Evaluation of the Antihyperalgesic Potential of Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata in Alloxan-Induced Diabetic Neuropathy in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts
2.2. Experimental Animals
2.3. Diabetes Mellitus Induction and Applied Treatments
2.4. Blood Glucose Levels
2.5. Experimental Assays for Evaluating Antihyperalgesic Effects
2.5.1. Heat Hypersensitivity
2.5.2. Cold Hypersensitivity
2.5.3. Tactile Hypersensitivity
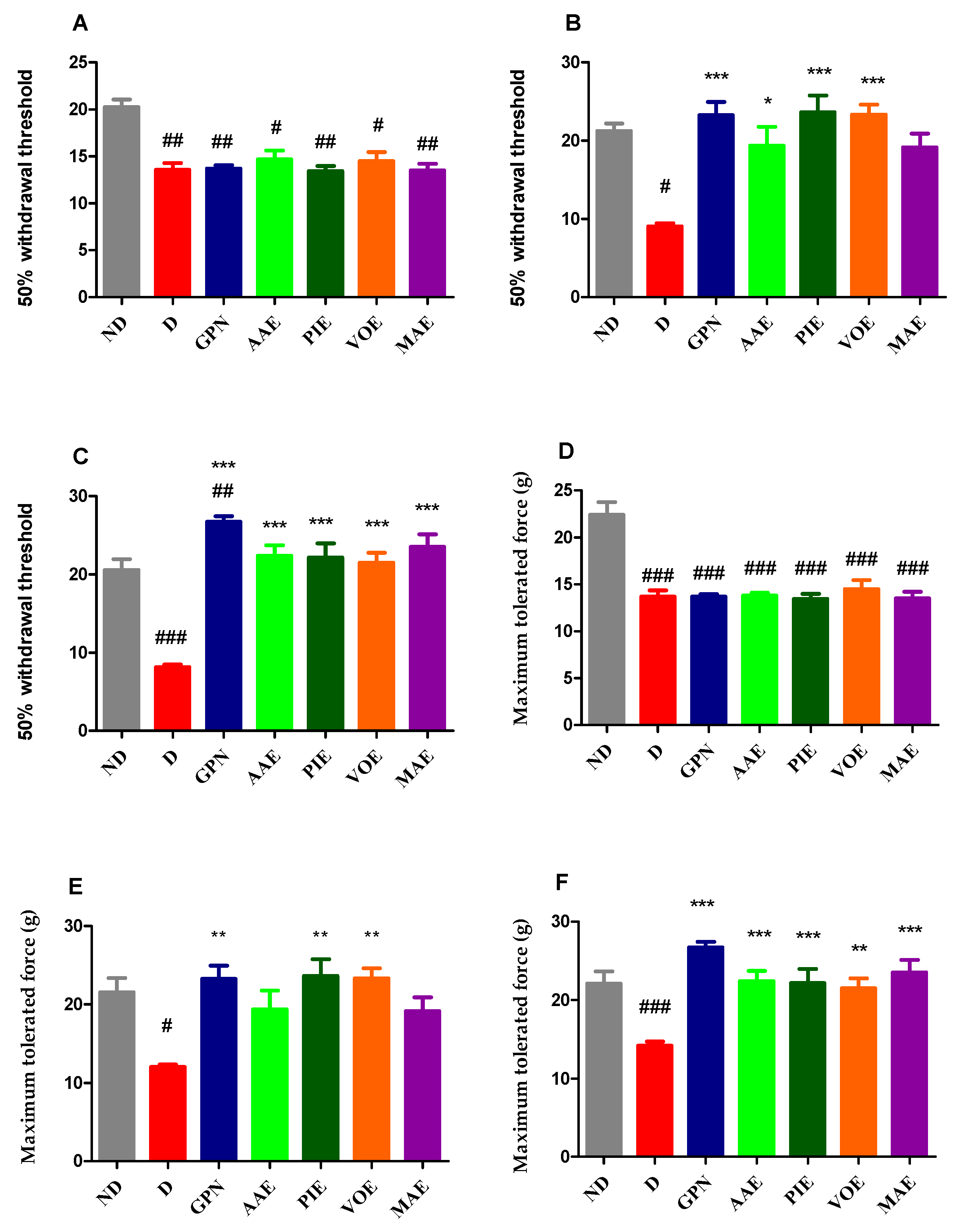
2.5.4. Mechanical Hyperalgesia
2.6. Biochemical Analysis of Brain and Liver Homogenates of Rats
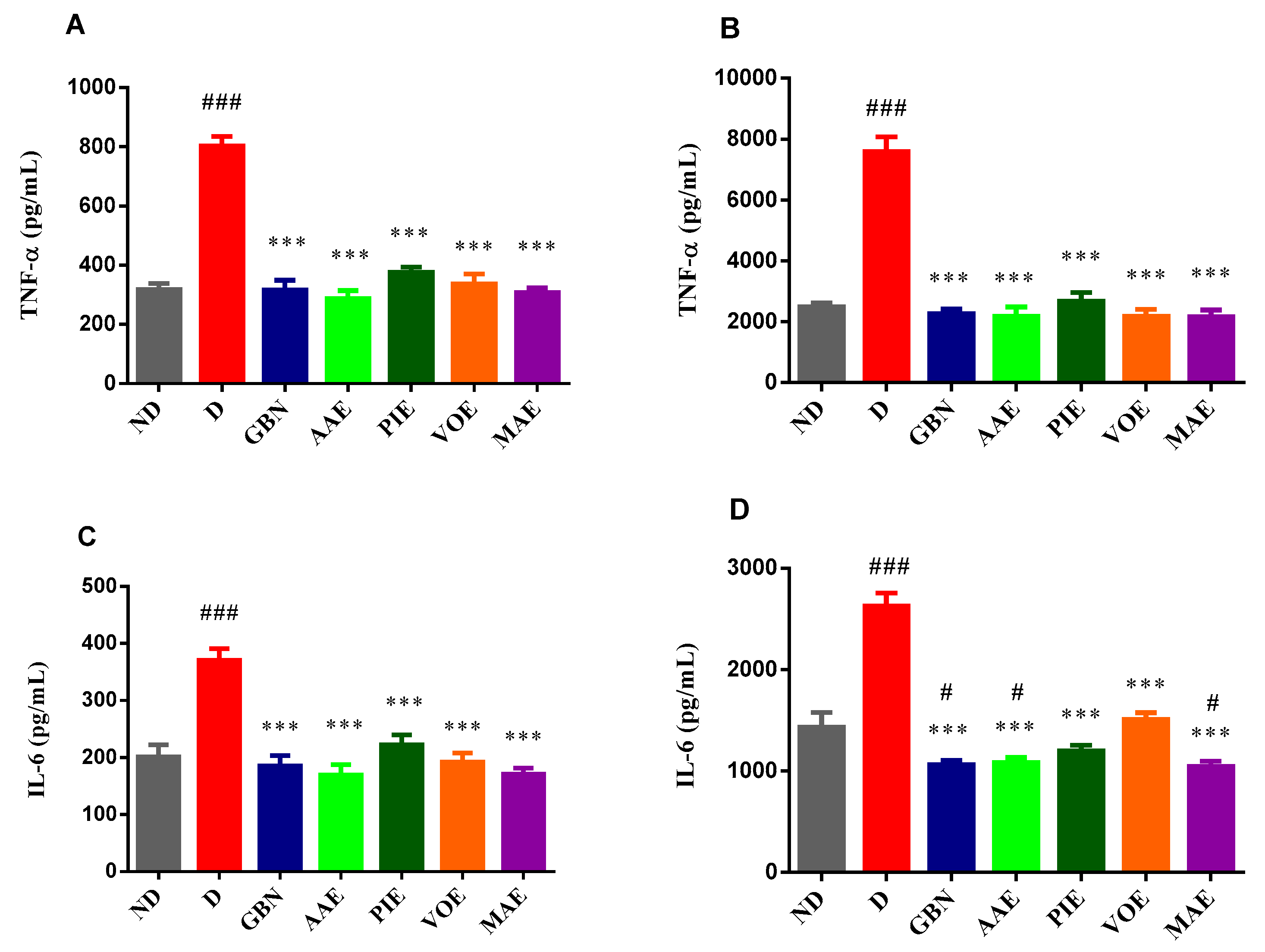
Evaluation of TNF-α (Tumor Necrosis Factor-α and IL-6 (Interleukin-6) Levels
2.7. Statistical Analysis
2.8. Computational Methods
2.8.1. Molecular Target Prediction
2.8.2. Molecular Docking Simulations
3. Results
3.1. Blood Glucose Levels
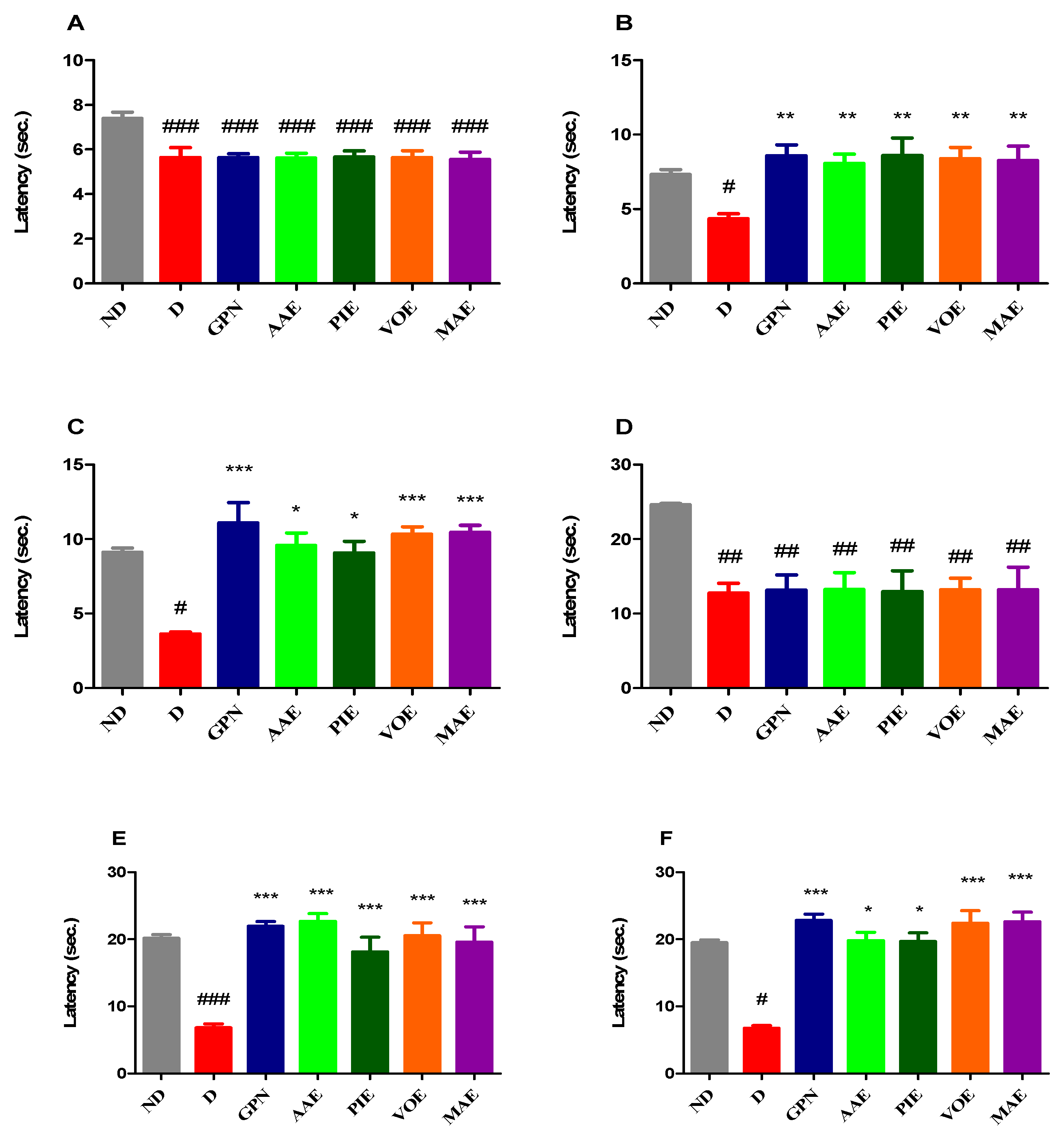
3.2. Experimental Tests for Evaluating Antihyperalgesic Effects
3.2.1. Heat Hypersensitivity
3.2.2. Cold Hypersensitivity
3.2.3. Tactile Hypersensitivity
3.2.4. Mechanical Hyperalgesia
3.3. Biochemical Analysis of Brain and Liver Homogenates
Evaluation of TNF-α and IL-6 Levels
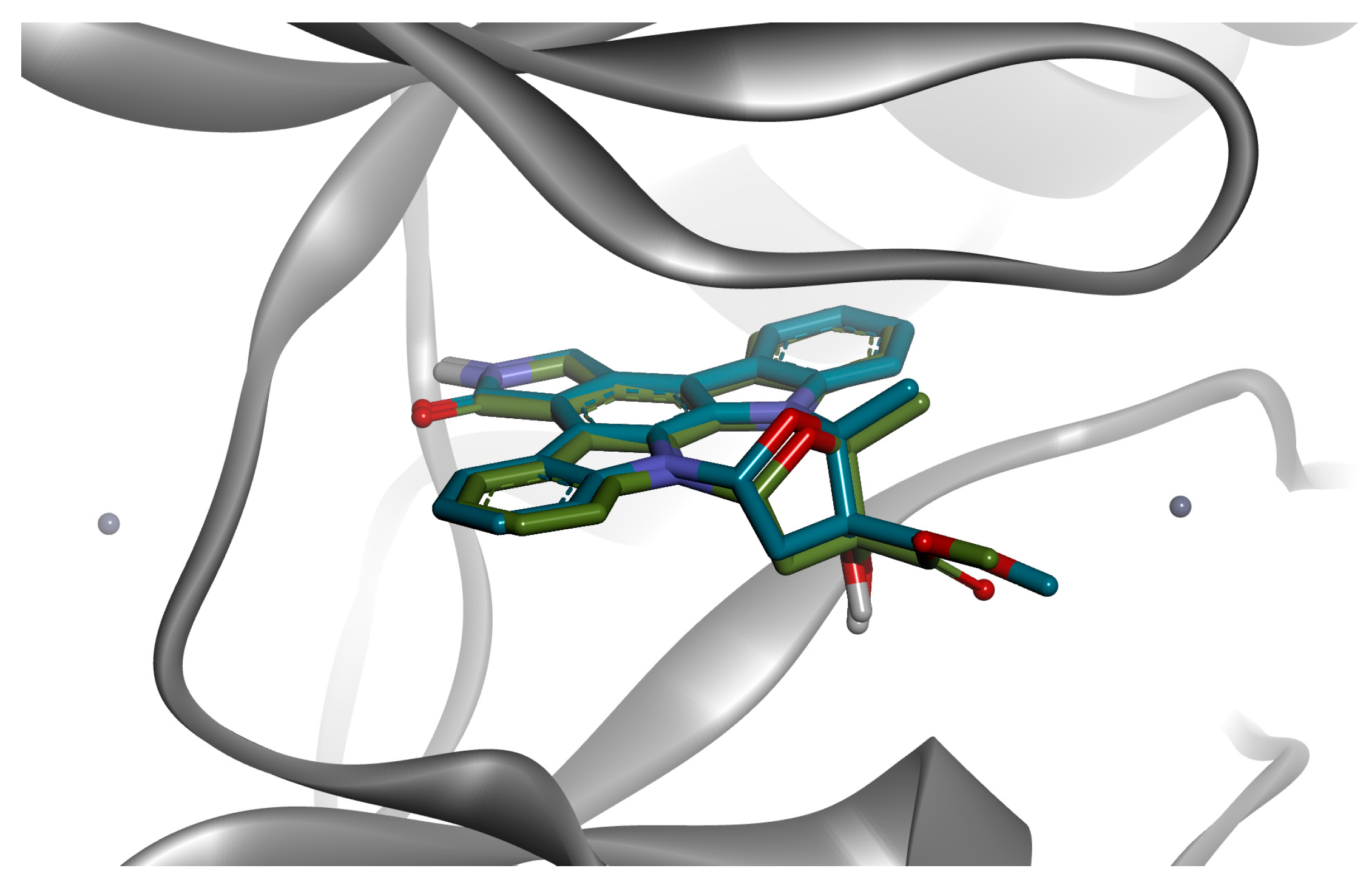
3.4. Computational Studies
3.4.1. Prediction of Neuropathy-Associated Targets
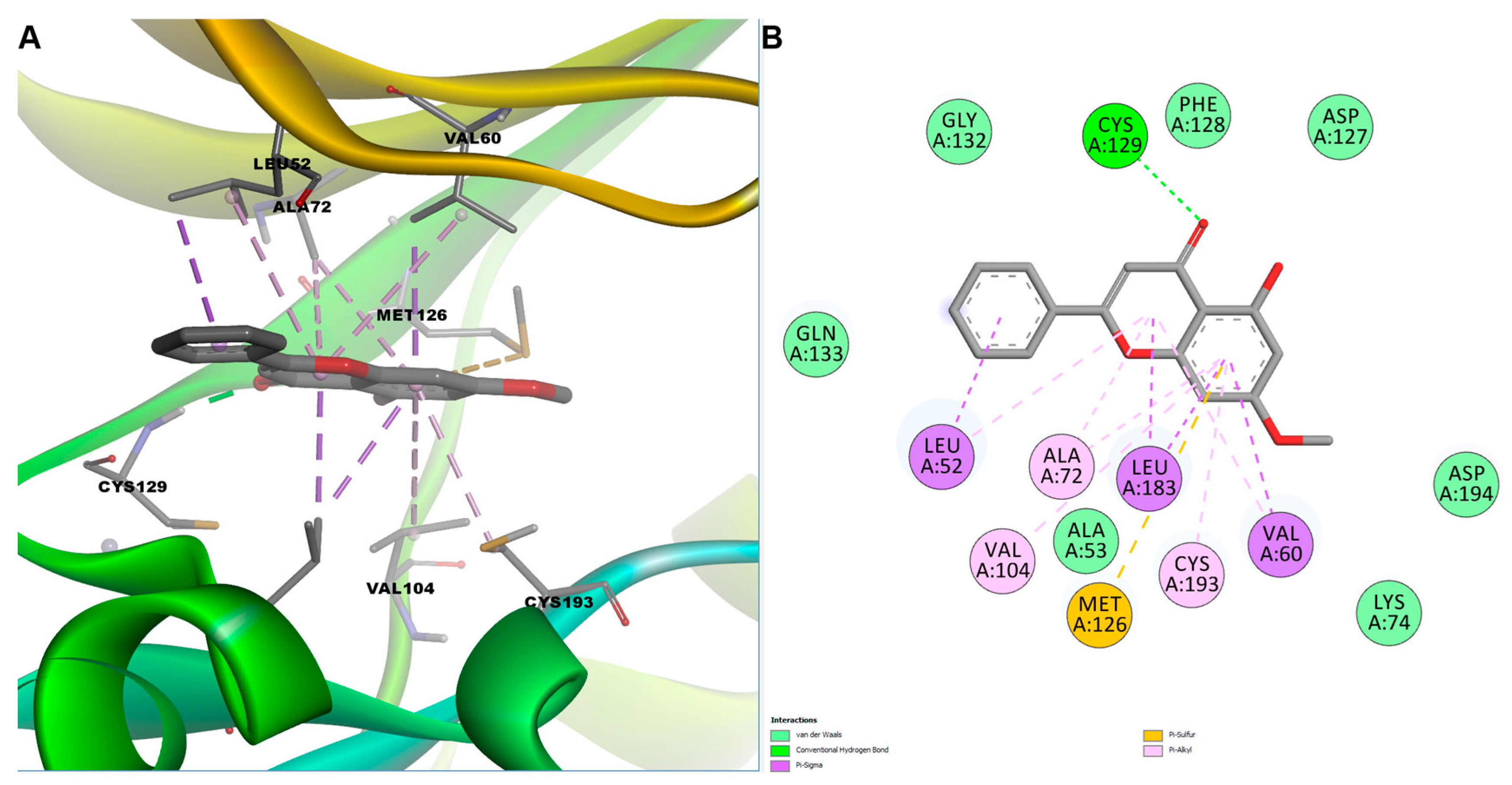
3.4.2. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2016, 49, 106–116. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2016, 40, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.-D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Misra, S.; Swarnkar, P.; Patel, P.; Das Kurmi, B.; Das Gupta, G.; Singh, A. Understanding the role of hyperglycemia and the molecular mechanism associated with diabetic neuropathy and possible therapeutic strategies. Biochem. Pharmacol. 2023, 215, 115723. [Google Scholar] [CrossRef]
- Jensen, T.S.; Karlsson, P.; Gylfadottir, S.S.; Andersen, S.T.; Bennett, D.L.; Tankisi, H.; Finnerup, N.B.; Terkelsen, A.J.; Khan, K.; Themistocleous, A.C.; et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021, 144, 1632–1645. [Google Scholar] [CrossRef]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12, Correction in Pain Med. 2023, 24, 219. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Moisset, X.; Bouhassira, D.; Couturier, J.A.; Alchaar, H.; Conradi, S.; Delmotte, M.; Lanteri-Minet, M.; Lefaucheur, J.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Hennemann-Krause, L.; Sredni, S. Systemic drug therapy for neuropathic pain. Rev. Dor 2016, 17, 91–94. [Google Scholar] [CrossRef]
- Rowbotham, M.C. Tricyclic antidepressants and opioids—Better together? Pain. 2015, 156, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Shinu, P.; Morsy, M.A.; Nair, A.B.; Al Mouslem, A.K.; Venugopala, K.N.; Goyal, M.; Bansal, M.; Jacob, S.; Deb, P.K. Novel Therapies for the Treatment of Neuropathic Pain: Potential and Pitfalls. J. Clin. Med. 2022, 11, 3002. [Google Scholar] [CrossRef] [PubMed]
- Kopsky, D.J.; Hesselink, J.M.K. High Doses of Topical Amitriptyline in Neuropathic Pain: Two Cases and Literature Review. Pain. Pr. 2011, 12, 148–153. [Google Scholar] [CrossRef]
