The Regulation of Catecholamine Biosynthesis by the Gas Transmitters Carbon Monoxide and Hydrogen Sulfide
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Design
2.3. RT-qPCR
2.4. Catecholamine ELISA
2.5. Data Analysis
3. Results
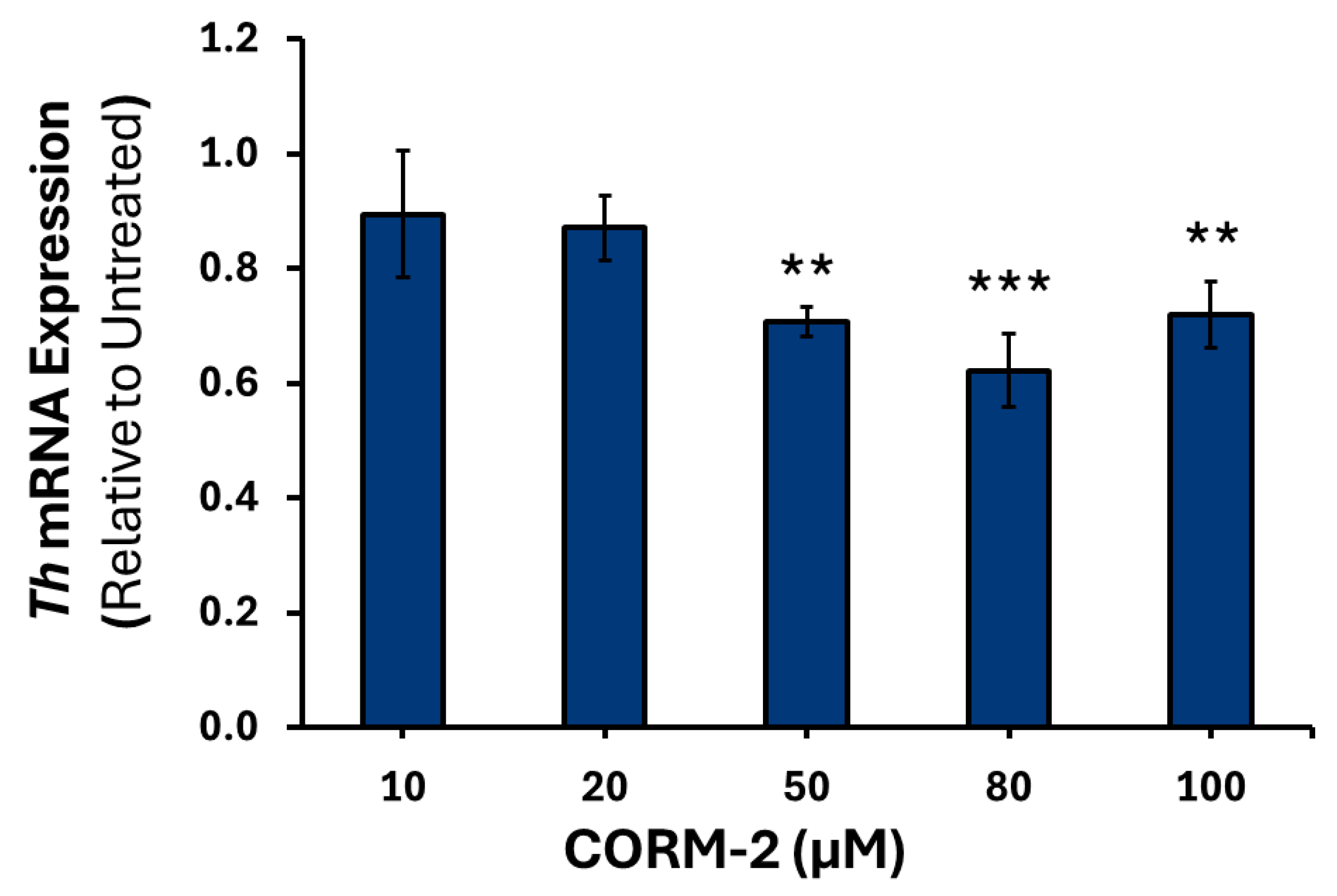
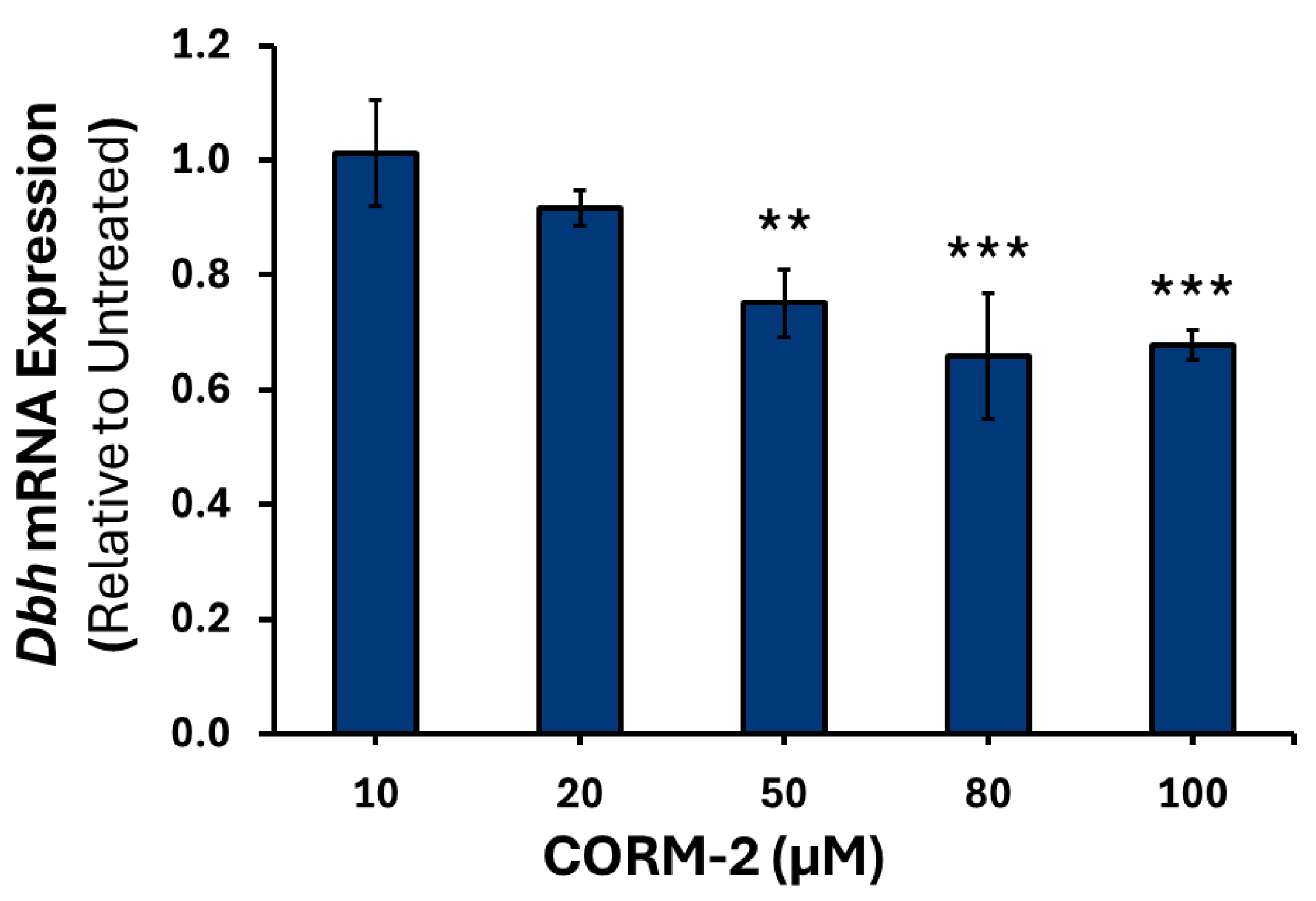
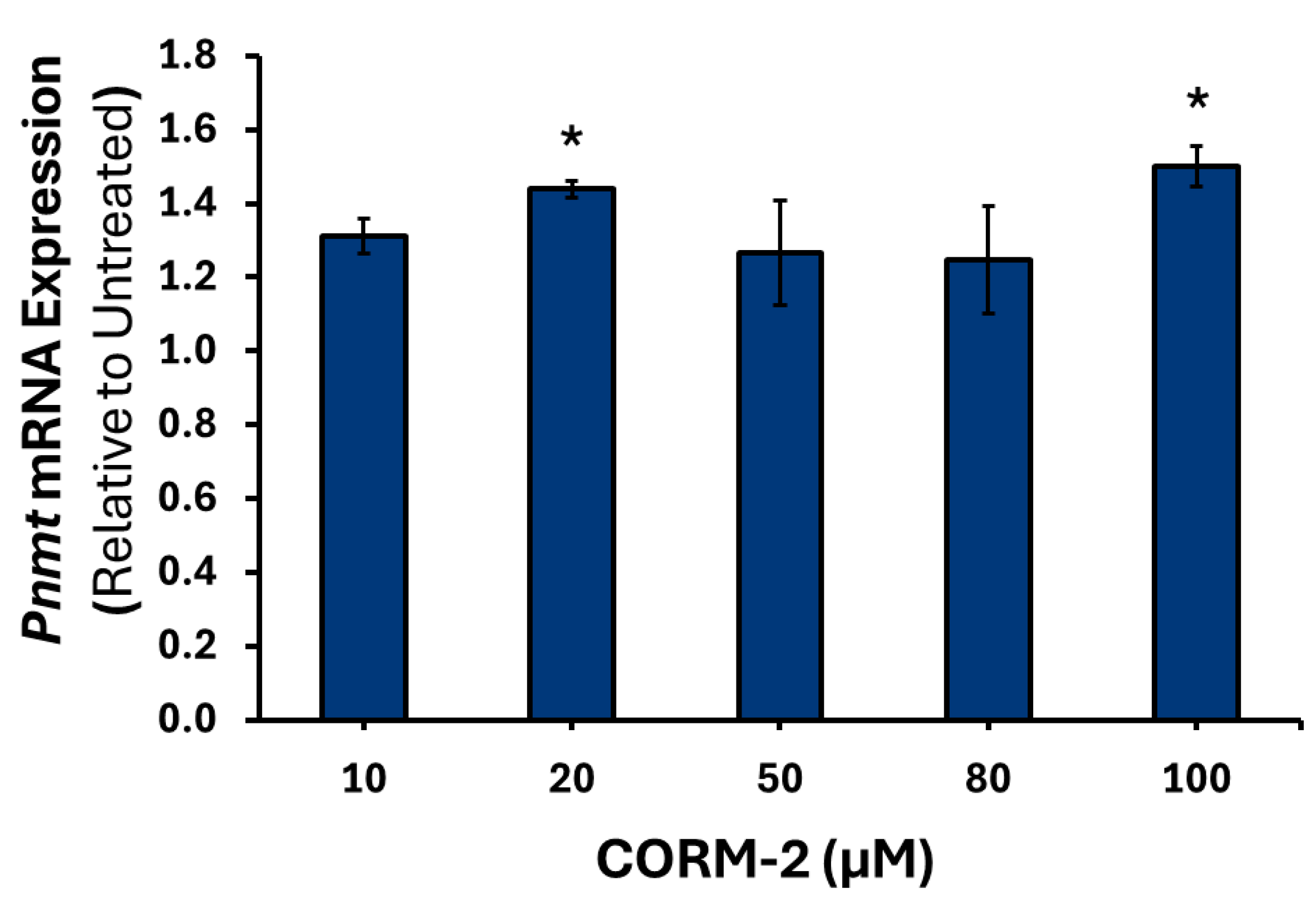
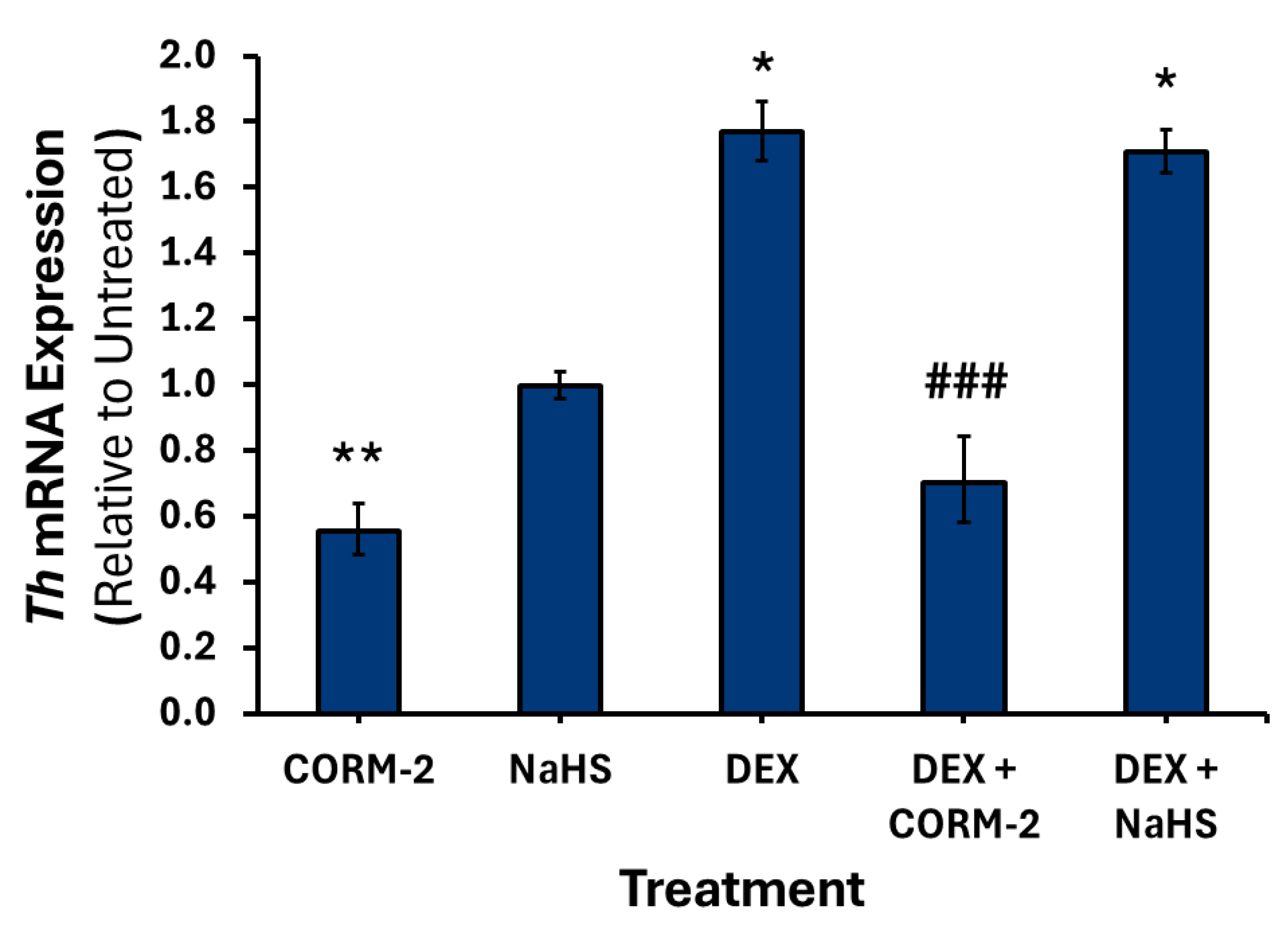
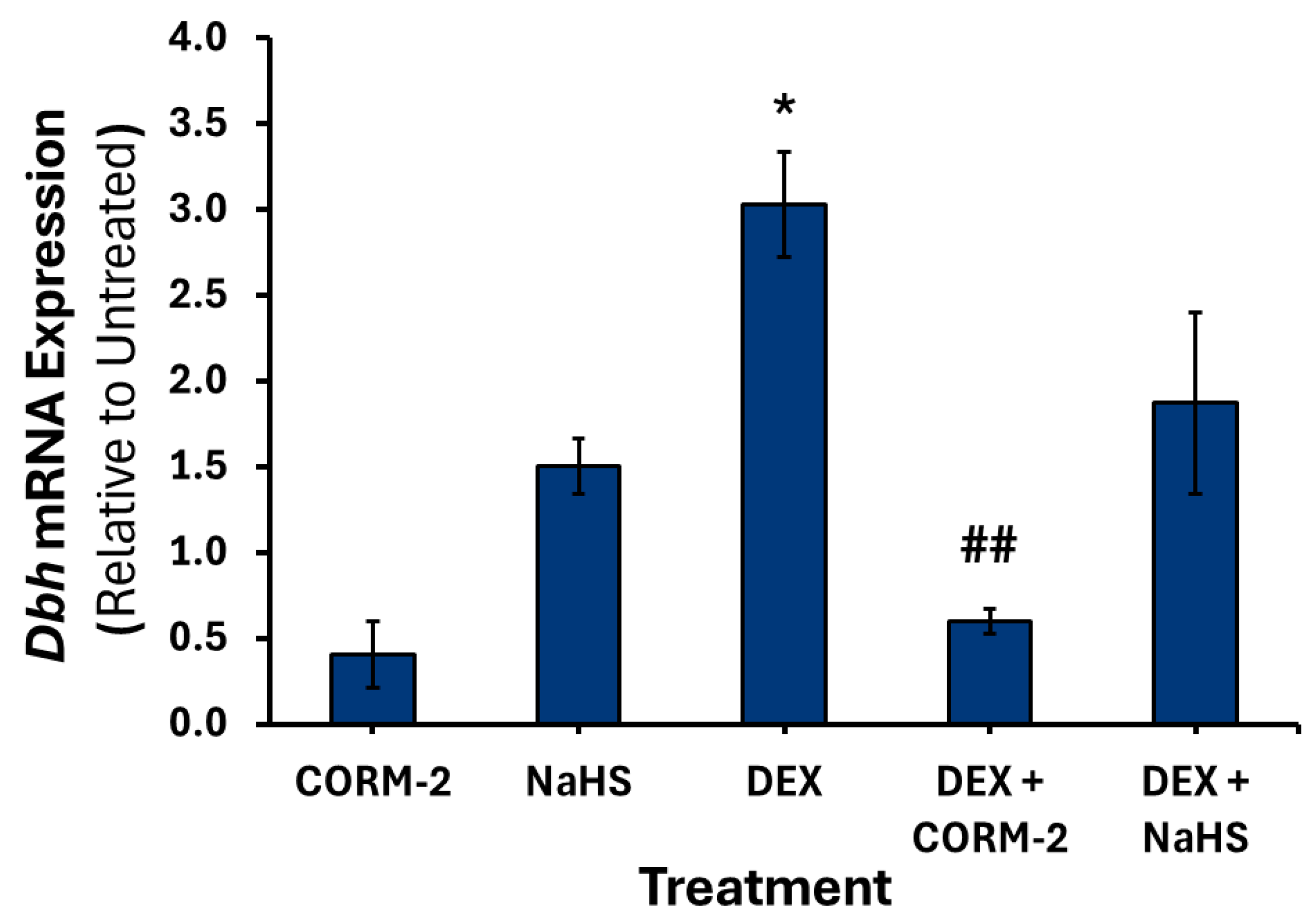
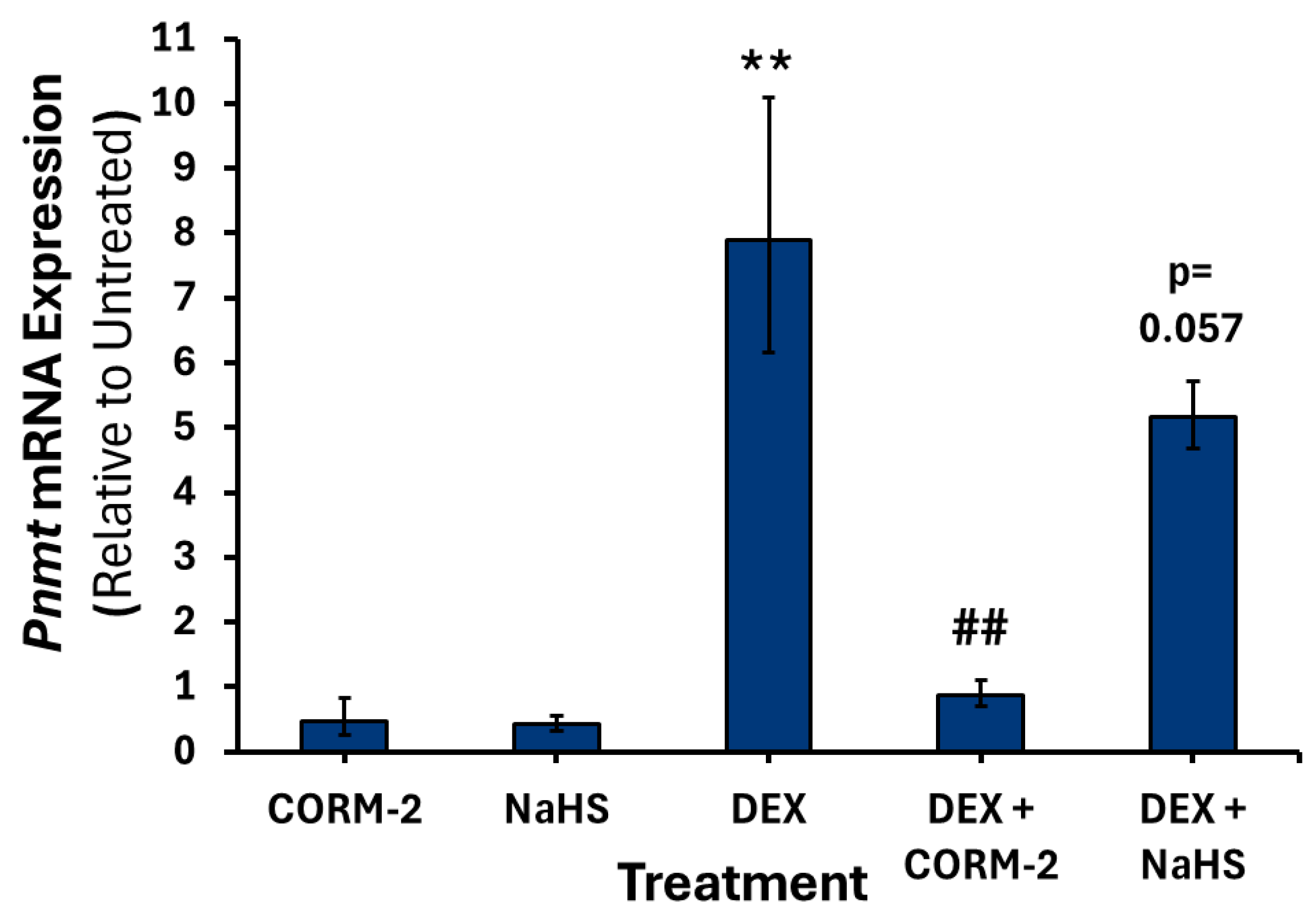
3.1. Effects of CO on the Expression of Catecholamine Biosynthesis Genes
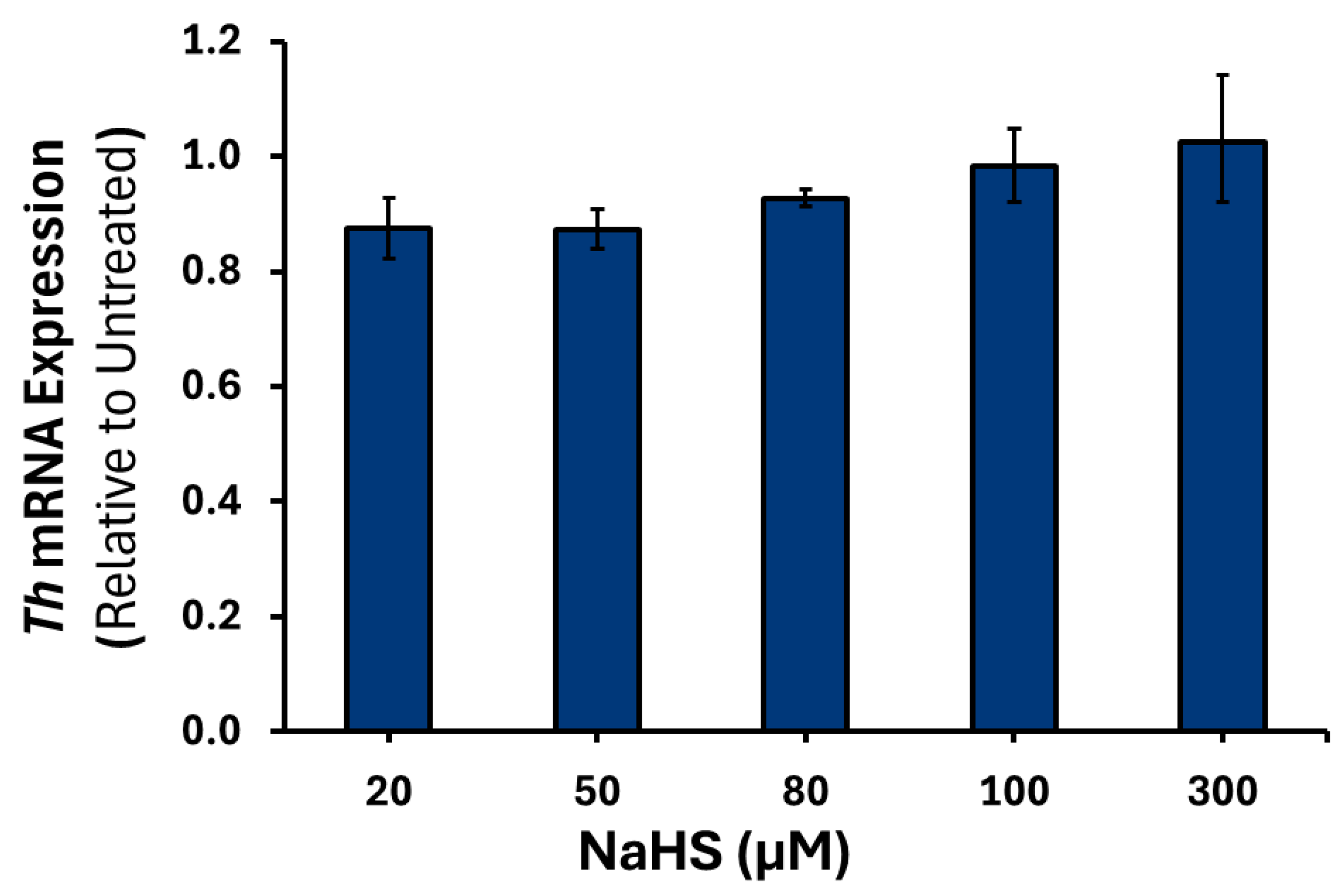
3.2. Effects of H2S on the Expression of Catecholamine Biosynthesis Genes
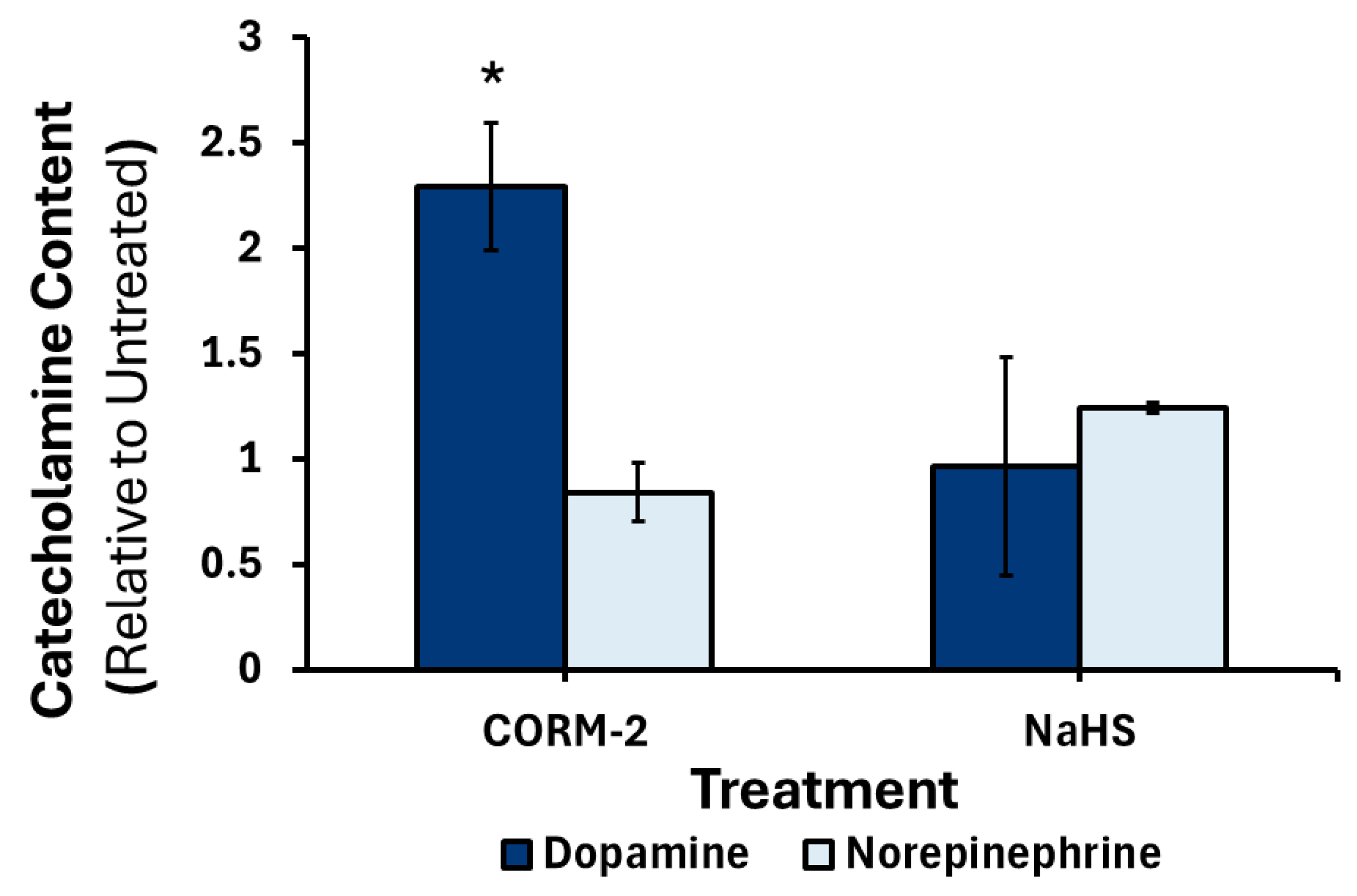
3.3. Effects of CO and H2S on the Production and Release of Catecholamines
3.4. Effects of CO and H2S on the Regulation of DEX-Associated Expression of Catecholamine Biosynthesis Genes
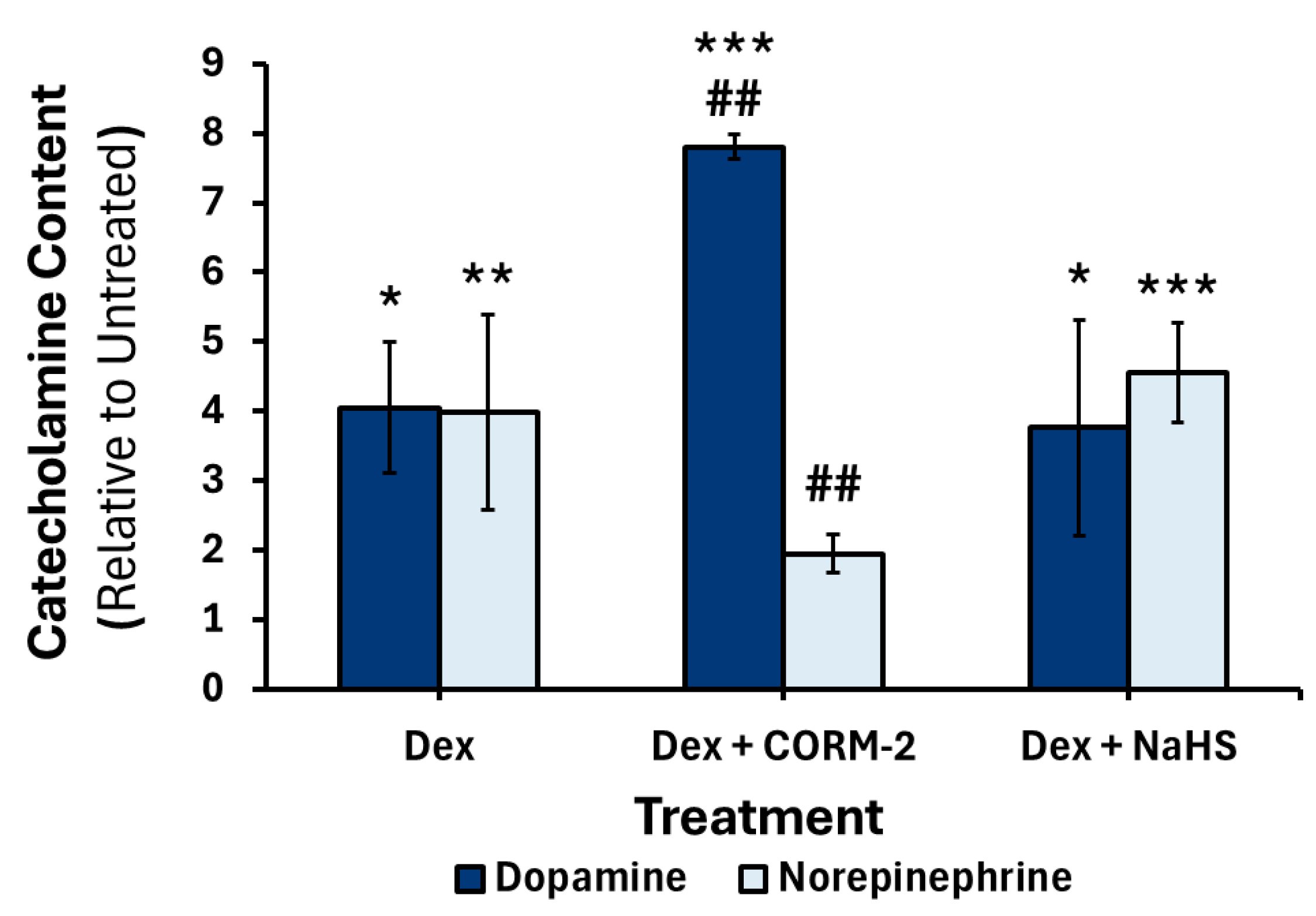
3.5. Effects of CO and H2S on the DEX-Mediated Catecholamine Content in Culture Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3MST | 3-Mercaptopyruvate Sulfurtransferase |
| AP-1 | Activating protein-1 |
| CRE | cAMP response element |
| CO | Carbon monoxide |
| CBS | Cystathionine β-synthase |
| CSE | Cystathionine γ-lyase |
| CYP3A4 | Cytochrome P450 3A4 |
| DEX | Dexamethasone |
| DBH | Dopamine β-hydroxylase |
| Egr1 | Early growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| GR | Glucocorticoid receptor |
| GRE | Glucocorticoid response element |
| GCs | Glucocorticoids |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Hsp70 | Heat shock protein 70 |
| Hsp90 | Heat shock protein 90 |
| HO | Heme oxygenase |
| HD | Homeodomain core recognition |
| H2S | Hydrogen sulfide |
| HPA | Hypothalamic–pituitary–adrenal |
| HIF-1α | Hypoxia-inducible transcription factor-1α |
| IL-1β | Interleukin-1 beta |
| mRNA | Messenger RNA |
| MAPK | Mitogen-activated protein kinase |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| NPAS2 | Neuronal PAS domain protein 2 |
| PNMT | Phenylethanolamine N-methyltransferase |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| Rpl32 | Ribosomal Protein L32 |
| NaHS | Sodium hydrosulfide |
| SNP | Sodium nitroprusside |
| sGC | Soluble guanylyl cyclase |
| SHR | Spontaneously hypertensive rat |
| TH | Tyrosine 3-hydroxylase |
References
- Pocock, G.; Richards, C.D.; Richards, D.A. Human Physiology; Oxford University Press: England, UK, 2018. [Google Scholar]
- Tank, A.W.; Lee Wong, D. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef]
- Wurtman, R.J. Stress and the Adrenocortical Control of Epinephrine Synthesis. Metab. Clin. Exp. 2002, 51, 11–14. [Google Scholar] [CrossRef]
- Sharara-Chami, R.I.; Joachim, M.; Pacak, K.; Majzoub, J.A. GLUCOCORTICOID TREATMENT-EFFECT ON ADRENAL MEDULLARY CATECHOLAMINE PRODUCTION. Shock 2010, 33, 213–217. [Google Scholar] [CrossRef]
- Kim, K.-T.; Park, D.H.; Joh, T.H. Parallel Up-Regulation of Catecholamine Biosynthetic Enzymes by Dexamethasone in PC12 Cells. J. Neurochem. 1993, 60, 946–951. [Google Scholar] [CrossRef]
