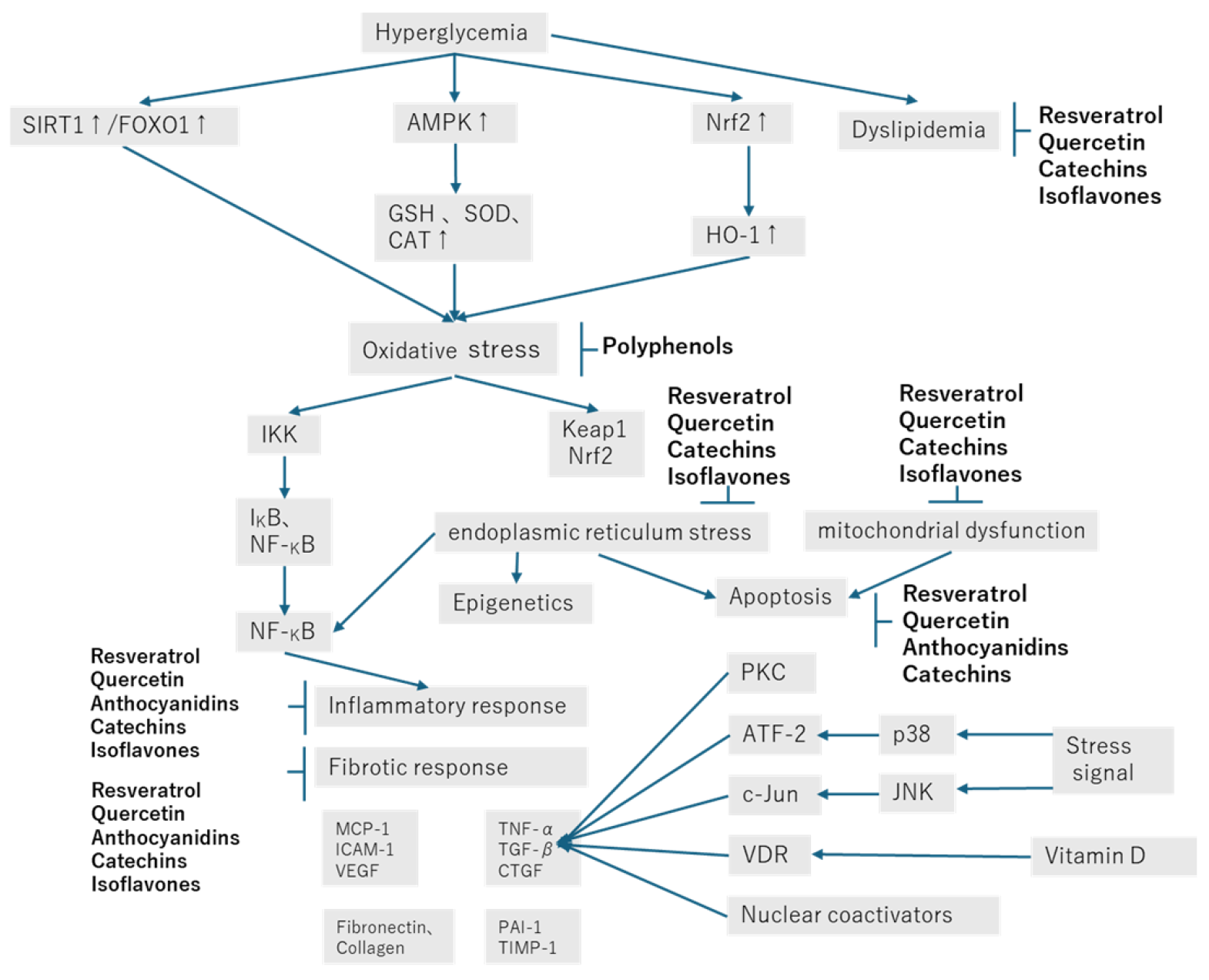
3. Renoprotective Effect of Resveratrol in Diabetic Nephropathy
4. Renoprotective Effect of Quercetin in Diabetic Nephropathy
5. Renoprotective Effect of Anthocyanidins and Anthocyanins in Diabetic Nephropathy
6. Renoprotective Effect of Catechin in Diabetic Nephropathy
7. Renoprotective Effect of Isoflavones in Diabetic Nephropathy
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. S1), 3–15. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Ansari, Z.; Chaurasia, A.; Neha; Sharma, N.; Bachheti, R.K.; Gupta, P.C. Exploring inflammatory and fibrotic mechanisms driving diabetic nephropathy progression. Cytokine Growth Factor. Rev. 2025, 84, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Epelde, F. Impact of DPP-4 Inhibitors in Patients with Diabetes Mellitus and Heart Failure: An In-Depth Review. Medicina 2024, 60, 1986. [Google Scholar] [CrossRef]
- Kawanami, D.; Takashi, Y.; Takahashi, H.; Motonaga, R.; Tanabe, M. Renoprotective Effects of DPP-4 Inhibitors. Antioxidants 2021, 10, 246. [Google Scholar] [CrossRef]
- Hou, G.; Dong, Y.; Jiang, Y.; Zhao, W.; Zhou, L.; Cao, S.; Li, W. Immune inflammation and metabolic interactions in the pathogenesis of diabetic nephropathy. Front. Endocrinol. 2025, 16, 1602594. [Google Scholar] [CrossRef]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef]
- Chu, A.J. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Front. Biosci. 2022, 27, 134. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Martiniakova, M.; Sarocka, A.; Penzes, N.; Biro, R.; Kovacova, V.; Mondockova, V.; Sevcikova, A.; Ciernikova, S.; Omelka, R. Protective Role of Dietary Polyphenols in the Management and Treatment of Type 2 Diabetes Mellitus. Nutrients 2025, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Natesan, V.; Kim, S.J. Natural Compounds in Kidney Disease: Therapeutic Potential and Drug Development. Biomol. Ther. 2025, 33, 39–53. [Google Scholar] [CrossRef]
- Sun, F.; Wang, X.H.; Fang, Z.; Wang, W.; Wang, D.; Teng, J. Mechanisms of resveratrol in alleviating diabetic nephropathy: Focus on tumor necrosis factor receptor-related factor expression and toll-like reeptor 4/nuclear factor-kappaB signaling pathway. J. Physiol. Pharmacol. 2024, 75, 637–651. [Google Scholar]
- Mladenov, M.; Bogdanov, J.; Bogdanov, B.; Hadzi-Petrushev, N.; Kamkin, A.; Stojchevski, R.; Avtanski, D. Efficacy of the monocarbonyl curcumin analog C66 in the reduction of diabetes-associated cardiovascular and kidney complications. Mol. Med. 2022, 28, 129. [Google Scholar] [CrossRef]
- Karim, N.; Rahman, A.; Chanudom, L.; Thongsom, M.; Tangpong, J. Mangosteen Vinegar Rind from Garcinia mangostana Prevents High-Fat Diet and Streptozotocin-Induced Type II Diabetes Nephropathy and Apoptosis. J. Food Sci. 2019, 84, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huo, Z.; Jia, X.; Xiong, Y.; Li, B.; Zhang, L.; Li, X.; Li, X.; Fang, Y.; Dong, X.; et al. (+)-Catechin ameliorates diabetic nephropathy injury by inhibiting endoplasmic reticulum stress-related NLRP3-mediated inflammation. Food Funct. 2024, 15, 5450–5465. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.; Salami, R.; Mafi, A.; Aarabi, M.H.; Vakili, O.; Asemi, Z. Therapeutic Potential of Resveratrol in Diabetic Nephropathy According to Molecular Signaling. Curr. Mol. Pharmacol. 2022, 15, 716–735. [Google Scholar] [CrossRef]
- Zhu, X.; Deng, Z.; Cao, Y.; Zhou, Z.; Sun, W.; Liu, C.; Fan, S.; Yin, X.X. Resveratrol prevents Drp1-mediated mitochondrial fission in the diabetic kidney through the PDE4D/PKA pathway. Phytother. Res. 2023, 37, 5916–5931. [Google Scholar] [CrossRef]
