A Liposomal Formulation Enhances the Anti-Senescence Properties of Nicotinamide Adenine-Dinucleotide (NAD+) in Endothelial Cells and Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Liposomal Formulation
2.2. Cell Culture
2.3. Intracellular NAD+/NADH Measurement
2.4. Cell Survival
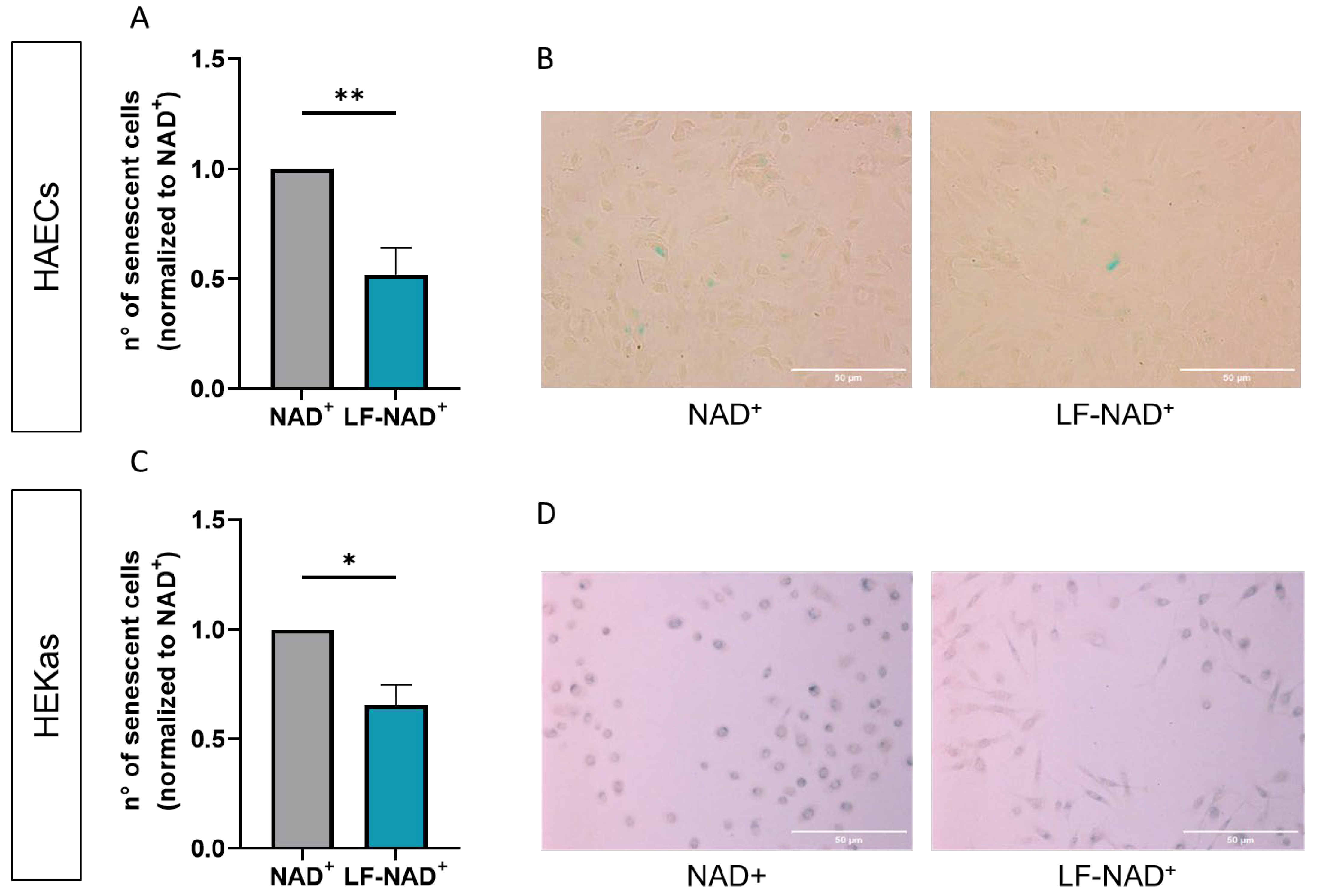
2.5. Cell Senescence
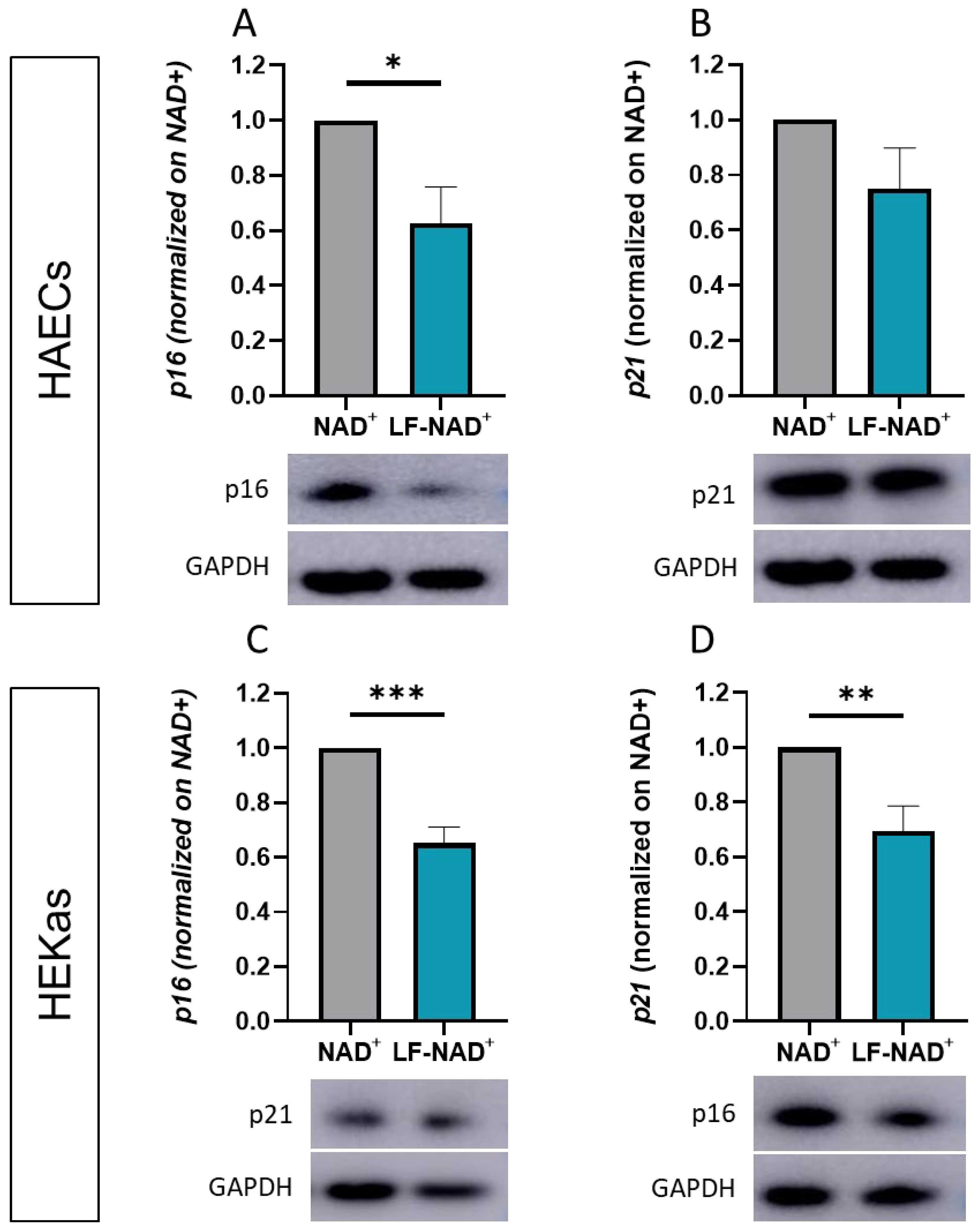
2.6. Western Blot
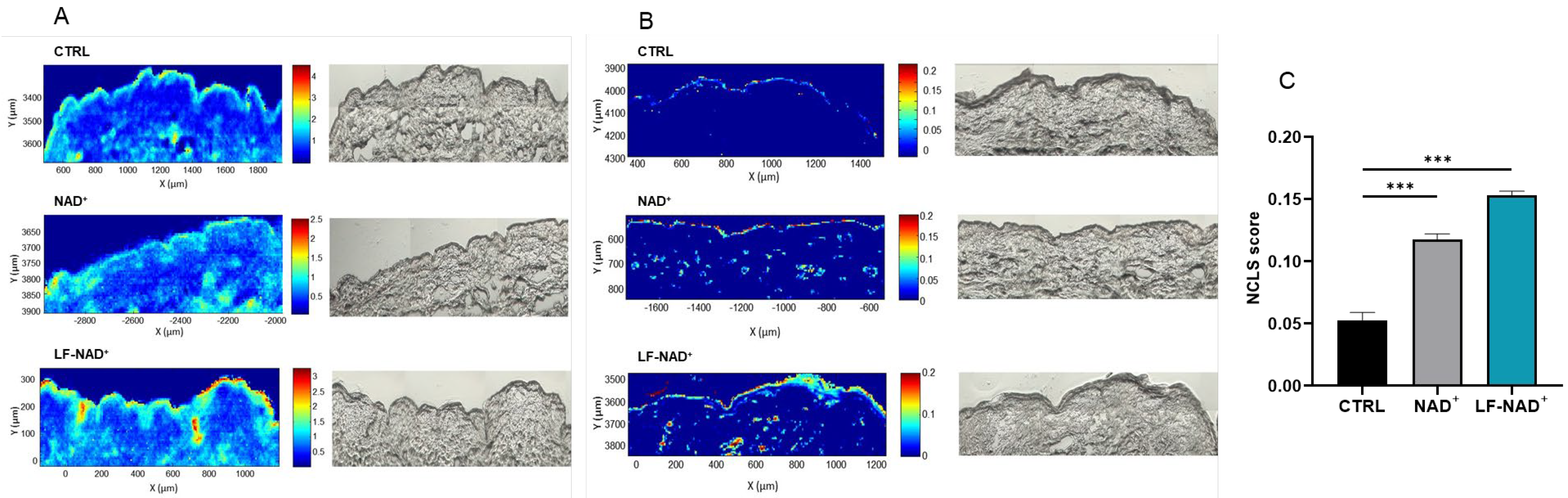
2.7. Ex Vivo Skin Penetration
2.8. Statistical Analysis
3. Results
3.1. Intracellular Delivery of NAD+ and NADH
3.2. Effect of LF-NAD+ on Cell Survival and Senescence
3.3. Effect of LF-NAD+ on Molecular Markers of Cell Senescence
3.4. Ex Vivo Skin Penetration of NAD+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine di-phosphate |
| ANOVA | Analysis of variance |
| CDK | Cyclin dependent kinases |
| DBCM | Dermal basal cell medium |
| DTT | Dithiothreitol |
| EDTA | Ethylene-diamine-tetra-acetic acid |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HAECs | Human aortic endothelial cells |
| HEKas | Human epidermal keratinocytes—adult |
| LDH | Lactate dehydrogenase |
| LF-NAD+ | Liposomal formulation of nicotinamide adenine-dinucleotide |
| NAD+ | Nicotinamide adenine-dinucleotide |
| NADH | Reduced nicotinamide adenine-dinucleotide |
| NAM | Nicotinamide |
| PARPs | Poly (ADP-ribose) polymerases |
| PMSF | Phenylmethylsulfonyl fluoride |
| ROS | Reactive oxygen species |
| SABG | Senescence-associated β-galactosidase |
| SASP | Senescence-associated secretive phenotype |
References
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Bürkle, A.; Diefenbach, J.; Brabeck, C.; Beneke, S. Ageing and PARP. Pharmacol. Res. 2005, 52, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 733696. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial Dysfunction in Cell Senescence and Aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD+ in Regulating Cellular and Metabolic Signaling Pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Gehring, W. Nicotinic Acid/Niacinamide and the Skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef]