- Nagatsu, T.; Stjärnet, L. Catecholamine Synthesis and Release. In Advances in Pharmacology; Goldstein, D.S., Eisenhofer, G., McCarty, R., Eds.; Academic Press: Cambridge, MA, USA, 1997; pp. 1–14. [Google Scholar]
- Goldstein, D.S. Plasma Catecholamines and Essential Hypertension. An Analytical Review. Hypertension 1983, 5, 86–99. [Google Scholar] [CrossRef]
- Axelrod, J. Catecholamines and Hypertension. Clin. Sci. Mol. Med. 1976, 51, 415s–421s. [Google Scholar] [CrossRef] [PubMed]
- Reja, V.; Goodchild, A.K.; Pilowsky, P.M. Catecholamine-Related Gene Expression Correlates with Blood Pressures in SHR. Hypertension 2002, 40, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hoehe, M.R.; Plaetke, R.; Otterud, B.; Stauffer, D.; Holik, J.; Byerley, W.F.; Baetge, E.E.; Gershon, E.S.; Lalouel, J.-M.; Leppert, M. Genetic Linkage of the Human Gene for Phenylethanol-Amine N-Methyltransferase (Pnmt), the Adrenaline-Synthesizing Enzyme, to DNA Markers on Chromosome 17q21–Q22. Hum. Mol. Genet. 1992, 1, 175–178. [Google Scholar] [CrossRef]
- Kaneda, N.; Ichinose, H.; Kobayashi, K.; Oka, K.; Kishi, F.; Nakazawa, A.; Kurosawa, Y.; Fujita, K.; Nagatsu, T. Molecular Cloning of cDNA and Chromosomal Assignment of the Gene for Human Phenylethanolamine N-Methyltransferase, the Enzyme for Epinephrine Biosynthesis. J. Biol. Chem. 1988, 263, 7672–7677. [Google Scholar] [CrossRef]
- Koike, G.; Jacob, H.J.; Krieger, J.E.; Szpirer, C.; Hoehe, M.R.; Horiuchi, M.; Dzau, V.J. Investigation of the Phenylethanolamine N-Methyltransferase Gene as a Candidate Gene for Hypertension. Hypertension 1995, 26, 595–601. [Google Scholar] [CrossRef]
- Nguyen, P.; Peltsch, H.; de Wit, J.; Crispo, J.; Ubriaco, G.; Eibl, J.; Tai, T.C. Regulation of the Phenylethanolamine N-Methyltransferase Gene in the Adrenal Gland of the Spontaneous Hypertensive Rat. Neurosci. Lett. 2009, 461, 280–284. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by Gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef]
- Feelisch, M.; te Poel, M.; Zamora, R.; Deussen, A.; Moncada, S. Understanding the Controversy over the Identity of EDRF. Nature 1994, 368, 62–65. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric Oxide in Hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, S.; Guo, Y.; Liu, S.; Xu, J.; Pan, L.; Hu, Y.; Liu, Y.; Cheng, Y. Association between eNOS Rs1799983 Polymorphism and Hypertension: A Meta-Analysis Involving 14,185 Cases and 13,407 Controls. BMC Cardiovasc. Disord. 2021, 21, 385. [Google Scholar] [CrossRef]
- Taha, H.; Skrzypek, K.; Guevara, I.; Nigisch, A.; Mustafa, S.; Grochot-Przeczek, A.; Ferdek, P.; Was, H.; Kotlinowski, J.; Kozakowska, M.; et al. Role of Heme Oxygenase-1 in Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1634–1641, Correction in Arterioscler Thromb Vasc Biol. 2010, 30, e166. [Google Scholar] [CrossRef] [PubMed]
- Martasek, P.; Schwartzman, M.L.; Goodman, A.I.; Solangi, K.B.; Levere, R.D.; Abraham, N.G. Hemin and L-Arginine Regulation of Blood Pressure in Spontaneous Hypertensive Rats. J. Am. Soc. Nephrol. 1991, 2, 1078. [Google Scholar] [CrossRef]