- Ahmed, Q.U.; Ali, A.H.M.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Sabere, A.S.M.; Nawi, M.S.M.; Khatib, A.; Siddiqui, M.J.; Umar, A.; et al. Medicinal Potential of Isoflavonoids: Polyphenols That May Cure Diabetes. Molecules 2020, 25, 5491. [Google Scholar] [CrossRef]
- Peng, X.; Su, H.; Liang, D.; Li, J.; Ting, W.J.; Liao, S.C.; Huang, C.Y. Ramipril and resveratrol co-treatment attenuates RhoA/ROCK pathway-regulated early-stage diabetic nephropathy-associated glomerulosclerosis in streptozotocin-induced diabetic rats. Environ. Toxicol. 2019, 34, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Lin, Y.; Cao, C.; Li, L.; Wang, J.; Niu, J.; Guo, Y.; Sun, Y.; Wang, Y.; Wang, W. Protective effect of umbilical cord mesenchymal stem cells combined with resveratrol against renal podocyte damage in NOD mice. Diabetes Res. Clin. Pract. 2019, 156, 107755. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Qi, X.; Zhang, X.; Ren, K. Resveratrol Protects Against Post-Contrast Acute Kidney Injury in Rabbits With Diabetic Nephropathy. Front. Pharmacol. 2019, 10, 833. [Google Scholar] [CrossRef]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, W.; Wang, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2009–2017. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Fan, Y.J. Resveratrol improves lipid metabolism in diabetic nephropathy rats. Front. Biosci. 2020, 25, 1913–1924. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.J.; Ding, M.R.; You, C.Y.; Lin, X.; Wang, Y.; Wu, M.J.; Xu, G.F.; Wang, G.D. Resveratrol decreases high glucose-induced apoptosis in renal tubular cells via suppressing endoplasmic reticulum stress. Mol. Med. Rep. 2020, 22, 4367–4375. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Chen, W.; Feng, F.; Deng, Y. Resveratrol Inhibits Lipopolysaccharide-Induced Extracellular Matrix Accumulation and Inflammation in Rat Glomerular Mesangial Cells by SphK1/S1P2/NF-kappaB Pathway. Diabetes Metab. Syndr. Obes. 2020, 13, 4495–4505. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Tsatsakis, A.; Skaperda, Z.; Kouretas, D.; Shahraki, J. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Abharzanjani, F.; Hemmati, M. Protective effects of Quercetin and Resveratrol on aging markers in kidney under high glucose condition: In vivo and in vitro analysis. Mol. Biol. Rep. 2021, 48, 5435–5442. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, X.; Zhao, H.; Geng, J.; Li, X.; Zheng, K.; Guan, Y.; Hou, X.; Wang, C.; Song, G. Resveratrol ameliorates diabetic kidney injury by reducing lipotoxicity and modulates expression of components of the junctional adhesion molecule-like/sirtuin 1 lipid metabolism pathway. Eur. J. Pharmacol. 2022, 918, 174776. [Google Scholar] [CrossRef]
- Gu, W.; Yang, L.; Wang, X.; Geng, J.; Li, X.; Zheng, K.; Guan, Y.; Hou, X.; Wang, C.; Song, G. Pterostilbene, a Resveratrol Derivative, Improves Ectopic Lipid Deposition in the Kidneys of Mice Induced by a High-Fat Diet. Kidney Blood Press. Res. 2022, 47, 514–522. [Google Scholar] [CrossRef]
- Lan, K.C.; Peng, P.J.; Chang, T.Y.; Liu, S.H. Resveratrol Alleviates Advanced Glycation End-Products-Related Renal Dysfunction in D-Galactose-Induced Aging Mice. Metabolites 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, Y.; Guo, X.; Shan, W.; Zhang, J.; Yuan, A.; Shi, Y. Resveratrol protects mesangial cells under high glucose by regulating the miR-1231/IGF1/ERK pathway. Environ. Toxicol. 2024, 39, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, Y.; Lin, X.; Huang, J.; Zhang, F.; Chen, C.; Ren, H.; Zheng, S.; Yang, J.; Hui, S. Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice. Int. J. Food Sci. Nutr. 2024, 75, 264–276. [Google Scholar] [CrossRef]