- Maloney, J.; Pew, S.; Wie, C.; Gupta, R.; Freeman, J.; Strand, N. Comprehensive Review of Topical Analgesics for Chronic Pain. Curr. Pain. Headache Rep. 2021, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; de Andrade, D.C.; Adam, F.; Ranoux, D.; Teixeira, M.J.; Galhardoni, R.; Raicher, I.; Üçeyler, N.; Sommer, C.; Bouhassira, D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016, 15, 555–565. [Google Scholar] [CrossRef]
- Ansari, P.; Reberio, A.D.; Ansari, N.J.; Kumar, S.; Khan, J.T.; Chowdhury, S.; El-Mordy, F.M.A.; Hannan, J.M.A.; Flatt, P.R.; Abdel-Wahab, Y.H.A.; et al. Therapeutic Potential of Medicinal Plants and Their Phytoconstituents in Diabetes, Cancer, Infections, Cardiovascular Diseases, Inflammation and Gastrointestinal Disorders. Biomedicines 2025, 13, 454. [Google Scholar] [CrossRef]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Chen, C.; Razali, U.H.M.; Saikim, F.H.; Mahyudin, A.; Noor, N.Q.I.M. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, M.; Tian, X.; Ye, J. Study on capillary electrophoresis–amperometric detection profiles of different parts of Morus alba L. J. Chromatogr. A 2006, 1116, 286–290. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nihei, M.; Nagai, H.; Fukushi, H.; Tabata, K.; Suzuki, T.; Akihisa, T. Albanol A from the Root Bark of Morus alba L. Induces Apoptotic Cell Death in HL60 Human Leukemia Cell Line. Chem. Pharm. Bull. 2010, 58, 568–571. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Bodensieck, A.; Seger, C.; Ellmerer, E.P.; Bauer, R.; Langer, T.; Stuppner, H. Discovering COX-Inhibiting Constituents of Morus Root Bark: Activity-Guided versus Computer-Aided Methods. Planta Medica 2005, 71, 399–405. [Google Scholar] [CrossRef]
- Kim, H.J.; Baburin, I.; Zaugg, J.; Ebrahimi, S.N.; Hering, S.; Hamburger, M. HPLC-based activity profiling–discovery of sanggenons as GABAA receptor modulators in the traditional Chinese drug sang bai pi (Morus alba root bark). Planta Medica 2012, 78, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Bhatti, R. Understanding the phytochemistry and molecular insights to the pharmacology of Angelica archangelica L. (garden angelica) and its bioactive components. Phytotherapy Res. 2021, 35, 5961–5979. [Google Scholar] [CrossRef] [PubMed]
- Wszelaki, N.; Paradowska, K.; Jamróz, M.K.; Granica, S.; Kiss, A.K. Bioactivity-Guided Fractionation for the Butyrylcholinesterase Inhibitory Activity of Furanocoumarins from Angelica archangelica L. Roots and Fruits. J. Agric. Food Chem. 2011, 59, 9186–9193. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Garg, S.; Shiekh, B.A.; Singh, N.; Singh, P.; Bhatti, R. In Silico Studies and In Vivo MAOA Inhibitory Activity of Coumarins Isolated from Angelica archangelica Extract: An Approach toward Antidepressant Activity. ACS Omega 2020, 5, 15069–15076. [Google Scholar] [CrossRef]
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Atas, U.; Erin, N.; Tazegul, G.; Elpek, G.O.; Yıldırım, B. Distribution of transient receptor potential vanilloid-1 channels in gastrointestinal tract of patients with morbid obesity. World J. Clin. Cases 2022, 10, 79–90. [Google Scholar] [CrossRef]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Żydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef]