- Sabaawy, H.E.; Zhang, F.; Nguyen, X.; ElHosseiny, A.; Nasjletti, A.; Schwartzman, M.; Dennery, P.; Kappas, A.; Abraham, N.G. Human Heme Oxygenase-1 Gene Transfer Lowers Blood Pressure and Promotes Growth in Spontaneously Hypertensive Rats. Hypertension 2001, 38, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Ishikawa, K.; Matsumoto, H.; Kimura, S.; Kamiyama, Y.; Maruyama, Y. Synergetic Antioxidant and Vasodilatory Action of Carbon Monoxide in Angiotensin II–Induced Cardiac Hypertrophy. Hypertension 2007, 50, 1040–1048. [Google Scholar] [CrossRef]
- Huang, C.W.; Moore, P.K. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–25. [Google Scholar]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of Vascular Tone Homeostasis by NO and H2S: Implications in Hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Yan, H.; Du, J.; Tang, C. The Possible Role of Hydrogen Sulfide on the Pathogenesis of Spontaneous Hypertension in Rats. Biochem. Biophys. Res. Commun. 2004, 313, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Navarra, P.; Preziosi, P. Roles of Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide in the Regulation of the Hypothalamic-Pituitary-Adrenal Axis. J. Neurochem. 2010, 113, 563–575. [Google Scholar] [CrossRef]
- Ansell, D.; Grandbois, J.; Tai, T.C. Gene Regulation of Catecholamine Biosynthetic Enzymes by Nitric Oxide in PC12 Cells. Open J. Endocr. Metab. Dis. 2014, 4, 77–84. [Google Scholar] [CrossRef][Green Version]
- Grion, N.; Repetto, E.M.; Pomeraniec, Y.; Calejman, C.M.; Astort, F.; Sanchez, R.; Pignataro, O.P.; Arias, P.; Cymeryng, C.B. Induction of Nitric Oxide Synthase and Heme Oxygenase Activities by Endotoxin in the Rat Adrenal Cortex: Involvement of Both Signaling Systems in the Modulation of ACTH-Dependent Steroid Production. J. Endocrinol. 2007, 194, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Buerk, D.G.; Chugh, D.K.; Osanai, S.; Mokashi, A.; Lahiri, S. Dopamine Increases in Cat Carotid Body during Excitation by Carbon Monoxide: Implications for a Chromophore Theory of Chemoreception. J. Auton. Nerv. Syst. 1997, 67, 130–136. [Google Scholar] [CrossRef]
- Lahiri, S.; Penney, D.G.; Mokashi, A.; Albertine, K.H. Chronic CO Inhalation and Carotid Body Catecholamines: Testing of Hypotheses. J. Appl. Physiol. 1989, 67, 239–242. [Google Scholar] [CrossRef]
- Wang, C.-N.; Liu, Y.-J.; Duan, G.-L.; Zhao, W.; Li, X.-H.; Zhu, X.-Y.; Ni, X. CBS and CSE Are Critical for Maintenance of Mitochondrial Function and Glucocorticoid Production in Adrenal Cortex. Antioxid. Redox Signal. 2014, 21, 2192–2207. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, X.; Sun, J.; Li, J.; Ma, X.; Yao, W. H2S Induces Catecholamine Secretion in Rat Adrenal Chromaffin Cells. Toxicology 2012, 302, 40–43. [Google Scholar] [CrossRef]
- de Pascual, R.; Baraibar, A.M.; Méndez-López, I.; Pérez-Ciria, M.; Polo-Vaquero, I.; Gandía, L.; Ohia, S.E.; García, A.G.; de Diego, A.M.G. Hydrogen Sulphide Facilitates Exocytosis by Regulating the Handling of Intracellular Calcium by Chromaffin Cells. Eur. J. Physiol. 2018, 470, 1255–1270. [Google Scholar] [CrossRef]