- Lei, D.; Chengcheng, L.; Xuan, Q.; Yibing, C.; Lei, W.; Hao, Y.; Xizhi, L.; Yuan, L.; Xiaoxing, Y.; Qian, L. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol. Res. 2019, 146, 104320. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, J.; Wang, X.; Ge, J.; Li, N. Quercetin improves lipid metabolism via SCAP-SREBP2-LDLr signaling pathway in early stage diabetic nephropathy. Diabetes Metab. Syndr. Obes. 2019, 12, 827–839. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Xu, L.; Shi, J.; Yu, X.; Wang, X.; Li, X.; Jiang, H.; Yang, T.; Yin, X.; et al. Quercetin Attenuates Podocyte Apoptosis of Diabetic Nephropathy Through Targeting EGFR Signaling. Front. Pharmacol. 2021, 12, 792777. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Pan, Q.; Chen, X.; Chen, S.; Li, X.; Yao, W. Quercetin attenuates the proliferation, inflammation, and oxidative stress of high glucose-induced human mesangial cells by regulating the miR-485-5p/YAP1 pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 20587384211066440. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin Ameliorates Diabetic Kidney Injury by Inhibiting Ferroptosis via Activating Nrf2/HO-1 Signaling Pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chang, L.; Ren, Y.; Sui, M.; Fu, Y.; Zhang, L.; Hao, L. Quercetin improves diabetic kidney disease by inhibiting ferroptosis and regulating the Nrf2 in streptozotocin-induced diabetic rats. Ren. Fail. 2024, 46, 2327495. [Google Scholar] [CrossRef]
- Dh, H.S.; Sultana, R.; Prabhu, A.; Pavan, S.R.; Mohanto, S.; Subramaniyan, V. Biomedicine and pharmacotherapeutic effectiveness of combinatorial atorvastatin and quercetin on diabetic nephropathy: An in vitro study. Biomed. Pharmacother. 2024, 174, 116533. [Google Scholar] [CrossRef]
- Liu, F.; Feng, Q.; Yang, M.; Yang, Y.; Nie, J.; Wang, S. Quercetin prevented diabetic nephropathy by inhibiting renal tubular epithelial cell apoptosis via the PI3K/AKT pathway. Phytother. Res. 2024, 38, 3594–3606. [Google Scholar] [CrossRef]
- Guo, X.; Wen, S.; Wang, J.; Zeng, X.; Yu, H.; Chen, Y.; Zhu, X.; Xu, L. Senolytic combination of dasatinib and quercetin attenuates renal damage in diabetic kidney disease. Phytomedicine 2024, 130, 155705. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Tan, R.Z.; Zhang, X.Q.; Yu, Y.; Yu, C. Calycosin Ameliorates Diabetes-Induced Renal Inflammation via the NF-kappaB Pathway In Vitro and In Vivo. Med. Sci. Monit. 2019, 25, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, C.; Wu, H.; Zhang, H.; Yuan, F.; Yang, G.; Yang, Q.; Jia, L.; Liang, Z.; Kang, L. Tectorigenin attenuates diabetic nephropathy by improving vascular endothelium dysfunction through activating AdipoR1/2 pathway. Pharmacol. Res. 2020, 153, 104678. [Google Scholar] [CrossRef]
- Jheng, H.F.; Hayashi, K.; Matsumura, Y.; Kawada, T.; Seno, S.; Matsuda, H.; Inoue, K.; Nomura, W.; Takahashi, H.; Goto, T. Anti-Inflammatory and Antioxidative Properties of Isoflavones Provide Renal Protective Effects Distinct from Those of Dietary Soy Proteins against Diabetic Nephropathy. Mol. Nutr. Food Res. 2020, 64, e2000015. [Google Scholar] [CrossRef]
- Li, Y.; Ou, S.; Liu, Q.; Gan, L.; Zhang, L.; Wang, Y.; Qin, J.; Liu, J.; Wu, W. Genistein improves mitochondrial function and inflammatory in rats with diabetic nephropathy via inhibiting MAPK/NF-kappaB pathway. Acta Cir. Bras. 2022, 37, e370601. [Google Scholar] [CrossRef] [PubMed]