- Aleo, M.F.; Giudici, M.L.; Sestini, S.; Danesi, P.; Pompucci, G.; Preti, A. Metabolic Fate of Extracellular NAD in Human Skin Fibroblasts. J. Cell. Biochem. 2001, 80, 360–366. [Google Scholar] [CrossRef]
- Fuchs, E. Epidermal Differentiation: The Bare Essentials. J. Cell Biol. 1990, 111, 2807–2814. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Roustit, M. Human Skin Microcirculation. Compr. Physiol. 2020, 10, 1105–1154. [Google Scholar] [CrossRef]
- Campisi, J. The Role of Cellular Senescence in Skin Aging. J. Investig. Dermatol. Symp. Proc. 1998, 3, 1–5. [Google Scholar] [CrossRef]
- Ghosh, K.; Capell, B.C. The Senescence-Associated Secretory Phenotype: Critical Effector in Skin Cancer and Aging. J. Investig. Dermatol. 2016, 136, 2133–2139. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C.; William, J. Characteristics and Pathomechanisms of Endogenously Aged Skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, Y. The Effect of Aging and Arteriosclerosis on Human Skin Blood Flow. J. Dermatol. Sci. 1993, 5, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Bentov, I.; Reed, M.J. The Effect of Aging on the Cutaneous Microvasculature. Microvasc. Res. 2015, 100, 25–31. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Campagna, R.; Mazzanti, L.; Pompei, V.; Alia, S.; Vignini, A.; Emanuelli, M. The Multifaceted Role of Endothelial Sirt1 in Vascular Aging: An Update. Cells 2024, 13, 1469. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Kang, S.; Park, J.; Cheng, Z.; Ye, S.; Jun, S.-H.; Kang, N.