- Tian, X.; Guan, T.; Guo, Y.; Zhang, G.; Kong, J. Selective Susceptibility of Oligodendrocytes to Carbon Monoxide Poisoning: Implication for Delayed Neurologic Sequelae (DNS). Front. Psychiatry 2020, 11, 815. [Google Scholar] [CrossRef]
- DeLeon, E.R.; Stoy, G.F.; Olson, K.R. Passive Loss of Hydrogen Sulfide in Biological Experiments. Anal. Biochem. 2012, 421, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Khurana, S.; Peltsch, H.; Grandbois, J.; Eibl, J.; Crispo, J.; Ansell, D.; Tai, T.C. Prenatal Glucocorticoid Exposure Programs Adrenal Pnmt Expression and Adult Hypertension. J. Endocrinol. 2015, 227, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sabban, E.L.; Kvetňanský, R. Stress-Triggered Activation of Gene Expression in Catecholaminergic Systems: Dynamics of Transcriptional Events. Trends Neurosci. 2001, 24, 91–98. [Google Scholar] [CrossRef]
- Lewis, E.J.; Harrington, C.A.; Chikaraishi, D.M. Transcriptional Regulation of the Tyrosine Hydroxylase Gene by Glucocorticoid and Cyclic AMP. Proc. Natl. Acad. Sci. USA 1987, 84, 3550–3554. [Google Scholar] [CrossRef]
- Swanson, D.J.; Zellmer, E.; Lewis, E.J. AP1 Proteins Mediate the cAMP Response of the Dopamine β-Hydroxylase Gene *. J. Biol. Chem. 1998, 273, 24065–24074. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.C.; Morita, K.; Wong, D.L. Role of Egr-1 in cAMP-Dependent Protein Kinase Regulation of the Phenylethanolamine N-Methyltransferase Gene. J. Neurochem. 2001, 76, 1851–1859. [Google Scholar] [CrossRef]
- Swanson, D.J.; Adachi, M.; Lewis, E.J. The Homeodomain Protein Arix Promotes Protein Kinase A-Dependent Activation of the Dopamine β-Hydroxylase Promoter through Multiple Elements and Interaction with the Coactivator cAMP-Response Element-Binding Protein-Binding Protein. J. Biol. Chem. 2000, 275, 2911–2923. [Google Scholar] [CrossRef]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.F. The Homeobox Gene Phox2b Is Essential for the Development of Autonomic Neural Crest Derivatives. Nature 1999, 399, 366–370. [Google Scholar] [CrossRef]
- Zellmer, E.; Zhang, Z.; Greco, D.; Rhodes, J.; Cassel, S.; Lewis, E.J. A Homeodomain Protein Selectively Expressed in Noradrenergic Tissue Regulates Transcription of Neurotransmitter Biosynthetic Genes. J. Neurosci. 1995, 15, 8109–8120. [Google Scholar] [CrossRef][Green Version]
- Swanson, D.J.; Zellmer, E.; Lewis, E.J. The Homeodomain Protein Arix Interacts Synergistically with Cyclic AMP to Regulate Expression of Neurotransmitter Biosynthetic Genes*. J. Biol. Chem. 1997, 272, 27382–27392. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a Noradrenergic Clonal Line of Rat Adrenal Pheochromocytoma Cells Which Respond to Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, J.; Duffourc, M.; Kao, R.L.; Ordway, G.A.; Huang, R.; Zhu, M.-Y. Transcription Factor Phox2 Upregulates Expression of Norepinephrine Transporter and Dopamine β-Hydroxylase in Adult Rat Brains. Neuroscience 2011, 192, 37–53. [Google Scholar] [CrossRef]
- Ryter, S.W.; Morse, D.; Choi, A.M.K. Carbon Monoxide: To Boldly Go Where NO Has Gone Before. Sci. STKE 2004, 2004, re6. [Google Scholar] [CrossRef]