- Yosri, H.; El-Kashef, D.H.; El-Sherbiny, M.; Said, E.; Salem, H.A. Calycosin modulates NLRP3 and TXNIP-mediated pyroptotic signaling and attenuates diabetic nephropathy progression in diabetic rats; An insight. Biomed. Pharmacother. 2022, 155, 113758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, L.; Wei, X.; Chen, M.; Zhang, X.; Mo, R.; Huang, R.; Liang, T.; Xu, X. Puerarin alleviates diabetic nephropathy by inhibiting Caspase-1-mediated pyroptosis. J. Pharm. Pharmacol. 2024, 76, 213–223. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yan, J.; He, J.C.; Li, Y.; Zhong, Y. Additive renal protective effects between arctigenin and puerarin in diabetic kidney disease. Biomed. Pharmacother. 2024, 171, 116107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W. Puerarin alleviates the high glucose-induced oxidative stress via the RAGE/PKC/NOX4 axis in renal mesangial cells. J. Toxicol. Sci. 2024, 49, 497–507. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Chen, Q.; Chen, Y.; Xu, X.; Liang, T. Puerarin reduces diabetic nephropathy-induced podocyte pyroptosis by modulating the SIRT1/NLRP3/caspase-1 pathway. Mol. Cell. Endocrinol. 2025, 595, 112409. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Yang, X.Y.; Zhang, X.J.; Yu, C.J.; Pang, Q.Q.; Huang, Y.W.; Wang, X.J.; Sheng, J. EGCG targeting Notch to attenuate renal fibrosis via inhibition of TGFbeta/Smad3 signaling pathway activation in streptozotocin-induced diabetic mice. Food Funct. 2020, 11, 9686–9695. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.; Jia, Q.; Yang, X.; Mehmood, S. Epigallocatechin-3-gallate ameliorates renal endoplasmic reticulum stress-mediated inflammation in type 2 diabetic rats. Exp. Biol. Med. 2022, 247, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhu, D.; Chen, C.; Wang, F.; Miu, Y.; Zhang, W. (+)-Catechins Play a Protective Role in Diabetic Kidney Disease by Alleviating EMT through Multiple Pathways. Mol. Nutr. Food Res. 2024, 68, e2400387. [Google Scholar]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes de Faria, J.M.; Lopes de Faria, J.B. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, D.L.; Yu, M.H.; Wang, C.Y.; Liang, H.W.; Lee, H.J. Apple Polyphenol Mitigates Diabetic Nephropathy via Attenuating Renal Dysfunction with Antioxidation in Streptozotocin-Induced Diabetic Rats. Antioxidants 2025, 14, 130. [Google Scholar] [CrossRef]
- Chtourou, Y.; Morjen, M.; Ammar, R.; Mhiri, R.; Jemaa, M.; Elbini-Dhouib, I.; Fetoui, H.; Srairi-Abid, N.; Marrakchi, N.; Jebali, J. Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats. Nutrients 2022, 14, 2867. [Google Scholar] [CrossRef]
- Dai, M.; Yuan, D.; Lei, Y.; Li, J.; Ren, Y.; Zhang, Y.; Cang, H.; Gao, W.; Tang, Y. Expression, purification and structural characterization of resveratrol synthase from Polygonum cuspidatum. Protein Expr. Purif. 2022, 191, 106024. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Farjana, M.; Moni, A.; Hossain, K.S.; Hannan, M.A.; Ha, H. Prospective Pharmacological Potential of Resveratrol in Delaying Kidney Aging. Int. J. Mol. Sci. 2021, 22, 8258. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, S.; Yan, H.; Wang, K.; Chen, L.; Zhou, M.; Li, Y. Resveratrol reverts Streptozotocin-induced diabetic nephropathy. Front. Biosci. 2020, 25, 699–709. [Google Scholar] [CrossRef]
- Hussein, M.M.; Mahfouz, M.K. Effect of resveratrol and rosuvastatin on experimental diabetic nephropathy in rats. Biomed. Pharmacother. 2016, 82, 685–692. [Google Scholar] [CrossRef]
- Fujita, T.; Uchida, K.; Maruyama, N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim. Biophys. Acta 1992, 1116, 122–128. [Google Scholar] [CrossRef]
- Malakoti, F.; Mohammadi, E.; Akbari Oryani, M.; Shanebandi, D.; Yousefi, B.; Salehi, A.; Asemi, Z. Polyphenols target miRNAs as a therapeutic strategy for diabetic complications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1865–1881. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Papakotsi, V.; Murphy, A.M.; Vagts, M.L.; Arsenault, E.J.; McGill, C.M.; Barth, B.M. Alaska’s Flora as a Treatment for Cancer. Int. J. Biopharm. Sci. 2024, 2, 120. [Google Scholar]