- Appel, K.; Rose, T.; Fiebich, B.; Kammler, T.; Hoffmann, C.; Weiss, G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytotherapy Res. 2010, 25, 838–843. [Google Scholar] [CrossRef]
- Aman, U.; Subhan, F.; Shahid, M.; Akbar, S.; Ahmad, N.; Ali, G.; Fawad, K.; Sewell, R.D.E. Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioural mechanisms. BMC Complement. Altern. Med. 2016, 16, 77. [Google Scholar] [CrossRef]
- Sanchez, M.; Burgos, E.G.; Iglesias, I.; Gómez-Serranillos, M.P. Updating the biological interest of ’Valeriana officinalis’. Mediterr. Bot. 2021, 42, e70280. [Google Scholar] [CrossRef]
- Felgentreff, F.; Becker, A.; Meier, B.; Brattström, A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine 2012, 19, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Khuda, F.; Iqbal, Z.; Khan, A.; Nasir, F.; Shah, Y. Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pak. J. Pharm. Sci. 2013, 26, 451–454. [Google Scholar] [PubMed]
- Wu, C.-R.; Lee, S.-Y.; Chen, C.-H.; Lin, S.-D. Bioactive compounds of underground valerian extracts and their effect on inhibiting metabolic syndrome-related enzymes activities. Foods 2023, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Marawne, H.; Mohammadhassan, R.; Mohammadalipour, Z.; Ahmadpour, S. Valerian (Valeriana officinalis) extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells. Tradit. Med. Res. 2022, 7, 47. [Google Scholar] [CrossRef]
- Magnaghi, V.; Castelnovo, L.F.; Faroni, A.; Cavalli, E.; Caffino, L.; Colciago, A.; Procacci, P.; Pajardi, G. Nerve Regenerative Effects of GABA-B Ligands in a Model of Neuropathic Pain. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Backonja, M.; Beydoun, A.; Edwards, K.R.; Schwartz, S.L.; Fonseca, V.; Hes, M.; LaMoreaux, L.; Garofalo, E. Gabapentin for the Symptomatic Treatment of Painful Neuropathy in Patients With Diabetes MellitusA Randomized Controlled Trial. JAMA 1998, 280, 1831–1836. [Google Scholar] [CrossRef]
- Zhou, H.; Rao, Z.; Zhang, Z.; Zhou, J. Function of the GABAergic System in Diabetic Encephalopathy. Cell. Mol. Neurobiol. 2022, 43, 605–619. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Liu, Y.; Li, J.; He, Q.; Zhang, C.; Wei, B.; Zhang, Y.; Wang, J. Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci. Rep. 2016, 6, 18933. [Google Scholar] [CrossRef]
- Elsas, S.-M.; Rossi, D.; Raber, J.; White, G.; Seeley, C.-A.; Gregory, W.; Mohr, C.; Pfankuch, T.; Soumyanath, A. Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method. Phytomedicine 2010, 17, 940–949. [Google Scholar] [CrossRef]
- Zaugg, J.; Eickmeier, E.; Rueda, D.C.; Hering, S.; Hamburger, M. HPLC-based activity profiling of Angelica pubescens roots for new positive GABAA receptor modulators in Xenopus oocytes. Fitoterapia 2011, 82, 434–440. [Google Scholar] [CrossRef]
- Yuan, C.-S.; Mehendale, S.; Xiao, Y.; Aung, H.H.; Xie, J.-T.; Ang-Lee, M.K. The Gamma-Aminobutyric Acidergic Effects of Valerian and Valerenic Acid on Rat Brainstem Neuronal Activity. Anesthesia Analg. 2004, 98, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Hovaneţ, M.; Viorel Ancuceanu, R.; Dinu, M.; Oprea, E.; Adriana Budura, E.; Negreş, S.; Ştefan Velescu, B.; Elena Duţu, L.; Adriana Anghel, I.; Ancu, I.; et al. Toxicity and anti-inflammatory activity of ziziphus jujuba mill. leaves. Farmacia 2016, 64, 802–808. [Google Scholar]