- Lian, S.; Xia, Y.; Ung, T.T.; Khoi, P.N.; Yoon, H.J.; Kim, N.H.; Kim, K.K.; Jung, Y.D. Carbon Monoxide Releasing Molecule-2 Ameliorates IL-1β-Induced IL-8 in Human Gastric Cancer Cells. Toxicology 2016, 361–362, 24–38. [Google Scholar] [CrossRef]
- Li, M.H.; Jang, J.H.; Na, H.K.; Cha, Y.N.; Surh, Y.J. Carbon Monoxide Produced by Heme Oxygenase-1 in Response to Nitrosative Stress Induces Expression of Glutamate-Cysteine Ligase in PC12 Cells via Activation of Phosphatidylinositol 3-Kinase and Nrf2 Signaling. J. Biol. Chem. 2007, 282, 28577–28586. [Google Scholar] [CrossRef]
- Schultess, J.; Danielewski, O.; Smolenski, A.P. Rap1GAP2 Is a New GTPase-Activating Protein of Rap1 Expressed in Human Platelets. Blood 2005, 105, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Hartsfield, C.L. Cross Talk Between Carbon Monoxide and Nitric Oxide. Antioxid. Redox Signal. 2002, 4, 301–307. [Google Scholar] [CrossRef]
- Roskoski, R.; Roskoski, L.M. Activation of Tyrosine Hydroxylase in PC12 Cells by the Cyclic GMP and Cyclic AMP Second Messenger Systems. J. Neurochem. 1987, 48, 236–242. [Google Scholar] [CrossRef]
- Kim, D.; Choi, H.J.; Kim, S.W.; Cho, S.-W.; Hwang, O. Upregulation of Catecholamine Biosynthetic Enzymes by Nitric Oxide. J. Neurosci. Res. 2003, 72, 98–104. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Wang, B. Carbon Monoxide Signaling and Soluble Guanylyl Cyclase: Facts, Myths, and Intriguing Possibilities. Biochem. Pharmacol. 2022, 200, 115041. [Google Scholar] [CrossRef]
- Friebe, A.; Schultz, G.; Koesling, D. Sensitizing Soluble Guanylyl Cyclase to Become a Highly CO-Sensitive Enzyme. EMBO J. 1996, 15, 6863–6868. [Google Scholar] [CrossRef]
- Gysbers, J.W.; Rathbone, M.P. Guanosine Enhances NGF-Stimulated Neurite Outgrowth in PC12 Cells. Neuroreport 1992, 3, 997–1000. [Google Scholar] [CrossRef]
- Bau, C.; Middlemiss, P.J.; Hindley, S.; Jiang, S.; Ciccarelli, R.; Caciagli, F.; DiIorio, P.; Werstiuk, E.S.; Rathbone, M.P. Guanosine Stimulates Neurite Outgrowth in PC12 Cells via Activation of Heme Oxygenase and Cyclic GMP. Purinergic Signal. 2005, 1, 161–172. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Sabban, E.L. Regulation of Expression of Dopamine β-Hydroxylase in PC12 Cells by Glucocorticoids and Cyclic AMP Analogues. J. Neurochem. 1992, 59, 2040–2047. [Google Scholar] [CrossRef]
- Rani, C.S.S.; Elango, N.; Wang, S.; Kobayashi, K.; Strong, R. Identification of an Activator Protein-1-Like Sequence as the Glucocorticoid Response Element in the Rat Tyrosine Hydroxylase Gene. Mol. Pharmacol. 2009, 75, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.C.; Claycomb, R.; Her, S.; Bloom, A.K.; Wong, D.L. Glucocorticoid Responsiveness of the Rat PhenylethanolamineN-Methyltransferase Gene. Mol. Pharmacol. 2002, 61, 1385–1392. [Google Scholar] [CrossRef]
- Leonardi, D.B.; Anselmino, N.; Brandani, J.N.; Jaworski, F.M.; Páez, A.V.; Mazaira, G.; Meiss, R.P.; Nuñez, M.; Nemirovsky, S.I.; Giudice, J.; et al. Heme Oxygenase 1 Impairs Glucocorticoid Receptor Activity in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1006. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.D.; Ozsan, I.; Rodriguez Ospina, S.; Gulick, D.; Blair, L.J. Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease. Int. J. Mol. Sci. 2019, 20, 79. [Google Scholar] [CrossRef]