- Islam, M.R.; Al-Imran, M.I.K.; Zehravi, M.; Sweilam, S.H.; Mortuza, M.R.; Gupta, J.K.; Shanmugarajan, T.S.; Devi, K.; Tummala, T.; Alshehri, M.A.; et al. Targeting signaling pathways in neurodegenerative diseases: Quercetin’s cellular and molecular mechanisms for neuroprotection. Anim. Model. Exp. Med. 2025, 8, 798–818. [Google Scholar] [CrossRef]
- Mustafa, N.H.; Siti, H.N.; Kamisah, Y. Role of Quercetin in Diabetic Cardiomyopathy. Plants 2024, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Atrahimovich, D.; Samson, A.O.; Barsheshet, Y.; Vaya, J.; Khatib, S.; Reuveni, E. Genome-wide localization of the polyphenol quercetin in human monocytes. BMC Genom. 2019, 20, 606. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; He, J.; Zhao, Y. Quercetin Regulates Lipid Metabolism and Fat Accumulation by Regulating Inflammatory Responses and Glycometabolism Pathways: A Review. Nutrients 2024, 16, 1102. [Google Scholar] [CrossRef]
- Noman, A.M.; Sultan, M.T.; Maaz, M.; Mazhar, A.; Tariq, N.; Imran, M.; Hussain, M.; Mujtaba, A.; Abdelgawad, M.A.; Mostafa, E.M.; et al. Nutraceutical Potential of Anthocyanins: A Comprehensive Treatise. Food Sci. Nutr. 2025, 13, e70164. [Google Scholar] [CrossRef]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from Black Rice Ameliorates Diabetic Nephropathy via Reducing Blood Glucose, Suppressing Oxidative Stress and Inflammation, and Regulating Transforming Growth Factor beta1/Smad Expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Luo, G.; Yang, X.; Huang, W. Cyanidin-3-O-glucoside attenuates high glucose-induced podocyte dysfunction by inhibiting apoptosis and promoting autophagy via activation of SIRT1/AMPK pathway. Can. J. Physiol. Pharmacol. 2021, 99, 589–598. [Google Scholar] [CrossRef]
- Li, Y.X.; Lu, Y.P.; Tang, D.; Hu, B.; Zhang, Z.Y.; Wu, H.W.; Fan, L.J.; Cai, K.W.; Tang, C.; Zhang, Y.Q.; et al. Anthocyanin improves kidney function in diabetic kidney disease by regulating amino acid metabolism. J. Transl. Med. 2022, 20, 510. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. The effect of epigallocatechin-3-gallate on the renal dysfunction in the obstructed kidney in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 119–126. [Google Scholar]
- Cao, J.; Dai, D.L.; Yao, L.; Yu, H.H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.H.; Shen, W.; Yang, Z.X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012, 364, 115–129. [Google Scholar] [PubMed]
- Fiore, M.; Tonchev, A.B.; Pancheva, R.Z.; Yamashima, T.; Venditti, S.; Ferraguti, G.; Terracina, S. Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets. Molecules 2025, 30, 2888. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Cheng, Y.; Xu, Z.; Cai, L. Role of sirtuin-1 in diabetic nephropathy. J. Mol. Med. 2019, 97, 291–309. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Horiike, K.; Chin-Kanasaki, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Haneda, M.; Kashiwagi, A.; Koya, D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 2010, 120, 1043–1055. [Google Scholar] [CrossRef]
- Zhuo, L.; Fu, B.; Bai, X.; Zhang, B.; Wu, L.; Cui, J.; Cui, S.; Wei, R.; Chen, X.; Cai, G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell. Physiol. Biochem. 2011, 27, 681–690. [Google Scholar] [CrossRef]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, M.; Qian, W.; Yin, H. Calycosin, a Phytoestrogen Isoflavone, Induces Apoptosis of Estrogen Receptor-Positive MG-63 Osteosarcoma Cells via the Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway. Med. Sci. Monit. 2018, 24, 6178–6186. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, A.; Cheng, K.; Deng, Q.; Zhang, S.; Lan, Z.; Wang, W.; Chen, J. New Insights into the Mechanisms of Pyroptosis and Implications for Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 7057. [Google Scholar] [CrossRef]