- Negreş, S.; Chiriţă, C.; Moroşan, E.; Arsene, A.L. Experimental pharmacological model of diabetes induction with aloxan in rat. Farmacia 2013, 61, 313–323. [Google Scholar]
- Sultana, T.; Hossain, M.; Chowdhury, S. Evaluation of Analgesic and Neuropharmacological Activity of the Bark of Morus Alba, L. (Family: Moraceae). Jordan J. Pharm. Sci. 2020, 13, 11–18. [Google Scholar]
- Shahidi, S.; Bathaei, A.; Pahlevani, P. Antinociceptive Effects of Valeriana Extract in Mice: Involvement of the Dopaminergic and Serotonergic Systems. Neurophysiology 2013, 45, 448–452. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Bhatti, M.S.; Bhatti, R. Optimization of extraction conditions of Angelica archangelica extract and activity evaluation in experimental fibromyalgia. J. Food Sci. 2020, 85, 3700–3710. [Google Scholar] [CrossRef]
- Pușcașu, C.; Ungurianu, A.; Șeremet, O.C.; Andrei, C.; Mihai, D.P.; Negreș, S. The Influence of Sildenafil–Metformin Combination on Hyperalgesia and Biochemical Markers in Diabetic Neuropathy in Mice. Medicina 2023, 59, 1375. [Google Scholar] [CrossRef]
- Back, S.K.; Won, S.Y.; Kil Hong, S.; Na, H.S. Gabapentin relieves mechanical, warm and cold allodynia in a rat model of peripheral neuropathy. Neurosci. Lett. 2004, 368, 341–344. [Google Scholar] [CrossRef]
- Pușcașu, C.; Negreș, S.; Zbârcea, C.E.; Ungurianu, A.; Ștefănescu, E.; Blebea, N.M.; Chiriță, C. Evaluating the Antihyperalgesic Potential of Sildenafil–Metformin Combination and Its Impact on Biochemical Markers in Alloxan-Induced Diabetic Neuropathy in Rats. Pharmaceuticals 2024, 17, 783. [Google Scholar] [CrossRef]
- Pușcașu, C.; Zanfirescu, A.; Negreș, S. Recent Progress in Gels for Neuropathic Pain. Gels 2023, 9, 417. [Google Scholar] [CrossRef]
- Blebea, N.M.; Mihai, D.P.; Andrei, C.; Stoica, D.M.; Chiriță, C.; Negreş, S. Evaluation of therapeutic potential of cannabidiol-based products in animal models of epileptic seizures, neuropathic pain and chronic inflammation. Farmacia 2022, 70, 1185–1193. [Google Scholar] [CrossRef]
- Dixon, W.J. The Up-and-Down Method for Small Samples. J. Am. Stat. Assoc. 1965, 60, 967–978. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Andrei, C.; Mihai, D.P.; Nitulescu, G.; Ungurianu, A.; Margina, D.M.; Nitulescu, G.M.; Olaru, O.T.; Busca, R.M.; Zanfirescu, A. Cetirizine and Levetiracetam as Inhibitors of Monoacylglycerol Lipase: Investigating Their Repurposing Potential as Novel Osteoarthritic Pain Therapies. Pharmaceuticals 2023, 16, 1563. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2021, 23, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Suciu, F.; Mihai, D.P.; Ungurianu, A.; Andrei, C.; Pușcașu, C.; Chițescu, C.L.; Ancuceanu, R.V.; Gird, C.E.; Stefanescu, E.; Blebea, N.M.; et al. Investigation of Anticonvulsant Potential of Morus Alba, Angelica Archangelica, Valeriana Officinalis, and Passiflora Incarnata Extracts: In Vivo and In Silico Studies. Int. J. Mol. Sci. 2025, 26, 6426. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, F.J.; Szklarz, M.; Azeez, K.R.A.; Elkins, J.M.; Knapp, S. Family-wide Structural Analysis of Human Numb-Associated Protein Kinases. Structure 2016, 24, 401–411. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations; Springer: Berlin, Germany, 2018; pp. 43–67. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Mir, M.S.; Darzi, M.M.; Khan, H.M.; Kamil, S.A.; Sofi, A.H.; Wani, S.A. Pathomorphological effects of Alloxan induced acute hypoglycaemia in rabbits. Alex. J. Med. 2013, 49, 343–353. [Google Scholar] [CrossRef][Green Version]
- Lin, J.; Chen, S.; Butt, U.D.; Yan, M.; Wu, B. A comprehensive review on ziconotide. Heliyon 2024, 10, e31105. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Zhang, J.; Chen, H.; Pan, H.-L. Streptozotocin-Induced Diabetic Neuropathic Pain Is Associated with Potentiated Calcium-Permeable AMPA Receptor Activity in the Spinal Cord. J. Pharmacol. Exp. Ther. 2019, 371, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, B.; Wang, Y.; Lan, H.; Liu, X.; Hu, Y.; Cao, P. Diabetic neuropathy: Cutting-edge research and future directions. Signal Transduct. Target. Ther. 2025, 10, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Gomez, K.; Chen, Y.; Allen, H.N.; Hestehave, S.; Rodríguez-Palma, E.J.; Loya-Lopez, S.; Calderon-Rivera, A.; Duran, P.; Nelson, T.S.; et al. C2230, a preferential use- and state-dependent CaV2.2 channel blocker, mitigates pain behaviors across multiple pain models. J. Clin. Investig. 2024, 135, e177429. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Sun, W.-T.; Lei, C.-L.; Bi, C.-C.; Chen, Z.-L.; Zhang, L. Effect of alloxan time administerDrug on establishing diabetic rabbit model. Int. J. Ophthalmol. 2010, 3, 200–202. [Google Scholar] [CrossRef]
- Yu, L.-N.; Yang, X.-S.; Hua, Z.; Xie, W. Serum levels of pro-inflammatory cytokines in diabetic patients with peripheral neuropathic pain and the correlation among them. Zhonghua yi xue za zhi 2009, 89, 469–471. [Google Scholar]
- Park, J.M.; Bong, H.Y.; Jeong, H.I.; Kim, Y.K.; Kim, J.Y.; Kwon, O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr. Res. Pr. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, J.S.; Park, S.-K.; Lee, K.; Kim, J.Y.; Kim, Y.J.; Hong, J.T.; Kim, Y.; Han, S.-B. Antidiabetic activity of angelan isolated from Angelica gigas Nakai. Arch. Pharmacal Res. 2008, 31, 1489–1496. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, D.; Chaudhary, A.K.; Maithani, M.; Singh, R. Antidiabetic activity of Passiflora incarnata Linn. in streptozotocin-induced diabetes in mice. J. Ethnopharmacol. 2012, 139, 801–806. [Google Scholar] [CrossRef]
- Harada, K.; Kato, Y.; Takahashi, J.; Imamura, H.; Nakamura, N.; Nishina, A.; Phay, N.; Tadaishi, M.; Shimizu, M.; Kobayashi-Hattori, K. The Effect of Methanolic Valeriana officinalis Root Extract on Adipocyte Differentiation and Adiponectin Production in 3T3-L1 Adipocytes. Plant Foods Hum. Nutr. 2020, 75, 103–109. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in vivo and in vitro. Front. Neurosci. 2021, 15, 636172. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Melhim, S.B.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Song-Tao, M.; Dong-Lian, L.; Jing-Jing, D.; Yan-Juan, P. Protective effect of mulberry flavonoids on sciatic nerve in alloxan-induced diabetic rats. Braz. J. Pharm. Sci. 2014, 50, 765–771. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S. Bioactive Compounds and Their Neuroprotective Effects in Diabetic Complications. Nutrients 2016, 8, 472. [Google Scholar] [CrossRef]