- Agyeman, A.S.; Jun, W.J.; Proia, D.A.; Kim, C.R.; Skor, M.N.; Kocherginsky, M.; Conzen, S.D. Hsp90 Inhibition Results in Glucocorticoid Receptor Degradation in Association with Increased Sensitivity to Paclitaxel in Triple-Negative Breast Cancer. Horm. Cancer 2016, 7, 114–126. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Chen, Y.-C.; Shih, C.-M.; Lin, C.-M.; Cheng, C.-H.; Chen, K.-C.; Lin, C.-W. The Induction of Heme Oxygenase-1 Suppresses Heat Shock Protein 90 and the Proliferation of Human Breast Cancer Cells through Its Byproduct Carbon Monoxide. Toxicol. Appl. Pharmacol. 2014, 274, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, C.K.; Lee, H.; Jeoung, D.; Ha, K.S.; Kwon, Y.G.; Kim, K.W.; Kim, Y.M. Carbon Monoxide Promotes VEGF Expression by Increasing HIF-1α Protein Level via Two Distinct Mechanisms, Translational Activation and Stabilization of HIF-1α Protein. J. Biol. Chem. 2010, 285, 32116–32125. [Google Scholar] [CrossRef]
- Tai, T.C.; Wong-Faull, D.C.; Claycomb, R.; Wong, D.L. Hypoxic Stress-Induced Changes in Adrenergic Function: Role of HIF1α. J. Neurochem. 2009, 109, 513–524. [Google Scholar] [CrossRef]
- Brown, S.T.; Kelly, K.F.; Daniel, J.M.; Nurse, C.A. Hypoxia Inducible Factor (HIF)-2α Is Required for the Development of the Catecholaminergic Phenotype of Sympathoadrenal Cells. J. Neurochem. 2009, 110, 622–630. [Google Scholar] [CrossRef]
- Richter, S.; Qin, N.; Pacak, K.; Eisenhofer, G. Role of Hypoxia and HIF2α in Development of the Sympathoadrenal Cell Lineage and Chromaffin Cell Tumours with Distinct Catecholamine Phenotypic Features. Adv. Pharmacol. 2013, 68, 285–317. [Google Scholar] [CrossRef]
- Watts, D.; Bechmann, N.; Meneses, A.; Poutakidou, I.K.; Kaden, D.; Conrad, C.; Krüger, A.; Stein, J.; El-Armouche, A.; Chavakis, T.; et al. HIF2α Regulates the Synthesis and Release of Epinephrine in the Adrenal Medulla. J. Mol. Med. 2021, 99, 1655–1666. [Google Scholar] [CrossRef]
- Hashimoto, H.; Toide, K.; Kitamura, R.; Fujita, M.; Tagawa, S.; Itoh, S.; Kamataki, T. Gene Structure of CYP3A4, an Adult-Specific Form of Cytochrome P450 in Human Livers, and Its Transcriptional Control. Eur. J. Biochem. 1993, 218, 585–595. [Google Scholar] [CrossRef]
- Kitzmiller, J.P.; Groen, D.K.; Phelps, M.A.; Sadee, W. Pharmacogenomic Testing: Relevance in Medical Practice: Why Drugs Work in Some Patients but Not in Others. Cleve. Clin. J. Med. 2011, 78, 243. [Google Scholar] [CrossRef] [PubMed]
- Hara, H. Inhibitory Effect of Nitric Oxide on the Induction of Cytochrome P450 3A4 mRNA by 1,25-Dihydroxyvitamin D3 in Caco-2 Cells. Free Radic. Res. 2000, 33, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, B.; Faull, K.F.; Janzen, C.; Mascharak, P.K. Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J. Med. Chem. 2021, 64, 8437–8446. [Google Scholar] [CrossRef]