-G. Novel Approach to Skin Anti-Aging: Boosting Pharmacological Effects of Exogenous Nicotinamide Adenine Dinucleotide (NAD+) by Synergistic Inhibition of CD38 Expression. Cells 2024, 13, 1799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Habiballa, L.; Aversa, Z.; Ng, Y.E.; Sakamoto, A.E.; Englund, D.A.; Pearsall, V.M.; White, T.A.; Robinson, M.M.; Rivas, D.A.; et al. Characterization of Cellular Senescence in Aging Skeletal Muscle. Nat. Aging 2022, 2, 601–615. [Google Scholar] [CrossRef]
- Yan, J.; Chen, S.; Yi, Z.; Zhao, R.; Zhu, J.; Ding, S.; Wu, J. The Role of P21 in Cellular Senescence and Aging-Related Diseases. Mol. Cells 2024, 47, 100113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Poi, M.J.; Tsai, M.-D. Regulatory Mechanisms of Tumor Suppressor P16(INK4A) and Their Relevance to Cancer. Biochemistry 2011, 50, 5566–5582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fisher, J.C.; Mathew, R.; Ou, L.; Otieno, S.; Sublet, J.; Xiao, L.; Chen, J.; Roussel, M.F.; Kriwacki, R.W. Intrinsic Disorder Mediates the Diverse Regulatory Functions of the Cdk Inhibitor P21. Nat. Chem. Biol. 2011, 7, 214–221. [Google Scholar] [CrossRef]
- Zaldua, N.; Llavero, F.; Artaso, A.; Gálvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. Rac1/P21-Activated Kinase Pathway Controls Retinoblastoma Protein Phosphorylation and E2F Transcription Factor Activation in B Lymphocytes. FEBS J. 2016, 283, 647–661. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Kanásová, M.; Nesměrák, K. Systematic Review of Liposomes’ Characterization Methods. Monatshefte Für Chem.—Chem. Mon. 2017, 148, 1581–1593. [Google Scholar] [CrossRef]
- Liberale, L.; Akhmedov, A.; Vlachogiannis, N.I.; Bonetti, N.R.; Nageswaran, V.; Miranda, M.X.; Puspitasari, Y.M.; Schwarz, L.; Costantino, S.; Paneni, F.; et al. Sirtuin 5 Promotes Arterial Thrombosis by Blunting the Fibrinolytic System. Cardiovasc. Res. 2021, 117, 2275–2288. [Google Scholar] [CrossRef]