- Bharti, A.; Kaur, J.; Kumar, A.; Singh, S.; Kumar, D. Efficacy of p-coumaric acid in chronic constriction injury induced neuropathic pain in rats. Indian. Drugs 2021, 58, 52–58. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.; Li, X.; Shi, H.; Zheng, Y.; Chang, S.; Gao, L.; Zhao, G. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, D.; Yu, X.; Xie, X.; Wang, L.; Lian, L.; Fei, N.; Chen, J.; Zhu, N.; Wang, G.; et al. The antinociceptive effects of ferulic acid on neuropathic pain: Involvement of descending monoaminergic system and opioid receptors. Oncotarget 2016, 7, 20455–20468. [Google Scholar] [CrossRef]
- Pușcașu, C.; Mihai, P.; Zbârcea, C.E.; Zanfirescu, A.; Chiriță, C.; Viorica Ghiță, C.I.; Negreş, S. Investigation of antihyperalgesic effects of different doses of sildenafil and metformin in alloxan-induced diabetic neuropathy in mice. Farmacia 2023, 71, 3. [Google Scholar] [CrossRef]
- Vashistha, B.; Sharma, A.; Jain, V. Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: An evidence of behavioral and biochemical examination. Nutr. Neurosci. 2016, 20, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2013, 63, 81–90. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Q.; Liang, X.; Xie, J.; Sun, Q. Quercetin reduces inflammation in a rat model of diabetic peripheral neuropathy by regulating the TLR4/MyD88/NF-κB signalling pathway. Eur. J. Pharmacol. 2021, 912, 174607. [Google Scholar] [CrossRef]
- Li, Y.; Chi, G.; Shen, B.; Tian, Y.; Feng, H. Isorhamnetin ameliorates LPS-induced inflammatory response through downregulation of NF-κB signaling. Inflammation 2016, 39, 1291–1301. [Google Scholar] [CrossRef]
- Kostich, W.; Hamman, B.D.; Li, Y.-W.; Naidu, S.; Dandapani, K.; Feng, J.; Easton, A.; Bourin, C.; Baker, K.; Allen, J.; et al. Inhibition of AAK1 Kinase as a Novel Therapeutic Approach to Treat Neuropathic Pain. J. Pharmacol. Exp. Ther. 2016, 358, 371–386. [Google Scholar] [CrossRef]
- Xin, X.; Wang, Y.; Zhang, L.; Zhang, D.; Sha, L.; Zhu, Z.; Huang, X.; Mao, W.; Zhang, J. Development and therapeutic potential of adaptor-associated kinase 1 inhibitors in human multifaceted diseases. Eur. J. Med. Chem. 2023, 248, 115102. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, N.E.; Bouziane, O.; Bouhrim, M.; Bnouham, M. Natural aldose reductase inhibitors for treatment and prevention of diabetic cataract: A review. Herba Pol. 2022, 68, 35–58. [Google Scholar] [CrossRef]
- Kim, S.B.; Hwang, S.H.; Wang, Z.; Yu, J.M.; Lim, S.S. Rapid Identification and Isolation of Inhibitors of Rat Lens Aldose Reductase and Antioxidant in Maackia amurensis. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Khan, J.T.; Chowdhury, S.; Reberio, A.D.; Kumar, S.; Seidel, V.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Plant-Based Diets and Phytochemicals in the Management of Diabetes Mellitus and Prevention of Its Complications: A Review. Nutrients 2024, 16, 3709. [Google Scholar] [CrossRef] [PubMed]