- Mulay, S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Huang, D.; Tang, C.; Lu, Y.; Huang, S.; Peng, C.; Hu, X. Pyroptosis in diabetes and diabetic nephropathy. Clin. Chim. Acta 2022, 531, 188–196. [Google Scholar] [CrossRef]
- Monack, D.M.; Raupach, B.; Hromockyj, A.E.; Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 9833–9838. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef]
- Hilbi, H.; Moss, J.E.; Hersh, D.; Chen, Y.; Arondel, J.; Banerjee, S.; Flavell, R.A.; Yuan, J.; Sansonetti, P.J.; Zychlinsky, A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 1998, 273, 32895–32900. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid. Med. Cell. Longev. 2017, 2017, 9743280. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, K.; Zhou, M.; Gao, H.; Wang, W.; Xiao, W. Natural compounds improve diabetic nephropathy by regulating the TLR4 signaling pathway. J. Pharm. Anal. 2024, 14, 100946. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Fan, J.; Jia, H.; Li, J. Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy. Molecules 2024, 30, 20. [Google Scholar] [CrossRef]
- Lam, H.N.; Lin, S.P.; Nguyen, D.H.N.; Chen, C.M.; Su, C.T.; Fang, T.C.; Li, S.C. Integrative Roles of Functional Foods, Microbiotics, Nutrigenetics, and Nutrigenomics in Managing Type 2 Diabetes and Obesity. Nutrients 2025, 17, 608. [Google Scholar] [CrossRef]
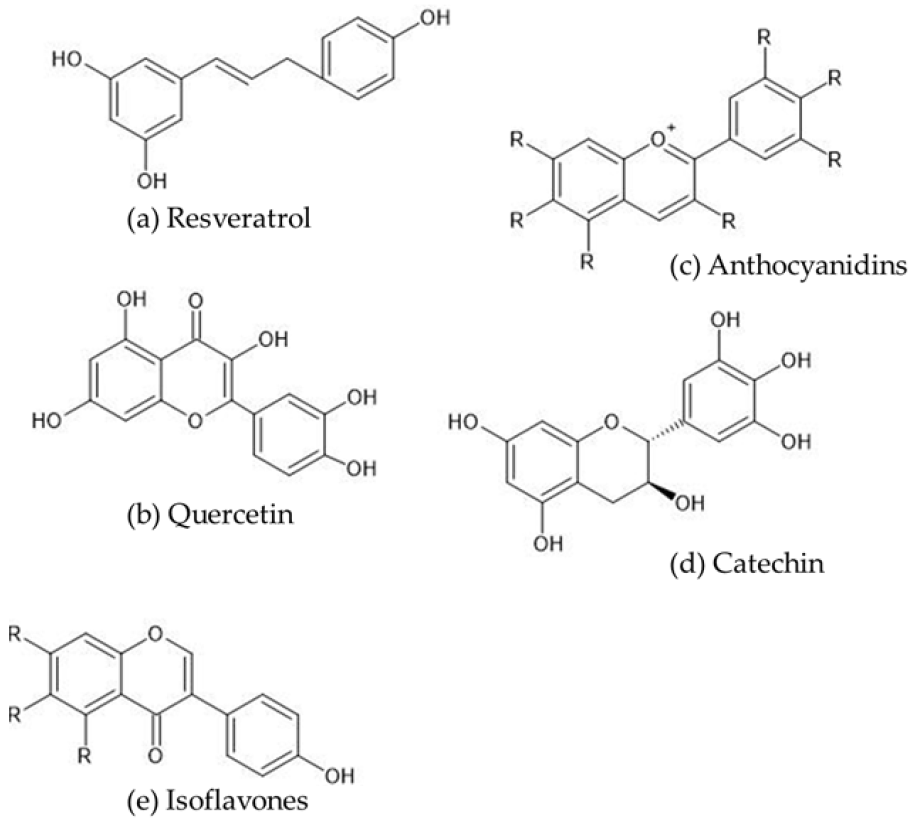
| Authors/Year/Reference No. | Polyphenols Used | Model Animals/Cells | Effects |
|---|---|---|---|
| Peng et al. (2019) [19] | Resveratrol and ramipril co-treatment | Rats with streptozotocin-induced diabetes | RhoA/ROCK pathway regulation of early-stage diabetic nephropathy-associated glomerulosclerosis. |
| Xian et al. (2019) [20] | Resveratrol | Human umbilical cord mesenchymal stem cells in combination with resveratrol to treat non-obese diabetic (NOD) mice | Increased levels of podocyte-associated proteins to better protect renal podocyte function. |
| Wang et al. (2019) [21] | Resveratrol | Rabbits with diabetic nephropathy and renal failure/renal tubular epithelial (HK-2) cells exposed to high-glucose conditions | Protection against post-contrast acute kidney injury and reduced renal hypoxia, mitochondrial dysfunction, and apoptosis of renal tubular cells. |
| Xian et al. (2020) [22] | Resveratrol | Non-obese diabetic (NOD) mice | An anti-inflammatory effect and improved renal function by improving metabolic memory of hyperglycemia. |
| Zhao et al. (2020) [23] | Resveratrol | Rats with streptozotocin-induced diabetes | Protection against diabetic nephropathy through several mechanisms, including improving lipid metabolism and alleviating insulin resistance by inducing autophagy. |
| Wang et al. (2020) [24] | Resveratrol | db/db mice | Suppression of oxidative stress-mediated apoptosis of podocytes dependent on 5′ adenosine monophosphate-activated protein kinase (AMPK) activation. |
| Zhang et al. (2020) [25] | Resveratrol | db/db mice/renal proximal tubule epithelial (NRK-52E) cells | Protection of renal tubular cells against hyperglycemia-induced apoptosis in diabetic nephropathy by suppressing ER stress. |
| Gong et al. (2020) [26] | Resveratrol | Lipopolysaccharide-induced rat glomerular mesangial cells | Inhibition of lipopolysaccharide-induced proliferation and inflammation of rat glomerular mesangial cells. |
| Hashemzaei et al. (2020) [27] | Resveratrol/curcumin/gallic acid | Renal proximal tubule cells | Toxic interactions between mitochondria and lysosomes exacerbated the oxidative stress/cytotoxicity produced by glyoxal. Resveratrol, curcumin, and gallic acid inhibited ROS formation and attenuated glyoxal-induced renal cell death. |