- Law, B.F.; Lin, C.-C.; Hettick, J.M. Human Keratinocyte Response to 4,4’-Methylene Diphenyl Diisocyanate-Glutathione Conjugate Exposure. Xenobiotica 2024, 54, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Szatkowska, R.; Furmanek, E.; Kierzek, A.M.; Ludwig, C.; Adamczyk, M. Mitochondrial Metabolism in the Spotlight: Maintaining Balanced RNAP III Activity Ensures Cellular Homeostasis. Int. J. Mol. Sci. 2023, 24, 14763. [Google Scholar] [CrossRef] [PubMed]
- van der Loo, B.; Fenton, M.J.; Erusalimsky, J.D. Cytochemical Detection of a Senescence-Associated β-Galactosidase in Endothelial and Smooth Muscle Cells from Human and Rabbit Blood Vessels. Exp. Cell Res. 1998, 241, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Peno-Mazzarino, L.; Jeanneton, O.; Scalvino, S.A.; Percoco, G.; Beauchef, G.; Nizard, C.; Pays, K. A New Ex Vivo Human Skin Model for the Topographic and Biological Analysis of Cosmetic Formulas. Int. J. Cosmet. Sci. 2025, 47, 305–320. [Google Scholar] [CrossRef]
- Tfayli, A.; Gobinet, C.; Vrabie, V.; Huez, R.; Manfait, M.; Piot, O. Digital Dewaxing of Raman Signals: Discrimination between Nevi and Melanoma Spectra Obtained from Paraffin-Embedded Skin Biopsies. Appl. Spectrosc. 2009, 63, 564–570. [Google Scholar] [CrossRef]
- Braverman, I.M. The Cutaneous Microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef]
- Tschan, T.; Steffen, H.; Supersaxo, A. Sebaceous-Gland Deposition of Isotretinoin after Topical Application: An in Vitro Study Using Human Facial Skin. Ski. Pharmacol. 1997, 10, 126–134. [Google Scholar] [CrossRef]
- Thiele, B.; Ghyczy, M.; Lunow, C.; Teichert, H.M.; Wolff, H.H. Influence of Phospholipid Liposomes (PLL) on UVB-Induced Erythema Formation. Arch. Dermatol. Res. 1993, 285, 428–431. [Google Scholar] [CrossRef]