- Balestri, F.; Poli, G.; Piazza, L.; Cappiello, M.; Moschini, R.; Signore, G.; Tuccinardi, T.; Mura, U.; Del Corso, A. Dissecting the Activity of Catechins as Incomplete Aldose Reductase Differential Inhibitors through Kinetic and Computational Approaches. Biology 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Roohi, T.F.; Mehdi, S.; Aarfi, S.; Krishna, K.L.; Pathak, S.; Suhail, S.M.; Faizan, S. Biomarkers and signaling pathways of diabetic nephropathy and peripheral neuropathy: Possible therapeutic intervention of rutin and quercetin. Diabetol. Int. 2023, 15, 145–169. [Google Scholar] [CrossRef]
- Hou, D.-X.; Kumamoto, T. Flavonoids as Protein Kinase Inhibitors for Cancer Chemoprevention: Direct Binding and Molecular Modeling. Antioxidants Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Hassan, H.M.; Alhadrami, A.H.; Rateb, M.E. Molecular insights and inhibitory dynamics of flavonoids in targeting Pim-1 kinase for cancer therapy. Front. Pharmacol. 2024, 15, 1440958. [Google Scholar] [CrossRef]


| Compound | Target Names | Probabilities | Model Accuracies |
|---|---|---|---|
| Abscisic acid | CaV2.2 | 0.5029 | 0.9714 |
| Apigenin | GluA2; TP; AAK1; CaV2.2; CatS | 0.7366; 0.5245; 0.7149; 0.5516; 0.5376 | 0.8692; 0.9262; 0.831; 0.9714; 0.9560 |
| Caffeic acid | CCR2; AAK1 | 0.5212; 0.5088 | 0.9857; 0.8310 |
| Catechin | AAK1; CaV2.2 | 0.6417; 0.6223 | 0.831; 0.9714 |
| Chlorogenic acid | GluA2; M1R; CaV2.2; CCR2; M1R | 0.5414; 0.8814; 0.584; 0.5515; 0.8814 | 0.8692; 0.9423; 0.9714; 0.9856; 0.9423 |
| Chrysin | TP; CaV2.2; M1R; CatS; M1R | 0.5181; 0.6227; 0.5997; 0.5864; 0.5997 | 0.9262; 0.9714; 0.9423; 0.956; 0.9423 |
| Cinnamic acid | M1R; M1R | 0.6955; 0.6955 | 0.9423; 0.9423 |
| Daidzein | GluA2; AAK1; CatS; CaV2.2; M1R; M1R | 0.7268; 0.6016; 0.5966; 0.5599; 0.5094 | 0.8692; 0.8310; 0.956; 0.9714; 0.9423 |
| Epicatechin gallate | TRPA1; FAAH; CaV2.2; M1R | 0.5279; 0.5352; 0.5608; 0.6111 | 0.9217; 0.9753; 0.9714; 0.9423 |
| Ferulic acid | GluA2; CaV2.2 | 0.5380; 0.5874 | 0.8692; 0.9714 |
| Formononetin | GluA2; CaV2.2 | 0.8539; 0.7292 | 0.8692; 0.9714 |
| Galangin | TP; M1R; AAK1; CaV2.2; CatS; M1R | 0.565; 0.649; 0.6297; 0.5833; 0.5129; 0.649 | 0.9262; 0.9423; 0.831; 0.9714; 0.956; 0.9423 |
| Gallic acid | M1R | 0.5275 | 0.9423; 0.9423 |
| Genistein | GluA2; TP; AAK1; CaV2.2; CatS | 0.7874; 0.5564; 0.6801; 0.5701; 0.5596 | 0.8692; 0.9262; 0.831; 0.9714; 0.9560 |
| Genistin | GluA2; TP; A1R; CaV2.2; FAAH; M1R | 0.9175; 0.5174; 0.7768; 0.6142; 0.5615; 0.5261 | 0.8692; 0.9262; 0.9593; 0.9714; 0.9753; 0.9423 |
| Glycitein | GluA2; CaV2.2; MC4R | 0.7938; 0.7667; 0.513 | 0.8692; 0.9714; 0.9538 |
| Hesperetin | GluA2; CaV2.2; AAK1; MC4R | 0.7343; 0.8026; 0.5834; 0.5328 | 0.8692; 0.9714; 0.831; 0.9538 |
| Hyperoside | GluA2; M1R; CaV2.2; A1R; M1R | 0.8577; 0.6167; 0.5644; 0.5231; 0.6167 | 0.8692; 0.9423; 0.9714; 0.9593; 0.9423 |
| Isorhamnetin | GluA2; AR; CaV2.2; AAK1 | 0.7553; 0.6451; 0.731; 0.6805 | 0.8692; 0.9238; 0.9714; 0.8310 |
| Kaempferol | GluA2; TP; AR; AAK1; CaV2.2 | 0.6827; 0.5713; 0.5024; 0.8143; 0.5106 | 0.8692; 0.9262; 0.9238; 0.831; 0.9714 |
| Naringenin | GluA2; AAK1; CaV2.2; CatS | 0.6438; 0.738; 0.6124; 0.5395 | 0.8692; 0.831; 0.9714; 0.956 |