- Kroiss, M.; Quinkler, M.; Lutz, W.K.; Allolio, B.; Fassnacht, M. Drug Interactions with Mitotane by Induction of CYP3A4 Metabolism in the Clinical Management of Adrenocortical Carcinoma. Clin. Endocrinol. 2011, 75, 585–591. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Khurana, S.; Murray, A.; Lamothe, J.; Tai, T.C. Whole Transcriptome Analysis of Adrenal Glands from Prenatal Glucocorticoid Programmed Hypertensive Rodents. Sci. Rep. 2020, 10, 18755. [Google Scholar] [CrossRef]
- Albrecht, U. Molecular Mechanisms in Mood Regulation Involving the Circadian Clock. Front. Neurol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Dioum, E.M.; Rutter, J.; Tuckerman, J.R.; Gonzalez, G.; Gilles-Gonzalez, M.-A.; McKnight, S.L. NPAS2: A Gas-Responsive Transcription Factor. Science 2002, 298, 2385–2387. [Google Scholar] [CrossRef]
- Murgo, E.; Colangelo, T.; Bellet, M.M.; Malatesta, F.; Mazzoccoli, G. Role of the Circadian Gas-Responsive Hemeprotein NPAS2 in Physiology and Pathology. Biology 2023, 12, 1354. [Google Scholar] [CrossRef]
- Molinoff, P.B.; Axelrod, J. Biochemistry of Catecholamines. Annu. Rev. Biochem. 1971, 40, 465–500. [Google Scholar] [CrossRef]
- Bauer, N.; Liu, D.; Nguyen, T.; Wang, B. Unraveling the Interplay of Dopamine, Carbon Monoxide, and Heme Oxygenase in Neuromodulation and Cognition. ACS Chem. Neurosci. 2024, 15, 400–407. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Yokoyama, S.; Nabeshima, T.; Kameyama, T. Changes in Concentrations of Dopamine, Serotonin, and Their Metabolites Induced by Carbon Monoxide (CO) in the Rat Striatum as Determined by in Vivo Microdialysis. Pharmacol. Biochem. Behav. 1994, 48, 9–15. [Google Scholar] [CrossRef]
- Taskiran, D.; Kutay, F.Z.; Pogun, S. Effect of Carbon Monoxide on Dopamine and Glutamate Uptake and cGMP Levels in Rat Brain. Neuropsychopharmacology 2003, 28, 1176–1181. [Google Scholar] [CrossRef]
- Dreyer-Andersen, N.; Almeida, A.S.; Jensen, P.; Kamand, M.; Okarmus, J.; Rosenberg, T.; Friis, S.D.; Serrano, A.M.; Blaabjerg, M.; Kristensen, B.W.; et al. Intermittent, Low Dose Carbon Monoxide Exposure Enhances Survival and Dopaminergic Differentiation of Human Neural Stem Cells. PLoS ONE 2018, 13, e0191207. [Google Scholar] [CrossRef]
- Faizan, M.; Muhammad, N.; Niazi, K.U.K.; Hu, Y.; Wang, Y.; Wu, Y.; Sun, H.; Liu, R.; Dong, W.; Zhang, W.; et al. CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials 2019, 12, 1643. [Google Scholar] [CrossRef]
- Sze, P.Y.; Hedrick, B.J. Effects of Dexamethasone and Other Glucocorticoid Steroids on Tyrosine Hydroxylase Activity in the Superior Cervical Ganglion. Brain Res. 1983, 265, 81–86. [Google Scholar] [CrossRef]
- Williams, L.R.; Sandquist, D.; Black AC, J.R.; Williams, T.H. Glucocorticoids Increase Tyrosine Hydroxylase Activity in Cultured Murine Neuroblastoma. J. Neurochem. 1981, 36, 2057–2062. [Google Scholar] [CrossRef]