- Gehring, W.; Ghyczy, M.; Gloor, M.; Scheer, T.; Röding, J. Enhancement of the Penetration of Dithranol and Increase of Effect of Dithranol on the Skin by Liposomes. Arzneimittelforschung 1992, 42, 983–985. [Google Scholar]
- Pan, X.; Heacock, M.L.; Abdulaziz, E.N.; Violante, S.; Zuckerman, A.L.; Shrestha, N.; Yao, C.; Goodman, R.P.; Cross, J.R.; Cracan, V. A Genetically Encoded Tool to Increase Cellular NADH/NAD+ Ratio in Living Cells. Nat. Chem. Biol. 2024, 20, 594–604. [Google Scholar] [CrossRef]
- Jokinen, M.J.; Luukkonen, P.K. Hepatic Mitochondrial Reductive Stress in the Pathogenesis and Treatment of Steatotic Liver Disease. Trends Pharmacol. Sci. 2024, 45, 319–334. [Google Scholar] [CrossRef]
- Lewińska, A.; Radoń, A.; Gil, K.; Błoniarz, D.; Ciuraszkiewicz, A.; Kubacki, J.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Krogul-Sobczak, A.; et al. Carbon-Coated Iron Oxide Nanoparticles Promote Reductive Stress-Mediated Cytotoxic Autophagy in Drug-Induced Senescent Breast Cancer Cells. ACS Appl. Mater. Interfaces 2024, 16, 15457–15478. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple Functions of P21 in Cell Cycle, Apoptosis and Transcriptional Regulation after DNA Damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Serra, S.; Chetty, R. P16. J. Clin. Pathol. 2018, 71, 853–858. [Google Scholar] [CrossRef]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression That Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, I.; Lain, S. Sirtuins and P53. Adv. Cancer Res. 2009, 102, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic Signalling and the p16INK4a-Rb Pathway Cooperate to Enforce Irreversible Cellular Senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular Senescence and Tumor Suppressor Gene P16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Bauwens, E.; Parée, T.; Meurant, S.; Bouriez, I.; Hannart, C.; Wéra, A.-C.; Khelfi, A.; Fattaccioli, A.; Burteau, S.; Demazy, C.; et al. Senescence Induced by UVB in Keratinocytes Impairs Amino Acids Balance. J. Investig. Dermatol. 2023, 143, 554–565.e9. [Google Scholar] [CrossRef]