| p-Coumaric acid | TP; M1R; CatS; AAK1; M1R | 0.5049; 0.5597; 0.5493; 0.5370; 0.5597 | 0.9262; 0.9423; 0.956; 0.831; 0.9423 |
| Pinocembrin | CaV2.2; M1R; AAK1; M1R | 0.6498; 0.5695; 0.5141; 0.5695 | 0.9714; 0.9423; 0.831; 0.9423 |
| Pinostrobin | CaV2.2; M1R | 0.7959; 0.5779 | 0.9714; 0.9423 |
| Quercetin | GluA2; TP; AAK1 | 0.6568; 0.5404; 0.8041 | 0.8692; 0.9262; 0.831 |
| Rutin | GluA2; AR; A1R; CaV2.2 | 0.7631; 0.6648; 0.6359; 0.5362 | 0.8692; 0.9238; 0.9593; 0.9714 |
| Syringic acid | CaV2.2 | 0.5062 | 0.9714 |
| Bioactive Phytochemicals | ΔG (kcal/mol) | LE |
|---|---|---|
| Hyperoside | –9.814 | 0.2974 |
| Naringenin | –9.113 | 0.4556 |
| Isorhamnetin | –8.955 | 0.3893 |
| Pinostrobin | –8.946 | 0.4473 |
| Quercetin | –8.945 | 0.4066 |
| Apigenin | –8.876 | 0.4438 |
| Kaempferol | –8.876 | 0.4227 |
| Pinocembrin | –8.760 | 0.4611 |
| Formononetin | –8.742 | 0.4163 |
| Chrysin | –8.737 | 0.4598 |
| Catechin | –8.727 | 0.4156 |
| Genistin | –8.712 | 0.2810 |
| Galangin | –8.700 | 0.4350 |
| Glycitein | –8.549 | 0.4071 |
| Hesperetin | –8.525 | 0.4060 |
| Daidzein | –8.509 | 0.4478 |
| Genistein | –8.496 | 0.4248 |
| Rutin | –8.029 | 0.1867 |
| Chlorogenic acid | –7.705 | 0.3082 |
| Epicatechin gallate | –7.182 | 0.2244 |
| Abscisic acid | –6.698 | 0.3525 |
| Caffeic acid | –6.581 | 0.5062 |
| Ferulic acid | –6.546 | 0.4676 |
| p-Coumaric acid | –6.329 | 0.5274 |
| Cinnamic acid | –6.327 | 0.5752 |
| Syringic acid | –5.625 | 0.4018 |
| Gallic acid | –5.537 | 0.4614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, F.; Pușcașu, C.; Mihai, D.P.; Ungurianu, A.; Andrei, C.; Ancuceanu, R.V.; Gîrd, C.E.; Ciobanu, A.-M.; Blebea, N.M.; Popovici, V.; et al. Evaluation of the Antihyperalgesic Potential of Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata in Alloxan-Induced Diabetic Neuropathy in Rats. Curr. Issues Mol. Biol. 2025, 47, 719. https://doi.org/10.3390/cimb47090719
Suciu F, Pușcașu C, Mihai DP, Ungurianu A, Andrei C, Ancuceanu RV, Gîrd CE, Ciobanu A-M, Blebea NM, Popovici V, et al. Evaluation of the Antihyperalgesic Potential of Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata in Alloxan-Induced Diabetic Neuropathy in Rats. Current Issues in Molecular Biology. 2025; 47(9):719. https://doi.org/10.3390/cimb47090719
Chicago/Turabian StyleSuciu, Felicia, Ciprian Pușcașu, Dragos Paul Mihai, Anca Ungurianu, Corina Andrei, Robert Viorel Ancuceanu, Cerasela Elena Gîrd, Anne-Marie Ciobanu, Nicoleta Mirela Blebea, Violeta Popovici, and et al. 2025. "Evaluation of the Antihyperalgesic Potential of Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata in Alloxan-Induced Diabetic Neuropathy in Rats" Current Issues in Molecular Biology 47, no. 9: 719. https://doi.org/10.3390/cimb47090719
APA StyleSuciu, F., Pușcașu, C., Mihai, D. P., Ungurianu, A., Andrei, C., Ancuceanu, R. V., Gîrd, C. E., Ciobanu, A.-M., Blebea, N. M., Popovici, V., Ghiță, C. I. V., & Negres, S. (2025). Evaluation of the Antihyperalgesic Potential of Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata in Alloxan-Induced Diabetic Neuropathy in Rats. Current Issues in Molecular Biology, 47(9), 719. https://doi.org/10.3390/cimb47090719