| Abhaizanjani et al. (2021) [28] | Resveratrol and/or quercetin | Male Wistar rats in hyperglycemic conditions/HEK293 cells | Significant dose-dependent reduction in the amount of methylglyoxal, which had a beneficial effect on aging markers. |
| Gu et al. (2022) [29] | Resveratrol | C57BL/6J mice fed a high-fat diet for 12 weeks | Reduced lipid deposition in the kidney and improved diabetic nephropathy. |
| Gu et al. (2022) [30] | Pterostilbene (resveratrol derivative) | C57BL/6J mice fed a high-fat diet for 12 weeks | Alleviation of renal fibrosis and ectopic lipid deposition in the kidneys. |
| Lan et al. (2023) [31] | Resveratrol | C57BL/6 mice fed galactose for 8 weeks | Alleviation of advanced glycation end product-related renal dysfunction through reduced renal cell senescence, apoptosis, and fibrosis. |
| Zhu et al. (2023) [17] | Resveratrol | db/db mice/glomerular mesangial cell line | Prevention of mitochondrial fission attenuated the progression of diabetic nephropathy. |
| Zhang et al. (2024) [32] | Resveratrol | Human mesangial cells | Suppression of proliferation by suppression of the hyperglycemia-induced miR-1231/IGF1/ERK pathway. |
| Yan et al. (2024) [33] | Resveratrol | db/db mice | Amelioration of the progression of diabetic kidney disease by suppression of tubulointerstitial fibrosis, which may be at least partially involved in regulating the gut microbiota–short-chain fatty acid axis. |
| Lei et al. (2019) [34] | Quercetin | db/db mice/glomerular mesangial cells | Inhibition of mesangial cell proliferation through reactivation of the Hippo pathway in high-dose glucose-treated mouse glomerular mesangial cells and diabetic nephropathy. |
| Jiang et al. (2019) [35] | Quercetin | db/db mice | Alleviation of early diabetic kidney damage by improved lipid metabolism. |
| Liu et al. (2022) [36] | Quercetin | db/db mice/mouse podocytes | Attenuation of podocyte apoptosis by inhibition of the EGFR signaling pathway. |
| Wan et al. (2022) [37] | Quercetin | Human mesangial cells/blood samples collected from diabetic nephropathy patients and healthy controls | Inhibition of HG-induced HMC proliferation, inflammation, and oxidative stress via the miR-485-5p/YAP1 axis. |
| Feng et al. (2023) [38] | Quercetin | db/db mice/human renal proximal tubule epithelial (HK-2) cells | Inhibition of ferroptosis in renal tubular epithelial cells by regulation of the Nrf2/HO1 signaling pathway. |
| Zhang et al. (2024) [39] | Quercetin | db/db mice/human renal proximal tubule epithelial (HK-2) cells | Inhibition of ferroptosis in renal tubular epithelial cells. |
| Shahin et al. (2024) [40] | Quercetin and atorvastatin co-treatment | NRK-52E rat kidney epithelial cells | Restoration of cell viability. |
| Liu et al. (2024) [41] | Quercetin | Mouse model of type 2 diabetes induced by a combination of high-fat diet and streptozotocin (STZ)/human renal tubular epithelial (HK-2) cells | Inhibition of renal tubular epithelial cell apoptosis via the PI3K/AKT pathway. |
| Guo et al. (2024) [42] | Quercetin and dasatinib co-treatment | db/db mice/db/db mice transfected with PPARα or shPPARα overexpression vector | Overexpression of PPARα upregulated the expression of PPARα, which targeted downstream FAO pathway-related proteins, restored renal function, and inhibited renal fibrosis in vitro and in vivo. |
| Oza et al. (2019) [43] | Formononetin | Rats with type 2 diabetes induced by a high-fat diet and low-dose streptozotocin | Increased expression of SIRT1 in kidney tissue. |