| Components | Quantity (%) |
|---|---|
| Pentylene Glycol | 4.95 |
| Lecithin | 4.21 |
| Sodium hydroxide | 0.32 (as 10% solution to adjust pH) |
| Tocopherol | 0.02 |
| Water | 24.10 |
| Glycerin | 66.40 (as a preservative) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ministrini, S.; Liberale, L.; Erle, H.-E.; Percoco, G.; Tfayli, A.; Assi, A.; Kapitonov, I.; Greiner, I.; Camici, G.G. A Liposomal Formulation Enhances the Anti-Senescence Properties of Nicotinamide Adenine-Dinucleotide (NAD+) in Endothelial Cells and Keratinocytes. Curr. Issues Mol. Biol. 2025, 47, 722. https://doi.org/10.3390/cimb47090722
Ministrini S, Liberale L, Erle H-E, Percoco G, Tfayli A, Assi A, Kapitonov I, Greiner I, Camici GG. A Liposomal Formulation Enhances the Anti-Senescence Properties of Nicotinamide Adenine-Dinucleotide (NAD+) in Endothelial Cells and Keratinocytes. Current Issues in Molecular Biology. 2025; 47(9):722. https://doi.org/10.3390/cimb47090722
Chicago/Turabian StyleMinistrini, Stefano, Luca Liberale, Hanns-Eberhard Erle, Giuseppe Percoco, Ali Tfayli, Ali Assi, Ivan Kapitonov, Isabel Greiner, and Giovanni Guido Camici. 2025. "A Liposomal Formulation Enhances the Anti-Senescence Properties of Nicotinamide Adenine-Dinucleotide (NAD+) in Endothelial Cells and Keratinocytes" Current Issues in Molecular Biology 47, no. 9: 722. https://doi.org/10.3390/cimb47090722
APA StyleMinistrini, S., Liberale, L., Erle, H.-E., Percoco, G., Tfayli, A., Assi, A., Kapitonov, I., Greiner, I., & Camici, G. G. (2025). A Liposomal Formulation Enhances the Anti-Senescence Properties of Nicotinamide Adenine-Dinucleotide (NAD+) in Endothelial Cells and Keratinocytes. Current Issues in Molecular Biology, 47(9), 722. https://doi.org/10.3390/cimb47090722