- Núñez, C.; Földes, A.; Pérez-Flores, D.; García-Borrón, J.C.; Laorden, M.L.; Kovács, K.J.; Milanés, M.V. Elevated Glucocorticoid Levels Are Responsible for Induction of Tyrosine Hydroxylase mRNA Expression, Phosphorylation, and Enzyme Activity in the Nucleus of the Solitary Tract during Morphine Withdrawal. Endocrinology 2009, 150, 3118–3127. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; Von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine Hydroxylase Phosphorylation: Regulation and Consequences. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Al-Wadei, H.A.N.; Takahasi, T.; Schuller, H.M. PKA-Dependent Growth Stimulation of Cells Derived from Human Pulmonary Adenocarcinoma and Small Airway Epithelium by Dexamethasone. Eur. J. Cancer 2005, 41, 2745–2753. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Pandey, G.N. Adrenal Glucocorticoids Modulate [3H]Cyclic AMP Binding to Protein Kinase A (PKA), Cyclic AMP-Dependent PKA Activity, and Protein Levels of Selective Regulatory and Catalytic Subunit Isoforms of PKA in Rat Brain. J. Pharmacol. Exp. Ther. 2000, 294, 103–116. [Google Scholar] [CrossRef]
- Zhang, R.G.; Yip, C.Y.; Ko, W.H. Carbon Monoxide Inhibits Cytokine and Chloride Secretion in Human Bronchial Epithelia. Cell Physiol. Biochem. 2018, 49, 626–637. [Google Scholar] [CrossRef]
- Schwer, C.I.; Stoll, P.; Rospert, S.; Schallner, N.; Bürkle, H.; Schmidt, R.; Humar, M. Carbon Monoxide Releasing Molecule-2 CORM-2 Represses Global Protein Synthesis by Inhibition of Eukaryotic Elongation Factor eEF2. Int. J. Biochem. Cell Biol. 2013, 45, 201–212. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Gene (Accession Number) | Sequence (5′–3′) | Annealing Temperature (°C) | |
|---|---|---|---|
| Gapdh (NM_017008.4) | Forward | GTCATCCCAGAGCTGAACGG | 60 |
| Reverse | ATACTTGGCAGGTTTCTCCAGG | ||
| Rpl32 (NM_013226.3) | Forward | GGTGGCTGCCATCTGTTTTG | 60 |
| Reverse | GTTTCCGCCAGTTTCGCTTAAT | ||
| Th (NM_012740.4) | Forward | GCGACAGAGTCTCATCGAGGAT | 58 |
| Reverse | AAGAGCAGGTTGAGAACAGCATT | ||
| Dbh (NM_013158.3) | Forward | CGGTTTCTCCGACTGGAAGT | 60 |
| Reverse | ATCAAGGGCGTGTACACCAG | ||
| Pnmt Intronless (NM_031526.2) | Forward | CGAGGACAAGGGAGAGTCCT | 60 |
| Reverse | GGGCTTGTGCACATCAATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dingley, R.; Hourtovenko, C.; Lee, J.; Tharmalingam, S.; Tai, T.C. The Regulation of Catecholamine Biosynthesis by the Gas Transmitters Carbon Monoxide and Hydrogen Sulfide. Curr. Issues Mol. Biol. 2025, 47, 725. https://doi.org/10.3390/cimb47090725
Dingley R, Hourtovenko C, Lee J, Tharmalingam S, Tai TC. The Regulation of Catecholamine Biosynthesis by the Gas Transmitters Carbon Monoxide and Hydrogen Sulfide. Current Issues in Molecular Biology. 2025; 47(9):725. https://doi.org/10.3390/cimb47090725
Chicago/Turabian StyleDingley, Robert, Cameron Hourtovenko, James Lee, Sujeenthar Tharmalingam, and T. C. Tai. 2025. "The Regulation of Catecholamine Biosynthesis by the Gas Transmitters Carbon Monoxide and Hydrogen Sulfide" Current Issues in Molecular Biology 47, no. 9: 725. https://doi.org/10.3390/cimb47090725
APA StyleDingley, R., Hourtovenko, C., Lee, J., Tharmalingam, S., & Tai, T. C. (2025). The Regulation of Catecholamine Biosynthesis by the Gas Transmitters Carbon Monoxide and Hydrogen Sulfide. Current Issues in Molecular Biology, 47(9), 725. https://doi.org/10.3390/cimb47090725