| Zhang et al. (2019) [44] | Calycosin | db/db mice/mouse renal tubular epithelial cells | Significant amelioration of diabetes-induced renal inflammation in diabetic nephropathy via inhibition of the NFκB-dependent signaling pathway in vivo and in vitro. |
| Yang et al. (2020) [45] | Tectorigenin | db/db mice/human glomerular endothelial cells | Reversal of diabetes-induced glucose and lipid metabolism disorders. |
| Jheng et al. (2020) [46] | Genistein | KK-Ay mice | Inhibition of the activation of albumin-induced activator protein 1 and the development of reactive oxidative stress, accompanied by a decrease in NADPH oxidase (NOX) gene expression. |
| Li et al. (2022) [47] | Genistein | Sprague Dawley rats | Inhibition of mesangial matrix expansion and oxidative stress, protected podocyte integrity, and increased mitochondrial membrane potential. |
| Yosri et al. (2022) [48] | Calycosin | Rats with streptozotocin-induced diabetes | Inhibition of the progression of DN through regulation of the NFκB p65/NLRP3/TXNIP inflammasome signaling pathway. |
| Chen et al. (2024) [49] | Puerarin | Mice with streptozotocin-induced diabetes | Association with the inhibition of caspase 1-mediated pyrexia. |
| Li et al. (2024) [50] | Arctigenin and puerarin co-treatment | db/db mice | Arctigenin and puerarin have an additive inhibitory effect on the activation of the inflammatory NFκB pathway. |
| Wang et al. (2024) [51] | Puerarin | Renal mesangial cells | Attenuation of hyperglycemia-induced oxidative stress via the RAGE/PKC/NOX4 axis. |
| Wang et al. (2025) [52] | Puerarin | Mice with streptozotocin-induced diabetes | Regulation of the SIRT1/NLRP3/Caspase 1 pathway to inhibit podocyte pyroptosis, reduce podocyte damage, and alleviate renal inflammatory damage. |
| Zhu et al. (2020) [53] | (-)-Epigallocatechin gallate | Mice with streptozotocin-induced diabetes | Amelioration of renal fibrosis by targeting Notch via inhibition of the TGFβ/SMAD3 pathway. |
| Yang et al. (2022) [54] | (-)-Epigallocatechin gallate | Rats with streptozotocin-induced diabetes | Suppression of ER stress-mediated NLRP3 inflammasome overactivation. |
| Zhang et al. (2024) [15] | (+)-Catechin | Mice with streptozotocin-induced diabetes | Suppression of endoplasmic reticulum stress and NLRP3-related inflammation and reduced renal tubule damage. |
| Ni et al. (2024) [55] | (+)-Catechin | db/db mice | Alleviation of diabetic nephropathy through its anti-inflammatory properties and regulation of EMT-related genes, such as Rage, Cav1, Grem2, Macrod2, and Kap. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiyama, M.; Iijima, K.; Okuzawa, R.; Kawata, R.; Kimura, A.; Shinohara, Y.; Shimada, A.; Yamanaka, M.; Youda, A.; Iwamoto, T. Mechanisms of the Effects of Polyphenols on Diabetic Nephropathy. Curr. Issues Mol. Biol. 2025, 47, 735. https://doi.org/10.3390/cimb47090735
Kamiyama M, Iijima K, Okuzawa R, Kawata R, Kimura A, Shinohara Y, Shimada A, Yamanaka M, Youda A, Iwamoto T. Mechanisms of the Effects of Polyphenols on Diabetic Nephropathy. Current Issues in Molecular Biology. 2025; 47(9):735. https://doi.org/10.3390/cimb47090735
Chicago/Turabian StyleKamiyama, Masumi, Kotoe Iijima, Rema Okuzawa, Ruka Kawata, Airi Kimura, Yuki Shinohara, Ayana Shimada, Mika Yamanaka, Ayuka Youda, and Tamami Iwamoto. 2025. "Mechanisms of the Effects of Polyphenols on Diabetic Nephropathy" Current Issues in Molecular Biology 47, no. 9: 735. https://doi.org/10.3390/cimb47090735
APA StyleKamiyama, M., Iijima, K., Okuzawa, R., Kawata, R., Kimura, A., Shinohara, Y., Shimada, A., Yamanaka, M., Youda, A., & Iwamoto, T. (2025). Mechanisms of the Effects of Polyphenols on Diabetic Nephropathy. Current Issues in Molecular Biology, 47(9), 735. https://doi.org/10.3390/cimb47090735






